Abstract
This report elucidates four aspects of the immunology of pulmonary tuberculosis produced in rabbits: (i) the virulence of bovine-type tubercle bacilli, strain Ravenel S, (ii) systemic factors influencing the generation of visible primary pulmonary tubercles, (iii) differences in tuberculin sensitivity of rabbits and humans, and (iv) the effect of Mycobacterium vaccae immunotherapy on cavitary tuberculosis. Laboratory strain Ravenel S (ATCC 35720) was not fully virulent. Fully virulent strains produce one visible primary pulmonary tubercle for each three bacillary units inhaled. Strain ATCC 35720 produced one such tubercle for each 18 to 107 bacillary units inhaled, indicating that its virulence was reduced by 6- to 36-fold. When a low dose of this Ravenel S strain was inhaled, the host resistance (measured by the number of inhaled bacilli needed to generate one visible primary pulmonary tubercle) was increased at least 3.5-fold compared to the host resistance when a high dose was inhaled. Rabbits and humans differ in the degree and in the maintenance of their dermal sensitivities to tuberculin. Compared to rabbits, humans are 100 times more sensitive to tuberculin. Also, at 33 weeks rabbits with well-controlled cavitary tuberculosis usually showed a decrease in their tuberculin reactions of about 50% from peak values, whereas humans with such well-controlled tuberculosis are thought to maintain strong reactions for many years. These species differences may be due to desensitization to group II mycobacterial antigens in the rabbits because they have a different diet and a different type of digestive tract. M. vaccae immunotherapy of rabbits with cavitary tuberculosis produced no statistically significant effects. Experiments with many more rabbits would be required to prove whether or not such immunotherapy is beneficial.
In most industrialized nations, tuberculosis (TB) declined throughout the 20th century, but it has recently reemerged as an important infectious disease. The increased prevalence is apparently due to (i) a relaxation of public health TB control efforts (because of other budgetary priorities); (ii) human immunodeficiency virus infection (AIDS), which greatly increases susceptibility to TB; (iii) increased poverty and homelessness in parts of the industrialized world; and (iv) the emergence of multidrug-resistant cases (because of inadequate treatment). In developing countries, TB has always been a common disease, and the above factors, as well as civil war and famine, have increased its prevalence. To control this disease, we need new vaccines and new antimicrobials supplemented by effective immunotherapy.
The most promising immunotherapeutic agent available today is heat-killed Mycobacterium vaccae, a rapidly growing bacillus, which was originally found in the pastures of cattle and in their milk (51). M. vaccae apparently enhances cell-mediated immunity (i.e., the ability of macrophages to kill tubercle bacilli) and reduces the tissue-damaging hypersensitivity response to these bacilli (38–40, 42, 48).
This report is a supplement to reference 7. In that reference, we published descriptions of rabbit cavitary TB without mentioning which animals were treated with M. vaccae and which were controls, because the disease seemed identical in both groups. Upon reexamination of the data in those experiments (including some that were not published), we found (i) new information on the virulence of the Ravenel S strain of bovine-type tubercle bacilli (section i of Results), (ii) new information concerning the effects of high vs low doses of inhaled bacilli on the early immune response (section ii of Results), (iii) new information about the maintenance of tuberculin sensitivity in rabbits with cavitary TB (section iii of Results), and (iv) new information on the effects of M. vaccae immunotherapy on cavitary TB (section iv of Results).
MATERIALS AND METHODS
Tubercle bacilli.
Virulent bovine-type tubercle bacilli, strain Ravenel S, were obtained from the American Type Culture Collection (ATCC) (ATCC 35720), grown on Löwenstein-Jensen solid media, and prepared for aerosolization as described in reference 7. It is a laboratory strain that has been used for over 80 years. In a personal communication from ATCC, we learned that the strain we received was an original aliquot of the strain obtained in 1985 from the Trudeau Mycobacterial Culture Collection in Saranac Lake, N.Y. In 1985, it was grown on Löwenstein-Jensen solid media, suspended in 2× sterile skim milk (made from a powder), aliquotted, quick frozen, lyophilized, and stored at 5°C until a vial was sent to us in 1993.
About three passages of our ATCC strain of Ravenel S on the solid media were made between experiments I and III. This strain did not lose virulence during the subculturing between our experiments, because the number of inhaled bacilli that generated one visible primary pulmonary tubercle, i.e., the “ratio” (12), did not increase during the 1.5-year lapse of time (see Results).
Rabbits and aerosol infection with virulent bovine-type tubercle bacilli.
New Zealand White rabbits were infected by aerosol with the Ravenel S strain at the United States Army Medical Research Institute of Infectious Diseases at Fort Detrick, Frederick, MD (7). The animals used in low-dose experiments I, III, and IV were commercially available Pasteurella-free New Zealand White rabbits from Covance Research Products, Inc. (formerly Hazelton Laboratories), Denver, Pa. (7). The animals used in high-dose experiment II were Pasteurella-free inbred New Zealand White rabbits (strain IIIVO/JU), which should have a high degree of native resistance to TB (see Results).
In conducting this research, the investigators adhered to reference 34a.
Four experiments were performed, but the rabbits in experiment I did not receive M. vaccae immunotherapy. In high-dose experiment II almost half of the rabbits were dead or dying by 9 weeks. In low-dose experiment III, all of the rabbits survived for 30 to 33 weeks, at which time they were sacrificed. Experiment III provided most of the data analyzed herein.
Tuberculin reactions.
Undiluted veterinary tuberculin (0.1 ml) (intradermic tuberculin, mammalian; Coopers Animal Health, Inc., Kansas City, Kans.; now available from Symbiotics, San Diego, Calif.) was injected intradermally, and 2 days later the volume of the resulting reaction was measured with calipers (13).
Euthanasia and necropsies.
The rabbits were weighed and then sacrificed by the intravenous injection of 2.5 to 3.0 ml of sodium pentobarbital (65 mg/ml) at the times listed in Table 1. Necropsy details on these rabbits (except for those listed in Tables 3 and 5) were presented in reference 7.
TABLE 1.
Experimental data for the M. vaccae immunotherapy experiments with rabbitsa
| Expt and group | Weeks 1 injection given | Rabbit no. | No. of inhaled bacillary unitsb | No. of grossly visible primary lesions in lungs (including those with cavities)b | No. of metastatic lesions in GALTb | Size of tuberculin reactions just before sacrifice (mm3)b | Weight at necropsy (kg)b |
|---|---|---|---|---|---|---|---|
| II (high dose) | |||||||
| M. vaccae treated | 5, 9, 13 | 1, 3, 5, 7, 9 | 5,400 ± 270 | 252 ± 26.3 | 428 ± 258 | 323 ± 71 | 2.5 ± 0.2 |
| Control | 2, 4, 6, 8, 10, 11 | 4,850 ± 230 (0.149) | 359 ± 56.8 (0.145) | 320 ± 50 (0.796) | 463 ± 61 (0.279) | 2.7 ± 0.2 (0.570) | |
| III (low dose) | |||||||
| M. vaccae treated | 7, 12, 17, 22, 27 | 2, 4, 6, 7, 9, 12 | 395 ± 39 | 8.0 ± 1.9 | 89 ± 47 | 1,038 ± 191 | 4.0 ± 0.3c |
| Control | 1, 3, 5, 8, 10, 11 | 440 ± 29 (0.417) | 9.5 ± 1.5 (0.545) | 149 ± 82 (0.539) | 1,283 ± 340 (0.567) | 4.2 ± 0.3 (0.578) |
This table summarizes the information published in reference 7, which did not mention that some of the rabbits had M. vaccae immunotherapy. Reference 7 gives detailed descriptions of the various types of TB found at necropsy. The mean number of primary lesions was derived from all of the rabbits, including those that died early. The other data were from the survivors at the times of sacrifice, i.e., at 18 weeks for experiment II and 33 weeks for experiment III (rabbit 12 was sacrificed at 30 weeks, before the data presented in the last two columns were obtained). The true number of primary lesions (cavitary plus noncavitary) is listed. An error was made in Fig. 8A of reference 7, where only the number of primary lesions which did not form cavities was listed.
Values are means ± standard errors. P values for data from the M. vaccae-treated group versus that from the control group (given in parentheses) were determined by Student’s t test. None were statistically significant.
Rabbit 7 was omitted from the tabulation of weights because it had an extensive purulent infection of the nasal turbinates (7), which probably interfered with its ingestion of food.
TABLE 3.
