Abstract
Objectives
To assess whether high- compared with low-dose corticosteroids started upon hospitalization reduce mortality in patients with severe COVID-19 pneumonia or in subgroups stratified by severity of respiratory impairment on admission.
Methods
We conducted a retrospective cohort study of patients with confirmed SARS-CoV-2 infection who required oxygen supplementation upon hospitalization between March 1 and December 31, 2020. In-hospital death was analyzed using logistic regression with inverse probability of treatment weighting of receiving low- or high-dose corticosteroid (dexamethasone 6-10 mg daily or >10-20 mg daily or other corticosteroid equivalents).
Results
We analyzed 13,366 patients who received low-dose and 948 who received high-dose corticosteroids, of whom 31.3% and 40.4% had severe respiratory impairment (>15 l/min of oxygen or mechanical ventilation) upon admission, respectively. There were no differences in the propensity score-adjusted odds of death (odds ratio 1.17, 95% CI 0.72-1.90) or infections (odds ratio 0.70, 95% CI 0.44-1.11) for patients who received high-dose compared with low-dose corticosteroids, beginning on the day of admission. No significant differences in subgroups stratified by severity of respiratory impairment were found.
Conclusion
Initiating high-dose compared with low-dose corticosteroids among newly hospitalized patients with COVID-19 pneumonia did not improve survival. However, benefit of high-dose corticosteroids in specific subgroups cannot be excluded.
Keywords: Corticosteroids, Anakinra, COVID-19, Mortality, Infections
Introduction
The optimal dose of corticosteroids in the treatment of SARS-CoV-2 infection is uncertain, particularly among patients with severe respiratory impairment (The WHO Rapid Evidence Appraisal for COVID-19 Therapies [REACT] Working Group, 2020), and practice remains varied. Recent randomized controlled trials (RCTs) that compared high (12 mg) to low (6 mg) doses of dexamethasone in patients hospitalized for severe COVID-19 pneumonia found no difference in mortality (Bouadma et al., 2022) or were inconclusive (COVID STEROID 2 Trial Group et al., 2021). The inconclusive study reported a statistically nonsignificant lower 28-day mortality in the high- compared with the low-dose groups (27.1% vs 32.3%, adjusted relative risk 0.86 [99% confidence interval (CI) 0.68-1.08]), but a high likelihood of benefit in the preplanned Bayesian analyses across multiple endpoints (Granholm et al., 2022).
Before this study, a relatively small, open-label RCT of high-dose dexamethasone (mean daily dose 15 mg) in patients with COVID-19 with moderate or severe acute respiratory distress syndrome (ARDS) (Tomazini et al., 2020) reported a statistically significant increased number of days alive and free from invasive mechanical ventilation (IMV) over 28 days and a statistically nonsignificant lower 28-day mortality compared with standard care (56.3% and 61.5%, respectively). Subsequently a large, open-label RCT of low-dose corticosteroids (6 mg daily) compared with usual care showed lower 28-day mortality in hospitalized patients requiring oxygen (23.3% vs 26.2%), even more so in those requiring IMV (29.3% vs 41.4%) but favored usual care in those who did not require respiratory support (17.8% vs 14.0%) (The RECOVERY Collaborative Group, 2021).
The two real-world studies comparing the effectiveness and safety of corticosteroid dose groups in relatively small samples of patients (n = 1379 and 573) (Kumar et al., 2022; Monreal et al., 2021), both reported significantly increased mortality in patients treated with high- compared with low-dose corticosteroids. But methodological limitations that increase the potential for unmeasured confounding make these findings difficult to interpret, such as excluding patients who died or were discharged within 7 days after admission (Kumar et al., 2022) and defining the dose group by last-used rather than first-started (Monreal et al., 2021). In addition, neither study reported results stratified by the degree of respiratory impairment.
The purpose of this study was to compare the effectiveness and safety of high- and low-dose corticosteroids upon admission for COVID-19 pneumonia, stratified by the severity of respiratory impairment. We hypothesized that high-dose corticosteroids would be associated with lower in-hospital mortality only in the sickest patients and may increase the risk of infections in patients with mild or moderate respiratory impairment. Using granular electronic health record data, we performed a retrospective, observational study to generate real-world evidence to address these clinically important questions.
Study design and methods
We performed this large, population-based, retrospective cohort study after receiving approval from the Kaiser Permanente Southern California (KPSC) Institutional Review Board (#12396).
