Abstract
Endothelial function (brachial artery flow-mediated dilation [FMD]) is reduced in estrogen-deficient postmenopausal women, mediated, in part, by reduced nitric oxide (NO) bioavailability, secondary to tetrahydrobiopterin (BH4) deficiency and oxidative stress. FMD is increased, but not fully restored, in postmenopausal women after acute intravenous vitamin C (VITC; superoxide scavenger) or oral BH4 supplementation. In vitro studies demonstrate that coadministration of VITC with BH4 prevents endothelial nitric oxide synthase (eNOS) uncoupling and reductions in NO by peroxynitrite. To investigate mechanisms of endothelial dysfunction in women, we assessed the separate and combined effects of VITC and BH4 to determine whether coadministration of VITC + BH4 improves FMD in healthy postmenopausal women (n = 19, 58 ± 5 yr) to premenopausal (n = 14, 36 ± 9 yr) levels, with exploratory testing in perimenopausal women (n = 8, 51 ± 3 yr). FMD was measured during acute intravenous infusions of saline (control) and VITC (∼2–3 g) ∼3 h after a single dose of oral BH4 (KUVAN, 10 mg/kg body wt) or placebo (randomized crossover, separated by ∼1 mo). Under the placebo condition, FMD was reduced in postmenopausal compared with premenopausal women during the saline infusion (5.6 ± 0.7 vs. 11.6 ± 0.9%, P < 0.001) and increased in postmenopausal women during VITC (+3.5 [1.4, 5.6]%, P = 0.001) and acute BH4 (+1.8 [0.37, 3.2]%, P = 0.01) alone. Coadministration of VITC + BH4 increased FMD in postmenopausal women (+3.0 [1.7, 4.3]%, P < 0.001), but FMD remained reduced compared with premenopausal women (P = 0.02). Exploratory analyses revealed that VITC + BH4 did not restore FMD in perimenopausal women to premenopausal levels (P = 0.045). Coadministration of VITC + BH4 does not restore FMD in menopausal women, suggesting that additional mechanisms may be involved.
NEW & NOTEWORTHY Endothelial function is reduced across the menopausal stages related to increased oxidative stress associated with estrogen deficiency. In vitro studies demonstrate that coadministration of VITC with BH4 prevents endothelial nitric oxide synthase (eNOS) uncoupling and reductions in NO by peroxynitrite; however, this remains untested in humans. We demonstrate that the coadministration of BH4 + VITC does not restore endothelial function in perimenopausal and postmenopausal women to the level of premenopausal women, suggesting that other mechanisms contribute.
Keywords: aging, endothelial function, menopause, nitric oxide bioavailability
INTRODUCTION
Menopause is a period of accelerated cardiovascular disease (CVD) risk in women related to changes in the hormone environment. The increased CVD risk during menopause is mediated, in part, by an acceleration in vascular aging, characterized by endothelial dysfunction, a key antecedent for the development of CVD (1). Endothelial function is progressively reduced across the stages of the menopause transition, independent of age and CVD risk factors (2), and is attributed to the loss of estrogen (3). In support of this, our group recently demonstrated that short-term pharmacological ovarian suppression (via gonadotropin-releasing hormone antagonist) reduced endothelial function measured by brachial artery flow-mediated dilation (FMD) in premenopausal and perimenopausal women receiving placebo but not estrogen add-back treatment. These and other data suggest that hormonal changes during the menopause transition are a triggering event for increased vascular vulnerability in women as they age.
Oxidative stress, characterized as excessive reactive oxygen species (ROS) production relative to antioxidant defense capacity, is a key mechanism underlying endothelial dysfunction with the menopause transition (3). Infusion of supraphysiological levels of the antioxidant vitamin C (VITC) is a commonly used experimental approach to temporarily and reversibly reduce ROS and remove the tonic suppression of vascular function by oxidative stress (3, 4). With this approach, endothelial function did not change in premenopausal and early perimenopausal women with VITC, likely related to sufficient levels of estrogen; however, VITC improved endothelial function in late perimenopausal and estrogen-deficient postmenopausal women, indicating tonic suppression of endothelial function by ROS. Importantly, VITC improved but did not fully rescue the impairment in endothelial function in late perimenopausal women and postmenopausal women, suggesting that other sources of ROS are not scavenged by VITC and/or other mechanisms contribute.
