Abstract
Objectives
To characterize the kinetics of humoral and T-cell responses in rheumatoid arthritis (RA)-patients followed up to 4-6 weeks (T3) after the SARS-CoV-2 vaccine booster dose.
Methods
Health care workers (HCWs, n = 38) and patients with RA (n = 52) completing the messenger RNA vaccination schedule were enrolled at T3. In each cohort, 25 subjects were sampled after 5 weeks (T1) and 6 months (T2) from the first vaccine dose. The humoral response was assessed by measuring anti-receptor-binding domain (RBD) and neutralizing antibodies, the T-cell response by interferon-γ-release assay (IGRA), T cell cytokine production, and B cell phenotype at T3 by flow cytometry.
Results
Patients with RA showed a significant reduction of antibody titers from T1 to T2 and a significant increase at T3. T-cell response by IGRA persisted over time in patients with RA, whereas it increased in HCWs. Most patients with RA scored positive for anti-RBD, neutralizing antibody and T-cell responses, although the magnitude was lower than HCWs. The spike-specific-cytokine response was mainly clusters of differentiation (CD)4+ T cells restricted in both cohorts and significantly lower with reduced interleukin-2 response and CD4-antigen-responding naïve T cells in patients with RA. Unswitched memory B cells were reduced in patients with RA compared with HCWs independently of vaccination.
Conclusion
COVID-19 vaccine booster strengthens the humoral immunity in patients with RA even with a reduced cytokine response.
Keywords: COVID-19 vaccine, SARS-CoV-2, Rheumatoid arthritis, T-cell response, Antibody response, Immunosuppressive therapy
Introduction
Since the COVID-19 pandemic appearance, a global effort has been made to develop effective vaccines to stem SARS-CoV-2 infection through the induction of a coordinated B and T cell immune response (Agrati et al., 2021; Aiello et al., 2022a; Sette and Crotty, 2021, 2022). Humoral immunity consists of antibodies binding the SARS-CoV-2 spike (S) protein that neutralizes the virus, whereas cellular immunity includes virus-specific B and T cells, which provide long-term memory and promptly expand following re-exposure to antigens (Sette and Crotty, 2021, 2022).
Current COVID-19 vaccines include four different platforms: messenger RNA (mRNA) vaccines, adenovirus vector-based vaccines, inactivated virus vaccines, and adjuvanted protein vaccines (Barouch, 2022).
Several studies have demonstrated the immunogenicity of COVID-19 vaccines in the healthy population (Angyal et al., 2022). However, vulnerable subjects, particularly those with immune-mediated inflammatory disease (IMID) such as rheumatoid arthritis (RA), needed special attention and were prioritized for vaccination to mitigate COVID-19 risk. mRNA vaccines are safe in patients with RA and, 1 month after the first vaccination cycle, induce both humoral and T-cell responses, although their magnitude is lower than controls (Jena et al., 2022; Picchianti-Diamanti et al., 2021). However, a progressive waning of the humoral response to the COVID-19 vaccine was observed in vaccinated individuals (Bar-On et al., 2021), including patients with RA (Dayam et al., 2022; Farroni et al., 2022; Le Moine et al., 2022).
Moreover, different waves of SARS-CoV-2 variants have emerged, replacing the previous variants, and are often associated with increased transmissibility and greater antibody escape (Barouch, 2022). Several studies have shown little cross-reactivity of neutralizing antibodies induced by primary vaccines with Omicron, whereas T-cell responses induced by vaccines show a good cross-reactivity, even to Omicron (Carreño et al., 2022; Keeton et al., 2022; Liu et al., 2022; Nemet et al., 2022; Tarke et al., 2022).
Except for a study in rituximab-treated patients with RA, few studies on a small and heterogeneous population are available on the immunogenicity and safety of the booster dose in IMID patients treated with therapies associated with nonresponse to two vaccine doses (Aikawa et al., 2022; Benucci et al., 2022; Jyssum et al., 2022; Schmiedeberg et al., 2022; van der Togt et al., 2022).
In this prospective multicenter longitudinal observational study, we characterized, for the first time, in patients with RA treated with different immunosuppressive agents the antibody and T-cell response, in terms of cytokine and/or memory T-cell profile, following the COVID-19 mRNA vaccine booster. A proportion of this cohort was followed up to investigate the evolution of the immune responses.
Material and methods
The extended version of the Material and methods is included as supplemental information.
Study design
This longitudinal multicenter prospective study was conducted on patients with a diagnosis of RA according to the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria (Aletaha et al., 2010). Inclusion criteria for patients with RA were: (i) booster dose of a SARS-CoV-2 mRNA vaccine within the previous 4-6 weeks; (ii) ongoing treatment with a biological drug, i.e., tumor necrosis factor (TNF-α), interleukin (IL)-6 or cytotoxic T-lymphocyte antigen 4 (CTLA-4)-immunoglobulin (Ig), or Janus Kinase (JAK) inhibitors with or without disease-modifying anti-rheumatic drugs (DMARDs), with only DMARDs, or low dosage of corticosteroids (CSs).