Pulmonary TB in rabbits dying of TB in high-dose experiment IIa
| Treatment (rabbit no.) | Approximate time of death (or sacrifice) (wk) | No. of inhaled virulent bacillary units | No. of visible primary lesions in lungsb | No. of cavities | Comments |
|---|---|---|---|---|---|
| Control (2) | 5 | 4,800 | 550 | 0 | Numerous 3- to 4-mm-diam lesions with minute caseous centers |
| M. vaccae (9) | 6 | 5,800 | 210 | Many | Over half of the lesions had cavitated |
| Control (11) | 7 | 4,700 | 260 | Many | Over half of the lesions had cavitated |
| Control (10) | 8.5 | 3,900 | 250 | Many | Over half of the lesions had cavitated |
| Control (4) | 9 | 5,300 | 500 | Many | Numerous cavities were present |
These 5 dead or dying rabbits were not included in reference 7. Ten days before death, rabbit 9 was given one M. vaccae injection. This injection was probably ineffectual in such a nearly terminal rabbit. The remaining six rabbits of this high-dose experiment were sacrificed at 18 weeks, and details of their necropsies were presented in Table 1 of reference 7.
Includes primary lesions with cavities.
TABLE 5.
Data from experiment IV, in which 1/10 the human dose of M. vaccae bacilli was used for immunotherapya
| Treatment (rabbit no.) | Approximate inhaled dose (bacillary units) | No. of visible primary pulmonary lesions | Ratio | Avg diam of primary pulmonary lesions (mm) | No. of secondary pulmonary lesions |
|---|---|---|---|---|---|
| M. vaccae (1) | 1,000 | 28 | 35.7 | 2.4 | 30 |
| M. vaccae (2) | 1,000 | 30 | 33.3 | 2.5 | 125 |
| M. vaccae (3) | 1,000 | 41 | 24.4 | 3.0 | 35 |
| Control (4) | 1,000 | 58 | 17.2 | 5.7 | >40 |
| Control (5) | 1,000 | 103 | 9.7 | 3.0 | Several hundred |
In this pilot study, the rabbits were infected with virulent bovine-type tubercle bacilli (Ravenel S). At both 9 and 13 weeks, they were injected intradermally with 108 heat-killed M. vaccae cells, and at 19 weeks they were sacrificed. The ratio is the number of inhaled bacilli required to generate one grossly visible primary lesion (mean ± standard error = 24 ± 5). Note that the inhaled dose and the ratios are intermediate between those listed in Table 2.
Determining the number of primary pulmonary lesions.
In low-dose experiment III at 33 weeks, we were usually able to determine the total number of primary pulmonary lesions, as well as the number of primary lesions that cavitated (Table 4). Such established primary pulmonary lesions did not regress, and secondary lesions were usually small (or absent) and easily distinguished from the primary lesions. Some of these lesions had solid caseous centers; some had liquefied; and still others not only had liquefied but also had eroded bronchi and formed cavities. In M. vaccae-treated rabbit 2 and control rabbit 5, the primary cavities gave rise to multiple (loculated) secondary cavities in a cluster that obscured the primary lesions located there (7). In these two rabbits, we had to estimate the number of primary lesions from the distribution of other primary lesions in the same lungs.
TABLE 4.
Primary lesions, cavities, and tuberculin skin tests in the rabbits of low-dose experiment IIIa
| Rabbit no. | Total no. of primary lesions (including cavitary lesions) | No. of primary lesions with cavities | % Primary lesions that cavitated | Dermal tuberculin sensitivityb in mm3 (loge) at:
|
% Tuberculin sensitivity for 33 vs 4 week (loge) | |
|---|---|---|---|---|---|---|
| 4 wk | 33 wk | |||||
| M. vaccae-treated rabbits | ||||||
| 2 | 13c | 5c | 38 | 1,890 (7.54) | 1,200 (7.09) | 63.5 (−0.45) |
| 4 | 14 | 4 | 29 | 1,040 (6.90) | 1,660 (7.42) | 159.6 (+0.47) |
| 6 | 2 | 0 | 0 | 3,500 (8.16) | 630 (6.43) | 18.0 (−1.72) |
| 7 | 7 | 1 | 14 | 1,900 (7.55) | 650 (6.48) | 34.2 (−1.07) |
| 9 | 6 | 5 | 83 | 4,700 (8.46) | 1,050 (6.96) | 22.3 (−1.50) |
| 12 | 6 | 3 | 50 | 1,620 (7.39) | ||
| Mean ± SE | 8.0 ± 1.9 | 3.0 ± 0.9d | 35.7 ± 11.9e | 2,606 ± 562f | 1,038 ± 191 | 59.5 ± 26.3g |
| Control rabbits | ||||||
| 1 | 15 | 3 | 20 | 1,890 (7.54) | 1,420 (7.26) | 75.1 (−0.29) |
| 3 | 4 | 0 | 0 | 4,500 (8.41) | 1,660 (7.42) | 36.9 (−1.00) |
| 5 | 8c | 5c | 63 | 2,600 (7.86) | 2,700 (7.90) | 103.8 (−0.04) |
| 8 | 9 | 1 | 11 | 2,700 (7.90) | 810 (6.70) | 30.0 (−1.20) |
| 10 | 10 | 0 | 0 | 1,850 (7.53) | 500 (6.22) | 27.0 (−1.31) |
| 11 | 11 | 2 | 18 | 2,400 (7.78) | 610 (6.41) | 25.4 (−1.37) |
| Mean ± SE | 9.5 ± 1.5 | 1.8 ± 0.8d | 18.7 ± 9.5e | 2,657 ± 396) | 1,283 ± 340 | 49.7 ± 13.2g |
Note that the data in this table differ from those in Fig. 8 of reference 7. In Fig. 8A, the number of cavitary lesions should have included primary lesions that had formed cavities but did not. And in Fig. 8B, the total number of cavities formed by both primary and secondary TB lesions was plotted, not just the number of cavities formed by primary lesions.
The mean tuberculin sensitivity at 33 weeks is 39.8% of the mean sensitivity at 4 weeks for M. vaccae-treated rabbits; the corresponding value for control rabbits is 48.3%.
Estimate; see the text.
Student’s t test showed no statistically significant differences between the M. vaccae-treated and control groups (P = 0.341).
See footnote d. P = 0.291.
Mean without rabbit 12; the mean with rabbit 12 is 2,442 mm3.
See footnote d. P = 0.732.
In high-dose experiment II, the secondary lesions (which contained no cavities) were much smaller than the primary lesions because the rabbits were sacrificed at 18 weeks.
Acid-fast stain for tubercle bacilli.
Paraffin-embedded tissue sections were stained for the bacillus by the Ziehl-Neelsen carbol-fuchsin method and were counterstained with methylene blue (3).
M. vaccae immunotherapy.
Autoclaved M. vaccae (strain NCTC 11659) bacilli were obtained from J. L. Stanford and G. A. W. Rook, Department of Bacteriology, Windeyer Institute of Medical Sciences, University College London Medical School, London, England (48). The M. vaccae bacilli (109 in 0.1 ml of diluent) were injected intradermally into the left flank of our tuberculous rabbits at the times listed in Table 1. Diluent alone was injected into the controls. Since 108 M. vaccae bacilli did not always produce distinct lesions in normal (tuberculin-negative) rabbits, we chose 109 bacilli (the human dose) for experiments II and III. In experiment IV, performed at Texas A & M University, the rabbits were given 1/10 the human dose of M. vaccae. This was a small pilot-type experiment with only three M. vaccae-treated rabbits and two controls. The dermal lesions produced by M. vaccae appeared to be larger in tuberculin-positive rabbits, but not enough M. vaccae lesions in normal tuberculin-negative rabbits were evaluated to make an accurate comparison.
Statistics.
The TB produced in these rabbits seemed to be influenced by several factors: (i) the number of primary lesions and the number of these lesions that formed cavities, (ii) the degree of tuberculin sensitivity, and, possibly, (iii) the M. vaccae immunotherapy. Multiple linear regression analysis (43, 55) in the form of y = b0 + b1x1 + b2x2 enabled us to evaluate relationships among these factors. In this equation, y is the outcome variable, b0 is the value of y where x1 and x2 are both equal to 0, b1 is the difference between the regression lines representing the M. vaccae-treated and the control groups, x1 equals 1 for the M. vaccae-treated group and 0 for the control group, b2 is the slope of the regression lines, and x2 is the input variable.