Excessive ROS production can oxidize endothelial nitric oxide synthase [eNOS, enzyme that produces nitric oxide (NO)] and its cofactors [i.e., tetrahydrobiopterin (BH4)], uncoupling eNOS and producing ROS, decreasing NO bioavailability, and increasing reactive nitrogen species including peroxynitrite in a vicious cycle to impair vascular function and induce cellular damage (5). Indeed, acute oral BH4 administration improved, but did not restore, endothelial function to premenopausal levels in estrogen-deficient postmenopausal women, suggesting that reduced BH4 bioavailability and eNOS uncoupling contribute to a portion of endothelial dysfunction in estrogen-deficient postmenopausal women (6). Interestingly, in vitro studies demonstrate that the coadministration of BH4 and VITC prevents eNOS uncoupling and reductions in NO bioavailability in bovine endothelial cells treated with a peroxynitrite donor compared with the administration of either one alone (7); however, this remains untranslated in humans. Accordingly, the purpose of this study was to investigate mechanisms of endothelial dysfunction in menopausal women. We tested the separate and combined effects of BH4 and VITC on endothelial function and whether the coadministration of BH4 + VITC would improve endothelial function in postmenopausal women back to levels of premenopausal women. Additionally, exploratory analyses were conducted in a subset of perimenopausal women.
METHODS
Women aged 18–75 yr were recruited from the Denver Metropolitan area to undergo testing at the University of Colorado Clinical Translational Science Institute (CCTSI) Clinical and Translational Research Center (CTRC). All women were considered healthy as determined by self-reported medical history, physical examination, standard blood chemistries (chemistry panel, complete blood count [CBC], thyroid stimulating hormone), and ECG at rest and during a maximal-exercise treadmill test. Additional inclusion criteria included fasted glucose < 126 mg/dL, resting blood pressure < 140/90 mmHg, nonsmoking, sedentary or recreationally active (<3 days/wk vigorous exercise), no use of vascular altering medications, no use of oral contraceptives or hormone therapy for at least 6 mo before study participation, and natural menopause in postmenopausal women. Participants had not used vitamins, supplements, or anti-inflammatory medications for at least 4 wk before vascular testing. All procedures were approved by the Colorado Multiple Institutional Review Board and conducted in conformance with the Declaration of Helsinki. The trial was registered on clinicaltrials.gov (NCT No. 2042196) before participant, and all participants provided their written informed consent to participate.
Menopausal Staging
Menopausal status was characterized in all women with the Stages of Reproductive Aging Workshop Survey (8). Perimenopausal women were classified as early (i.e., 2 or more cycles with cycle length changes of 7 days or more) or late (i.e., amenorrhea for 2 mo or more) perimenopausal for the purposes of vascular testing but were collapsed into one group because of exploratory analyses. Postmenopausal women had gone 1 yr or longer since their final menstrual period and were further characterized as early (i.e., ≤5 yr) or late (i.e., >5 yr) postmenopausal for subject characteristics only.
Study Design
Eligible participants were enrolled in a randomized, placebo-controlled, crossover design study (∼1 mo between visits) to determine the separate and combined effects of BH4 and VITC. Vascular testing was conducted in the supine position after an overnight fast (including caffeine) with proper hydration. Women refrained from exercise for at least 20 h before testing. Premenopausal and early perimenopausal (when possible) women were studied during the 7–10 days (i.e., midfollicular phase) after the onset of menses so that vascular testing was performed at a similar cycle phase (3). Late perimenopausal women were tested regardless of menstrual cycle phase after 2 mo of amenorrhea, and postmenopausal women were studied at any time.
Acute Oral BH4 and VITC Infusion Intervention
Participants were randomized to consume either placebo (compounded by Belmar Pharmacy, Colorado) or BH4 (10 mg/kg body wt; KUVAN, BioMarin, Novato, CA) dissolved in 8 oz of water 3 h before reporting for vascular testing based on prior studies demonstrating maximal plasma concentrations peaking at ∼3 h (9). This dose has been shown to increase plasma biopterin levels by 50-fold (9) and to improve endothelial function in estrogen-deficient postmenopausal women (6). The difference in FMD during BH4 versus saline infusion was interpreted as modulation of FMD by eNOS uncoupling.