Health care workers (HCWs) were enrolled at the INMI-Lazzaro Spallanzani-IRCCS (Rome, Italy).
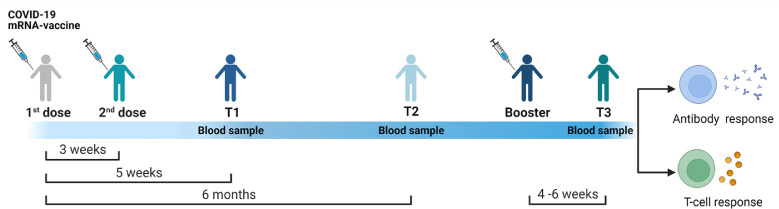
A follow-up study was performed on the subjects providing a blood sample after 5 weeks (T1) and 6 months (T2) from the first vaccine dose and after 4-6 weeks from the booster dose (T3) (Figure 1 ).
Figure 1.
Timing of COVID-19 vaccination and planning of the study enrollment.
Both health care workers and patients with rheumatoid arthritis received three doses of a COVID-19 mRNA vaccine. Blood sampling were performed after 5 weeks (T1) and 6 months (T2) from the first vaccine dose, and after 4-6 weeks from the booster dose (T3).
mRNA, messenger RNA.
Demographical and clinical data were collected at enrollment, and any clinical adverse event after vaccination was registered. RA disease activity was assessed by disease activity score 28 based on C-reactive protein (DAS28crp hereafter referred to as DAS28).
The protocol was approved by the Ethical Committee of INMI-Lazzaro Spallanzani-IRCCS (approval numbers 297/2021, 247/2021, and 318/2021). All participants signed written informed consent.
Experimental design
Antibody response was evaluated by anti-nucleoprotein-IgG (anti-N-IgG) and anti-receptor-binding domain-(RBD)-IgG. A microneutralization assay was performed as described (Matusali et al., 2021) using the SARS-CoV-2/Human/ITA/PAVIA10734/2020. For the interferon (IFN)-γ-release assay, whole blood was stimulated overnight with a peptide mix covering the entire SARS-CoV-2 S protein and with staphylococcal enterotoxin B (SEB) as a positive control, as described (Aiello et al., 2021, 2022b). Plasma IFN-γ levels were measured using an automatic enzyme-linked immunosorbent assay (ELLA, protein simple). For flow cytometry, whole blood was stimulated overnight with S peptide mix (Farroni et al., 2022), and cells were stained for the intracellular cytokines and T and B cell phenotypes (Carsetti et al., 2020) (Supplemenatary Table 1 and Supplementary Figure 1).
Statistical analysis
Continuous measures were reported as median and interquartile range. We performed Kruskal-Wallis and Friedman tests to compare several groups (for unpaired and paired data, respectively) adjusted with Dunn's multiple comparisons test; Mann-Whitney U-test for pairwise comparisons, chi-square, Fisher tests, and Cochran's Q test for proportions. Bonferroni correction was used when appropriate. Correlations were evaluated by nonparametric Spearman's rank test and reported with rho coefficient.
Results
Characteristics of the enrolled population
We prospectively enrolled 52 patients with RA and 38 HCWs. The two cohorts significantly differed in age (P <0.0001) (Table 1 ). Among patients with RA, we enrolled seven subjects treated with TNF-α inhibitors, 13 with IL-6 inhibitors with or without DMARDs/CSs (named IL-6 inhibitors), 12 with CTLA-4-Ig with or without DMARDs/CSs (named CTLA-4-Ig), 12 with DMARDs with or without CSs (named DMARDs) and eight under JAK inhibitors with or without DMARDs/CSs (named JAK inhibitors). Treatment subgroups did not differ in lymphocyte count or treatment duration (Table 1).
Table 1.
Demographical and clinical information of the 90 enrolled subjects at T3.