Study population
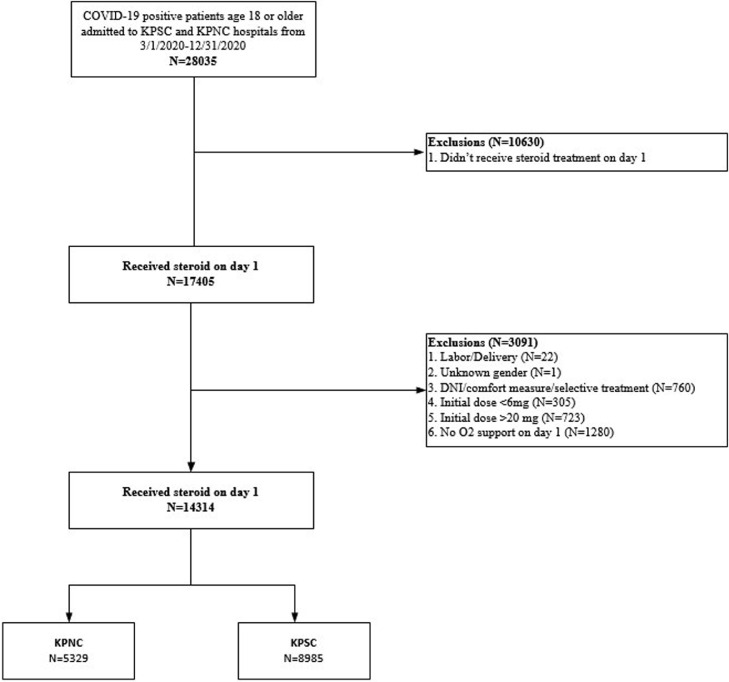
KPSC and Kaiser Permanente Northern California (KPNC) provide care for >9 million members and operate 36 medical centers across California. To assemble the study cohorts, we identified adults (aged ≥18 years) admitted between March 1, 2020 and December 31, 2020, with a positive SARS-CoV-2 polymerase chain reaction result in the 3 weeks before admission or during the hospitalization who required oxygen supplementation (a proxy for COVID-19 pneumonia) within 24 hours of admission. We excluded patients who were admitted for labor and delivery; did not receive corticosteroid treatment or oxygen supplementation within 24 hours of admission; received corticosteroid doses less than 6 mg or greater than 20 mg of dexamethasone or equivalent, and patients with “do not intubate” orders, comfort care status, or unknown sex (Figure 1 ).
Figure 1.
Consort diagram of cohort derivation.
Abbreviations: AC, anticoagulation; DNI, do not intubate; KPSC, Kaiser Permanente Southern California; KPNC, Kaiser Permanente Northern California.
Data collection
We retrieved data from the KPSC and the KPNC electronic medical records on exposures, outcomes, and covariates of interest, the latter of which included patient demographic characteristics, comorbidity burden as reflected by the Elixhauser comorbidity score, corticosteroid use in the 12 months before admission, body mass index (BMI), inpatient, outpatient and emergency department use in the year before admission, other inpatient treatments (including anakinra, tocilizumab, convalescent plasma, and hemodialysis), and selected inflammatory markers, including d-dimer and c-reactive protein (CRP) values. We also calculated values for the Epic Deterioration Index (DI), a score based on inpatient vital signs and laboratory values that has been shown to predict hospital mortality in hospitalized patients with and without COVID-19 (Singh et al., 2021).
We categorized patients according to their respiratory status, defined by the intensity of respiratory support. The categories included four tiers: mild impairment (>0-<6 l/min using a nasal cannula or <40% fraction of inspired oxygen [FiO2] using a face mask), moderate (6-15 l/min using a nasal cannula or 40-60% FiO2 using a mask), severe (>15 l/min using a nasal cannula or high-flow system, >60% FiO2 using a mask, or noninvasive ventilation), or very severe (IMV).
Corticosteroid groups
Low-dose corticosteroid use was defined as a total daily dose of 6-10 mg of dexamethasone or equivalent doses of other corticosteroids. High-dose corticosteroid use was defined as a total daily dose of >10 up to 20 mg of dexamethasone or equivalent doses of other corticosteroids. We did not distinguish between oral or intravenous administration or whether doses were divided into twice or more daily doses. The corticosteroid group assignment was based on the highest prescribed corticosteroid dose (e.g., dexamethasone 6 mg once a day for 10 days) within 24 hours of admission. All analyses followed the intention-to-treat principle, with patients remaining in their day 1 group assignment even if corticosteroid doses were subsequently increased or decreased.
Outcomes
The primary outcome was in-hospital death. Secondary outcomes were hospital length of stay, the proportion of patients subsequently requiring mechanical ventilation after day 1, the duration of mechanical ventilation among those patients who required it, and infectious complications. We assessed the frequency of all hospital-acquired infections as well as specific infections (bloodstream, urinary tract, soft tissue, and pulmonary infections other than COVID-19) using the International Classification of Diseases, 10th Revision codes (Appendix Table 1) entered by professional medical coders on discharge.