For Fig. 1 through 4, we have assumed that the slopes of the regression lines for the M. vaccae and control groups would be the same. (There was no statistically significant evidence that they were not.) Then, we determined whether the two regression lines together (representing all 11 or 12 rabbits) showed a statistically significant slope and also whether the difference on the y axis of the regression lines for M. vaccae-treated and control groups was statistically significant.
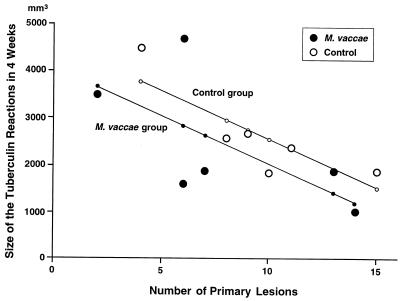
FIG. 1.
The relation between the number of primary pulmonary lesions and the size of the dermal tuberculin reactions in the control and M. vaccae-treated groups at 4 weeks (in experiment III) before M. vaccae immunotherapy was begun. The multiple linear regression equation (see Statistics in Materials and Methods) was y = 4591.53 − 520.51x1 − 203.67x2, where y is the predicted size of the tuberculin reaction, x1 equals 1 for the M. vaccae-treated group and 0 for the control group, and x2 equals the number of primary lesions. (No rabbit failed to develop primary lesions.) This regression model shows (i) that the slope of the lines, i.e., the decrease in the mean size of the tuberculin reactions for each additional primary lesions present, was 203.67 mm3, which was statistically significant (P = 0.015), and (ii) that the difference in the predicted sizes of the tuberculin reactions between the control and M. vaccae-treated groups was 520.51 mm3, which was not statistically significant (P = 0.344). R2 was found to be 0.4524 when the M. vaccae and control data were pooled.
FIG. 4.
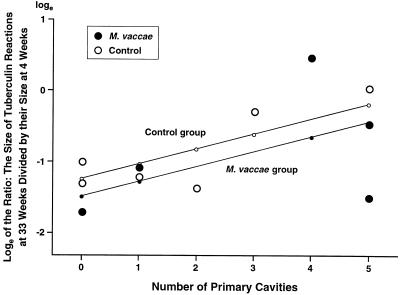
The relation between the number of primary pulmonary cavities and the maintenance of tuberculin sensitivities represented by the ratios of the size of the dermal tuberculin reactions at 33 weeks to their size at 4 weeks (in experiment III). The ratios were converted into natural logs (see Table 4). The multiple linear regression equation (see Materials and Methods) was y = −1.24 + 0.25x1 + 0.21x2, where y is the predicted loge of the ratio of the two tuberculin reactions, x1 equals 1 for the M. vaccae-treated group and 0 for the control group, and x2 equals the number of primary cavities. The graph shows that the tuberculin sensitivity tended to be maintained at higher levels when more primary cavities were present (P = 0.059). No statistically significant difference between the M. vaccae-treated and control groups was found in the maintenance of their predicted tuberculin sensitivities (P = 0.543). R2 was found to be 0.3773 when the M. vaccae and control data were pooled.
For these analyses, we used (in our mainframe computer) STATA statistical software, version 5.0 (1997) from Stata, Inc., College Station, Tex.
RESULTS
Rabbits were infected by aerosol with virulent bovine-type tubercle bacilli, strain Ravenel S (ATCC 35720). In high-dose experiment II, the rabbits inhaled an average of 5,100 U of one to three bacilli. In low-dose experiment III, the rabbits inhaled an average of 420 bacillary units. At 6 to 10 weeks after infection, the lungs of such rabbits are known (from previous experiments) to contain liquefied caseous and early cavitary tuberculous lesions, similar to those usually present in immunocompetent patients with TB. Liquefied and cavitary lesions do not occur in tuberculous mice and only rarely occur in tuberculous guinea pigs.
The tuberculous rabbits received monthly injections of heat-killed M. vaccae. These injections began at 5 to 7 weeks (when cavities were developing) and continued until the animals died or were sacrificed at 18 or 33 weeks (depending on the experiment). At necropsy, most of the rabbits in these experiments had cavitary TB (7).
Table 1 summarizes our findings in these two experiments and lists which rabbits received M. vaccae immunotherapy and which rabbits did not.
Virulence (for rabbits) of the bovine-type tubercle bacilli, strain Ravenel S.
Counts of the primary pulmonary lesions indicated that this Ravenel S strain was reduced in virulence by 6- to 36-fold (Table 2). The number of inhaled bacillary units of one to three bacilli required to produce one primary lesion (i.e., the ratio) was 107 in high-dose experiment I, 18 in high-dose experiment II, and 63 in low-dose experiment III (Table 2). If the Ravenel S strain had been fully virulent, only 3 bacillary units would have been required (26, 27). Nonetheless, this Ravenel S bovine-type bacillus was much more virulent for rabbits than H37Rv, a common laboratory strain of virulent human-type bacillus. For H37Rv to produce one primary lesion in commercially available New Zealand White rabbits, about 1,500 inhaled bacillary units would be required (unpublished observations).
TABLE 2.
Number of inhaled units (of one to three bacilli) that generate one grossly visible primary pulmonary tuberclea
| Expt and no. of rabbits | Inhaled dose (bacillary units) | No. of visible primary pulmonary lesions (including those with cavities) | Ratio |
|---|---|---|---|
| I (low dose), 6 | 523 ± 137 | 6.7 ± 2.3 | 107 ± 29 |
| II (high dose), 11 | 5,100 ± 186 | 310 ± 36 | 18 ± 2 |
| III (low dose), 12 | 418 ± 24 | 8.8 ± 1.2 | 63 ± 13 |
The numbers of grossly visible primary lesions in the M. vaccae and control groups, presented in Tables 1, 3, and 4 and in reference 7, were pooled to prepare this table. The means and their standard errors are listed. The ratio for each rabbit is the number of inhaled bacillary units required to generate one grossly visible primary lesion. The means in the ratio column are derived from ratios for individual rabbits in each experiment and are not derived from the means listed here. The P values for the ratios are as follows: (i) between experiments I and II, P = 0.001; (ii) between experiments I and III, P = 0.124; and (iii) between experiments II and III, P = 0.003. Student’s t test was used. The significant differences in the ratios between experiment II and experiments I and III indicate that a large number of primary lesions (simultaneously developing in the lungs) decreased the early immune response or that a small number of lesions increased this response (see text).
The Ravenel S bacilli (ATCC 35720) were not reduced in virulence by several passages on Löwenstein-Jensen media during the 1.5-year period between experiments I and III. The ratio was 107 ± 29 in experiment I and 63 ± 13 in experiment III, i.e., an insignificant trend in the opposite direction was present (Table 2). The inhaled dose for each rabbit was not determined in experiment IV, performed at Texas A & M University rather than at the U.S. Army Medical Research Institute of Infectious Diseases at Fort Detrick (see below). Therefore, the ratios in experiment IV cannot be accurately compared to those in the first three experiments.
Ratios are one of the most accurate methods of measuring the virulence of bovine-type tubercle bacilli in rabbits because ratios reflect the ability of the bacillus to establish a progressive disease (12).
Effect of inhaled dose on the initial (very early) immune response.
The rabbits in high-dose experiment II inhaled, on the average, 5,100 bacillary units and developed 310 visible primary pulmonary lesions (Table 2). The rabbits in low-dose experiment III inhaled, on the average, 418 bacillary units and developed 8.8 primary lesions (Table 2). The ratios, i.e., the numbers of inhaled bacillary units required to generate one visible primary lesion (12), were 18.2 and 63.3, respectively (Table 2). In other words, the low dose of inhaled bacilli apparently increased host resistance 3.5-fold (63/18) or the high dose of inhaled bacilli decreased the host resistance 3.5-fold. The 12-fold difference in inhaled dose (5,100/310 bacillary units) would not have affected the alveolar macrophages (AM), which determine the fate of many of the inhaled bacilli during the first few days (see Discussion). Therefore, the 3.5-fold difference in host resistance was probably due to the effect of dosage on the host’s native and early acquired immune responses. This difference in ratio was highly significant (P = 0.003). The mean ratio in low-dose experiment I gives additional support to this result (Table 2).
We always thought that early microscopic pulmonary tubercles developed independently of each other, because they were so small and were usually separated by numerous alveolar spaces. The influence of the inhaled bacillary dose on the progression of such microscopic tubercles to grossly visible size clearly indicates that early-developing tubercles have systemic effects (see Discussion).