| Characteristics | Rheumatoid arthritis-patients | Health care workers | P-value | |
|---|---|---|---|---|
| N (%) | 52 (57.8) | 38 (42.2) | ||
| Age median (IQR) | 61 (55-69) | 44 (30-51) | <0.0001a | |
| Female N (%) | 43 (82.7) | 29 (76.3) | 0.594b | |
| Origin N (%) | West Europe | 44 (84.6) | 37 (97.4) | 0.135b |
| East Europe | 5 (9.6) | 0 (0) | ||
| Asia | 1 (1.9) | 1 (2.6) | ||
| South America | 2 (3.9) | 0 (0) | ||
| Rheumatologic treatment N (%) | TNF-α inhibitors | 7 (13.4) | - | |
| IL-6 inhibitors +/-DMARDs/CSs | 13 (25) | - | ||
| CTLA-4-Ig +/-DMARDs/CSs | 12 (23.1) | - | ||
| DMARDs +/- CSs | 12 (23.1) | - | ||
| JAK inhibitors +/-DMARDs/CSs | 8 (15.4) | - | ||
| Disease activity median (IQR) | DAS28crp | 3.2 (2.3-3.5) | - | |
| Lymphocytes count N (%) | 42 (80.8) | 0 (0) | ||
| Lymphocytes count N (%)Median x 103/µl (IQR) | TNF-α inhibitors | 6 (14.3)2.5 (2.0-3.3) | - | 0.174c |
| IL-6 inhibitors +/-DMARDs/CSs | 11 (26.2)1.8 (1.3-2.3) | - | ||
| CTLA-4-Ig +/-DMARDs/CSs | 12 (28.6)2.2 (1.8-2.8) | - | ||
| DMARDs +/- CSs | 7 (16.7)2.1 (1.4-2.3) | - | ||
| JAK inhibitors +/-DMARDs/CSs | 6 (14.3)1.9 (1.3-2.5) | - | ||
| Therapy, median years (IQR) | 5.4 (2.4-8.4)d | - | ||
| TNF-α inhibitors | 2.9 (1.8-12) | - | ||
| IL-6 inhibitors +/-DMARDs/CSs | 6.1 (5.2-7.8) | - | ||
| CTLA-4-Ig +/-DMARDs/CSs | 7.4 (2.3-10.9) | - | 0.250c | |
| DMARDs +/- CSs | 5.4 (2.5-6.6) | - | ||
| JAK inhibitors +/-DMARDs/CSs | 3.4 (1.1-4.2) | - | ||
Abbreviations: CSs, corticosteroids; CTLA-4, Cytotoxic T-lymphocyte antigen 4; DAS28crp, disease activity score 28 based on C-reactive protein; DMARDs, disease-modifying anti-rheumatic drugs; Ig, immunoglobulin; IL, interleukin; IQR, interquartile range; JAK, Janus Kinase; N, number; TNF, tumor necrosis factor.
Mann-Whitney U-statistic test; bChi-square test; cKruskal-Wallis test; davailable only for 39/52 subjects.
Neither disease flares nor severe adverse reactions were observed in our cohorts, as also reported by others (Connolly et al., 2022; Spinelli et al., 2022). Mild, transient, systemic, and local side effects (pain at the injection site, mild fever, arthromyalgia, fatigue) were reported by 12 patients following COVID-19 vaccination.
Humoral response after the booster dose
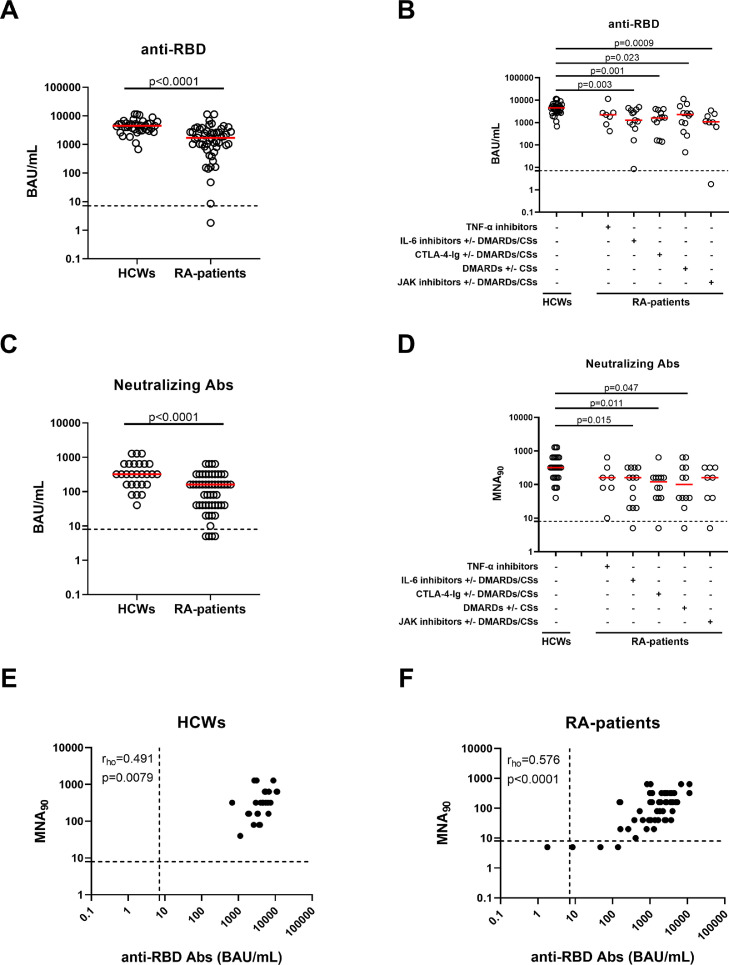
Enrolled subjects were naïve to SARS-CoV-2 infection as confirmed by undetectable anti-N antibodies (data not shown). At T3, all HCWs and almost all patients with RA (51/52, 98.1%) had a positive anti-RBD-IgG response (Figure 2a and Table 2 ). Within the RA cohort, the quantitative-specific response was not significantly different among treatment subgroups. However, compared with HCWs, significantly lower anti-RBD-IgG titers were found in patients under IL-6 (P-value = 0.003), CTLA-4-Ig (P-value = 0.001), or JAK inhibitors (P-value = 0.0009) and DMARDs (P-value = 0.023) (Figure 2b and Table 2).