Statistical analysis
We described continuous and categorical variables using means and standard deviations and counts and percentages, respectively. To reduce the imbalance in demographic and clinical characteristics among the treatment groups, we fit propensity score models using logistic regression to estimate the probability of receiving each treatment as a function of predictor variables that were plausibly associated with both exposure and outcome (Imbens, 2000). The covariates included the continuous variables: inpatient and emergency department use in the year before admission, age, Elixhauser comorbidity score, and Epic DI upon admission and categorical variables: sex; race and ethnicity; smoking; BMI; respiratory status (mild, moderate, severe, and very severe); treatment with remdesivir (yes/no), anakinra (yes/no), dialysis (yes/no), or convalescent plasma (yes/no); medical center on day 1 of admission; and month of admission. Tocilizumab use on day 1 of admission and oral corticosteroid prescriptions in the year before admission were not included in the propensity score because they were associated with the corticosteroid treatment group but not with the primary outcome in our dataset (Brookhart et al., 2006).
We analyzed in-hospital mortality by fitting logistic regression models with inverse probability of treatment weights based on propensity scores. We used robust sandwich estimators to obtain appropriate standard errors of treatment effects because the weighting induced a within-subject correlation in the outcomes and an inflated sample size (Hernán et al., 2000; van der Wal and Geskus, 2011; Xu et al., 2010). Propensity score-adjusted odds ratios (ORs) were reported with low-dose corticosteroids as the reference group. To examine the heterogeneity of treatment effect, we performed prespecified subgroup analyses, comparing treatment groups stratified by age, sex, BMI, CRP levels, and respiratory status. We displayed these subgroup results as forest plots.
All analyses were conducted using SAS 9.4 (Cary, NC, USA). Two-tailed tests were used with the threshold of 0.05 for statistical significance.
Results
Among 28,035 patients admitted between March 1, 2020 and December 31, 2020, 13,721 were excluded for a final study population of 14,314, of whom 8985 were from KPSC and 5329 were from KPNC. The main reasons for exclusion were initial daily dexamethasone or corticosteroid equivalent dose of 0->6 mg or >20 mg, no supplemental oxygen requirements within 24 hours of admission, or do not intubate/comfort measures only (Figure 1).
Most of the included patients (n = 13,366, 93.4%) received low-dose corticosteroids, whereas 948 (6.6%) patients received high-dose corticosteroids within 24 hours of admission (Table 1). The median daily dose of dexamethasone or equivalent was 6 mg (interquartile range [IQR] 6-6 mg) in the low-dose group and 15 mg (IQR 7.5-15 mg) in the high-dose group. Of the patients who were started on low-dose corticosteroids, 12.1% (n = 1620) were switched to high-dose corticosteroids after a median of 2.2 days. The median duration of corticosteroid treatment was 6 days (IQR 4-10) in the low-dose group and 6 days (IQR 4-10) in the high-dose group.
Table 1.
Patient characteristics on day 1 of hospital admission.
| 6-10 mg DEXA | >10-20 mg DEXA | Total | |
|---|---|---|---|
| N = 13,366 | N = 948 | N = 14,314 | |
| Age, mean (SD), years | 59.6 (15.5) | 59.7 (13.9) | 59.6 (15.4) |
| Male gender, n (%) | 7876 (58.9) | 596 (62.9) | 8472 (59.2) |
| Race and ethnicity, n (%) | |||
| Asian | 1562 (11.7%) | 118 (12.4%) | 1680 (11.7%) |
| Black | 1015 (7.6%) | 29 (3.1%) | 1044 (7.3%) |
| Hispanic | 7598 (56.8%) | 661 (69.7%) | 8259 (57.7%) |
| White | 2512 (18.8%) | 100 (10.5%) | 2612 (18.2%) |
| Multiple | 209 (1.6%) | 4 (0.4%) | 213 (1.5%) |
| Native American Alaskan | 35 (0.3%) | 2 (0.2%) | 37 (0.3%) |