In high-dose experiment II, inbred New Zealand White rabbits were used. In low-dose experiments I and III, commercially available noninbred New Zealand White rabbits were used. Since the inbred rabbits came from the same lineage as Lurie’s highly resistant Race III rabbits, they needed to inhale a greater (not smaller) number of tubercle bacilli to produce one primary lesion. For this reason, the adverse effects of large numbers of inhaled bacilli were probably somewhat more pronounced than those we observed.
Levels and maintenance of tuberculin sensitivity in rabbits with TB. (i) Overview.
The levels and maintenance of tuberculin sensitivity are primarily determined by the number of bacilli and by the host’s immune response. We did not directly measure the number of bacilli or the levels of serologic or cellular immunity, but we did measure the effects of the number of primary lesions, the number of cavities, and M. vaccae immunotherapy on tuberculin sensitivity. All of these factors reflect interactions between the bacillus and the host.
The data from our low-dose experiment III were used with multiple linear regression analysis. The rabbits in this experiment inhaled about 400 U of the virulent bovine-type bacilli. They did not die of TB but were sacrificed at 33 weeks before the disease had run its full course (Table 1) (7). At that time, the pulmonary lesions showed considerable fibrosis. Therefore, at least some of the rabbits would probably have arrested their disease. This slowly progressing TB is similar to that found in adult humans.
(ii) Effects of the number of primary lesions on tuberculin sensitivity at 4 weeks.
In experiment III, the rabbits were skin tested with tuberculin 4 weeks after the onset of the infection. (M. vaccae immunotherapy was begun afterwards, i.e., at 7 weeks.) The rabbits with few primary lesions had the largest tuberculin reactions, and, conversely, rabbits with many primary lesions had the smallest tuberculin reactions (Fig. 1).
By 4 weeks the tuberculin skin reactions for rabbits with the greatest antigenic stimulus may already have been reduced from presumably higher peak values, i.e., the down-regulatory mechanisms for tuberculin sensitivity occurred earlier when the antigenic load in the host was larger. Alternatively, the rabbits with an innate ability to reduce the number of grossly visible primary lesions may have been rabbits that produced a more vigorous immune response including more sensitivity to tuberculin.
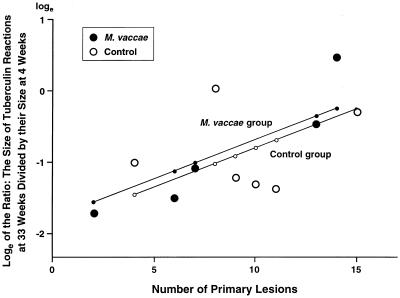
(iii) Effects of the number of primary lesions on the drop in tuberculin sensitivity between 4 and 33 weeks.
In low-dose experiment III, the mean tuberculin sensitivity decreased over 50% between 4 and 33 weeks (Table 4). The tuberculin sensitivities of rabbits with the largest numbers of primary lesions decreased the least by 33 weeks (P = 0.044) (Fig. 2). In other words, rabbits with more extensive pulmonary TB (i.e., greater antigenic loads) seemed to maintain their 4-week tuberculin reactivities better than rabbits with less extensive TB.
FIG. 2.
The relation between the number of primary pulmonary lesions and the maintenance of tuberculin sensitivity (in experiment III). The maintenance of tuberculin sensitivity was represented by the ratios of the size of the dermal tuberculin reactions at 33 weeks to their size at 4 weeks. The ratios were converted into natural logs (see Table 4). The multiple linear regression equation (see Materials and Methods) was y = −1.90 + 0.12x1 + 0.11x2, where y is the predicted loge of the ratio of the two tuberculin reactions, x1 equal 1 for the M. vaccae-treated group and 0 for the control group, and x2 equals the number of primary lesions (see Fig. 1). The graph shows that when more primary lesions were present tuberculin sensitivity was maintained at higher levels (P = 0.044). No statistically significant difference between the M. vaccae-treated and control groups was found in the maintenance of their predicted tuberculin sensitivities (P = 0.747). R2 was found to be 0.4089 when the M. vaccae and control data were pooled.
A log scale was used in Fig. 2 to show proportional decreases as straight lines, because the volumes (in cubic millimeters) of large skin reactions could decrease more than could the volumes of small skin reactions.
(iv) Relationships of tuberculin sensitivity to cavity formation.
After infection of rabbits by aerosolized virulent bovine-type tubercle bacilli, many of the resulting primary pulmonary lesions liquefied and cavitated at between 6 and 10 weeks (7). The studies of Yamamura et al. (56, 57; see reference 16) indicated that sensitivity to tuberculin was necessary for cavities to form. In these studies, he injected tubercle bacilli and their components through the chest wall directly into the lung. Our aerosol inhalation experiment III provided a more natural model of TB with which to determine whether or not there was an association between tuberculin sensitivity and cavity formation.
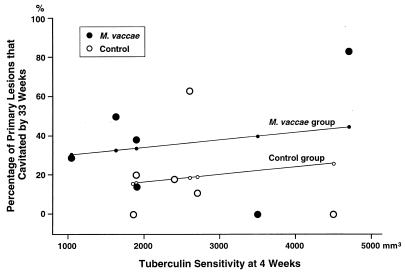
We tried to answer two questions. (i) Was the amount of tuberculin sensitivity at 4 weeks correlated with the percentage of primary lesions that cavitated by 33 weeks? (ii) Was the number of cavities (in primary lesions) correlated with the degree of tuberculin sensitivity at 33 weeks?
Concerning the first question, the tuberculin sensitivity at 4 weeks did not show a statistically significant correlation with the percentage of primary lesions that cavitated (Fig. 3) (P = 0.612). These results do not negate Yamamura’s findings because there was tremendous variability among our individual rabbits. Our studies did, however, provide a model for future experiments to address this subject.
FIG. 3.
The relation between the dermal tuberculin sensitivity at 4 weeks and the percentage of the primary pulmonary lesions that had formed cavities by 33 weeks (in experiment III). The multiple linear regression equation (see Materials and Methods) was y = 0.0852 + 0.1782x1 + 0.0000382x2, where y is the predicted percentage of primary lesions that cavitated by 33 weeks, x1 equals 1 for the M. vaccae-treated group and 0 for the control group, and x2 is the size of the tuberculin reaction at 4 weeks. The graph shows (i) that there was no statistically significant correlation between the 4-week tuberculin sensitivity and the percentage of primary pulmonary lesions with cavities at 33 weeks (P = 0.612) and (ii) that there was no statistically significant difference between M. vaccae-treated and control groups in the percentage of primary lesions that cavitated (P = 0.291). R2 was found to be 0.3188 when the M. vaccae and control data were pooled. See Results for the importance of these findings.
Concerning the second question, Fig. 4 shows that rabbits with large numbers of primary cavities tended to maintain tuberculin sensitivity at higher levels than did rabbits with small numbers (P = 0.059). Although not statistically significant, this P value is suggestive. Figure 8 of reference 7 shows that when both primary and secondary cavities (at 33 weeks) were added together, a significant correlation between the number of cavities and the degree of tuberculin sensitivity existed (P < 0.025).
When liquefaction occurs and cavities form, the bacilli grow extracellularly in the liquefied caseum of the inner cavity wall (see Fig. 5, discussed below) and from there spread via the bronchial tree to other sites in the lung. New lesions that boost the levels of tuberculin sensitivity are formed. Therefore, one would expect less decrease in the size of the tuberculin reaction in animals with more cavities. Another interpretation of our results would be that cavity formation occurred more frequently in the rabbits innately able to maintain high levels of tuberculin sensitivity.
FIG. 5.
