Abstract
Background
The COVID-19 virus is believed to increase the risk of diffusing intravascular coagulation. Total joint arthroplasty (TJA) is one of the most common elective surgeries and is also associated with a temporarily increased risk of venous thromboembolism (VTE). However, the influence of a history of COVID-19 infection on perioperative outcomes following TJA remains unknown. Therefore, this study sought to determine what effect a history of COVID-19 infection had on outcomes following primary TJA.
Methods
A retrospective case-control study using the national database was performed to identify all patients who had a history of COVID-19 and had undergone TJA, between 2019 and 2020. Patients who had a history of both were 1:1 matched to those who did not have a history of COVID-19, and 90-day outcomes were compared. A total of 661 TKA and 635 THA patients who had a history of COVID-19 were 1:1 matched to controls. There were no differences in demographics and comorbidities between the propensity-matched pairs in both TKAs and THAs studied. Previous COVID-19 diagnosis was noted in 28.3% of patients 5 days within TJA and in 78.6%, 90 days before TJA.
Results
Patients who had a previous diagnosis of COVID-19 had a higher risk of pneumonia during the postoperative period for both THA and TKA (6.9% versus 3.5%, P < .001 and 2.27% versus 1.21%, P = .04, respectively). Mean lengths of stay were also greater for those with a previous COVID-19 infection in both cohorts (TKA: 3.12 versus 2.57, P = .027, THA: 4.52 versus 3.62, P < .001). Other postoperative outcomes were similar between the 2 groups.
Conclusion
COVID-19 infection history does not appear to increase the risk of VTE following primary TJA, but appears to increase the risk of pneumonia in addition to lengths of stay postoperatively. Individual risk factors should be discussed with patients, to set reasonable expectations regarding perioperative outcomes.
Keywords: total hip arthroplasty, total knee arthroplasty, COVID-19, thromboembolism, complications
The SARS-CoV-2 (COVID-19) virus came with many unknowns, and for the safety of patients and providers, elective surgeries came to a halt. Now, with surgeries resumed, and with many people having a history of COVID-19, it is important to understand the implications of previous COVID-19 infection on perioperative outcomes. There is a paucity of data on the nature of these outcomes among patients who have a history of COVID-19 infection.
It is suspected that COVID-19 can cause systematic coagulation activation, excessive inflammation, platelet activation, hypoxia, and diffuse intravascular coagulation leading to thrombotic disease [[1], [2], [3], [4], [5], [6], [7]]. It remains unknown how long these hyperinflammatory and hypercoagulopathic states remain following COVID-19 infection [4,5]. Furthermore, little evidence on the incidence of venous thromboembolism (VTE) in patients undergoing orthopaedic procedures who have a history of COVID-19 infection exists [8].
Total joint arthroplasty (TJA) is one of the most common surgical procedures performed in the United States and is associated, temporally, with an increased risk of VTE. Only one study has previously evaluated the additive effect of hip surgery and COVID-19 infection on increasing the risk of VTE and lowering survival [9], with a reported 7 to 10-fold increased mortality risk in those patients. However, the small cohort of this study may limit its generalizability. Current clinical practice at many institutions requires patients undergoing surgery to have a negative test preoperatively. However, there are no clear guidelines as to the timing and/or safety of surgery following COVID-19 infection. Therefore, this study sought to analyze the effect of previous COVID-19 infection on outcomes following TJA, including orthopaedic and non-orthopaedic complications. Specifically, the aims of this study were to evaluate 90-day post-operative outcomes following TJA among patients who did and did not have a history of prior COVID-19 infection. We hypothesized that patients who have a history of a COVID-19 infection are at a higher risk of VTE following TJA. The results of this study might impact the clinical recommendations for VTE prophylaxis and/or timing of TJA surgery and could meaningfully impact patient morbidity and mortality among a major section of our population.
Methods
We conducted a retrospective case-control study with registry data within the Mariner database of the PearlDiver Server (Colorado Springs, CO) which allows queries to be Institutional Review Boards exempt. In this study, 2 separate queries were performed by separating THA and TKA.