| Pacific Islander | 210 (1.6%) | 14 (1.5%) | 224 (1.6%) |
| Other | 61 (0.5%) | 9 (0.9%) | 70 (0.5%) |
| Unknown | 164 (1.2%) | 11 (1.2%) | 175 (1.2%) |
| Body mass index categories, n (%) | |||
| Underweight | 109 (0.8%) | 8 (0.8%) | 117 (0.8%) |
| Normal | 1421 (10.6%) | 112 (11.8%) | 1533 (10.7%) |
| Overweight | 3705 (27.7%) | 287 (30.3%) | 3992 (27.9%) |
| Moderate obese | 5709 (42.7%) | 403 (42.5%) | 6112 (42.7%) |
| Severe obese | 2166 (16.2%) | 130 (13.7%) | 2296 (16.0%) |
| Unknown | 256 (1.9%) | 8 (0.8%) | 264 (1.8%) |
| Smoking, n (%) | |||
| Never/Passive | 7584 (56.7%) | 519 (54.7%) | 8103 (56.6%) |
| Quit | 3603 (27.0%) | 237 (25.0%) | 3840 (26.8%) |
| Active | 326 (2.4%) | 18 (1.9%) | 344 (2.4%) |
| Unknown | 1853 (13.9%) | 174 (18.4%) | 2027 (14.2%) |
| Oral corticosteroids in the past 12 months, n (%) | 2229 (16.7%) | 206 (21.7%) | 2435 (17.0%) |
| Elixhauser comorbidities, median (IQR) | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.0) |
| Healthcare utilizations in the past 12 months | |||
| Inpatient visit count, n (%) | |||
| 0 visit | 12,044 (90.1%) | 852 (89.9%) | 12,896 (90.1%) |
| 1-2 visits | 1127 (8.4%) | 86 (9.1%) | 1213 (8.5%) |
| 3 or more visits | 195 (1.5%) | 10 (1.1%) | 205 (1.4%) |
| Emergency department visit count, n (%) | |||
| 0 visit | 8258 (61.8%) | 658 (69.4%) | 8916 (62.3%) |
| 1-2 visits | 4282 (32.0%) | 244 (25.7%) | 4526 (31.6%) |
| 3 or more visits | 826 (6.2%) | 46 (4.9%) | 872 (6.1%) |
| Urgent care visit count, n (%) | |||
| 0 visit | 9923 (74.2%) | 646 (68.1%) | 10,569 (73.8%) |
| 1-2 visits | 2712 (20.3%) | 227 (23.9%) | 2939 (20.5%) |
| 3 or more visits | 731 (5.5%) | 75 (7.9%) | 806 (5.6%) |
| COVID severity on day 1 of hospital admissiona | |||
| Epic deterioration index, n | 13,353 | 948 | 14,301 |
| Median (IQR) | 44.0 (36.9, 54.0) | 45.5 (37.0, 57.0) | 44.0 (36.9, 54.0) |
| Respiratory impairment, n (%) | |||
| Mild (>0 and <6 l/min via N/C or 40% fraction of inspired oxygen via mask) | 7749 (58.0%) | 447 (47.2%) | 8196 (57.3%) |
| Moderate (6-15 l/min via N/C or 40%-60% via mask) | 1429 (10.7%) | 118 (12.4%) | 1547 (10.8%) |
| Severe (high-flow, noninvasive ventilation, >15 l/min via N/C or >60% via mask) | 3745 (28.0%) | 294 (31.0%) | 4039 (28.2%) |
| Invasive mechanical ventilation | 443 (3.3%) | 89 (9.4%) | 532 (3.7%) |
| Other treatments started on day 1 of hospital admission | |||
| Convalescent plasma, n (%) | 618 (4.6%) | 77 (8.1%) | 695 (4.9%) |
| Tocilizumab, n (%) | 9 (0.1%) | 1 (0.1%) | 10 (0.1%) |
| Dialysis, n (%) | 185 (1.4%) | 10 (1.1%) | 195 (1.4%) |
| Remdesivir, n (%) | 8724 (65.3%) | 547 (57.7%) | 9271 (64.8%) |
| Anakinra, n (%) | 417 (3.1%) | 416 (43.9%) | 833 (5.8%) |
| Selected laboratory values on day 1 of hospital admissiona | |||
| C-reactive protein, n | 12305 | 902 | 13207 |
| Median (IQR), mg/l | 125.6 (68.0,188.0) | 142.3 (75.4, 221.5) | 126.3 (68.6,190.0) |
| D-dimer, n | 11643 | 881 | 12524 |
| Median (IQR), mcg fibrinogen equivalent units /ml | 1.0 (0.6, 1.7) | 1.1 (0.7, 2.1) | 1.0 (0.6, 1.7) |
Based on highest value within 24 hours of admission
Abbreviations: DEXA, dexamethasone or equivalent corticosteroid dosing; N/C, nasal cannula.
Patients started on high-dose corticosteroids were more often of Hispanic ethnicity, more likely to receive anakinra, had slightly higher CRP values and had more severe respiratory impairment (>15 l/min of oxygen or mechanical ventilation) upon admission compared with patients who received low-dose corticosteroids (40.4% and 31.3%, respectively) (Table 1). High- and low-dose groups were similar in age, sex, BMI, smoking behavior, Elixhauser comorbidity indices, inpatient emergency department, and urgent care use in the 12 months before admission, and d-dimer levels and Epic DI scores upon admission. Treatment on day 1 with remdesivir was common, whereas tocilizumab treatment was rare and neither differed significantly between groups.