Vascular assessments were obtained after a 20-min bolus of normal isovolumic saline, followed by a “drip” infused systemically (control), and then repeated after a bolus of 100 mL of VITC solution (dosed at 0.06 g VITC per kg fat-free mass per 100 mL normal saline) followed by a “drip infusion” until testing was completed as previously described (3). This dose of VITC has been shown to improve vascular function in estrogen-deficient postmenopausal women (4, 10–12). The difference in FMD during VITC versus saline infusion was interpreted as modulation of FMD by oxidative stress.
Measurements
Clinical characteristics.
Seated blood pressure was measured in triplicate and averaged with a semiautomated device (Dinamap; Johnson & Johnson) after 10 min of rest (3). Total body fat was calculated as a percentage by dual-energy X-ray absorptiometry (Hologic Discovery). Waist and hip circumferences were measured in triplicate and averaged and used to calculate a waist-to-hip ratio.
Vascular testing.
Brachial artery FMD was measured as previously described (3, 6) in response to 5 min of forearm cuff occlusion (250 mmHg) with duplex ultrasonography (GE Vivid I) using a multifrequency linear-array transducer according to established guidelines (13). FMD was measured during intravenous administration of normal saline (control) and a supraphysiological dose of VITC as previously described as a relative change (%, primary outcome) and an absolute change (millimeter, secondary outcome) (3, 4). Brachial artery diameters were analyzed in end diastole with a commercially available software package (Vascular Analysis Tools 5.5.1; Medical Imaging Applications, Iowa City, IA) (6).
Blood sampling.
An intravenous catheter was placed in an antecubital vein for blood sampling and infusion of normal saline and VITC. Fasted blood glucose, insulin, and total (Roche Diagnostics Systems, Indianapolis, IN) and high-density lipoprotein (HDL) cholesterol were measured by enzymatic/colorimetric methods as previously described (3). Low-density lipoprotein was calculated with the Friedewald equation. Estradiol, follicle-stimulating hormone (FSH), progesterone, and sex hormone-binding globulin (SHBG) were determined by chemiluminescence (3). Estrone was measured by radioimmunoassay, and testosterone was calculated by a one-step competitive assay (Beckman Coulter). Endothelin-1 was measured by enzyme-linked immunoassay (3). Oxidized LDL was measured by ELISA plate assay (Alpco Diagnostics, Windham, NH). Total antioxidant status (TAS) was calculated in serum samples with the Randox Laboratories enzymatic kit (Oceanside, CA).
Statistical Analysis
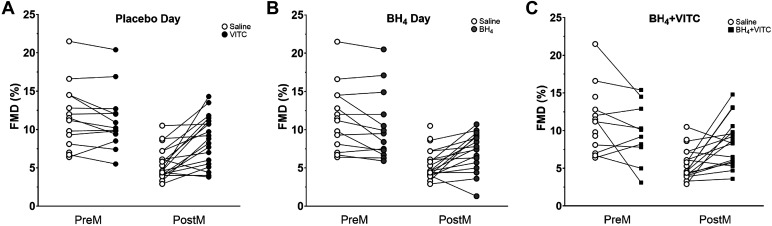
Analyses were conducted with R software (v.4.1.2; The R Foundation for Statistical Computing). One-way analysis of variance was used to determine menopausal group (premenopausal, perimenopausal, postmenopausal) differences in baseline participant characteristics. A linear mixed model was built using all combinations of menopause group and treatment as fixed effects and a random intercept on individual to account for repeated measures. Student’s t test was used for within-group contrasts and for between-group contrasts when a difference in means was detected with an F test. The Satterthwaite approximation of denominator degrees of freedom was used in contrasts of treatment effects to adjust for dependent and unequally sized samples. Results of the linear mixed models are reported in Table 2. Figure 1 illustrates individual change in FMD between menopausal groups by treatment and is qualitative in representation.
Table 2.