Figure 2.
Patients with RA have a reduced antibody response compared with HCWs after the booster dose.
SARS-CoV-2-specific anti-RBD-IgG (a, b) and neutralizing (c, d) antibodies were detected in all HCWs (n = 38) and in the majority of patients with RA (51/52). Anti-RBD-IgG (b) and neutralizing (d) antibodies were reported by stratifying patients with RA according to the ongoing drug treatment: TNF-α inhibitors (n = 7), IL-6 inhibitors with or without DMARDs/CSs (n = 13), CTLA-4-Ig with or without DMARDs/CSs (n = 12), DMARDs with or without CSs (n = 12) and JAK inhibitors with or without DMARDs/CSs (n = 8). Anti-RBD-IgG and neutralizing antibodies were determined in sera samples, and reported as BAU/ml and reciprocal of dilution (MNA90), respectively. Anti-RBD-IgG titers and neutralizing antibodies analyzed in 28 HCWs (e) and 48 patients with RA (f) correlate each other. (a-d) Red horizontal lines indicate medians. Statistical analysis was performed using Mann-Whitney U-test for pairwise comparisons (a, c), whereas Kruskal-Wallis test adjusted with Dunn's multiple comparisons test to compare groups (b, d) and P <0.05 was considered significant. Dashed lines in the figure indicate the threshold value of each test (anti-RBD: 7.1 BAU/mL and MNA90: 8). Nonparametric Spearman's rank test was used for correlations (rho coefficient).
Abs, antibodies; BAU, binding antibody units; CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; Ig, immunoglobulin; IL, interleukin; JAK, Janus Kinase; MNA, microneutralization assay; RA, rheumatoid arthritis; RBD, receptor-binding domain; TNF, tumor necrosis factor.
Table 2.
Serological and T cell specific response at T3.
| Characteristics | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| patients with RA | HCWs | Within RA cohort | patients with RA vs HCWs | |||||
| N (%) | 52 (57.8) | 38 (42.2) | ||||||
| Antibody response | Qualitative response | Anti-RBD antibodies responders N (%) | 51 (98.1) | 38 (100) | >0.999b | |||
| Anti-RBD antibodies responders within the subgroups N (%) | TNF-α-inhibitors | 7/7 (100) | - | 0.213a | >0.999b | |||
| IL-6 inhibitors+/-DMARDs/CSs | 13/13 (100) | - | >0.999b | |||||
| CTLA-4-Ig inhibitors +/-DMARDs/CSs | 12/12 (100) | - | >0.999b | |||||
| DMARDs +/- CSs | 12/12 (100) | - | >0.999b | |||||
| JAK inhibitors+/-DMARDs/CSs | 7/8 (87.5) | - | 0.174b | |||||
| Quantitative response | Anti-RBD titersbinding antibody units /ml median (IQR) | 1701 (816-2843) | 4516 (3098-5477) | <0.0001c | ||||
| TNF-α inhibitors | 2214 (835-2767) | - | 0.829c | 0.126d | ||||
| IL-6 inhibitors+/-DMARDs/CSs | 1272 (670-3691) | - | 0.003d | |||||
| CTLA-4-Ig inhibitors +/-DMARDs/CSs | 1598 (379-3434) | - | 0.001d | |||||
| DMARDs +/- CSs | 2264 (521-4671) | - | 0.023d | |||||
| JAK inhibitors+/-DMARDs/CSs | 1082 (735-2309) | 0.0009d | ||||||
| S-specific interferon-γ T cell response | Qualitative response | S responders N (%) | 36 (69.2) | 38 (100) | <0.0001b | |||
| S responders within the subgroups N (%) | TNF-α inhibitors | 6/7 (85.7) | - | 0.211a | 0.156b | |||
| IL-6 inhibitors+/-DMARDs/CSs | 10/13 (76.9) | - | 0.014b | |||||
| CTLA-4-Ig inhibitors +/-DMARDs/CSs | 5/12 (41.7) | - | <0.0001b | |||||
| DMARDs +/- CSs | 9/12 (75) | - | 0.011b | |||||
| JAK inhibitors+/-DMARDs/CSs | 6/8 (75) | - | 0.027b | |||||
| Quantitative response | S interferon-γ levelspg/ml median (IQR) | 34.5 (12.4-141.9) | 425.9 (196-792) | <0.0001c | ||||
| TNF-α inhibitors | 43.5 (29.6-144.6) | - | 0.164c | 0.024d | ||||
| IL-6 inhibitors+/-DMARDs/CSs | 48.5 (14.9-168.5) | - | 0.0002d | |||||
| CTLA-4-Ig inhibitors +/-DMARDs/CSs | 12.8 (0.26-34.3) | - | <0.0001d | |||||
| DMARDs +/-CSs | 34.5 (15.6 -219) | - | 0.0015d | |||||
| JAK inhibitors+/-DMARDs/CSs | 40.4 (15.4-85.4) | - | 0.0010d | |||||
Abbreviations: CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; Ig, immunoglobulin; IL, interleukin; IQR, interquartile range; JAK, Janus Kinase; N, number; RA, rheumatoid arthritis; RBD, receptor-binding domain; S, spike; TNF, tumor necrosis factor.
