Abstract
The period of efficacious immune reactivity afforded by tetanus immunization and the need for continuing some forms of tetanus vaccination programs have been the subjects of recent debates. Our studies demonstrate that the level of antitetanus immunity based on immunological memory (i.e., cellular immune responsiveness) varies dramatically as a function of age, with older individuals constituting a population which is increasingly susceptible to tetanus infection.
In the United States, tetanus is an increasingly rare disorder, with only 40 to 60 cases reported on an annual basis (9). Of those, most cases occur in individuals over 50 years of age who are unvaccinated or whose history of vaccination is unknown. Indeed, the most effective mechanism for tetanus prevention involves immunization with tetanus toxoid (5). However, the continued practice for routine “tetanus booster” vaccination in adults has recently been questioned because of the increasing rarity of tetanus-associated diseases, the medical costs associated with vaccination programs, and the potential for adverse sensitivity reactions upon immunization (3). This argument has been countered by others who cite observations for near universal protective efficacy of tetanus toxoid in children, markedly enhanced antitoxin levels afforded through booster administration, and the scarcity of fatal responses to vaccination (10, 11). Critical to resolving these arguments is an understanding of the levels of humoral immunity necessary to provide effective prevention as well as knowledge regarding the cellular immune system in response to tetanus antigen, the latter indicating the potential for stimulating immunological memory upon environmental antigenic encounters. Previous studies have demonstrated reduced frequencies and concentrations of anti-tetanus toxoid antibody titers with increasing age (6, 7, 12). However, large-scale investigations addressing the question of whether the levels of cellular immunity remain elevated in the age groups previously identified as having an absence or waning of humoral immune response against tetanus have not been reported previously, and therefore they are the basis for this study.
Serum samples were obtained from 461 randomly selected individuals (age range, 2 to 73 years; 199 males and 262 females) seen at the University of Florida outpatient clinics and Clinical Research Center from 1992 to 1997. No selection was made for tetanus toxoid immunization histories, racial or ethnic groupings, or socioeconomic status. Informed consent was obtained from the subjects and their parents as approved by the University of Florida Institutional Review Board.
Peripheral blood mononuclear cells were isolated from heparinized whole blood by Ficoll-Hypaque density centrifugation (1). The peripheral blood mononuclear cells (105 per well) were cultured in round-bottom 96-well tissue culture trays in RPMI 1640 (10% human AB+ sera) for seven days (95% air–5% CO2). The cells were incubated with 10 μg of tetanus toxoid (Massachusetts Public Health Biological Laboratories) per ml (0.875 Lyons flocculating units/ml) in triplicate cultures. Eighteen hours prior to harvest, 1 μCi of [3H]thymidine was added to each well. Thymidine incorporation was assessed by Matrix 96 beta-particle (Packard Instruments) counting, and the mean value of each triplicate stimulation was determined. Cellular proliferation was expressed as the stimulation index (SI): mean counts per minute incorporated in the presence of antigen divided by the mean counts per minute incorporated in antigen absence (medium alone). An SI of ≥3 was defined as positive. Analysis of the relationships between age and cellular immunity was performed by using both analysis of variance (Bonferroni corrected) and chi-square (two-tailed) testing (GraphPad Prism).
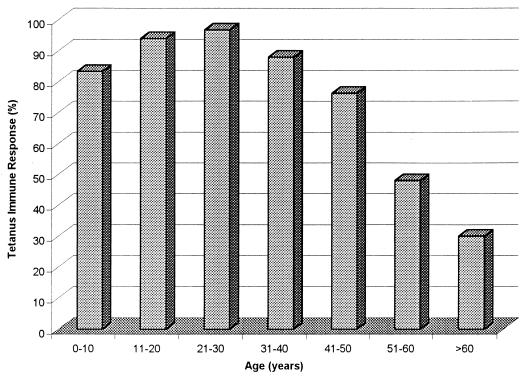
A summary of the results is presented in Table 1. Overall, 81% (372 of 461) of individuals 2 years old and older demonstrated a positive cellular immune response to tetanus toxoid. The frequency of a positive cellular immune response varied dramatically according to age (Fig. 1). Positive responses were observed in over 80% of individuals in their first through fourth decades of life. Beginning at age 41, the frequency of cellular immune reactivity to tetanus dropped dramatically, falling to less than 30% in subjects over 60 years of age.
TABLE 1.