Patient Population
Between January 1, 2019 and December 31, 2020, all patients who had a history of a diagnosis of COVID-19 within the database were identified. A second cohort of patients was created consisting of all patients within the database who had undergone primary THA or TKA. We excluded patients who had a diagnosis of COVID-19 before 2019 (miscode) and patients under the age of 50 years. Our “cases” were those patients who had a diagnosis of COVID-19 and had also undergone a primary TJA. Only patients who had a COVID-19 diagnosis before 90 days of their TJA were included. We later analyzed the cohort to be matched as described below to determine COVID-19 infection rates before surgery at different time points. To evaluate for rates of COVID before surgeries both TKA and TJA were combined into a group labeled TJA. Individual comorbidity burdens within each cohort were then acquired and used to propensity score match, at a 1:1 ratio, those who had previous COVID-19 to controls who did not have a history of COVID-19. Matching was performed to evaluate whether COVID-19 had a significant effect on 90-day outcomes. This was done by nearest neighbor matching at a 1:1 ratio. This was achieved by matching age distribution, sex distribution, region where the surgery was performed (ie, North, East, Midwest, and South based on National Census Data), year of surgery, state within the United States where the surgery was performed, and 49 comorbidities.
Outcome Measure
Postoperative outcomes were identified as those events occurring within 90 days of the date of surgery. Primary outcomes of interest included: deep vein thrombosis, pulmonary embolism, and prosthetic joint infection. Secondary outcomes included acute kidney injury, cardiac arrest, nerve injury, pneumonia, urinary tract infection (UTI), wound complication, hematoma, and transfusion rate. Lengths of stay by age group were also compared to minimize confounding on lengths of stay comparisons.
Data Analyses
The statistical analyses were performed using descriptive and comparative statistics including unpaired t-tests, paired t-tests, chi-squares, and odds ratios as well as Cox regression models. Normality was analyzed on continuous data to determine normality a priori. The statistical software R that is housed within the PearlDiver server was used for this analysis. An alpha value of less than 0.05 was determined to be statistically significant. The Family-Wise Error Rate (FWER) was reported for grouped outcomes compared. The formula used herein to report on this was = 1–(1-α)n where α represents 0.05 and n the number of comparisons of outcomes.
The Mariner Dataset contained 857,830 patients who had a diagnosis of COVID-19 from 2019 to 2020. During this period, there were a total of 222,663 TKA and 159,890 THA patients who were 50 years old and above. Our query of patients who were 50 years old and above and had a previous diagnosis of COVID-19 identified a total of 661 patients who underwent TKA and a separate cohort of 635 THA. When evaluating COVID-19 diagnosis rates days before the TJA, we found that 28.3% of those who underwent TJA had a diagnosis within 5 days of surgery, 34.2% within 10 days, 42.6% during 20 days before surgery, and 78.6% within the 90-day period before the TJA.
Excluding patients who had a history of COVID-19, our control groups were comprised of 220,038 patients for TKA and 157,191 for THA. Table 1 illustrates the demographic differences between the groups before and after matching. Patients who had a previous COVID-19 diagnosis had lower rates of the 49 comorbidities used to propensity score match. Matching was successful in creating 2 populations with similar age distributions, sex distributions, and region of surgery (P > .05 for all in both TKA and THA cohorts).
Table 1.
Study Demographics.
