Abstract
Objective
to evaluate the prevalence of cardiac involvement after COVID-19 in competitive athletes at return-to-play (RTP) evaluation, following the recommended Italian protocol including cardiopulmonary exercise test (CPET) and 24-Hour Holter monitoring.
Design and methods
this is a single centre observational, cross-sectional study. Since October 2020, all competitive athletes (age ≥ 14 years) evaluated in our Institute after COVID-19, prior RTP were enrolled. The protocol dictated by the Italian governing bodies included: 12‑lead ECG, blood test, CPET, 24-h ECG monitoring, spirometry. Cardiovascular Magnetic Resonance (CMR) was performed based on clinical indication.
Results
219 consecutive athletes were examined (59% male), age 23 years (IQR 19–27), 21% asymptomatic, 77% mildly symptomatic, 2% with previous pneumonia. The evaluation was performed after a median of 10 (6–17) days from negative SARS-CoV-2 swab.
All athletes showed a good exercise capacity at CPET without cardiovascular and respiratory limitations. Uncommon premature ventricular contractions (PVCs) were found in 9.5% (n = 21) at CPET/Holter ECG monitoring. Two athletes (0.9%) were diagnosed with acute myocarditis (by CMR) and another one with new pericardial effusion. All the three athletes were temporally restricted from sport participation.
Conclusions
Myocarditis in competitive athletes screened after COVID-19 resolution was detected in a low minority of the cases (0.9%). However, a non-negligible prevalence of uncommon PVCs (9%) was observed, either at CPET and/or Holter ECG monitoring, including all athletes with COVID-19 related cardiovascular abnormalities.
Keywords: COVID-19, SARS-CoV-2, Athletes, Cardiovascular evaluation, Cardiopulmonary exercise test (CPET), Return-to-play (RTP)
Practical implications
-
-
All athletes had good performance at cardiopulmonary exercise test after COVID-19
-
-
The prevalence of myocarditis in athletes after COVID-19 is remarkably low.
-
-
The prevalence of uncommon premature ventricular beats detected by cardiopulmonary exercise test/ECG Holter after COVID-19 is not negligible.
-
-
Cardiopulmonary exercise test had additional value in the return-to-play evaluation
1. Introduction
Cardiovascular diseases can occur during hospitalized COVID-19 patients, comprising a wide spectrum of disorders including myocarditis, pericarditis, pulmonary embolism, myocardial infarction, Takotsubo cardiomyopathy and, rarely, multisystemic inflammatory syndrome in pediatric population.1 , 2 The reports of myocarditis and pericarditis occurring in asymptomatic or mildly symptomatic young patients generated large clinical concern regarding the risk of resuming sport activity after the infection. Several screening strategies for a safe return-to-play (RTP) of competitive athletes have been proposed, mainly based on experts' opinion.3, 4, 5, 6., 7 Data accumulated so far, derived from cross-sectional studies, rely usually on the approach based on symptoms, Troponin test, 12‑lead electrocardiogram and echocardiogram.8, 9, 10, 11, 12, 13 Strategies integrating exercise stress test and/or Holter ECG in the RTP were less investigated, although ideally relevant also to confirm the normal cardiac response to effort in the healed athletes.