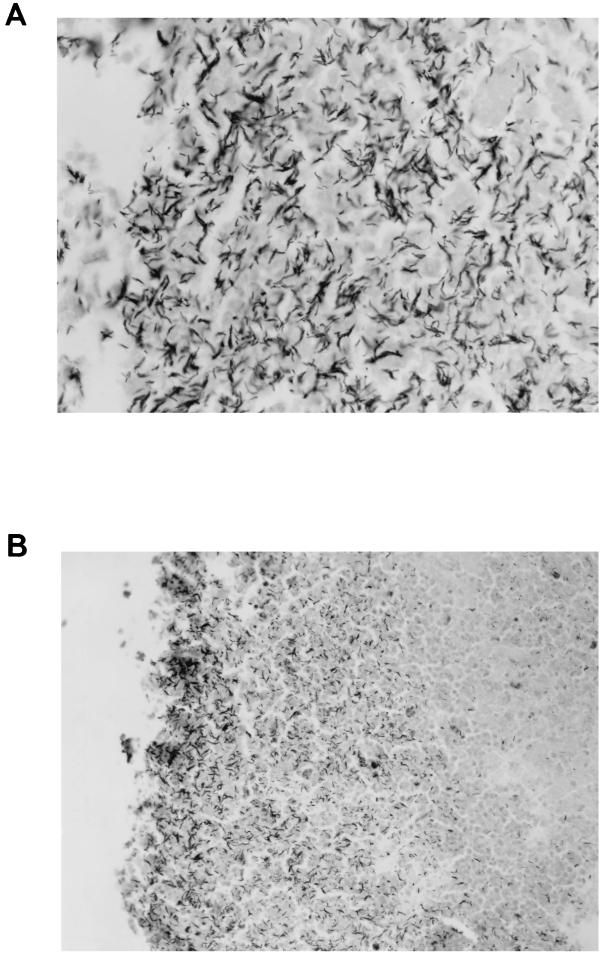
(A) Tissue section of a pulmonary tuberculous lesion of M. vaccae-treated rabbit 4 in low-dose experiment III showing tubercle bacilli that had grown profusely in the liquefied caseum. This lesion was probably beginning to cavitate because some of the spaces in the liquefied caseum were larger than usual. Such profuse growth occurs only in some lesions with liquefied centers, presumably where the composition of the liquefied caseum is most favorable and/or the adaptation of the bacillus to extracellular growth is most complete. Carbol-fuchsin, counterstained with methylene blue. Magnification, ×600. (B) Tissue section of the wall of a pulmonary cavity of control rabbit 5 in low-dose experiment III showing tubercle bacilli that had grown profusely in the liquefied caseum. The bacilli are more numerous near the lumen of the cavity (on the left), presumably because the oxygen tension there is highest. As stated in the legend for panel A, such profuse growth occurs only in the walls of some, not all, cavities. Carbol-fuchsin, counterstained with methylene blue. Magnification, ×250.
Effects of M. vaccae immunotherapy on the extent and progression of TB. (i) M. vaccae skin lesions.
In our experiments II, III, and IV, the first intradermal injection of M. vaccae was 5, 7, and 9 weeks, respectively, after the rabbits were infected by aerosol with virulent bovine-type tubercle bacilli (Tables 1 and 5). The skin lesions produced by M. vaccae were well tolerated. In some tuberculous rabbits, M. vaccae produced a prominent lesion about 10 mm in diameter with a 1- to 2-mm central ulcer that healed in about 4 weeks. In other tuberculous rabbits, the lesions healed without ulceration. We made no study of whether M. vaccae injections converted the tuberculin skin tests of tuberculin-negative rabbits.
(ii) Mortality in our high-dose experiment II.
To date, experiment II has been our only experiment in which many rabbits died or were dying of their TB. None of the five rabbits that died by 9 weeks had immunotherapy for more than 10 days (Table 3). The six remaining rabbits, two in the control group and four in the M. vaccae-treated group, survived for 18 weeks (Table 1) (7). There were too few survivors to determine whether or not M. vaccae had an effect, but the trend was in the same direction as and consistent with that of the human M. vaccae trials in which immunotherapy improved the survival rate (35).
(iii) Effects of M. vaccae on gross pathology and histopathology.
At the time of necropsy, a slowly progressing cavitary-type pulmonary TB with considerable fibrosis was present in both the M. vaccae-treated and the control groups (7). The lesions of the two groups were also similar in their gross and microscopic characteristics.
The numbers of metastatic lesions in the gut-associated lymphoid tissue (GALT) of the appendices and ileocecal junctions for the M. vaccae-treated and control groups were likewise not significantly different (Table 1) (P = 0.567). (The GALT lesions apparently came from the bacilli in cavities that ascended the mucociliary escalator of the bronchial tree and were swallowed.) At necropsy, the weights of the rabbits in the immunotherapy and control groups were also comparable (Table 1) (P = 0.578).
(iv) Effects of M. vaccae immunotherapy on tuberculin sensitivity.
In low-dose experiment III, intradermal injections of M. vaccae bacilli were given at 7, 12, 17, 22, and 27 weeks after infection (Table 1). Such immunotherapy had no statistically significant effect on maintaining the tuberculin reactions at 33 weeks (Fig. 2 and 4), i.e., the immunotherapy had little tuberculin-sensitizing power (if any). In Fig. 2, the x axis represents the number of primary lesions (including cavities). In Fig. 4, the x axis represents the number of primary cavities. Both figures show that the tuberculin sensitivities of rabbits receiving M. vaccae were not statistically different from those of the controls (P = 0.747 and 0.543 for Fig. 2 and 4, respectively). Multiple linear regression statistical analyses were necessary, because the numbers of primary lesions and primary cavities affect the degree of tuberculin sensitivity and could obscure whatever effect M. vaccae immunotherapy might have had.
That M. vaccae did not appreciably affect the tuberculin sensitivities of the tuberculous rabbits is similar to what was found for humans (8, 35, 50): in individuals with active TB, M. vaccae injections were a consistently safe therapeutic measure because the M. vaccae (unlike Koch’s tuberculin “therapeutic” injections [25]) did not cause systemic hypersensitivity reactions (8, 35, 50).
(v) Effects of M. vaccae immunotherapy on cavity formation.
The mean numbers of primary lesions (including those that formed cavities) for the M. vaccae-treated and control groups were comparable: 8.0 ± 1.9 and 9.5 ± 1.5 lesions, respectively (Table 4). The mean numbers of these primary cavitary lesions that cavitated in the M. vaccae-treated and control groups were 3.0 ± 0.9 and 1.8 ± 0.8, respectively (Table 4). However, due to the large variation among the rabbits, the difference in these means was not statistically significant (P = 0.341). The percentage of primary lesions that cavitated is a better index of M. vaccae’s effect on cavitation (Table 4 and Fig. 3). It averaged 35.7% ± 11.9% for the M. vaccae-treated group and 18.7% ± 9.5% for the control group. However, the large variability within the two groups showed that again this difference was of no statistical significance (P = 0.291). If we assume that the difference between the average percentages for the two groups (35.7% − 18.7%) was truly 17.0%, then 350 rabbits (175 + 175) would be required to produce a result that would be statistically significant at the 95% certainty level. (For this computation, we have treated the data as simple random samples and used an α error of 0.05 [two-tailed] and a β error of 0.05 [one-tailed]).
Although the P values were high enough to assure us that the observed M. vaccae effect on cavity formation was a chance phenomenon, the difference in means is a bit worrisome. Cavity formation is an immune reaction (16, 56, 57) that is a major cause of the spread of tubercle bacilli from person to person as well as a major factor in the development of multidrug-resistant strains of bacilli. (They multiply profusely [and extracellularly] in the well-oxygenated cavity wall [Fig. 5].) An immunotherapeutic agent that favors cavity formation would definitely be contraindicated.
(vi) Number of bacilli in liquefied and cavitary lesions: its variability masked any M. vaccae effect.
In most lesions with liquefied centers, appreciable numbers of acid-fast bacilli were visible microscopically (7, 26), but in lesions with solid caseous centers only a few bacilli were visible (26). The number of bacilli probably increases substantially after cavities form. However, to date no careful comparisons between the number of bacilli in the liquefied caseum of cavitary lesions and the number of bacilli in the liquefied caseum of noncavitary lesions have been made. The increased oxygen provided by the connection to the bronchial tree has always been considered to be a stimulus for bacillary multiplication (26), and Fig. 5B clearly demonstrates this phenomenon: the most numerous bacilli exist in the liquefied caseum adjacent to the air within the cavity.
The multiplication of tubercle bacilli within individual liquefied caseous lesions is extremely variable (7), probably depending on the composition of the liquefied caseum and on the rate at which the bacilli adapt to the new extracellular environment. Because of this variability, the effects of M. vaccae immunotherapy on the extracellular growth of tubercle bacilli could not be determined.
(vii) Experiment IV: immunotherapy with 1/10 the human dose of M. vaccae.
Because the human dose of 109 M. vaccae cells may have been excessive for rabbits, a small pilot-type experiment was performed at Texas A & M University. In this experiment 108 M. vaccae baccilli (1/10 the human dose) was given intradermally at 9 and 13 weeks after the rabbits inhaled about 1,000 U of one to three virulent M. bovis bacilli (Ravenel S). The inhaled dose was intermediate between those in experiments II and III (Tables 1 and 5). The Texas A & M aerosol exposure system is described in references 32 and 54.