Crude in-hospital mortality was higher among patients who received high-dose corticosteroids (18.8%, 95% CI 16.3-21.3%) than the low-dose group (14.8%, 95% CI 14.2-15.4%), but this difference was not significant in the propensity score-adjusted model (OR 1.17, 95% CI 0.72-1.90) (Table 2 ). Similarly, there was no significant difference in length of hospital stay, duration of mechanical ventilation, and total or specific types of hospital-acquired infections between groups in the adjusted models; although, the crude rates were somewhat higher in the high-dose group (Table 2).
Table 2.
Corticosteroid dose on day 1 of admission and primary and secondary outcomes.
| 6-10 mg DEXA | >10-20 mg DEXA | |
|---|---|---|
| N | 13,366 | 948 |
| Mortality, n (%) | 1974 (14.8) | 178 (18.8) |
| Unadjusted, OR (95% CI) | Reference | 1.33 (1.13, 1.58) |
| Propensity score adjustment (IPTW) with robust standard errors, OR (95% CI)a |
Reference | 1.17 (0.72, 1.90) |
| Hospital length of stay, mean (SD), days | 9.8 (10.5) | 11.6 (12.3) |
| Unadjusted mean difference (95% CI) |
Reference | 1.8 (1.0, 2.6) |
| Propensity score adjustment (IPTW) with robust standard errors, mean difference (95% CI)a |
Reference | 0.1 (-1.7, 1.9) |
| Ventilator days, mean (SD) | 2.4 (7.9) | 3.8 (10.1) |
| Unadjusted mean difference (95% CI) |
Reference | 1.4 (0.8, 2.1) |
| Propensity score adjustment (IPTW) with robust standard errors, mean difference (95% CI)a |
Reference | 0.3 (-1.0, 1.6) |
| Hospital-acquired infection (%) | 9.5 | 13.2 |
| Unadjusted, OR (95% CI) | Reference | 1.45 (1.19, 1.76) |
| Propensity score adjustment (IPTW) with robust standard errors, OR (95% CI)a |
Reference | 0.70 (0.44, 1.11) |
| Bloodstream infection (%) | 7.2 | 9.2 |
| Unadjusted, OR (95% CI) | Reference | 1.31 (1.04, 1.64) |
| Propensity score adjustment (IPTW) with robust standard errors, OR (95% CI)a |
Reference | 0.67 (0.38, 1.15) |
| Pulmonary infection (%) | 2.9 | 5.2 |
| Unadjusted, OR (95% CI) | Reference | 1.83 (1.35, 2.48) |
| Propensity score adjustment (IPTW) with robust standard errors, OR (95% CI)a |
Reference | 0.63 (0.35, 1.11) |
| Urinary tract infection (%) | 1.7 | 3.1 |
| Unadjusted, OR (95% CI) | Reference | 1.84 (1.24, 2.72) |
| Propensity score adjustment (IPTW) with robust standard errors, OR (95% CI)a |
Reference |
|
The propensity score model for all outcomes included age, gender, race and ethnicity, smoking, body mass index, Elixhauser comorbidity index, Epic Deterioration Index score, severity of respiratory impairment and dialysis on day 1 of admission; remdesivir, convalescent plasma, or anakinra use on day 1 of admission; and inpatient, outpatient and urgent care utilizations in the 12 months prior to admission.
Abbreviations: DEXA= dexamethasone or equivalent corticosteroid dosing; IPTW, inverse probability of treatment weights; OR, odds ratio.
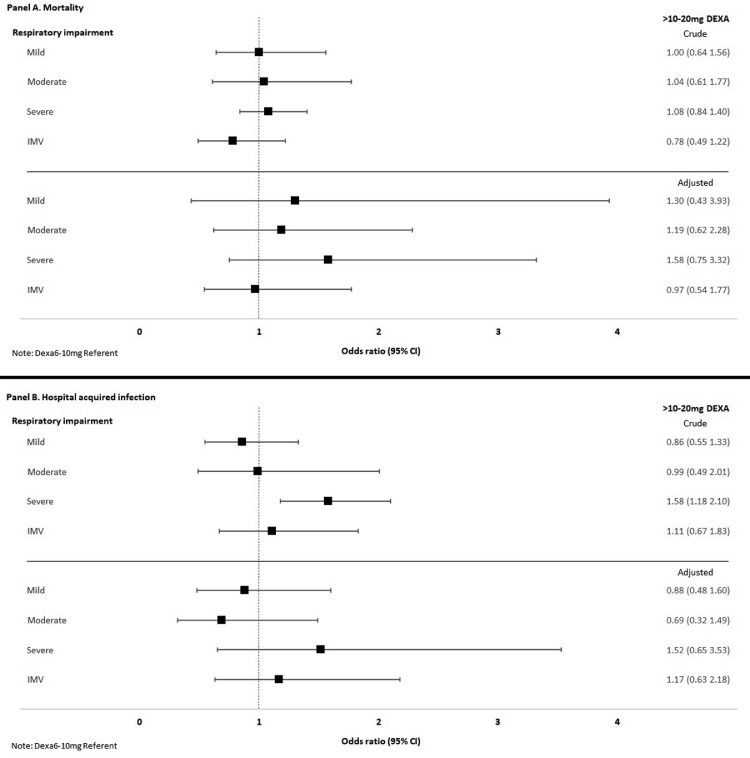
In the subgroup analyses stratified by severity of respiratory impairment upon admission, the unadjusted frequencies of both mortality and hospital-acquired infections increased with increasing severity of impairment (Table 3 ). However, the adjusted models showed no significant difference between the high- and low-dose corticosteroid-treated groups in either mortality or hospital-acquired infections, regardless of the severity of respiratory impairment (Figure 2 , panels a and b). Among patients receiving mechanical ventilation upon admission (n = 532), the crude mortality rate was lower among patients treated with high- than low-dose corticosteroids (49.4% and 55.8% respectively, Table 3). However, this difference was not statistically significant after propensity score adjustment (adjusted OR 0.97, 95% CI 0.54-1.77; Figure 2, panel a). In the mild and moderate respiratory impairment groups, the crude mortality and hospital-acquired infection rates were almost identical among patients treated with high- or low-dose corticosteroids (Table 3) and the adjusted analyses showed no significant differences (Figure 2, panels a and b).