Separate and combined effects of BH4 and VITC on endothelial function in pre-, peri-, and postmenopausal women
| Placebo |
BH4 |
|||
|---|---|---|---|---|
| Saline | VITC | Saline | VITC | |
| Premenopausal | ||||
| Baseline diameter, mm | 3.20 ± 0.11 | 3.23 ± 0.11 | 3.13 ± 0.11 | 3.27 ± 0.12 |
| Absolute diameter change, mm | 0.36 ± 0.03 | 0.35 ± 0.03 | 0.31 ± 0.03 | 0.29 ± 0.03 |
| FMD, % | 11.6 ± 0.9 | 11.0 ± 0.8 | 10.3 ± 0.8 | 9.7 ± 1.2 |
| Perimenopausal | ||||
| Baseline diameter, mm | 3.51 ± 0.15 | 3.61 ± 0.15 | 3.58 ± 0.15 | 3.59 ± 0.15 |
| Absolute diameter change, mm | 0.26 ± 0.04* | 0.36 ± 0.04# | 0.33 ± 0.04# | 0.30 ± 0.04* |
| FMD, % | 7.6 ± 1.2* | 10.3 ± 1.1# | 9.4 ± 1.1 | 8.5 ± 1.5* |
| Postmenopausal | ||||
| Baseline diameter, mm | 3.69 ± 0.10* | 3.58 ± 0.10*# | 3.61 ± 0.10* | 3.62 ± 0.10* |
| Absolute diameter change, mm | 0.20 ± 0.02* | 0.30 ± 0.02*# | 0.26 ± 0.02# | 0.31 ± 0.02# |
| FMD, % | 5.6 ± 0.7* | 8.6 ± 0.6*# | 7.4 ± 0.7*# | 8.6 ± 0.9*# |
Values are mean ± SE baseline diameter and interventional brachial artery flow-mediated dilation (FMD) expressed as absolute change in diameter (mm) and % change in diameter in premenopausal (n = 14), perimenopausal (n = 8), and postmenopausal (n = 19) women. BH4, tetrahydrobiopterin; VITC, vitamin C. A linear mixed model was built using all combinations of menopause group and treatment as fixed effects and a random intercept on individual to account for repeated measures. Student’s t test was used for within-group contrasts and for between-group contrasts when a difference in means was detected with an F test. *P < 0.05 compared with premenopausal women within same condition; #P < 0.05 compared with saline condition on the placebo day (reference) within the same menopausal group.
Figure 1.
Individual change in endothelial function measured by flow-mediated dilation (FMD%) in premenopausal (PreM, n = 14) and postmenopausal (PostM, n = 19) women on oral placebo during saline (reference condition) and vitamin C (VITC) infusion (A); reference condition vs. oral tetrahydrobiopterin (BH4) supplementation (B); reference condition vs. coadministration of BH4 + VITC (C). Data are qualitative in nature. Quantitative data analyzed with a mixed-linear model to account for missing data are reported in text and Table 2.
RESULTS
Participant Characteristics
Seventy-one women consented and underwent eligibility testing in which 23 did not qualify, and 11 women dropped out during or after screening, resulting in a total enrollment of 41 participants including 14 premenopausal, 8 perimenopausal (n = 4 early perimenopausal; n = 4 late perimenopausal), and 19 postmenopausal (n = 9, early postmenopausal; n = 10, late postmenopausal) women. Participant characteristics are provided in Table 1. Perimenopausal and postmenopausal women were older (P < 0.001) and had higher fasted glucose (P = 0.001), triglycerides (P = 0.02), and total and LDL cholesterol (P ≤ 0.001) levels compared with premenopausal women.
Table 1.