Three rabbits received M. vaccae, and two received diluent alone. When the rabbits were sacrificed at 19 weeks, the M. vaccae-treated group had fewer and smaller primary lesions and fewer secondary lesions (Table 5). The low number of primary lesions in the M. vaccae-treated group was probably not due to immunotherapy, because such therapy began at 9 weeks, long after these lesions were well established. (Grossly visible primary pulmonary lesions produced by virulent Mycobacterium bovis in rabbits have never been shown to regress [26]). The reduced size of the primary lesions and the lower number of secondary pulmonary lesions could have been due to M. vaccae immunotherapy, but such differences from the control group were not statistically significant. These suggestive results are, however, consistent with those found in human M. vaccae trials (see Discussion).
DISCUSSION
Rabbit model.
Pulmonary TB in rabbits (7, 9, 11, 15, 26) can be an excellent model for TB in human beings (17), in that both species are relatively resistant to TB and both species readily form liquefied caseous foci and cavities. Neither species is inbred, so considerable variation in the amount and extent of the disease occurs. Much variation among the individual lesions within a given host also occurs (7). These factors make studies of both rabbits and humans more difficult and more expensive than studies of inbred mice, but cavitary TB cannot be produced in mice and only rarely in guinea pigs.
Virulence of the tubercle bacillus, strain Ravenel S (ATCC 35720).
This Ravenel S strain of virulent bovine-type tubercle bacilli was not fully virulent. With fully virulent (bovine-type) Ravenel S tubercle bacilli, one-third of the bacillary units inhaled produce one grossly visible tubercle in both resistant and susceptible inbred rabbits (1, 26, 27, 30). With Ravenel S, 18 to 107 inhaled bacillary units were required to produce one grossly visible primary pulmonary tubercle (Table 2). About every 5 to 10 years, Lurie (26) maintained the full virulence of the Ravenel S strain that he used by infecting rabbits and recovering the bacilli from the tuberculous lesions produced. This procedure was successful because only the most virulent bacilli seemed to survive in such lesions after the immune response developed.
The ATCC personnel kept Ravenel S lyophilized after they received it from the Trudeau Mycobacterial Culture Collection in 1985 (see Materials and Methods). It was obtained in 1910 from M. P. Ravenel of the University of Wisconsin in Madison. Ravenel had isolated it from a tuberculous bovine. At the Trudeau Institute, it was periodically cultured on Proskauer-Beck solid media until 1970, when it was rapidly frozen and stored (unlyophilized) at −70°C until it was sent to ATCC. At the Trudeau Institute, the infection of mice, guinea pigs, and rabbits was used to study virulence, and strains of reduced virulence were usually replaced by bacilli from early cultures that had been stored in a frozen state. However, the aerosol method of infection and subsequent pulmonary tubercle counts (12) were not apparently used for virulence testing.
This history was supplied by Frank M. Collins, Mycobacterial Laboratory, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Rockville, Maryland, and from reference 5a. The repeated subculturing could have reduced the virulence of the Ravenel S strain for rabbits, but lyophilization was the most likely cause. Lyophilization apparently reduced the virulence of H37Rv mycobacteria in the mouse (6).
The initial (very early) immune response.
The initial immune response determines whether a microscopic pulmonary tubercle develops into a grossly visible tubercle, if the infecting strain of tubercle bacillus is not 100% virulent for the species involved (which is the case for humans). The studies herein reported provide new insight into this early host immune response.
In the lung, each site of bacillary lodgment is usually separated by numerous alveolar spaces. Therefore, we always thought that early sites of infection were completely independent of each other. However, Table 2 shows (i) that large numbers of developing lesions had systemic effects that apparently reduced the efficacy of the host’s early innate and acquired immune responses or (ii) that low numbers of developing lesions had systemic effects that increased the efficacy of these responses.
The efficacy of alveolar macrophages (AM) could not have been reduced or increased, because AM determine the fate of the developing lesion only during the first few days before the blood-borne monocytes/macrophages take over (9, 10, 15, 17). AM are highly activated macrophages (14). They are apparently activated by the continuous ingestion of a variety of inhaled particles (18). The ability of these phagocytes to destroy or inhibit tubercle bacilli exists before the infection begins and would not be influenced by a large or small inhaled bacillary dose.
Therefore, the large inhaled dose of bacilli must have affected the blood-borne macrophages that control the fate of the very early tubercle after the AM cease to play a role. Blood-borne macrophages are activated (i) by the host’s innate immune response, i.e., by natural killer cells and the gamma interferon (IFN-γ) that they produce (23, 33), and (ii) by the host’s acquired immune response, i.e., by Th1 lymphocytes (34, 36) and the cytokines (including the IFN-γ) that they produce (17). Once activated, the blood-borne macrophages can also inhibit the intracellular growth of the bacillus, thereby preventing some of the early microscopic tubercles from reaching grossly visible size.
Thus, both the AM and the infiltrating blood-borne mononuclear cells, including natural killer cells, lymphocytes, macrophages, and probably dendritic cells, can together stop inhaled tubercle bacilli from producing grossly visible pulmonary lesions (12). Once established, grossly visible tubercles can heal with fibrosis, stabilize, or progress, but they will usually be recognizable at necropsy, even years later.
The dose effects discussed above were probably due to changes in the Th1/Th2 lymphocyte ratios, as first described by Bretscher et al. (5). After being infected with a very low dose of Leishmania major, fully susceptible BALB/c mice produced a “resistant” Th1 response and survived. After a standard dose, such mice always produced a “susceptible” Th2 response and succumbed to the infection. The low infecting doses of L. major apparently locked in the beneficial Th1 response, so much so that these BALB/c mice could survive a subsequent challenge with the standard dose of L. major (5).
Because only few bacilli are usually inhaled over a period of months, pulmonary TB in most human beings begins as a single primary tubercle. This initial low dose would favor a high Th1/Th2 ratio, a fact which partly explains why humans usually must inhale an estimated 20 to 200 bacillary units before they develop the disease.
Unfortunately, Lurie (26) did not expose his susceptible and resistant strains of rabbits to very low aerosolized doses of tubercle bacilli, but he did prove that the very early developing primary pulmonary lesions were controlled by systemic factors. Decreasing the immune response by cortisone treatment increased the number of such grossly visible lesions (29), probably (at least in part) due to a decrease in the Th1/Th2 ratio (19, 37). Increasing the immune response by a previous vaccination with M. bovis BCG decreased the number of primary lesions that were grossly visible 5 weeks after the inhalation of virulent human-type tubercle bacilli (28). In this BCG experiment, many primary microscopic tubercles were evidently eliminated or arrested by the developing immune response before they became grossly visible. In fact, such early immune-mediated inhibition of bacillary growth is the way in which clinically evident disease is prevented by vaccination. The destruction of tubercle bacilli by AM is immunologically nonspecific and would not be increased after the nonspecific adjuvant effects of the vaccine have subsided.
All of these studies indicate that systemic factors affect the progression and regression of early-developing primary tubercles. In these tubercles, the ratio of Th1 to Th2 lymphocytes appears to be under systemic control. Studies of the composition and functions of peripheral blood mononuclear cells (monocytes and lymphocytes) (20–22, 52) may therefore give some indication of what is occurring in the local tuberculous lesions. However, it remains to be investigated whether microscopic primary tubercles that regress or stabilize have a higher local Th1/Th2 ratio than do microscopic primary tubercles that progress to a grossly visible size.
Tuberculin sensitivity in hosts with stabilized disease.
The studies reported herein show that rabbits and human beings probably differ in maintaining their tuberculin sensitivity as well as in the amount of this sensitivity. In humans, when TB is not extensive, the tuberculin sensitivity seems to be maintained at rather high levels for years, even after the disease has regressed or stabilized (7a). In the rabbits of our low-dose experiment III, the peak tuberculin sensitivity was not maintained at high levels, even though their pulmonary TB was stabilized or only slowly progressing at 33 weeks, and they appeared overtly healthy (Table 4). This decrease in tuberculin sensitivity is consistent with what is found for many immunologic reactions, which peak and then regress. Unfortunately, no sequential study of tuberculin reactions in humans with TB seems to have been made. Once such a patient is found to be tuberculin positive, further skin tests are usually stopped because of the danger of severe reactions (including necrosis) at the test site. Thus, we have no information on whether tuberculin sensitivity peaked and partly regressed in patients whose TB was well controlled, but we do know that it remains strong for many years.