Fisher test; bFisher test with Bonferroni correction (α/5, P ≤0.01); cKruskal-Wallis test; dKruskal-Wallis test adjusted with Dunn's multiple comparisons test (P <0.05). In bold are reported values that are significant.
Neutralizing serum antibodies were evaluated in patients with RA and in 28/38 HCWs. All HCWs and most patients with RA (48/52, 92.3%) showed a detectable neutralizing activity (Figure 2c, d). Significant different neutralizing titers were found comparing IL-6 inhibitors, CTLA-4-Ig- and DMARDs-treated patients, and HCWs (P-value = 0.015, P-value = 0.011, and P-value = 0.047, respectively). In both cohorts, anti-RBD-IgG and neutralizing antibody titers significantly correlated (HCWs: rho = 0.491, P-value = 0.0079 and RA: rho = 0.576, P <0.0001) (Figure 2e, f). In contrast, no correlation was found between antibody titers and lymphocyte count, DAS28, or years of therapy in patients with RA (Supplementary Figure 2a-c).
Kinetic of the antibody-specific response after COVID-19 vaccination
Antibody response to the COVID-19 vaccine was monitored in patients with RA (n = 25), and HCWs (n = 25) longitudinally sampled at T1-T3 (Figure 1). JAK inhibitors-treated patients with RA were excluded due to their limited number.
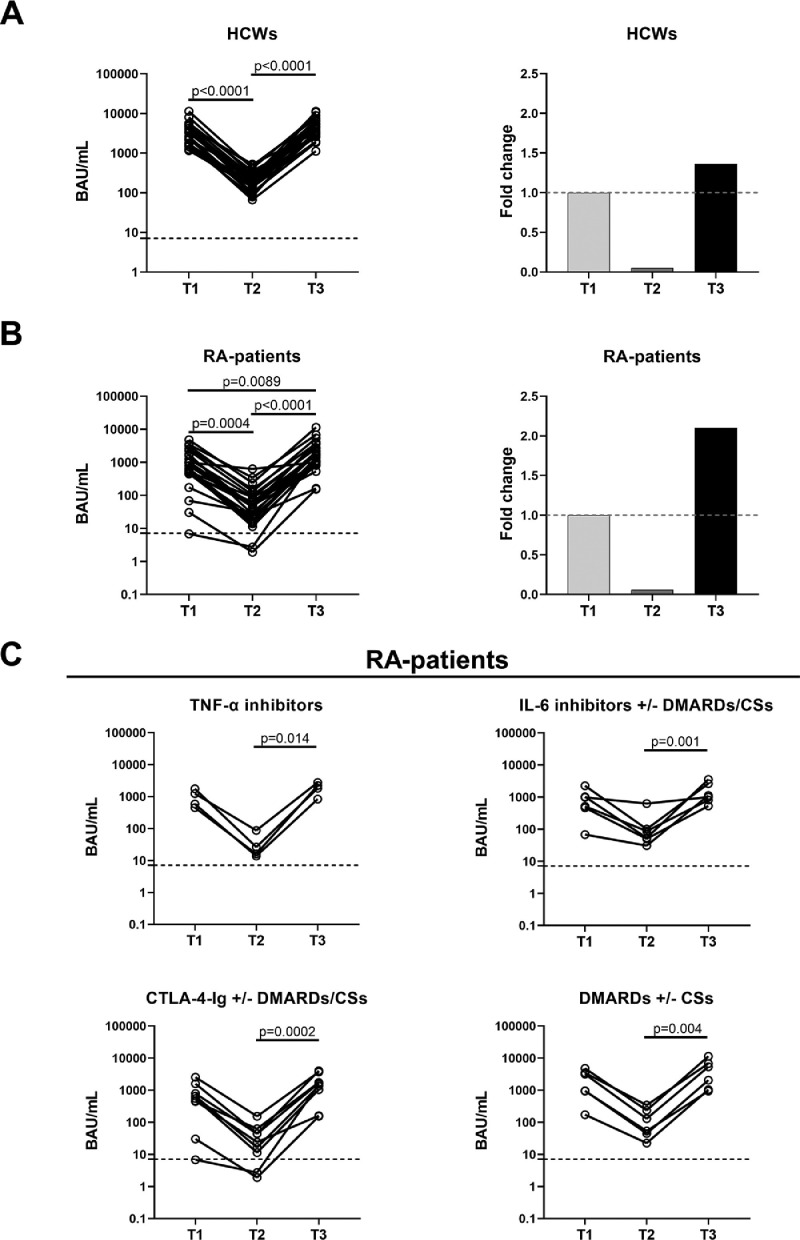
In HCWs, we found a significant reduction of anti-RBD-IgG titers from T1 to T2, followed by a significant increase from T2 to T3 (for both P <0.0001) (Figure 3a and Table 3 ). Similarly, in patients with RA, we observed a significant reduction in anti-RBD-IgG titers from T1 to T2 (P-value = 0.0004) and a significant 2-fold and 37-fold increase at T3 from T1 (P-value = 0.0089) and T2 (P <0.0001), respectively (Figure 3b and Table 3). In stratifying patients with RA, the antibody response significantly varied from T2 to T3 regardless of the ongoing treatment (Figure 3c). In contrast, no difference was observed over time in the proportion of antibody responders for both cohorts (Table 3).
Figure 3.
The antibody response to COVID-19 vaccine increases in HCWs and patients with RA after the booster dose.