Participant characteristics
| Premenopause | Perimenopause | Postmenopause | P Value | |
|---|---|---|---|---|
| N | 14 | 8 | 19 | |
| Age, yr | 36 ± 9 | 51 ± 3* | 58 ± 5*† | <0.001 |
| Weight, kg | 65.8 ± 15.1 | 69.6 ± 9.7 | 66.4 ± 13.8 | 0.81 |
| Body mass index, kg/m2 | 24.6 ± 5.0 | 25.5 ± 3.9 | 25.2 ± 4.9 | 0.89 |
| Total body fat, % | 31.6 ± 8.0 | 33.6 ± 6.3 | 34.6 ± 8.0 | 0.54 |
| Clinic systolic BP, mmHgb | 104 ± 11 | 109 ± 5 | 119 ± 13*† | 0.002 |
| Clinic diastolic BP, mmHgb | 63 ± 7 | 67 ± 6 | 71 ± 10* | 0.03 |
| Relative V̇o2peak, mL/kg/minb | 35.1 ± 8.3 | 28.8 ± 4.7 | 29.2 ± 7.0 | 0.06 |
| Estrogen, pg/mLψ | 110.2 [66.8–141.0] | 95.4 [28.0–156.0] | 17.1 [10.0–23.0] | <0.001 |
| Follicle-stimulating hormone, mIU/mLψ | 7.6 [4.8–9.5] | 32.8 [11.9–62.3] | 63.1 [52.9–74.0] | <0.001 |
| Progesterone, ng/dLψ | 0.38 [0.15–0.60] | 0.26 [0.20–0.30] | 0.20 [0.10–0.30] | 0.562 |
| Testosterone, ng/dLψ | 30.0 [17.0–41.5] | 28.0 [17.0- 41.0] | 24.6 [17.0–33.0] | 0.320 |
| Cholesterol, mg/dLa | 159 ± 28 | 191 ± 20* | 213 ± 30* | <0.001 |
| LDL, mg/dL | 89 ± 23 | 113 ± 16* | 124 ± 26* | 0.001 |
| HDL, mg/dLa | 55 ± 12 | 55 ± 6 | 68 ± 18* | 0.02 |
| Triglycerides, mg/dLψ | 69.4 [51.2–87.6] | 110.6 [86.1–135.0]* | 92.9 [77.5–108.3]* | 0.02 |
| Glucose, mg/L | 81 ± 5 | 89 ± 6* | 88 ± 5* | 0.001 |
| Insulin, µIU/dL | 8.1 ± 4.1 | 9.8 ± 3.9 | 8.5 ± 4.0 | 0.85 |
| Endothelin-1, pg/mLd | 5.2 ± 0.7 | 5.7 ± 0.8 | 5.5 ± 1.1 | 0.64 |
| Oxidized LDL, U/Lc | 47.2 ± 10.3 | 59.6 ± 10.3* | 63.6 ± 10.0* | <0.001 |
| Total antioxidant status, mmol/L | 1.56 ± 0.11 | 1.50 ± 0.06 | 1.56 ± 0.16 | 0.85 |
| C-reactive protein, mg/Laψ | 1.02 [0.31–1.73] | 1.00 [0.16–1.84] | 1.49 [0.58–2.40] | 0.42 |
Values are means ± SD, except in the case of nonnormally distributed data (ψ); n participants: an = 40, bn = 39, cn = 38, and dn = 36. BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; V̇o2peak, peak oxygen consumption. *P < 0.05 compared with premenopause; †P < 0.05 compared with perimenopause. Significant P values are in bold.
Effect of BH4 on FMD
Results are presented in Table 2 and Fig. 1A. On the placebo day and during the infusion of normal saline (reference condition), FMD was lower in postmenopausal women compared with premenopausal women (5.6 ± 0.7 vs. 11.6 ± 0.9%, P < 0.001). After acute oral BH4 treatment alone, there was a greater change in FMD in postmenopausal compared with premenopausal women (+3.0 [0.89, 5.1]%, P = 0.006). Within-group comparisons showed that FMD was significantly increased in postmenopausal women (FMD change: +1.8 [0.4, 3.2]%, P = 0.01) after acute BH4 treatment but did not change significantly in premenopausal women (−1.2 [−2.8, 0.4]%, P = 0.13). FMD remained significantly reduced in postmenopausal women compared with premenopausal women when expressed as a percent change (7.4 ± 0.8 vs. 10.3 ± 0.9%, P = 0.02) but not expressed as an absolute change (P = 0.15; Table 2).
Effect of VITC on FMD
Results are presented in Table 2 and Fig. 1B. The change in FMD with VITC treatment was greater in postmenopausal compared with premenopausal women (+3.5 [1.4, 5.6]%, P = 0.001). Within-group comparisons showed that FMD did not change in premenopausal women (−0.5 [−2.1, 1.1]%, P = 0.52) but was significantly increased in postmenopausal women (+3.0 [1.8, 4.3]%, P < 0.001). After VITC treatment, FMD remained different between premenopausal and postmenopausal women (8.6 ± 0.8 vs. 11.0 ± 0.9%, P = 0.04). Expressing FMD as an absolute did not alter the results.