Humans are at least 100 times more sensitive to tuberculin than are rabbits (unpublished experiments from our laboratory with 250 tuberculin units of purified protein derivative [PPD]). (Guinea pigs are only about two times more sensitive than rabbits to tuberculin [26a].) The differences in the tuberculin sensitivities of rabbits and humans (when both are infected with tubercle bacilli) may be due to differences in their diets and in their bowel physiologies. Wild rabbits are in constant contact with the soil mycobacteria, and even laboratory rabbits are probably exposed to such mycobacteria. Their diet in the wild consists of uncooked vegetation, and sometimes in captivity raw vegetables are included. Rabbits have a huge cecum, a blind pouch (8 to 9 in. long and about 1 in. in diameter) that ends in a well-developed appendix. In this cecum, bacteria break down the vegetable cellulose providing nourishment for the host. Therefore, soil mycobacteria probably exist in the bacterial flora of the cecum and other parts of the rabbit intestine (44).
Group I (common) antigens are produced by all mycobacteria (46). Group II antigens are produced by all slow-growing mycobacteria including both virulent TB bacilli and slow growers from the soil (31, 45, 46), and group II antigens are major components of tuberculin, including PPD (31, 45). Rabbits are continuously exposed to the group II antigens in the intestines and probably become at least partially desensitized to these antigens (45). They remain sensitive, however, to the group IV species-specific antigens of virulent mycobacteria (45). These concepts may explain why tuberculous rabbits react more weakly than humans to tuberculin and why their tuberculin skin tests may not be maintained as well as those of infected human beings. They also imply that the group II antigens (present in both virulent and nonvirulent tubercle bacilli) are important in maintaining in human beings both tuberculin sensitivity and the resistance produced by BCG vaccination (31, 44, 45).
In humans with extensive TB, the tuberculin reactions may become negative (20–22, 52). In our high-dose experiment II, rabbits with extensive TB had tuberculin reactions that were about one-third the size of those of rabbits in low-dose experiment III with less extensive TB (Table 1). These findings indicate that extensive pulmonary TB can reduce the tuberculin reactions in both rabbits and human beings.
Effects of M. vaccae immunotherapy.
In tuberculin-negative healthy humans, injections of M. vaccae usually did not convert the PPD skin test (53). Also, BCG mixed with M. vaccae produced the same percentage of positive PPD skin tests as did BCG alone (2), but the diameters of the skin reactions tended to be smaller in people receiving the mixture (4).
Studies of M. vaccae effects in humans have involved much larger numbers of individuals than are practical with rabbits, and statistically significant results were obtained (8, 35, 47, 50). A single injection of 109 M. vaccae bacilli (given early during antimicrobial therapy) halved the treatment failure rates and reduced the death rates. Repeated injections of M. vaccae at 2-month intervals appeared promising in patients with multidrug-resistant TB, in patients coinfected with human immunodeficiency virus, and in patients receiving inadequate antimicrobial therapy (49). M. vaccae also improved the patients’ clinical condition and helped clear the sputum of bacilli. These effects were thought to be due to the enhancement of the ratio of Th1 lymphocytes to Th2 lymphocytes (40–42) and to the decrease in the amount of tissue destruction caused by the Koch phenomenon (24, 38, 40–42, 47). In these human studies, a reduction in tuberculin sensitivity was produced by M. vaccae immunotherapy (48), and this reduction was again considered to be due to the enhancement of the Th1/Th2 cell ratio. Yamamura (57) showed that no cavities developed in rabbits desensitized to a tuberculin-active peptide. Therefore, a decrease in tuberculin sensitivity produced by M. vaccae might reduce the progression of existing cavities and reduce the formation of new cavities. The above-listed favorable effects for the host were evidently produced by group I antigens common to M. vaccae and Mycobacterium tuberculosis coupled with the absence in M. vaccae of the species-specific antigens present in M. tuberculosis (40–42, 47, 48).
In our M. vaccae rabbit experiments, no antimicrobial drugs were administered, whereas in the human M. vaccae trials antimicrobials were simultaneously given. This difference in experimental design may have partly contributed to our inability to confirm in rabbits the results of the human trials that showed M. vaccae immunotherapy to be beneficial.
ACKNOWLEDGMENTS
We appreciate the editorial assistance of both Ilse M. Harrop and Rena Ashworth; the statistical help of Haydar Sengul, Department of Oncology; and the review of Robert Koch’s 1890 article by Johan Otterhaus, Department of Epidemiology, all at Johns Hopkins University, Baltimore, Md.
This work was supported by grant AI-35195 for the National Institute of Allergy and Infectious Diseases, Bethesda, Md., and in part by grant ES-03819 (for the Johns Hopkins Environmental Health Sciences Center) from the National Institute of Environmental Health Sciences, Research Triangle Park, N.C., and grant HL-10342 from the National Heart, Lung and Blood Institute, Bethesda, Md.
REFERENCES
- 1.Allison M J, Zappasodi P, Lurie M B. Host-parasite relationships in natively resistant and susceptible rabbits on quantitative inhalation of tubercle bacilli. Am Rev Respir Dis. 1962;85:553–569. doi: 10.1164/arrd.1962.85.4.553. [DOI] [PubMed] [Google Scholar]
- 2.Bahr G M, Stanford J L, Rook G A W, Rees R J W, Frayha G J, Abdelnoor A M. Two potential improvements to BCG and their effect on skin test reactivity in Lebanon. Tubercle. 1986;67:205–218. doi: 10.1016/s0041-3879(86)80027-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew J W. Stains for microorganisms in sections. In: Conn H J, Darrow M A, Emmel V M, editors. Staining procedures. 2nd ed. Baltimore, Md: Williams & Wilkins; 1981. pp. 446–447. [Google Scholar]
- 4.Bottasso O, Merlin V, Cannon L, Cannon H, Ingledew N, Keni M, Hartopp R, Stanford C, Stanford J. Studies of vaccination of persons in close contact with leprosy patients in Argentina. Vaccine. 1998;16:1166–1171. doi: 10.1016/s0264-410x(98)80115-1. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 5a.Catalog of the Mycobacterial Culture Collection. National Institutes of Health publication 80-289. Bethesda, Md: National Institutes of Health; 1980. [Google Scholar]
- 6.Collins F M, Smith M M. A comparative study of the virulence of Mycobacterium tuberculosis measured in mice and guinea pigs. Am Rev Respir Dis. 1969;100:631–639. doi: 10.1164/arrd.1969.100.5.631. [DOI] [PubMed] [Google Scholar]
- 7.Converse P J, Dannenberg A M, Jr, Estep J E, Sugisaki K, Abe Y, Schofield B H, Pitt M L M. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect Immun. 1996;64:4776–4787. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Comstock, G. W. Personal communication.
- 8.Corlan E, Marica C, Macavei C, Stanford J L, Stanford C A. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. 2. Chronic or relapsed disease. Respir Med. 1997;91:21–29. doi: 10.1016/s0954-6111(97)90133-5. [DOI] [PubMed] [Google Scholar]
- 9.Dannenberg A M., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 10.Dannenberg A M., Jr Immunopathogenesis of pulmonary tuberculosis. Hosp Pract. 1993;28:33–40. doi: 10.1080/21548331.1993.11442738. , 51–58. [DOI] [PubMed] [Google Scholar]
- 11.Dannenberg A M., Jr . Rabbit model of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 149–156. [Google Scholar]
- 12.Dannenberg A M., Jr Lurie’s tubercle-count method to test TB vaccine efficacy in rabbits. Front Biosci. 1998;3:c27–33. doi: 10.2741/a261. [DOI] [PubMed] [Google Scholar]
- 13.Dannenberg A M, Jr, Ando M, Shima K. Macrophage accumulation, division, maturation, and digestive and microbial capacities in tuberculous lesions. III. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972;109:1109–1121. [PubMed] [Google Scholar]
- 14.Dannenberg A M, Jr, Burstone M S, Walter P C, Kinsley J W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dannenberg A M, Jr, Rook G A W. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 459–483. [Google Scholar]
- 16.Dannenberg A M, Jr, Sugimoto M. Liquefaction of caseous foci in tuberculosis. Am Rev Respir Dis. 1976;113:257–259. doi: 10.1164/arrd.1976.113.3.257. [DOI] [PubMed] [Google Scholar]
- 17.Dannenberg A M, Jr, Tomashefski J F., Jr . Pathogenesis of pulmonary tuberculosis. In: Fishman A P, et al., editors. Fishman’s pulmonary diseases and disorders. 3rd ed. Vol. 2. New York, N.Y: McGraw-Hill Co., Inc.; 1997. pp. 2447–2471. [Google Scholar]
- 18.Dannenberg A M, Jr, Walter P C, Kapral F A. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. II. The effect of particle ingestion on enzyme activity; two phases of in vitro activation. J Immunol. 1963;90:448–465. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daynes R A, Araneo B A, Dowell T A, Hung K, Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellner J J. Immunosuppression in tuberculosis. Infect Agents Dis. 1996;5:62–72. [PubMed] [Google Scholar]
- 21.Ellner J J. Regulation of the human immune response during tuberculosis. J Lab Clin Med. 1997;130:469–475. doi: 10.1016/s0022-2143(97)90123-2. [DOI] [PubMed] [Google Scholar]
- 22.Ellner J J. Review: the immune response in human tuberculosis—implications for tuberculosis control. J Infect Dis. 1997;176:1351–1959. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 23.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Pando R, Rook G A W. The role of TNF-alpha in T-cell-mediated inflammation depends upon the Th1/Th2 cytokine balance. Immunology. 1994;82:591–595. [PMC free article] [PubMed] [Google Scholar]
- 25.Koch R. Weitere Mittheilungen über ein Heilmittel gegen Tuberkulose. Dtsch Med Wochenschr. 1890;16:1029–1032. [Google Scholar]
- 26.Lurie M B. Resistance to tuberculosis: experimental studies in native and acquired defensive mechanisms. Cambridge, Mass: Harvard University Press; 1964. [Google Scholar]
- 26a.Lurie, M. B. Personal communication.