The antibody response was evaluated in the HCWs (a) and patients with RA (b) followed over time (n = 25 for each group). Blood sampling were performed after 5 weeks (T1) and 6 months (T2) from the first vaccine dose and after 4-6 weeks from the booster dose (T3). On the left of both panels A and B we reported the histograms showing the fold change calculated with respect to T1. (c) The antibody response was reported by stratifying patients with RA based on drug treatment: TNF-α inhibitors (n = 4), IL-6 inhibitors with or without DMARDs/CSs (n = 6), CTLA-4-Ig with or without DMARDs/CSs (n=9) and DMARDs with or without CSs (n = 6). Anti-RBD-IgG were determined in sera samples and expressed as BAU/ml. Black dashed lines identify the cut-off used to define a positive responder (anti-RBD: 7.1 BAU/ml). Statistical analysis was performed using Friedman test adjusted with Dunn's multiple comparisons test. A P <0.05 was considered significant.
BAU, binding antibody units; CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; Ig, immunoglobulin; IL, interleukin; RA, rheumatoid arthritis; RBD, receptor-binding domain; TNF, tumor necrosis factor.
Table 3.
Longitudinal observation of the T cell and antibody responses.
| T1 | T2 | T3 | P-value | |||
|---|---|---|---|---|---|---|
| N (%) | 25 (100%) | 25 (100%) | 25 (100%) | |||
| Qualitative response | Antibody responders N (%) | HCWs | 25/25 (100%) | 25/25 (100%) | 25/25 (100%) | - |
| RA | 24/25 (96%) | 23/25 (92%) | 25/25 (100%) | 0.223a | ||
| T cell responders N (%) | HCWs | 25/25 (100%) | 25/25 (100%) | 25/25 (100%) | - | |
| RA | 17/25 (68%) | 17/25 (68%) | 18/25 (72%) | 0.819a | ||
| Quantitative response | Anti-RBD titersbinding antibody units/ml median (IQR) | HCWs | 3377 (1647-4839) | 178 (132-271) | 4608 (3040-6408) | <0.0001b |
| RA | 785 (458-1678) | 45 (19-94) | 1653 (969-3166) | <0.0001b | ||
| Spike interferon-γ levelspg/ml median (IQR) | HCWs | 262 (118-531) | 196 (66-490) | 525 (234-812) | 0.012b | |
| RA | 39.4 (5.3-177) | 70.6 (8.7-180) | 43.5 (14.5-168.5) | 0.961b |
Abbreviations: HCW, health care worker; IQR, interquartile range; N, number; RA, rheumatoid arthritis; RBD, receptor-binding domain.
Cochran's Q test; bFriedman test were performed for the statistical analysis.