| TKA | Prematch |
Postmatch |
||||
|---|---|---|---|---|---|---|
| Covid | Control | P Value | Covid | Control | P Value | |
| N= | 661 | 220,038 | t test | 660 | 660 | t test |
| Age | ||||||
| Mean | 66.19 | 68.14 | 66.21 | 66.00 | ||
| Range | 50-84 | 50-84 | <.001 | 50-84 | 50-84 | .648 |
| Sex | % | % | Chi square | % | % | Chi square |
| Women | 69.1 | 62.3 | <.001 | 69.1 | 69.7 | .811 |
| Men | 30.9 | 37.7 | 30.9 | 30.3 | ||
| Age Distribution | % | % | Chi square | % | % | Chi square |
| 50 to 54 | 8.8 | 5.8 | <.001 | 8.7 | 8.6 | .259 |
| 55 to 59 | 12.7 | 11.4 | 12.7 | 15.7 | ||
| 60 to 64 | 25.7 | 16.9 | 25.7 | 21.3 | ||
| 65 to 69 | 18.9 | 20.7 | 18.7 | 19.4 | ||
| 70 to 74 | 14.5 | 20.3 | 14.8 | 17.4 | ||
| 75 to 79 | 11.3 | 14.9 | 11.4 | 11.3 | ||
| 80 to 84 | 8.0 | 9.9 | 7.9 | 6.2 | ||
| Region of Surgery | % | % | Chi square | % | % | Chi square |
| Midwest | 30.6 | 27.2 | <.001 | 30.1 | 30.9 | .975 |
| Northeast | 30.7 | 19.1 | 30.5 | 31.3 | ||
| South | 30.9 | 38.6 | 30.4 | 29.5 | ||
| Unknown | 0.3 | 0.6 | 1.5 | 1.5 | ||
| West | 7.6 | 14.5 | 7.5 | 6.7 | ||
| THA | Prematch | Postmatch | ||||
| N= | 635 | 157,191 | t test | 634 | 634 | t test |
| Age | ||||||
| Mean | 70.50 | 70.01 | 70.42 | 70.01 | ||
| Range | 50-84 | 50-84 | .1796 | 50-84 | 50-84 | .45 |
| Gender | % | % | Chi square | % | % | Chi square |
| Female | 59.0 | 59.2 | .913 | 60.4 | 59.0 | .606 |
| Male | 41.0 | 40.8 | 39.6 | 41.0 | ||
| Age Distribution | % | % | Chi square | % | % | Chi square |
| 50 to 54 | 6.6 | 5.5 | <.001 | 6.7 | 6.6 | .355 |
| 55 to 59 | 9.1 | 10.1 | 9.2 | 9.9 | ||
| 60 to 64 | 16.9 | 14.4 | 17.1 | 14.8 | ||
| 65 to 69 | 12.3 | 16.6 | 12.2 | 15.9 | ||
| 70 to 74 | 13.5 | 17.1 | 13.3 | 14.7 | ||
| 75 to 79 | 13.4 | 15.0 | 13.3 | 13.9 | ||
| 80 to 84 | 28.2 | 21.4 | 28.1 | 24.1 | ||
| Region of Surgery | % | % | Chi square | % | % | Chi square |
| Midwest | 25.8 | 25.8 | <.001 | 25.9 | 28.5 | .542 |
| Northeast | 29.9 | 20.1 | 29.7 | 28.5 | ||
| South | 35.0 | 37.2 | 34.9 | 33.8 | ||
| Unknown | 0.0 | 0.5 | 0.3 | 0.0 | ||
| West | 9.3 | 16.4 | 9.3 | 9.1 | ||
TKA, total knee arthroplasty; THA, total hip arthroplasty.
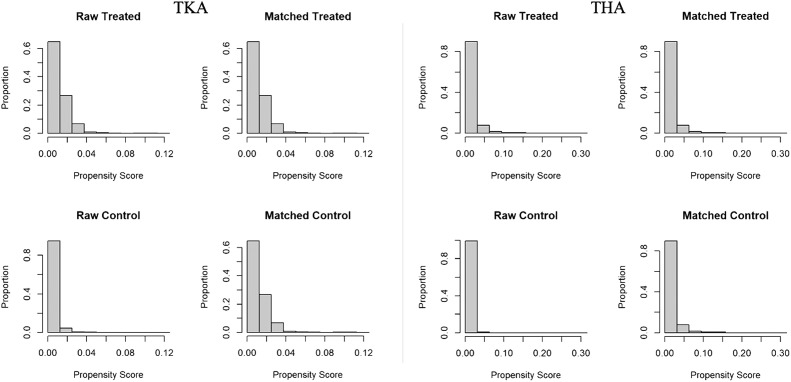
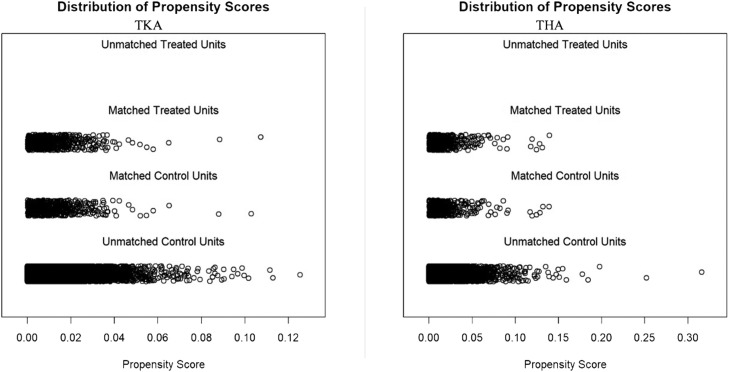