Thus, the aim of this study was to evaluate the results of the comprehensive screening program of competitive athletes at RTP, based on the recommended Italian protocol which included cardiopulmonary exercise test (CPET) and Holter ECG monitoring.6
2. Methods
All competitive athletes who were evaluated at the Institute of Sport Medicine between October 2020 and May 2021 after COVID-19, prior resuming their training, were consecutively enrolled. Diagnosis of COVID-19 was made by a positive oro-naso-pharyngeal throat swab for SARS-CoV-2 by reverse-transcriptase-polymerase chain reaction. All athletes were unvaccinated at that time of COVID-19 infection. The cardiovascular (CV) evaluation of RTP was performed within 30 days from first negative swab in agreement with the Italian Ministerial Decree.6 The protocol included blood tests, pulmonary function tests (PFTs), 12‑lead resting electrocardiogram (ECG), Transthoracic Echocardiogram (TTE), Cardiopulmonary exercise test (CPET) independently by the severity of the disease. Data regarding the presence of infection-related symptoms, any prescribed medication and the time from the first negative swabs to the RTP evaluation were recorded. COVID-19 severity course was graded.14
Blood tests comprised: full blood count, alanine (ALT) and aspartate (AST) aminotransferase, gamma-glutamyl transferase (GGT), creatinine, creatinine phosphokinase (CPK), high-sensitivity Troponin (hs-Trop), lactic acid dehydrogenase (LDH), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), protein electrophoresis, D-dimer. PFTs evaluated Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1) and the FEV1/FVC ratio. All parameters were measured, expressed as percent of the predicted, and compared with values derived from normal subjects of the same age, gender, height, weight and ethnicity. ECG was recorded with the subject in supine position during quiet respiration, at 25 mm/s, using a Cardioline ClickECG (Cardioline, Italy). ECG patterns were analyzed according to the international criteria.15 In case of abnormalities, ECGs post-COVID-19 were compared to the ones previously recorded. TTE was performed using a Philips Epiq7 (Philips Medical Systems, Andover, Massachusetts) equipped with an S3 probe (2 to 4 MHz). Left (LV) and right ventricle (RV) dimension, wall thickness, global and regional systolic function, and indexes of diastolic function were evaluated. The presence of pericardial effusion was evaluated. Imaging interpretation was based on the international recommendation.16 , 17 CPET was a maximal, symptom-limited continuous ramp CPET, using a cycle ergometer (MORTARA) connected with Quark CPET (COSMED). A COSMED V2 silicon Oro-Nasal Mask was worn by each athlete during testing. Each test included the recording of cardiac and ventilator parameters for about 1 min at rest; subsequently the ramp protocol was started until the exhaustion and followed by five minutes of recovery. The following parameters were collected: resting heart rate (HR rest), resting Systolic and Diastolic Blood Pressure (SBP and DBP rest), Maximal Heart Rate (HR max), Maximal Systolic and Diastolic Blood Pressure (SBP and DBP max), Maximal workload (Watt/Kg max), Maximal Ventilation (VE max), Maximal Oxygen Uptake (VO2 max), Ventilator Efficiency Slope (VE/VCO2 slope), Oxygen Pulse (VO2/HR) and Peak Respiratory Exchange Ratio (RER max). The peak VO2 was the highest VO2 during a 10-s interval obtained at the end of exercise. The Lactate Threshold (LT) was determined using both the V-slope and the ventilatory equivalent for O2 graphs. Oxygen saturation measures were collected with a digital pulse oximeter (KAARSEN) at rest, at maximum physical effort and at the end of the recovery phase. Any supraventricular and/or ventricular arrhythmias, ST/T changes and symptoms during the exercise were recorded. All athletes underwent 24-h Holter ECG monitoring (12‑leads). Any supraventricular and ventricular arrhythmias were investigated. The burden of premature ventricular contractions (PVCs) was arbitrarily classified as <50, 50–500, >500 PVCs/24 h. Ventricular arrhythmias were classified as common or uncommon, as previously defined.18 Newly diagnosed PVCs were those detected for the first time after COVID-19 and not observed in previous evaluations. CMR was performed upon clinical indication. CMR protocol included LV and RV volumes and ejection fraction assessment and tissue characterization by late gadolinium enhancement and parametric techniques (T1 and T2 Mapping).19 , 20 The diagnosis of myocarditis by CMR was made in accordance with expert recommendations of CMR in non-ischemic myocardial inflammation in case of both positive T1 and T2 criteria.21
The study design of the present investigation was evaluated and approved by the Review Board of the Institute. All athletes included in this study were fully informed of the collection of the clinical data and signed the consent form, pursuant to Italian law and the Institute policy.
All clinical data assembled from the study population are maintained in an institutional database.
Continuous variables were presented as mean ± standard deviation (SD) if normally distributed or median and 25th–75th percentiles if not. Normality distribution was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Discrete variables were expressed as count (n) and percentages (%). Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 21.0.V.20).
3. Results
Two-hundred nineteen athletes were enrolled in the RTP program (Table 1 ). Mean age was 23 (25th–75th percentile 19–27, range 14–58) years, 129 (59%) were male. Athletes were engaged in different disciplines: mixed 84 (39%), power 73 (33%), endurance 57 (26%) and skill 5 (2%). Seventy-nine (36%) were Olympic athletes, while the other 64% were national or regional-level athletes. Almost half of the cohort (n = 105, 48%) had been previously evaluated at our Institute, as part of the annual screening program, before the post COVID-19 re-evaluation.