B cell phenotype in patients with RA and HCWs
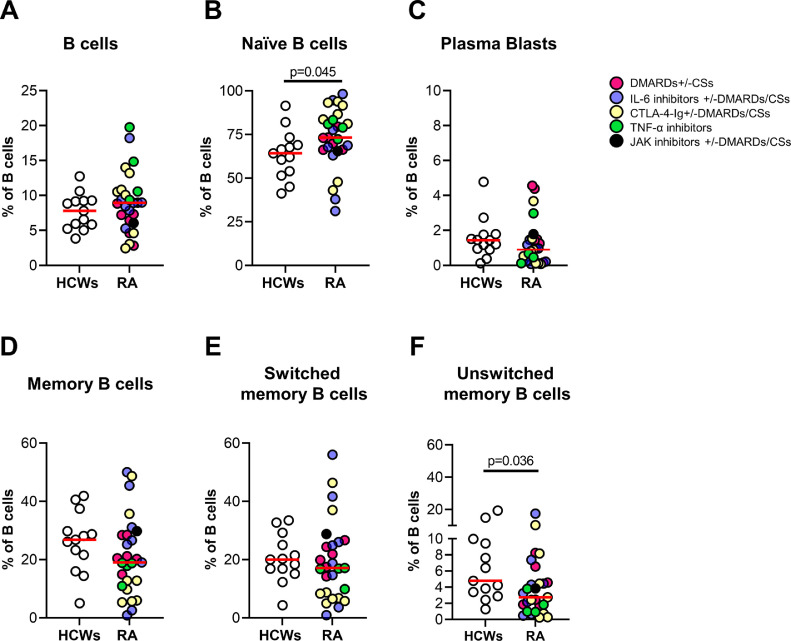
By flow cytometry, we observed a comparable frequency of total B cells between patients with RA (n = 28) and HCWs (n = 13) after the booster dose (Figure 4a ). Naïve B cells were significantly increased in patients with RA (P-value = 0.045) (Figure 4b), whereas a reduction trend of plasma blasts was observed (Figure 4c). Moreover, the unswitched memory B cells were significantly reduced (P-value = 0.036) compared with HCWs, whereas memory B cells and switched memory B cells showed only a trend of reduction in patients with RA (Figure 4d-f). No significant differences were found among patients under different treatments, probably due to the small sample investigated.
Figure 4.
Evaluation of the B cell phenotype by flow cytometry.
B cell subpopulations were evaluated by flow cytometry in HCWs (n = 13) and patients with RA (n = 28). Patients with RA were color-coded according to the treatment. B cells were gated as CD19+CD3− (a), naïve B cells as CD24+CD27− (b), plasma blasts as CD27+CD24− (c), memory B cells as CD24highCD27+ (d), switched memory B cells as CD27+IgD− (e) and unswitched memory B cells as CD27+IgD+ (f). Each dot represents an individual, and the red horizontal line represents the median. Mann-Whitney U-test was used for the statistical analysis and P <0.05 was considered significant.
CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; Ig, immunoglobulin; IL, interleukin; JAK, Janus Kinase; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
SARS-CoV-2-spike-specific T-cell response after the booster dose
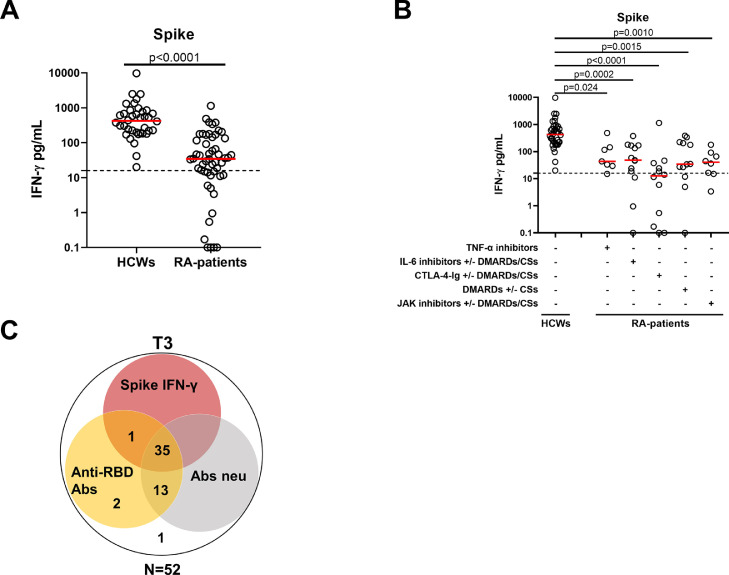
At T3, most patients with RA (36/52, 69.2%) showed an IFN-γ-S-specific response (Table 2). However, significantly different proportions of responders were observed compared with HCWs (38/38, 100%, P <0.0001) (Figure 5a and Table 2). In particular, the response rate of CTLA-4-Ig-treated patients (5/12, 41.7%) was significantly lower than that of HCWs (P <0.0001), whereas patients with RA under TNF-α, IL-6 or JAK inhibitors and DMARDs did not show significant differences in the number of responders compared with HCWs (P-value = 0.156, P-value = 0.014, P-value = 0.011, and P-value = 0.027) (Table 2). In contrast, IFN-γ levels were significantly lower in all patients with RA than in HCWs regardless of treatment (Figure 5a, b, and Table 2).