Table 2 illustrates the successful matching. In TKA, both cohorts were found to have comparable comorbidity rates among 44 of the 49 comorbidities evaluated. The only unbalanced comorbidities were asthma, diabetes, uncomplicated diabetes, drug abuse, and fluid and electrolyte disorders (P < .05 for all). In THA patients, following the matching process with propensity scores, the majority of comorbidities were found to occur at similar rates. There were 46 of 49 matches rates except for coagulopathy, dementia, and fluid and electrolyte disorders (P < .05 for all). Figure 1 demonstrates scoring before and after matching of the groups. Figure 2 demonstrates the well-balanced distribution of the matched cohorts and their variance over the propensity score.
Table 2.
Comparison of Comorbidities after Matching.
| TKA Odds Ratio |
THA Odds Ratio |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X2 | df | P-Value | Estimate | Lower | Upper | X2 | df | P-Value | Estimate | Lower | Upper | |
| Acute Myocardial Infarction | 1.06 | 1 | .303 | 0.81 | 0.56 | 1.17 | 0.09 | 1 | .762 | 1.06 | 0.79 | 1.43 |
| Alcohol Abuse | 0 | 1 | 1.000 | 1.00 | 0.65 | 1.54 | 0.00 | 1 | 1 | 0.99 | 0.71 | 1.37 |
| Asthma | 4.29 | 1 | .038 | 1.33 | 1.02 | 1.73 | 0.50 | 1 | .48 | 0.90 | 0.68 | 1.18 |
| Blood Loss Anemia | 0.88 | 1 | .348 | 1.28 | 0.81 | 2.04 | 1.32 | 1 | .251 | 0.79 | 0.54 | 1.15 |
| COPD | 2.08 | 1 | .149 | 1.19 | 0.95 | 1.49 | 0.74 | 1 | .39 | 1.11 | 0.89 | 1.39 |
| Cancer | 0.01 | 1 | .940 | 1.02 | 0.76 | 1.38 | 1.33 | 1 | .249 | 1.19 | 0.90 | 1.58 |
| Cardiac Arrhythmia | 3.07 | 1 | .080 | 1.25 | 0.98 | 1.58 | 0.84 | 1 | .361 | 1.12 | 0.89 | 1.40 |
| Cerebrovascular Disease | 0.04 | 1 | .843 | 0.97 | 0.75 | 1.25 | 0.01 | 1 | .907 | 0.98 | 0.78 | 1.23 |
| Chronic Kidney Disease | 1.9 | 1 | .168 | 1.22 | 0.93 | 1.60 | 0.18 | 1 | .671 | 0.94 | 0.74 | 1.20 |
| Chronic Pulmonary Disease | 3.24 | 1 | .072 | 1.23 | 0.99 | 1.54 | 0.92 | 1 | .337 | 1.12 | 0.90 | 1.40 |
| Coagulopathy | 0.81 | 1 | .367 | 1.20 | 0.84 | 1.71 | 7.22 | 1 | .007 | 1.50 | 1.12 | 2.00 |
| Congestive Heart Failure | 1.11 | 1 | .292 | 1.28 | 0.84 | 1.94 | 0.64 | 1 | .423 | 0.85 | 0.60 | 1.21 |
| Coronary Artery Disease | 0.13 | 1 | .723 | 1.05 | 0.83 | 1.32 | 0.00 | 1 | .954 | 0.99 | 0.79 | 1.24 |
| Deficiency Anemia | 2.78 | 1 | .096 | 1.26 | 0.97 | 1.64 | 0.99 | 1 | .321 | 1.14 | 0.89 | 1.45 |