Table 3.
Severity of respiratory impairment on day 1 of admission, corticosteroid dose, mortality and hospital acquired infections.
| 6-10mg DEXA | >10-20mg DEXA | |
|---|---|---|
| Mild, n | 7749 | 447 |
| Mortality, n (%) | 381 (4.9%) | 22 (4.9%) |
| Hospital-acquired infection (%) | 5.7 | 4.9 |
| Moderate, n | 1429 | 118 |
| Mortality, n (%) | 200 (14.0) | 17 (14.4) |
| Hospital acquired infection (%) | 7.7 | 7.6 |
| Severe, n | 3745 | 294 |
| Mortality, n (%) | 1146 (30.6) | 95 (32.3) |
| Hospital acquired infection (%) | 15.8 | 22.8 |
| Invasive mechanical ventilation, n | 443 | 89 |
| Mortality, n (%) | 247 (55.8) | 44 (49.4) |
| Hospital acquired infection (%) | 28.2 | 30.3 |
Abbreviations: DEXA, dexamethasone or equivalent corticosteroid dosing.
Figure 2.
Severity of respiratory impairment, high-dose corticosteroids and ORs for death or hospital acquired infections.
The forest plots depict the crude and adjusted ORs (squares) for in-hospital death (panel a) or hospital acquired infections (panel b) in subgroups stratified by severity of respiratory impairment on day 1 of hospitalization comparing high-dose (10-20 mg of dexamethasone or other corticosteroid equivalent, >10-20 mg DEXA) to low-dose corticosteroids (6-10 mg of dexamethasone or equivalent). High-dose corticosteroids were not associated with increased or decreased odds of death or infection in any of these pre-specified subgroups in crude or adjusted analyses.
Abbreviations: DEXA, dexamethasone or equivalent corticosteroid dosing; IMV, invasive mechanical ventilation; OR, odds ratio.
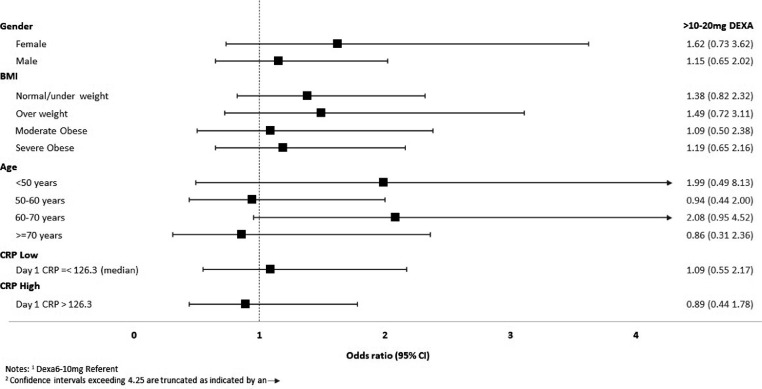
Treatment with high-dose corticosteroids also showed no significant association with mortality in subgroups stratified by sex, age, BMI, or CRP values in either the crude or adjusted analyses compared with the low-dose-treated group (Figure 3 ).
Figure 3.
Age, gender, BMI, high-dose corticosteroids and OR for death or hospital acquired infections.