Table 1.
Study population and clinical presentation of SARS-CoV-2 infection.
| Parameter | Athletes (n = 219) |
|---|---|
| Age, y.o. | 23 (19–27)a |
| Male sex, n (%) | 129 (59) |
| Caucasian, n (%) Afro-Caribbean, n (%) |
212 (97) 7 (3) |
| Weight, kg | 72 (61–85)a |
| Height, cm | 177 ± 13 |
| BSA, m2 | 1.9 (1.7–2.1)a |
| BMI, kg/m2 | 23 (21.5–25)a |
| Mild Symptoms during COVID-19, n (%) | 170 (77) |
| Ageusia/anosmia, n (%) | 92 (42) |
| Fever, n (%) | 97 (44) |
| Dyspnoea, n (%) | 18 (8) |
| Diarrhoea, n (%) | 15 (7) |
| Chest pain, n (%) | 0 (0) |
| Faintness, n (%) | 84 (38) |
| Headache, n (%) | 32 (15) |
| Cough, n (%) | 29 (13) |
| Cold, n (%) | 49 (22) |
| Palpitations, n (%) | 2 (1) |
BMI: body mass index; BSA: body surface area; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure.
Median (IQR).
The time between the first negative NPS and the RTP evaluation was 10 (6–17) days. Most of the athletes during COVID-19 were only mildly symptomatic (77%), and 21% were completely asymptomatic (Table 1). Only a small subset (n = 4, or 2%) had pneumonia, that was treated by antibiotics and corticosteroids without the need of hospitalization.
At the time of the RTP no one of the 219 athletes reported symptoms, except one male athlete with previous pneumonia complaining of persistent fatigability, and two female athletes reporting palpitations. Two athletes (1%) presented increased Troponin value. Increased value of both IL-6 and CRP was observed in 2 (1%) athletes while isolated increase of IL-6 or CRP was observed, respectively, in 17 (8%) and 5 (2%).
Spirometry was negative in all subjects, except for a swimmer with known allergic asthma who presented with a modest obstructive pattern, already present a year before the COVID-19 infection.
Rest 12‑leads ECGs revealed abnormality in 9 (4%) athletes: all of them had T-wave inversion. Five of these athletes had previously been screened in our Institute and had already shown the same pattern.
The CPET did not reveal any pulmonary or cardiac limitation to exercise. Peak exercise oxygen pulse and chronotropic competence was normal in all athletes and no one had ST-T abnormalities. The maximum heart rate, systolic and diastolic blood pressure were respectively 172 ± 12 bpm, 170 ± 18 mmHg, 76 ± 8 mmHg. Maximum workload was 3.5 W/Kg (3.1–4.0). VO2 max, maximum respiratory exchange ratio and ventilatory efficiency slope were 39 ± 8 L/Kg/min, 1.2 ± 0.1 and 25 ± 3.
During CPET n = 23 (10.5%) athletes presented PVCs, of whom 20 (9%) showed uncommon pattern (Table 2 , Fig. 1 ). Of those, 10 (5%) were polymorphic, 6 (2.5%) showed LBBB morphology with superior axis and 4 (1.5%) had RBBB morphology. Among those with uncommon PVCs during CPET with a previous evaluation available at the Institute (16/20), arrhythmia was newly diagnosed after COVID-19 in 13 (81%). Among athletes with uncommon PVCs during CPET, echocardiography showed isolated pericardial effusion in one case, while in others two, who had also increased serum Troponin, the CMR showed evidence for myocarditis.
Table 2.