VITC + BH4 Condition
Results are presented in Table 2 and Fig. 1C. The coadministration of BH4 + VITC significantly improved FMD in postmenopausal women (+3.0 [1.7, 4.3]%, P < 0.001); however, FMD values remained reduced in postmenopausal compared with premenopausal women when expressed as a percentage (8.6 ± 0.8 vs. 11.6 ± 0.9%, P = 0.02) but not as an absolute change (P = 0.15, Table 2). FMD was reduced in premenopausal women after BH4 + VITC (FMD change: −1.9 [−3.6, −0.2]%, P = 0.03).
Exploratory Analysis in Perimenopausal Women
BH4 alone.
Results of the exploratory analyses are presented in Table 2. FMD was reduced progressively across menopausal stages (P < 0.001) during the reference condition. FMD was marginally increased in perimenopausal women (+1.8 [−0.2, 3.9]%, P = 0.08) after BH4. The increase in perimenopausal women was greater than in premenopausal women (+3.1 [0.4, 5.7]%, P = 0.02) but was similar to postmenopausal women (−0.1 [−2.4, 2.6]%, P = 0.95). Simultaneous comparison of the three menopausal groups found a marginally significant difference in FMD following BH4 supplementation (P = 0.047); however, FMD in perimenopausal women did not differ compared with premenopausal women (−0.9 [−3.9, 2.1]%, P = 0.55) or postmenopausal women (−2.1, [−4.9, 0.8]%, P = 0.15).
VITC alone.
FMD was significantly increased in perimenopausal women (+2.7 [0.6, 4.8], P = 0.01) after VITC. The increase in FMD in the perimenopausal group was greater compared with premenopausal (+3.2 [0.6, 5.8]%, P = 0.02) but not postmenopausal (+0.3 [−2.1, 2.9]%, P = 0.80) women. There was no difference in FMD across the menopausal stages after VITC (P = 0.12).
BH4 + VITC.
The coadministration of BH4 + VITC did not improve FMD in perimenopausal women (+0.9 [−1.1, 3.0]%, P = 0.38). FMD remained significantly reduced in perimenopausal women after the coadministration of BH4 + VITC compared with premenopausal women during the reference condition (−3.1 [0.1, 6.0]%), P = 0.045).
DISCUSSION
We conducted a randomized placebo-controlled crossover study to determine whether the coadministration of BH4 + VITC reverses the effects of aging and menopause on endothelial function in apparently healthy menopausal women. Consistent with prior studies (3, 6), we demonstrate that the administration of either BH4 or VITC alone improves FMD in healthy postmenopausal women but does not significantly alter FMD in premenopausal women. However, FMD remained reduced in postmenopausal women compared with premenopausal women after the coadministration of BH4 + VITC. Exploratory analyses demonstrate that the coadministration of BH4 + VITC did not significantly improve FMD in perimenopausal women. However, the administration of VITC, but not BH4, alone improved endothelial function in perimenopausal women to levels consistent with premenopausal women. These data demonstrate that menopause-related oxidative stress contributes to reductions in endothelial function in perimenopausal and postmenopausal women that are not rescued by the acute coadministration of BH4 + VITC.
Oxidative Stress and Endothelial Function
Oxidative stress has been identified as a key mechanism contributing to the reduced NO and endothelial dysfunction with estrogen deficiency (14, 15) and aging (16, 17). Estrogen preserves endothelial function in ovariectomized animals by protecting NO from scavenging by ROS (15). Estrogen has been shown to inhibit nicotinamide adenine dinucleotide phosphatase (NADPH) oxidase, a major source of superoxide, which reacts with NO to generate peroxynitrite (18) in human umbilical endothelial cells (14). Prior studies demonstrate that VITC infusion increases NO bioavailability in older adults (17). We recently demonstrated that endothelial function was reduced after short-term ovarian hormone suppression in premenopausal and perimenopausal women but not postmenopausal women (3). In this earlier study, VITC infusion reversed the effects of ovarian hormone suppression in premenopausal women and improved FMD above baseline in perimenopausal and postmenopausal women. VITC had no effect on FMD in women randomized to receive estrogen added back during the ovarian hormone suppression intervention in any menopausal group (3). Importantly, VITC did not restore FMD in postmenopausal women to premenopausal levels in either study. Exploratory analysis in the present study showed that VITC improved FMD in perimenopausal women to levels of the premenopausal women in the reference condition. Collectively, these data suggest that oxidative stress contributes to a portion of the reduction in endothelial function in postmenopausal women but, however, that other sources of ROS not scavenged by VITC and/or other mechanisms (e.g., those associated with chronological aging, inflammation, etc.) also contribute.