The forest plots depict the adjusted ORs (squares) and 95% CIs lines for in-hospital death (panel a) or hospital acquired infections (panel b) in pre-specified subgroups comparing high-dose (10-20 mg of dexamethasone or other corticosteroid equivalent, >10-20 mg DEXA) to low-dose corticosteroids (6-10 mg of dexamethasone or equivalent). High-dose corticosteroids were not associated with an increased or decreased odds of death or infection in subgroups defined by gender, BMI, age, or CRP levels. Upper CI is truncated at OR of 4.5 for ease of presentation.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; DEXA, dexamethasone or equivalent corticosteroid dosing; OR, odds ratio.
Discussion
In this large observational, comparative effectiveness study of hospitalized patients with COVID-19 pneumonia, we found that high-dose corticosteroid use upon admission was not associated with reduced in-hospital mortality or increased hospital-acquired infections compared with low-dose corticosteroid use. As expected, the risk of in-hospital mortality and hospital-acquired infections increased with increasing severity of respiratory impairment upon admission. However, high- compared with low-dose corticosteroids did not significantly reduce mortality in any subgroup of respiratory impairment; although, in patients with very severe impairment requiring IMV, the crude but not adjusted mortality rate was lower in the high-dose-treated group. Among the patients with mild or moderate respiratory impairment upon admission, mortality and infection rates were low and very similar in patients treated with high- or low-dose corticosteroids. However, because switching from low- to high- and even more so from high- to low-dose corticosteroids after admission occurred rather frequently, this study cannot exclude the possibility of a benefit of high-dose corticosteroids in a specific subgroup.
Our findings are consistent with a recent RCT that compared 12 mg with 6 mg of dexamethasone, in addition to oxygen support strategies, and found no difference in mortality between treatment groups, regardless of the severity of respiratory status at randomization (Bouadma et al., 2022). Our findings in patients requiring IMV upon admission in the high-dose-treated group are also consistent, strictly speaking, with the open-label RCT that compared high-dose dexamethasone (20 mg daily for 5 days, followed by 10 mg daily for 5 days) with standard care in patients with COVID-19 with moderate or severe ARDS and found no significant difference in 28-day mortality between the groups (Tomazini et al., 2020).
However, the possibility of a benefit of high-dose corticosteroids, particularly in the severe respiratory or IMV groups, remains. The RCT in patients with COVID-19 ARDS did observe a lower 28-day mortality in the high-dose group than in standard care (56.3% and 61.5%, respectively); although, this was not statistically significant (Tomazini et al., 2020). In addition, the preplanned Bayesian analyses of the RCT that compared 12 mg to 6 mg of dexamethasone in patients hospitalized for severe COVID-19 pneumonia found a high probability of benefit across all outcome measures (Granholm et al., 2022). This leaves open the possibility that both studies were underpowered. Furthermore, the RCT comparing 12 mg to 6 mg of dexamethasone has not yet reported treatment effects stratified by respiratory impairment (COVID STEROID 2 Trial Group et al., 2021); thus, it is unclear whether the trend toward the beneficial effect of high-dose corticosteroids was seen across multiple subgroups or only in the 22% of who required IMV at the time of corticosteroid initiation. Should post hoc analyses show a potentially beneficial effect in the IMV group, this would bolster the rationale for repeating a low- versus high-dose corticosteroid RCT in patients with very severe respiratory impairment from COVID-19 pneumonia.
Interestingly, a long-term (180 day) follow-up of COVID STEROID 2 RCT population found that fewer patients in the 12 mg group had died than the 6 mg group (33.7% vs 38.6%, respectively) and quality of life scores were higher; although, neither finding reached statistical significance (Granholm et al., 2022). The higher quality of life scores is consistent with the RCT's primary end point, days alive without life support, which also favored the high-dose group but did not reach statistical significance (adjusted mean difference, 1.3 days [95% CI 0-2.6 days]; P-value = 0.07) (COVID STEROID 2 Trial Group et al., 2021). This suggests that an underexplored benefit of high-dose corticosteroids may be in lowering the risk of postacute COVID-19 syndrome.
This study has several limitations. First, residual confounding by indication could explain the null result of high-dose corticosteroids in the IMV subgroup, despite lower unadjusted in-hospital mortality. High-dose corticosteroid-treated patients had more respiratory impairment and higher Epic DI scores upon admission. This, coupled with unmeasured practice trends, including timing of IMV, could have obscured a potentially beneficial effect in patients with the most severe respiratory impairment upon admission. During the study, some clinicians questioned whether patients with COVID-19 pneumonia were being intubated too early, contributing to poor outcomes, and shifted toward high-flow nasal cannula respiratory support rather than early intubation (Papoutsi et al., 2021). Although we incorporated month of admission in the propensity score models, it is unlikely to have completely captured this practice trend. Second, assigning corticosteroid dosing group upon admission when 12.1% in the low-dose group switched to high-dose corticosteroids and over 25% in the high-dose group switched to the low-dose group may have obscured a potential benefit of high-dose corticosteroids in a specific subgroup and/or a potentially increased risk of hospital-acquired infections in patients with severe respiratory impairment or IMV where the crude infection rate was higher. Other limitations are the relatively small number of high-dose-treated patients with IMV and combining various preparations and dosing regimens of corticosteroids into two groups. Corticosteroids vary in their anti-inflammatory and mineralocorticoid potency, which could theoretically have different effects on patients with COVID-19; although, evidence to support this is lacking. Lastly, whether these results can be extrapolated to vaccinated populations where far fewer patients experience severe respiratory impairment or to the Omicron variant, or any future strain is uncertain.