Screening results: ECG, 24-h Holter monitoring and CPET.
| Parameter | Athletes (n = 219) | |
|---|---|---|
| 12‑lead ECG | ||
| TWI, n (%) | 9 (4) | |
| 24-h ECG Holter monitoring (12 lead) | ||
| SVPCs, n (%) | 128 (58) | |
| SVPCs >500/h, n (%) | 3 (1.4) | |
| PVCs, n (%) | 89 (41) | |
| PVCs | <50/24 h, n (%) | 78 (36) |
| 50–500/24 h, n (%) | 6 (2.8) | |
| >500/24 h, n (%) | 5 (2.2) | |
| NSVT, n (%) | 3 (1.6) | |
| Cardiopulmonary test (CPET) | ||
| VA during CPET, n (%) | 23 (10.5) | |
| Uncommon VA, n (%) | 20 (9) | |
DPB: diastolic blood pressure; HR: heart rate; LBBB: Left anterior fascicular block; NSVT: non sustained ventricular tachycardia; PVC: premature ventricular contraction; RBBB: right bundle branch block; RER: respiratory exchange ratio; SBP max: maximal systolic blood pressure; SVPCs: supra-ventricular premature contraction; TWI: T wave inversion; VE max: maximal ventilation; VE/VCO2 slope: ventilatory efficiency slope VO2 max: maximal oxygen uptake.
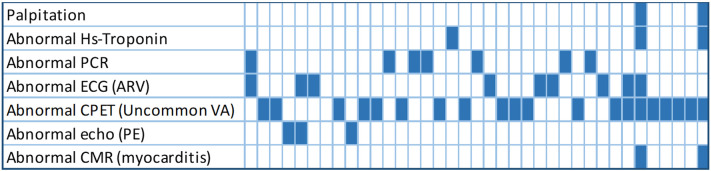
Fig. 1.
The figure shows graphically the summary of abnormal findings at cardiovascular evaluation. Every column represents an athletes and the blue boxes represent abnormal findings for the examination listed in the rows. CMR: cardiovascular magnetic resonance; CRP: C-reactive protein; ECG: electrocardiogram; HS: high-sensitive; TWI: T wave inversion; CPET cardiopulmonary exercise testing.
ECG-Holter monitoring revealed isolated supraventricular beats in 128 (58%) athletes, of which 125 were less than 500 over 24 h. PVCs were detected in 89 athletes (41%), of which 78 (36%) were less than 50 isolated over 24 h (Table 2). Non-sustained ventricular tachycardia (NSVT) was recorded in only 3 (1.4%) athletes. Of note, all these 3 athletes had been evaluated at the Institute prior SARS-CoV-2 infection and had already shown uncommon PVCs (but not NSVT) before the COVID-19. All athletes with NSVT recorded at ECG-Holter monitoring showed uncommon PVCs during CPET.
Echocardiogram showed normal systolic and diastolic function in all athletes. Mild pericardial effusion was detected in four athletes.
CMR was performed 16 (13–24) days after their first positive NPS. CMR detected two cases of acute myocarditis (0.9%) in the Olympic group. These two athletes were female, had symptoms (palpitation), NSVT on effort and/or 24-h ECG monitoring and Troponin raise. Diagnosis of myocarditis by CMR relied on increased native T1 and T2 Mapping values. No focal post contrast enhancement was detected on LGE sequences. No isolated T1 or T2 abnormalities were detected. No case of pericarditis were diagnosed.
Based on the RTP evaluation, five (2%) athletes were temporally considered not eligible for sport after COVID-19. Specifically, in two Olympic athletes because of myocarditis. Both athletes had complex ventricular arrhythmias, troponin raise and CMR fulfilling criteria for myocarditis (Fig. 2 ). Another Olympic male athlete presented with frequent exercise-induced PVCs, often as R-on-T, and couplets. The 12‑lead ECG showed T-wave inversion in V2-V3 (already present before COVID-19). Eventually, blood test, TTE and CMR were all normal and the athlete entered a close follow-up program. In these three athletes the arrhythmic burden was unchanged at the evaluation after three months and the follow-up was prolonged. Finally, other two competitive athletes were temporarily withdrawn from sport because of the heavy burden of newly diagnosed uncommon PVCs and entered a close follow-up program. Three months after, the ventricular arrhythmias had disappeared, and athletes resumed their competitive activities.