Reduced BH4 Bioavailability
Under physiological conditions, eNOS produces NO from the interaction with l-arginine and BH4, an essential cofactor for normal eNOS function. However, if BH4 is limited, eNOS can become uncoupled, resulting in increased production of superoxide instead of NO (19). BH4 can be limited through decreased synthesis from guanosine 5′-triphosphate (GTP) via the rate-limiting enzyme GTP cyclohydrolase I (GTPCH I) and/or via reduction to the inactive form BH2 by dihydrofolate reductase (20). Additionally, BH4 can be reduced by oxidation by peroxynitrite and other oxidases via the BH3 radical to BH2 (19). Prior studies demonstrate that estrogen administration counteracts the downregulation of GTPCH I in aortic endothelial cells incubated with high glucose (21). Moreover, estrogen administration improved endothelial function in female rats after ovariectomy mediated through an improvement in BH4 content, decreased production of superoxide, and increased NO bioavailability (22). Prior studies by our group demonstrate that vascular function (e.g., arterial stiffness and endothelial function) is not altered after acute oral BH4 supplementation in premenopausal women but is significantly improved in estrogen-deficient but not estrogen-treated postmenopausal women (6). In the present study, oral BH4 supplementation increased but did not rescue endothelial function in postmenopausal women back to the level of premenopausal women. Moreover, oral BH4 administration improved FMD in perimenopausal women by 1.8%, a clinically significant improvement in endothelial function (23); however, this change was marginally statistically significant. FMD remained reduced in perimenopausal women compared with premenopausal women after BH4 supplementation, suggesting that other mechanisms in addition to reduced BH4 bioavailability contribute to endothelial dysfunction in postmenopausal and perimenopausal women.
Coadministration of BH4 + VITC
VITC is a potent scavenger of superoxide and other ROS; however, it is a relatively weak peroxynitrite scavenger (7). BH4 reacts with peroxynitrite 6–10 times faster than VITC, suggesting that VITC may not fully protect BH4 from oxidation by peroxynitrite (7). VITC can stabilize eNOS by recycling BH3 to BH4; therefore, part of the VITC effect that we and others (16) have demonstrated may be related to increased intracellular BH4 recycling that prevents eNOS uncoupling. However, in contrast to prior in vitro data, the coadministration of BH4 + VITC did not improve endothelial function in postmenopausal and perimenopausal women back to the level of premenopausal women. Although the reasons for the discrepancy between studies are unclear, it is possible that other mechanisms (e.g., inflammation) associated with either estrogen deficiency or chronological aging contribute.
Endothelial function begins to decline in early perimenopause, with the greatest reductions occurring in the late perimenopausal and early postmenopausal periods, suggesting that changes in other ovarian hormones may also contribute (2). Exploratory analyses in the present study revealed that endothelial function was restored in perimenopausal, but not postmenopausal, women to premenopausal levels after VITC, but not BH4, treatment alone. These data suggest that the vascular endothelium may be more acutely modifiable during the perimenopause period or that additional mechanisms are playing a role in estrogen-deficient postmenopausal women (e.g., chronological aging, inflammation, changes in receptor activity). In support of this, endothelial function was improved after short-term administration of a tumor necrosis factor-α inhibitor (etanercept) in estrogen-deficient, but not estrogen-supplemented, postmenopausal women (24), suggesting that inflammation may also contribute. Alternatively, functional changes in the endothelin α- or β-receptors on endothelial cells (25, 26) and increased sympathetic tone (27, 28) have also been implicated. Finally, it should also be noted that the sex of the bovine aortic endothelial cells was not reported by Kuzkaya et al., nor was the aim of their study (7) to evaluate the role of estrogen deficiency on the effects of combined treatment, making comparisons between studies difficult.