Age and the cellular immune response to tetanus
| Parameter | SI for age group (yr) (n)a
|
||||||
|---|---|---|---|---|---|---|---|
| 0–10 (76) | 11–20 (132) | 21–30 (63) | 31–40 (86) | 41–50 (64) | 51–60 (29) | 61–73 (11) | |
| Mean SIb | 22.2 (3.1) | 29.3 (3.0) | 36.0 (4.9) | 29.9 (3.9) | 15.0 (2.4) | 9.7 (2.8) | 8.9 (4.4) |
| Median SI | 10.8 | 19.8 | 28.5 | 15.4 | 6.3 | 2.4 | 2.2 |
| SI range (CIc) | 0.6–133.0 (6.2) | 0.7–206.7 (5.9) | 0.7–199.2 (9.8) | 0.4–181.1 (7.7) | 0.4–74.4 (4.8) | 0.4–60.8 (5.7) | 0.4–39.4 (9.8) |
Values are SIs obtained by tetanus toxoid stimulation in indicated age group.
Standard errors are in parentheses.
CI, 95% confidence interval.
FIG. 1.
Age and the frequency of cellular immunity to tetanus. Bars indicate the percentage of individuals providing a positive SI within each age group.
As observed in Table 1, age also had a strong effect on the level of cellular immune responsiveness to tetanus toxoid (P < 0.0001). The mean SI for the entire study population was 25.4 (95% confidence interval, 2.9; range, 0.7 to 206.7). The levels of proliferation rose from the first through third decades of life (Table 1), with a dramatic decline occurring after age 41. Indeed, persons in the 41 to 50-, 51 to 60-, and >60-year-old age groups demonstrated significantly lower levels of immunity to tetanus toxoid than the peak 21- to 30-year-old age group (P = 0.04, 0.01, and 0.01, respectively).
Our studies support previous investigations suggesting that the prevalence and intensity of the immune response to tetanus vary according to subject age (6, 7, 12). For example, the third National Health and Nutrition Examination Survey of a United States population suggested that the prevalence of immunity to tetanus, assessed by the levels of tetanus antibodies, declined rapidly starting at the age of 40 years (7). Our studies of cellular immunity report similar findings, observing a significant and rapid decline in anti-tetanus toxoid immunity, beginning with the 41- to 50-year-old age group and extending to subsequent ages. Hence, immunological memory in terms of T cells does not appear to exceed that observed by monitoring tetanus antibody levels. In terms of younger individuals, both investigations revealed positive immune responses to tetanus at frequencies in the 80- to-96% range within the first and second decades of life, findings supporting favorable compliance and clinical effectiveness of early vaccination programs. The potential reasons for not observing near-100% coverage in these younger individuals have been reviewed elsewhere (7).
The need for understanding the role of cellular immune responsiveness to tetanus toxoid is not new. In 1978, Peel and coworkers monitored about 40 subjects and observed an absolute decline in cellular immunity to tetanus toxoid in persons beginning at 20 years of age (8). Our studies, however, while revealing a decline in cellular immunity with increasing age, did not indicate a rapid decline beginning at that age. One possible explanation for this variance is that in the 15- to 20-year period since the publication of that study (8) individuals in their third and fourth decades of life may have improved rates of compliance with booster immunization programs. This contention (i.e., an increased compliance) is also supported by the third National Health and Nutrition Examination Survey demonstrating higher levels of humoral immunity in the third decade of life (7). Indeed, the implementation and enforcement of school vaccination requirements beginning in the late 1960s, as well as the Childhood Immunization Act of 1978, establishing school entry immunization requirements in states that did not already have them, strongly suggest that the decreased antitetanus immunity levels in the older age groups result from the lack of a primary immunization series as well as a lack of compliance with booster doses.