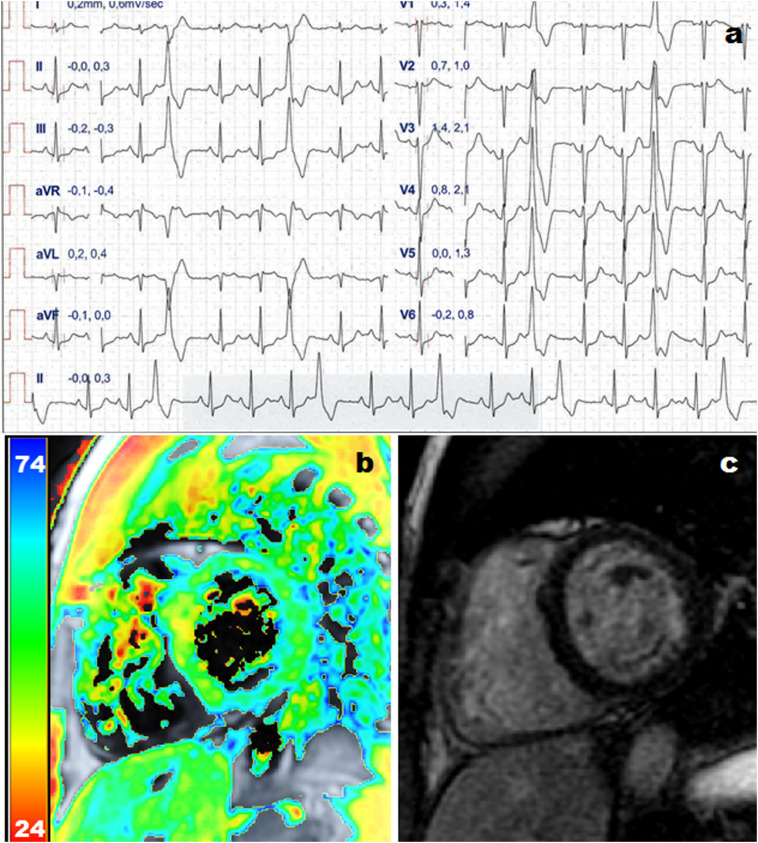
Fig. 2.
Example of an athletes presented with uncommon ventricular arrhythmias ultimately diagnosed with myocarditis based on troponin raise and increased T1 and T2 mapping values by cardiac magnetic resonance. Panel A shows ECG during cardiopulmonary exercise test showing premature ventricular contractions with right ventricle bundle branch block morphology, inferior axis. Panel B shows increased T2 mapping values at the mid infero-septum. Panel C shows no late gadolinium enhancement.
4. Discussion
This study investigated the prevalence of cardiac involvement after SARS-CoV-2 infection in competitive athletes evaluated at the RTP. The major finding was the low prevalence of myocarditis (0.9%) detected in this study group. In all the other athletes no significant abnormalities were detected and the return to play was allowed without further restrictions. Importantly, all athletes had a good performance after the recovery from the infection without functional limitation. In 9% of athletes uncommon PVCs during CPET and/or 24-h tape monitoring were detected. Based on the pre-COVID-19 evaluation (available in half of the athletes), we could assess that most of the uncommon PVCs occurred after the infection. However, based on the overall RTP results, only a small minority (2%) of athletes were temporally considered restricted from sport after COVID-19 and entered a close follow-up (only 0.9% were diagnosed with acute myocarditis).