| Dementia | 1 | 1 | .317 | 1.48 | 0.76 | 2.88 | 38.01 | 1 | 0 | 2.47 | 1.85 | 3.30 |
| Depression | 0.01 | 1 | .912 | 1.02 | 0.82 | 1.27 | 0.26 | 1 | .613 | 0.94 | 0.75 | 1.17 |
| Diabetes | 11.28 | 1 | .001 | 1.46 | 1.17 | 1.81 | 0.16 | 1 | .694 | 0.95 | 0.76 | 1.19 |
| Complicated Diabetes | 1.89 | 1 | .169 | 1.20 | 0.94 | 1.53 | 0.51 | 1 | .475 | 1.11 | 0.86 | 1.43 |
| Uncomplicated Diabetes | 4.27 | 1 | .039 | 1.27 | 1.02 | 1.58 | 0.65 | 1 | .419 | 1.11 | 0.88 | 1.39 |
| Drug Abuse | 6.49 | 1 | .011 | 1.66 | 1.14 | 2.42 | 0.11 | 1 | .745 | 0.94 | 0.68 | 1.29 |
| Fluid and Electrolyte Disorder | 22.15 | 1 | .000 | 1.74 | 1.38 | 2.18 | 11.09 | 1 | .001 | 1.47 | 1.18 | 1.83 |
| HIV | 3.59 | 1 | .096 | 1.17 | 0.02 | 1.38 | 0.33 | 1 | .566 | 0.75 | 0.35 | 1.59 |
| Hypertension | 0.01 | 1 | .937 | 0.98 | 0.72 | 1.33 | 0.17 | 1 | .682 | 0.92 | 0.67 | 1.27 |
| Hypothyroidism | 0.42 | 1 | .516 | 1.09 | 0.86 | 1.37 | 0.99 | 1 | .32 | 0.88 | 0.70 | 1.11 |
| Ischemic Heart Disease | 0 | 1 | 1.000 | 0.99 | 0.75 | 1.31 | 0.06 | 1 | .803 | 1.04 | 0.81 | 1.33 |
| Liver Disease | 1.1 | 1 | .294 | 1.16 | 0.90 | 1.50 | 0.02 | 1 | .895 | 0.97 | 0.75 | 1.26 |
| Lymphoma | 0 | 1 | 1.000 | 0.90 | 0.36 | 2.23 | 0.03 | 1 | .853 | 1.15 | 0.56 | 2.37 |
| Metastatic Cancer | 1.47 | 1 | .226 | 1.80 | 0.79 | 4.10 | 0.02 | 1 | .887 | 0.92 | 0.53 | 1.61 |
| Mild Liver Disease | 0.54 | 1 | .464 | 1.11 | 0.86 | 1.45 | 0.00 | 1 | 1 | 1.01 | 0.77 | 1.32 |
| Obesity | 1.37 | 1 | .243 | 1.15 | 0.92 | 1.45 | 0.00 | 1 | 1 | 0.99 | 0.79 | 1.24 |
| Neurological Disorders | 0.74 | 1 | .390 | 1.23 | 0.81 | 1.88 | 0.21 | 1 | .65 | 1.08 | 0.81 | 1.46 |
| Peripheral Vascular Disease | 0.64 | 1 | .423 | 1.11 | 0.87 | 1.42 | 1.21 | 1 | .271 | 0.87 | 0.70 | 1.10 |
| Paralysis | 0.26 | 1 | .612 | 1.26 | 0.65 | 2.45 | 1.23 | 1 | .268 | 1.36 | 0.83 | 2.21 |
| Peptic Ulcer Disease | 0 | 1 | 1.000 | 1.03 | 0.66 | 1.58 | 0.44 | 1 | .505 | 0.87 | 0.60 | 1.26 |
| Peptic Ulcer Disease without bleeding | 0.02 | 1 | .897 | 1.07 | 0.64 | 1.78 | 0.12 | 1 | .735 | 0.90 | 0.58 | 1.41 |
| Psychoses | 0.33 | 1 | .568 | 1.22 | 0.71 | 2.09 | 0.00 | 1 | 1 | 1.02 | 0.70 | 1.49 |
| Pulmonary Heart Disease | 0.07 | 1 | .791 | 1.06 | 0.75 | 1.51 | 0.05 | 1 | .817 | 0.95 | 0.71 | 1.29 |
| Renal Disease | 1.35 | 1 | .246 | 1.18 | 0.91 | 1.55 | 0.06 | 1 | .809 | 0.96 | 0.76 | 1.22 |