In addition, the previous work on cellular immunity (8) did not monitor individuals in the first and second decades of life. Our studies reveal a rapid increase in levels of antitetanus immunity in those periods. Hence, we believe the most likely explanation is that our large-scale studies (i.e., over 450 individuals) may represent a more accurate investigation of the cellular immune response to tetanus toxoid over a normal life span in a developed country. Future studies by our research group will monitor levels of humoral and cellular immunity to tetanus simultaneously. In addition to providing information regarding the specific relationship between these two immunological variables, such knowledge will be critical for identifying a potential level of cellular immunity associated with true protection (versus the SI value of ≥3 utilized herein). Indeed, the absolute level of humoral immunity and the corresponding challenge dose associated with disease protection and lack of progression are unclear (10, 11). Finally, ascertainment of tetanus immunization histories (which were unfortunately not available in this study design) as well as the addition of more individuals will also allow for analysis of variables (e.g., race, sex, and socioeconomic status) or biases (e.g., recruitment through outpatient clinics and effects of chronic illnesses in elderly subjects) which may impact the frequency and/or levels of cellular immune responsiveness to tetanus. In addition, the important question of whether the observed decline in tetanus immunity with increasing age results from immunological senescence or poor immunization practices will come only from expanded investigations of individuals whose tetanus immunization histories are available and who have received tetanus boosters.
In terms of the implications of this study, our findings clearly indicate the effectiveness of tetanus immunization programs in a majority of individuals. When one considers the impossibility of an environmental eradication of tetanus and the morbidity and mortality associated with the disease, our findings would support a continuance of this highly effective vaccination program. Indeed, rather than discontinuance (3), our studies reporting a loss of cellular immune reactivity in older individuals support the enhancement of immunization programs for adults. Most immunization guidelines suggest tetanus toxoid boosters at 10-year intervals throughout adult life (4). Our results suggest that compliance with this program is decreased after the fourth decade of life. We believe that our studies would support the recommendations of Balestra and Littenberg (2) as well as Gergen et al. (9); their proposals suggest booster immunizations for elderly individuals who already completed a primary immunization regimen and a primary series of tetanus toxoid vaccination for unvaccinated individuals, respectively.
Acknowledgments
This work was supported by Public Health Service grants DK45342, AI39250, and AI42288 from the National Institutes of Health, the Juvenile Diabetes Foundation International, and the American Diabetes Association.
REFERENCES
- 1.Atkinson M A, Bowman M A, Kao K J, Campbell L C, Dush P J, Simell O, Maclaren N K. Lack of immune responsiveness to bovine serum albumin in insulin dependent diabetes mellitus. N Engl J Med. 1993;329:1853–1858. doi: 10.1056/NEJM199312163292505. [DOI] [PubMed] [Google Scholar]
- 2.Balestra D J, Littenberg B. Should adult tetanus immunization be given as a single vaccination at age 65? A cost-effectiveness analysis. J Gen Intern Med. 1993;8:405–412. doi: 10.1007/BF02599616. [DOI] [PubMed] [Google Scholar]
- 3.Bowie C. Tetanus toxoid for adults—too much of a good thing. Lancet. 1996;348:1185–1186. doi: 10.1016/S0140-6736(05)65476-1. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1994;43:1–38. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Reported vaccine-preventable diseases—United States, 1993, and the Childhood Immunization Initiative. Morbid Mortal Weekly Rep. 1994;43:57–60. [PubMed] [Google Scholar]
- 6.Crossley K, Irvine P, Warren J B, Lee B K, Mead K. Tetanus and diphtheria immunity in urban Minnesota adults. JAMA. 1979;242:2298–3000. [PubMed] [Google Scholar]
- 7.Gergen P J, McQuillan G M, Kelly M, Essati-Rice T M, Sutter M S, Virella G. A population-based survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–766. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]
- 8.Peel M M, Edsall G, White W G, Barnes G M. Relationship between lymphocyte responses to tetanus toxoid and age of lymphocyte donor. J Hyg. 1978;80:259–265. doi: 10.1017/s0022172400053614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevots R, Sutter R W, Strebel P M, Cochi S L, Hadler S. Tetanus surveillance—United States, 1989–1990. Morbid Mortal Weekly Rep. 1992;41:1–9. [PubMed] [Google Scholar]
- 10.Rethy L A, Rethy L. Can tetanus boosting be rejected? Lancet. 1997;349:359–360. doi: 10.1016/S0140-6736(05)62862-0. [DOI] [PubMed] [Google Scholar]
- 11.Sehgal R. Tetanus toxoid for adults. Lancet. 1997;349:573. doi: 10.1016/S0140-6736(97)80123-7. [DOI] [PubMed] [Google Scholar]
- 12.Weiss B P, Strassburg M A, Feeley J C. Tetanus and diphtheria immunity in an elderly population in Los Angeles county. Am J Public Health. 1983;73:802–804. doi: 10.2105/ajph.73.7.802. [DOI] [PMC free article] [PubMed] [Google Scholar]