- 27.Lurie M B, Heppleston A G, Abramson S, Swartz I B. An evaluation of the method of quantitative airborne infection and its use in the study of the pathogenesis of tuberculosis. Am Rev Tuberc. 1950;61:765–797. [PubMed] [Google Scholar]
- 28.Lurie M B, Zappasodi P, Cardona-Lynch E, Dannenberg A M., Jr The response to the intracutaneous inoculation of BCG as an index of native resistance to tuberculosis. J Immunol. 1952;68:369–387. [PubMed] [Google Scholar]
- 29.Lurie M B, Zappasodi P, Dannenberg A M, Jr, Swartz I B. Constitutional factors in resistance to infection: the effect of cortisone on the pathogenesis of tuberculosis. Science. 1951;113:234–237. doi: 10.1126/science.113.2931.234. [DOI] [PubMed] [Google Scholar]
- 30.Lurie M B, Zappasodi P, Tickner C. On the nature of genetic resistance to tuberculosis in the light of host-parasite relationships in natively resistant and susceptible rabbits. Am Rev Tuberc Pulm Dis. 1955;72:297–329. doi: 10.1164/artpd.1955.72.3.297. [DOI] [PubMed] [Google Scholar]
- 31.McManus I C, Lockwood D N J, Stanford J L, Shaaban M A, Abdul-Ati M, Bahr G M. Recognition of a category of responders to group II, slow-grower associated, antigens amongst Kuwaiti senior school children, using a statistical model. Tubercle. 1988;69:275–281. doi: 10.1016/0041-3879(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 32.McMurray D N, Collins F M, Dannenberg A M, Jr, Smith D W. Pathogenesis of experimental tuberculosis in animal models. In: Shinnick T M, editor. Tuberculosis. Berlin, Germany: Springer-Verlag; 1996. pp. 157–179. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov R, Janeway C A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 34a.National Research Council. Guide for the care and use of laboratory animals. National Institutes of Health publication 86-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 35.Onyebujoh P, Abdulmumini T, Robinson S, Rook G A W, Stanford J L. Immunotherapy with Mycobacterium vaccae as an addition to chemotherapy for the treatment of pulmonary tuberculosis under difficult conditions in Africa. Respir Med. 1995;89:199–207. doi: 10.1016/0954-6111(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 37.Rook G A W. TH1/TH2 switching and loss of CD4+ T cells in chronic infections: an immunoendocrinological hypothesis not exclusive to HIV. Immunol Today. 1993;14:568–569. doi: 10.1016/0167-5699(93)90190-V. [DOI] [PubMed] [Google Scholar]
- 38.Rook G A W, al Attiyah R. Cytokines and the Koch phenomenon. Tubercle. 1991;72:13–20. doi: 10.1016/0041-3879(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 39.Rook G A W, Bahr G M, Stanford J L. The effect of two distinct forms of cell-mediated response to mycobacteria on the protective efficacy of BCG. Tubercle. 1981;62:63–68. doi: 10.1016/0041-3879(81)90038-6. [DOI] [PubMed] [Google Scholar]
- 40.Rook G A W, Bloom B R. Mechanisms of pathogenesis in tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 485–501. [Google Scholar]
- 41.Rook G A W, Hernandez-Pando R. T cell helper types and endocrines in the regulation of tissue-damaging mechanisms in tuberculosis. Immunobiology. 1994;191:478–492. doi: 10.1016/S0171-2985(11)80454-7. [DOI] [PubMed] [Google Scholar]
- 42.Rook G A W, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 43.Rosner B. Fundamentals of biostatistics. 4th ed. Belmont, Calif: Wadsworth Publishing Co.; 1995. pp. 443–550. [Google Scholar]
- 44.Shield M J. The importance of immunologically effective contact with environmental mycobacteria. In: Ratledge C, Stanford J L, editors. The biology of mycobacteria. Vol. 2. London, United Kingdom: Academic Press; 1983. pp. 343–415. [Google Scholar]
- 45.Stanford J L. Newer tuberculins: profile in developing countries. In: Seth V, editor. Essentials of tuberculosis in children. New Delhi, India: Jaypee Brothers; 1997. pp. 58–72. [Google Scholar]
- 46.Stanford J L, Grange J M. The meaning and structure of species as applied to mycobacteria. Tubercle. 1974;55:143–152. doi: 10.1016/0041-3879(74)90008-7. [DOI] [PubMed] [Google Scholar]
- 47.Stanford J L, Grange J M. New concepts for the control of tuberculosis in the twenty-first century. J R Coll Physicians Lond. 1993;27:218–223. [PMC free article] [PubMed] [Google Scholar]
- 48.Stanford J L, Rook G A W, Bahr G M, Dowlati Y, Ganapati R, Ghazi-Saidi K, Lucas S, Ramu G, Torres P, Minh-Ly H, Anstey N. Mycobacterium vaccae in immunoprophylaxis and immunotherapy of leprosy and tuberculosis. Vaccine. 1990;8:525–530. doi: 10.1016/0264-410x(90)90002-4. [DOI] [PubMed] [Google Scholar]
- 49.Stanford J L, Stanford C A. Immunotherapy with Mycobacterium vaccae and the treatment of tuberculosis. J Appl Bacteriol. 1996;81:81S–86S. [PubMed] [Google Scholar]
- 50.Stanford J L, Stanford C A, Rook G A W, Grange J M. Immunotherapy for tuberculosis: investigative and practical aspects. Clin Immunother. 1994;1:430–440. [Google Scholar]
- 51.Tsukamura M. The “non-pathogenic” species of mycobacteria: their distribution and ecology in non-living reservoirs. In: Kubicka G P, Wayne L G, editors. The mycobacteria: a sourcebook. Vol. 15. New York, N.Y: Marcel Dekker; 1984. pp. 1339–1359. [Google Scholar]
- 52.Tsuyuguchi I. Regulation of the human response in tuberculosis. Infect Agents Dis. 1996;5:82–97. [PubMed] [Google Scholar]
- 53.von Reyn C F, Arbeit R D, Yeaman G, Waddell R D, Marsh B J, Morin P, Modlin J F, Remold H G. Immunization of healthy adult subjects in the United States with inactivated Mycobacterium vaccae administered in a three-dose series. Clin Infect Dis. 1997;24:843–848. doi: 10.1093/clinids/24.5.843. [DOI] [PubMed] [Google Scholar]
- 54.Wiegeshaus E H, McMurray D N, Grover A A, Harding G E, Smith D W. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970;102:422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]
- 55.Woolson R F. Statistical methods for the analysis of biomedical data. New York, N.Y: John Wiley and Sons; 1987. pp. 280–313. [Google Scholar]
- 56.Yamamura Y. The pathogenesis of tuberculous cavities. Adv Tuberc Res. 1958;9:13–37. [PubMed] [Google Scholar]
- 57.Yamamura Y, Ogawa Y, Maeda H, Yamamura Y. Prevention of tuberculosis cavity formation by desensitization with tuberculin-active peptide. Am Rev Respir Dis. 1974;109:594–601. doi: 10.1164/arrd.1974.109.6.594. [DOI] [PubMed] [Google Scholar]