| Renal Failure | 1.24 | 1 | .265 | 1.28 | 0.86 | 1.91 | 0.07 | 1 | .788 | 1.07 | 0.75 | 1.52 |
| Rheumatoid Arthritis | 0.12 | 1 | .732 | 1.11 | 0.71 | 1.74 | 0.04 | 1 | .836 | 0.94 | 0.62 | 1.41 |
| Severe Liver Disease | 0 | 1 | 1.000 | 1.00 | 0.32 | 3.12 | 0.19 | 1 | .66 | 0.75 | 0.31 | 1.78 |
| Solid Tumor w/o metastasis | 0 | 1 | 1.000 | 1.00 | 0.74 | 1.36 | 1.22 | 1 | .269 | 1.19 | 0.89 | 1.59 |
| Tobacco Use | 0.61 | 1 | .434 | 1.10 | 0.88 | 1.37 | 0.00 | 1 | .955 | 1.01 | 0.81 | 1.26 |
| Valvular Disease | 0.9 | 1 | .342 | 1.14 | 0.89 | 1.46 | 0.80 | 1 | .37 | 0.89 | 0.71 | 1.13 |
| Weight Loss | 0 | 1 | 1.000 | 1.02 | 0.72 | 1.43 | 0.25 | 1 | .617 | 1.07 | 0.84 | 1.37 |
| History of DVT | 1.86 | 1 | .170 | 1.36 | 0.90 | 2.07 | 0.01 | 1 | .927 | 1.03 | 0.72 | 1.48 |
| Chronic Anticoagulation | 0.28 | 1 | .600 | 1.08 | 0.84 | 1.40 | 1.16 | 1 | .281 | 0.87 | 0.69 | 1.10 |
| Hypercoagulability Diagnosis | 0 | 1 | 1.000 | 2.00 | 0.18 | 22.14 | 0.00 | 1 | 1 | 0.80 | 0.21 | 2.99 |
| On Hormone replacement therapy | 0.23 | 1 | .200 | 0.86 | 0.54 | 1.39 | 0.93 | 1 | .335 | 0.79 | 0.52 | 1.21 |
Bold highlights statistically significant differences.
TKA, total knee arthroplasty; THA, total hip arthroplasty; DF, degrees of freedom; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; DVT, deep vein thrombosis.
Fig. 1.
Propensity Scores THA and TKA.
Fig. 2.
Distribution of propensity scores TKA and THA.
Results
Total Knee Arthroplasty
The 90-day outcomes of both groups undergoing TKA were similar albeit pneumonia occurred at a greater rate among those patients who had a previous diagnosis of Covid, (2.27% versus 1.21%, P = .04). Other complications occurred at similar rates in both cohorts.
The mean lengths of stay was significantly greater for those with previous Covid infection mean 3.12 days (SD 0.59) versus matched controls 2.62 (SD 0.60, P = .027). Given the 13 comparisons performed of both cohorts the FWER was 48.6%.
Total Hip Arthroplasty
The rates of pneumonia 6.9% versus 3.5% and hematoma (0.6% versus 0%) were statistically greater for those with prior COVID (P = <.001 and P = .004 respectively). Other complications occurred at similar rates between cohorts.
Mean lengths of stay were greater for those with prior COVID-19 (P < .001 for both). Same as with TKA, the FWER was also 48.6% (See Table 3 ).
Table 3.