These results are in line with those reporting a low prevalence of myocarditis after the SARS-CoV-2 infection. The very first study on the RTP evaluation of asymptomatic or mildly symptomatic athletes reported higher prevalence of myocarditis (15%).8 However, this observation was limited to 26 collegiate athletes and such large prevalence was not later confirmed. Subsequent studies described a prevalence of detected myocarditis from 0 to 3%.9 , 11, 12, 13 , 22 These large differences with regard to prevalence of myocarditis after COVID-19 are mainly related to the applied diagnostic algorithms. Recently, the widest study published so far, including 1597 athletes, reported a prevalence of myocarditis from 0.31% to 2.3% according to the adopted screening strategy.10 Specifically, the lowest prevalence was reported using a symptom based-diagnostic strategy, while the prevalence increased up to 1.1% adding resting ECG, Troponin and echocardiogram. Finally, using CMR in all as a screening tool, the prevalence of myocarditis increased up to 2.3%. Despite performing CMR routinely could increase the detection of myocarditis, the cost-effectiveness, and the clinical meaning of isolated positive CMR findings in otherwise asymptomatic subjects remain to be defined and the impact on the subsequent athlete's management questionable.23
In addition, all data reported so far are based on the so called “cardiac triad” (ECG, troponin, echocardiography) while data coming from different strategies and specifically from exercise test are scarce. Only few studies included the exercise stress in the RTP evaluation.24, 25, 26 Those studies demonstrated a normal exercise capacity and the absence or low prevalence of ventricle arrhythmias. However, in these studies no data on pre-COVID cardiovascular evaluation were available and the number of subjects recruited was low. More recently, a report from the Italian Federation of Sport Medicine collecting data on more than 4100 athletes reported a 2.4% of ventricular arrhythmias by exercise test or 24 h ECG monitoring and myocarditis diagnosed in 0.12%.27
In the present study, CPET and 24-h ECG monitoring were performed in addition to ECG, echocardiography, and lab tests, according to the protocol dictated by the Italian governing bodies after the first wave of the pandemic. This protocol was mandatory in every athletes regardless the severity of SARS-CoV-2. This study was not designed to assess the diagnostic yield of CPET and ECG Holter monitoring in athletes after SARS-CoV-2 infection and we cannot provide this information. However, a non-negligible prevalence of ventricular arrhythmias was documented and most of them were newly diagnosed after COVID-19. Despite arrhythmias are not necessarily expression of underlying structural heart disease, PVCs, especially of uncommon pattern, could be the only sign of otherwise clinically silent cardiac disease. Thus, cardiopulmonary exercise test has an undoubtful additional value in the RTP evaluation, because can identify, or raise suspicion, for subjects with COVID-19 related cardiovascular abnormalities in addition to the performance fitness evaluation. As matter of fact, those athletes that were eventually considered at risk and were eventually withdrawn from competitions had evidence of uncommon PVCs at CPET and ECG Holter monitoring. Therefore, exercise test may reasonably be advised in athletes recovering from COVID-19. Despite the number of athletes with a confirmed diagnosis of myocarditis was low, athletes with uncommon PVCs entered in a follow-up program. To be noticed, the protocol was later modified and stratified on disease severity, with accumulating evidence of low prevalence of myocarditis related to COVID-19. Finally, our results confirmed that CMR should be considered at RTP in case of high likelihood of myocarditis (i.e., symptoms, suspicious clinical findings including PVCs of uncommon morphology).
Our investigation has several limitations. Firstly, our study was a single-center cross-sectional study and the evaluation prior the infection was available for only half of the cohort. Secondly, our population was made by young, mostly Caucasian, asymptomatic or mildly symptomatic athletes. Consequently this cohort could not be representative of older subjects or those with more severe COVID-19 course. CMR was not performed in all athletes, possibly preventing the evaluation of the overall prevalence of isolated tissue characterization abnormalities and the real diagnostic yield of its use as a screening tool.
In addition, this study was not designed to evaluate the diagnostic yield od CPET and ECG Holter monitoring. A comparative group was not recruited, thus a defined association between SARS-CoV-2 infection and arrhythmias cannot be proved.
5. Conclusion
The prevalence of myocarditis after asymptomatic or mildly symptomatic COVID-19 in the athletic population is remarkably low (0.9%). On the other hand, the prevalence of uncommon premature ventricular beats was not negligible (9%). Uncommon PVC were detected by CPET, and were present in all athletes with COVID-19 related cardiovascular abnormalities.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not–for–profit sectors.
Confirmation of ethical compliance
The study design of the present investigation was evaluated and approved by the Review Board of the Institute. All athletes included in this study were fully informed of the types and nature of the evaluation and signed the consent form, pursuant to Italian law and the Institute policy. All clinical data assembled from the study population are maintained in an institutional database.
All authors have no conflict of interest to disclose.
Acknowledgment
All authors would like to acknowledge the technical support of Floriana Filannino and Alessandra Barreca.
Footnotes
Disclosure: All the authors have nothing to disclose.