Strengths of this study include the importance of the question, the very large number of patients, particularly those treated with low-dose corticosteroids, and restricting the analysis to corticosteroids started on day 1. Since release of The RECOVERY Collaborative Group (2021) trial results, starting patients with COVID-19 pneumonia requiring oxygen support on 6 mg of dexamethasone (or higher) upon admission rapidly became the standard of care in our hospitals. Before this, the risks and benefits were debated and if corticosteroids were started, it was often not until a patient's respiratory status had declined. In the cohort included in these analyses, the dose chosen in patients, especially in those with mild or moderate respiratory impairment, appears to be largely driven by clinician preference because there was sufficient uncertainty whether doses higher than 6 mg of dexamethasone could further reduce mortality.
Interpretation
Our findings are consistent with a recently published RCT (Bouadma et al., 2022), which does not support the use of high-dose corticosteroids in patients with mild (Swaminathan et al., 2022), moderate, or severe respiratory impairment from COVID-19 pneumonia. However, due to the observational study design and the frequency of switching dose groups in our population, our findings do not exclude the possibility that high-dose corticosteroids in patients with severe respiratory impairment particularly those requiring IMV upon admission, or other specific subgroups, provide a small mortality benefit or reduced risk of postacute COVID-19 syndrome. These questions should be addressed in future studies.
Author contribution
ALG drafted the manuscript. SX performed the analysis. AC and JG extracted the data. BC coordinated the IRB approval and data transfer. MKG, LCM, VXL, KB, and JLA advised on the methods and interpretation of the results. ALG, VXL and MKG conceived of the idea, obtained the funding and oversaw the project. All authors critically reviewed the manuscript.
Funding
This work was funded by the Sidney Garfield Memorial Fund, Kaiser Foundation Hospitals, Inc., the Southern California Permanente Medical Group, Inc., and The Permanente Medical Group, Inc. VXL was funded in part by NIH R35GM128672. The funders were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethical approval
We performed this retrospective cohort study after receiving approval from the KPSC Institutional Review Board (#12396).
Declaration of competing interest
KB has received research support unrelated to this study from Moderna/Pfizer. LCM has received research support unrelated to this study from Boehringer Ingelheim. The other authors have no conflict of interest.
Acknowledgments
The authors wish to thank Fernando Barreda (Project Manager, Kaiser Permanente Northern California) for facilitating the data transfer agreement and IRB approval and Jessica B. Smith (Research Associate IV, Kaiser Permanente Southern California) with manuscript preparation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.10.023.
Appendix. Supplementary materials
References
- Bouadma L, Mekontso-Dessap A, Burdet C, Merdji H, Poissy J, Dupuis C, et al. High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS randomized clinical trial. JAMA Intern Med. 2022;182:906–916. doi: 10.1001/jamainternmed.2022.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm A, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45–55. doi: 10.1007/s00134-021-06573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–710. [Google Scholar]
- Kumar G, Patel D, Hererra M, Jefferies D, Sakhuja A, Meersman M, Dalton D, Nanchal R, Guddati AK. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J Med Virol. 2022;94:372–379. doi: 10.1002/jmv.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal E, S Sainz de la Maza, Natera-Villalba E, Á Beltrán-Corbellini, Rodríguez-Jorge F, Fernández-Velasco JI, Walo-Delgado P, Muriel A, Zamora J, Alonso-Canovas A, Fortún J, Manzano L, Montero-Errasquín B, Costa-Frossard L, Masjuan J, Villar LM, group COVID-HRC. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021;40:761–769. doi: 10.1007/s10096-020-04078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25:121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Valley TS, Tang S, Li BY, Kamran F, Sjoding MW, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc. 2021;18:1129–1137. doi: 10.1513/AnnalsATS.202006-698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan L, Kaatz S, Chubb H, Tae K, Ramesh MS, Fadel R, et al. Impact of early corticosteroids on preventing clinical deterioration in non-critically ill patients hospitalized with COVID-19: a multi-hospital cohort study. Infect Dis Ther. 2022;11:887–898. doi: 10.1007/s40121-022-00615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP. COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw. 2011;43:1–23. [Google Scholar]
- Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.