90-Day Outcome Comparisons.
| TKA | Covid Cohort |
Matched Controls |
P Value | THA | Covid Cohort |
Matched Controls |
P Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Rate (%) | Events | Rate (%) | Events | Rate (%) | Events | Rate (%) | ||||
| Prosthetic Joint Infection | 1 | 0.15 | 1 | 0.15 | 1 | 2 | 0.30 | 1 | 0.20 | .5 | |
| AKI | 24 | 3.64 | 20 | 3.03 | .2 | 41 | 6.50 | 37 | 5.80 | .3 | |
| Cardiac Arrest | 0 | 0.00 | 0 | 0.00 | n/a | 4 | 0.60 | 4 | 0.60 | .8 | |
| DVT | 7 | 1.06 | 7 | 1.06 | .8 | 11 | 1.70 | 11 | 1.70 | .8 | |
| Nerve Injury | 0 | 0.00 | 0 | 0.00 | n/a | 0 | 0.00 | 1 | 0.20 | .3 | |
| PNA | 15 | 2.27 | 8 | 1.21 | .04 | 44 | 6.90 | 22 | 3.50 | <.001 | |
| PE | 1 | 0.15 | 0 | 0.00 | .3 | 0 | 0.00 | 1 | 0.20 | .3 | |
| UTI | 27 | 4.09 | 23 | 3.48 | .8 | 60 | 9.50 | 65 | 10.30 | .7 | |
| Wound Complication | 6 | 0.91 | 2 | 0.30 | .09 | 11 | 1.70 | 7 | 1.10 | .2 | |
| Hematoma | 1 | 0.15 | 0 | 0.00 | .3 | 4 | 0.60 | 0 | 0.00 | .04 | |
| Transfusion | 7 | 1.06 | 3 | 0.45 | .3 | 28 | 4.40 | 18 | 2.80 | .05 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Length Of Stay | 3.12 | 0.59 | 2.57 | 0.6 | .027 | 4.52 | 1.28 | 3.62 | 1.22 | <.001 | |
Bold highlights statistically significant differences.
TKA, total knee arthroplasty; THA, total hip arthroplasty; AKI, acute kidney injury; DVT, deep vein thrombosis; PNA, Pneumonia; PE, pulmonary embolism; UTI, urinary tract infection.
Discussion
This large, retrospective study, to the best of our knowledge, is the first of its magnitude to evaluate 90-day outcomes in patients who did and did not have a history of COVID-19 undergoing primary TJA. Despite prior studies demonstrating COVID-19 as a cause of systematic coagulation activation, excessive inflammation, platelet activation, hypoxia, and diffuse intravascular coagulation leading to thrombotic disease, this controlled study demonstrated there is no increased incidence of VTE among patients who have a previous COVID-19 infection, at multiple time points, undergoing primary TJA. This is an important finding as, while relatively rare, VTE following TJA has substantial patient morbidity and costs associated with their occurrence [[10], [11], [12]].
Outside of orthopaedics multiple studies have demonstrated an increased risk of VTE in patients with a history of COVID-19 infection [2,13,14]. For example, in a study of 184 Intensive Care Unit patients with proven COVID-19 pneumonia, there was a 31% incidence of thrombotic complication despite adequate prophylaxis, which is “remarkably high” [3]. Another study from Wuhan, China measured D-dimer levels in patients with COVID-19 [6] and found that among the patients with COVID-19 those with high D-dimer levels had an 18-fold increased risk of death; specifically, D-dimers greater than 1μg/mL were found to be associated with a fatal outcome. In another study, patients who died of COVID-19 were observed to have higher D-dimers upon admission compared to those who survived [7]. However, in a recent study, the authors found that there is no difference in D-dimer levels between SARS-CoV-2 IgG positive and negative patients that undergo TJA [8]. Regardless, DVT after TJA is relatively rare in the modern era (0.9% and 1.5%) for TJA [15], and this is consistent with the results of our study, which demonstrated an overall rate of VTE of 1.5% for patients who had a previous diagnosis of COVID-19 compared to 0.9% for those who did not have a previous COVID-19 diagnosis. It is possible that modern perioperative protocols that focus on enhanced recovery after surgery, as well as effective chemical and mechanical prophylaxis postoperatively mitigate the negative effect COVID-19 infection may have.