Author contribution statement: Viviana Maestrini: Conceptualization, Methodology, Investigation; Writing- Original draft; Marco Penza: Data Curation, Investigation; Domenico Filomena: Formal analysis, Investigation, Writing - Original Draft; Lucia Ilaria Birtolo: Investigation; Sara Monosilio: Investigation; Erika Lemme: Investigation; Maria Rosaria Squeo Investigation; Ruggiero Mango: Investigation; Giuseppe Di Gioia: Investigation; Andrea Serdoz: Investigation; Roberto Fiore: Investigation; Francesco Fedele: Investigation; Antonio Pelliccia: Investigation, Writing – Review & Editing, Supervision; Barbara Di Giacinto: Investigation, Writing – Review & Editing, Supervision.
References
- 1.Alsaied T., Tremoulet A.H., Burns J.C., et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 2.Maestrini V., Birtolo L.I., Francone M., et al. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: in-hospital evaluation and one-year follow-up. Int J Cardiol. 2021;339:235–242. doi: 10.1016/j.ijcard.2021.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia R.T., Marwaha S., Malhotra A., et al. Exercise in the severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European Association of Preventive Cardiology (EAPC) Eur J Prev Cardiol. 2020;27:1242–1251. doi: 10.1177/2047487320930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan D., Kim J.H., Elliott M.D., et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. J Am Coll Cardiol Img. 2020;13:2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson M.G., Hull J.H., Rogers J., et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br J Sports Med. 2020;54:1157–1161. doi: 10.1136/bjsports-2020-102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.http://www.fmsi.it/images/pdf/archivio_news/CS_Raccomandazioni_FMSI_2020.04.04-2.pdf. http://wwwfmsiit/images/pdf/archivio_news/CS_Raccomandazioni_FMSI_20200404-2pdf 2020.
- 7.Gluckman T.J.A.N., Bhave N.M., et al. ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol Mar. 2022;16:2022. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark D.E., Parikh A., Dendy J.M., et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrickson B.S., Stephens R.E., Chang J.V., et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143:1926–1928. doi: 10.1161/CIRCULATIONAHA.121.053982. [DOI] [PubMed] [Google Scholar]
- 12.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starekova J., Bluemke D.A., Bradham W.S., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S., Drezner J.A., Baggish A., et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39:1466–1480. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 16.Galderisi M., Cosyns B., Edvardsen T., et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia A., Caselli S., Sharma S., et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur Heart J. 2018;39:1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 18.Heidbuchel H., Adami P.E., Antz M., et al. Recommendations for participation in leisure-time physical activity and competitive sports in patients with arrhythmias and potentially arrhythmogenic conditions: part 1: supraventricular arrhythmias. A position statement of the section of sports cardiology and exercise from the European Association of Preventive Cardiology (EAPC) and the European heart rhythm association (EHRA), both associations of the European Society of Cardiology. Eur J Prev Cardiol. 2021;28:1539–1551. doi: 10.1177/2047487320925635. [DOI] [PubMed] [Google Scholar]
- 19.Kelle S., Bucciarelli-Ducci C., Judd R.M., et al. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magnet Reson. 2020;22:61. doi: 10.1186/s12968-020-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messroghli D.R., Moon J.C., Ferreira V.M., et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magnet Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 22.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filomena D., Birtolo L.I., Penza M., et al. The role of cardiovascular magnetic resonance in the screening before the return-to-play of elite athletes after COVID-19: utility o futility? J Sports Med Phys Fitness. 2021;61:1137–1143. doi: 10.23736/S0022-4707.21.12764-1. [DOI] [PubMed] [Google Scholar]
- 24.Gervasi S.F., Pengue L., Damato L., et al. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br J Sports Med. 2021;55:54–61. doi: 10.1136/bjsports-2020-102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavigli L., Frascaro F., Turchini F., et al. A prospective study on the consequences of SARS-CoV-2 infection on the heart of young adult competitive athletes: implications for a safe return-to-play. Int J Cardiol. 2021;336:130–136. doi: 10.1016/j.ijcard.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komici K., Bianco A., Perrotta F., et al. Clinical characteristics, exercise capacity and pulmonary function in post-COVID-19 competitive athletes. J Clin Med. 2021:10. doi: 10.3390/jcm10143053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casasco M., Iellamo F., Scorcu M., et al. Return to play after SARS-CoV-2 infection in competitive athletes of distinct sport disciplines in Italy: a FMSI (Italian Federation of Sports Medicine) Study J Cardiovasc Develop Dis. 2022:9. doi: 10.3390/jcdd9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]