Experimental Considerations
The present study should be evaluated within the context of the following limitations. We did not measure plasma or vascular levels of BH4 and therefore cannot evaluate whether the groups differed by BH4 level or whether BH4 levels were replenished after acute supplementation. Second, treatment effects may be underestimated in perimenopausal women because we collapsed early and late perimenopausal women into one group and because of the small sample size. Additionally, we did not measure smooth muscle cell function via endothelium-independent vasodilation, limiting our ability to evaluate whether BH4 or VITC increased FMD through improvements in vascular smooth muscle cell function. Plasma sex hormones were only analyzed at one time point (placebo visit). Whether the variability in treatment response between premenopausal and early perimenopausal women may be related to changes in plasma sex hormones is unknown. This study evaluated mechanisms of vascular dysfunction, and therefore we recognize that the supraphysiological doses used in the present study are not feasible as a potential therapeutic treatment. Finally, women enrolled in this study were healthy and predominantly non-Hispanic Caucasian, therefore limiting the generalizability to men, those with chronic diseases, or other races and ethnicities.
Summary and Conclusions
The American Heart Association recently identified the menopause transition as a period of accelerated CVD risk and a critical period for therapeutic intervention to mitigate future CVD event risk in women (29). Despite increased overall public awareness of CVD risk in menopausal women, the mechanisms underlying the pathogenesis of endothelial dysfunction with the menopause transition remain incompletely understood and, therefore, limit the ability to develop efficacious treatments for reducing CVD risk in menopausal women. Data from the present study demonstrate that the coadministration of BH4 + VITC to increase NO bioavailability via reductions in ROS and increased BH4 bioavailability does not restore endothelial function in postmenopausal or perimenopausal women. Although either BH4 or VITC alone improved endothelial function in estrogen-deficient postmenopausal women, FMD remained reduced compared with premenopausal women. These findings reinforce that mechanisms regulating endothelial function become dysfunctional during perimenopause and therefore it may be an ideal period to intervene on vascular function with treatments to prevent reductions in vascular function with the menopause transition.
GRANTS
This study was funded by National Institutes of Health Grants R01 AG027678 and R56 HL114073-06 (to K. L. Moreau) and F32AG071273 and T32AG000279 (to L. E. DuBose) and Clinical and Translational Science Awards UL1 DK048520 and UL1 TR002535 and Ludeman Family Center for Women’s Health Research (to L. E. DuBose and K. L. Moreau) and NIH Grant U54 AG062319.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L.M. conceived and designed research; C.O., K.L.H., and K.L.M. performed experiments; L.E.D., T.W., V.R., and K.L.M. analyzed data; L.E.D., T.W., V.R., K.L.H., and K.L.M. interpreted results of experiments; L.E.D. prepared figures; L.E.D. drafted manuscript; L.E.D., C.O., T.W., V.R., K.L.H., and K.L.M. edited and revised manuscript; L.E.D., C.O., T.W., V.R., K.L.H., and K.L.M. approved final version of manuscript.
REFERENCES
- 1. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. GeroScience 42: 1699–1714, 2020. doi: 10.1007/s11357-020-00236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreau KL, Gavin KM, Plum AE, Seals DR. ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 5. Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 6. Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 8. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97: 1159–1168, 2012. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriouniv D, Schircks B, Thöny B, Blau N. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab 81: 45–51, 2004. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 10. Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol (1985) 102: 890–895, 2007. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- 11. Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. doi: 10.1097/GME.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thijssen DH, Bruno RM, van Mil AC, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 14. Wagner AH, Schroeter MR, Hecker M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J 15: 2121–2130, 2001. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 15. Keaney JF Jr, Shwaery GT, Xu A, Nicolosi RJ, Loscalzo J, Foxall TL, Vita JA. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 89: 2251–2259, 1994. doi: 10.1161/01.CIR.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 16. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. doi: 10.1161/01.HYP.38.2.274. [DOI] [PubMed] [Google Scholar]
- 18. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 19. Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 20. Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem 284: 28128–28136, 2009. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause 13: 294–302, 2006. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- 23. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 24. Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT, Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 313: R51–R57, 2017. doi: 10.1152/ajpregu.00410.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplon RE, Walker AE, Seals DR. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol (1985) 111: 1416–1421, 2011. doi: 10.1152/japplphysiol.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hogarth AJ, Graham LN, Corrigan JH, Deuchars J, Mary DA, Greenwood JP. Sympathetic nerve hyperactivity and its effect in postmenopausal women. J Hypertens 29: 2167–2175, 2011. doi: 10.1097/HJH.0b013e32834b8014. [DOI] [PubMed] [Google Scholar]
- 29. Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 142: e506–e532, 2020. doi: 10.1161/CIR.0000000000000912. [DOI] [PubMed] [Google Scholar]