Considering the systemic effects that COVID-19 has been found to have on patients, it is also important to note that the results of our study suggest the incidence of infection, cardiac arrest, and other complications as well as LOS are comparable in patients who did and did not have a history of COVID-19 infection. There is no evidence to indicate there is a greater risk of any of these outcomes in patients who have a COVID-19 history undergoing TJA. While further research is necessary to determine any causal relationship behind these findings, they warrant further consideration for physicians caring for patients undergoing a TJA after previously having a COVID-19 infection. Moreover, the various COVID-19 variants and sub-variants could also affect how the disease affects patients and later has effects on outcomes such as DVTs.
This study is not without limitations. Most importantly, elective surgeries resumed mid-2020, which allows for only an approximately 19-month overlap of COVID-19 and TJA. Because of this, the number of patients was limited. It is possible that matching the 2 cohorts for surgical factors and comorbidities associated with VTE/bleeding could have given more accurate results albeit the numbers remain low at this time. In addition, we do not have the granularity in our dataset to control for chemical prophylaxis regimen. That is, we do not know what chemical VTE prophylaxis was used for each patient or what the adherence was with that medication by the patients themselves [16]. While we suspect any variance in this regard would likely be controlled for inherently by the study design, it is possible this could confound our results. Also, following up with patients beyond the 90-day interval could be beneficial, although the majority of our outcomes of interest occur in the early to mid-term postoperatively.
In conclusion, the data from this study suggest that patients who have a history of COVID-19 infection are not at a significantly increased risk of most perioperative complications following primary TJA, but are likely to require longer hospital stays and have greater chances of suffering from pneumonia. Additional follow up is required, but at least with the largest dataset available, patients and surgeons should feel comfortable resuming elective arthroplasty knowing that if they adhere to the most evidence-based clinical practices to date, COVID-19 infection history should not significantly increase their perioperative risk.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2022.10.041.
Appendix A. Supplementary Data
References
- 1.Gundtoft P.H., Pedersen A.B., Varnum C., Overgaard S. Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res. 2017;475:2623–2631. doi: 10.1007/s11999-017-5289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Magnusson M., Mackman N., et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5:756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan K.H., Lim S.L., Shaaban H., Guron G., Slim J. Persistent hypercoagulable state in COVID-19: a case series of COVID-19 associated pulmonary embolism. J Glob Infect Dis. 2021;13:38–41. doi: 10.4103/jgid.jgid_180_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Wang H., Yin P., Li D., Wang D.L., Peng P., et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J Thromb Haemost. 2021;19:1038–1048. doi: 10.1111/jth.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungwirth-Weinberger A., Oezel L., Morgenstern R., Shue J., Hanreich C., Sama A.A., et al. D-dimer levels are not elevated in SARS-CoV-2 IgG positive patients undergoing elective orthopedic surgery. J Clin Med. 2021;10:3508. doi: 10.3390/jcm10163508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsantes A.G., Papadopoulos D.V., Trikoupis I.G., Goumenos S., Piovani D., Tsante K.A., et al. The procoagulant effect of COVID-19 on the thrombotic risk of patients with hip fractures due to enhanced clot strength and fibrinolysis shutdown. J Clin Med. 2021;10:3397. doi: 10.3390/jcm10153397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svetitsky S., Shuaib R., McAdoo S., Thomas D.C. Long-term effects of COVID-19 on the kidney. QJM. 2021;114:621–622. doi: 10.1093/qjmed/hcab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahi A., Chen A.F., Tan T.L., Maltenfort M.G., Kucukdurmaz F., Parvizi J. The incidence and economic burden of in-hospital venous thromboembolism in the United States. J Arthroplasty. 2017;32:1063–1066. doi: 10.1016/j.arth.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almegren M.O., Alhedaithy A.A., Alomri A.S., Albawardy N.F., Mesmar R.S., Qahtani M.A.A. Venous thromboembolism after total knee and hip arthroplasty. Saudi Med J. 2018;39:1096–1101. doi: 10.15537/smj.2018.11.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaghan J.J., Mont M.A., Bozic K., Maloney W., Berry D. Rules of engagement using large databases: read the fine print. J Arthroplasty. 2018;33:1987. doi: 10.1016/j.arth.2018.04.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.