Figure 5.
IFN-γ T cell response detected by IGRA is reduced in patients with RA compared with HCWs after the booster dose.
(a) Spike-specific IFN-γ T cell response evaluated by IGRA was detected in all HCWs (n = 38) and in the majority of patients with RA (36/52). (b) T cell response was showed also stratifying patients with RA according to drug treatment: TNF-α inhibitors (n = 7), IL-6 inhibitors with or without DMARDs/CSs (n = 13), CTLA-4-Ig with or without DMARDs/CSs (n = 12), DMARDs with or without CSs (n = 12) and JAK inhibitors with or without DMARDs/CSs (n = 8). IFN-γ levels were quantified by automatic enzyme-linked immunoassay and reported after subtracting the unstimulated control value. The cut-off was set at 16 pg/ml (dashed line). (c) Venn diagram represents the number of responders for anti-RBD IgG, neutralizing antibodies and IFN-γ T cell response. Statistical analysis was performed using Mann-Whitney U-test for pairwise comparisons (a), whereas Kruskal-Wallis test adjusted with Dunn's multiple comparisons test to compare groups (b). A P-value <0.05 was considered significant.
Abs, antibodies; CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; IFN, interferon; IGRA, IFN-γ-release assay; Ig, immunoglobulin; IL, interleukin; JAK, Janus Kinase; RA, rheumatoid arthritis; RBD, receptor-binding domain; TNF, tumor necrosis factor.
No correlation was found between IFN-γ-S-specific response and lymphocyte count, DAS28, or years of therapy in patients with RA (Supplementary Figure 2d-f). Moreover, in both cohorts, no significant correlations were observed between the IFN-γ-S-specific response and anti-RBD-IgG or neutralizing titers (Supplementary Figure 3a-d). Among patients with RA, 35 subjects were full responders showing neutralizing activity, anti-RBD antibody, and T-cell responses; only one was a nonresponder, whereas 16 were partial responders (presenting at least one response). Among them, the majority had neutralizing and anti-RBD antibody response but not T-cell response (Figure 5c and Supplementary Table 2).
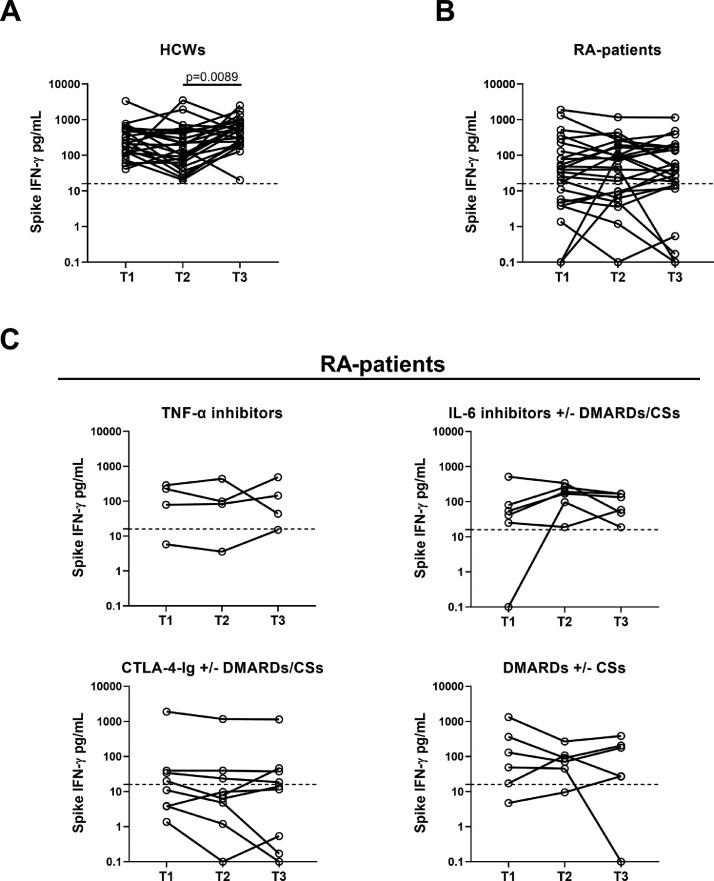
SARS-CoV-2-spike-specific T-cell response persists over time
Cell-mediated response to the COVID-19 vaccine was monitored over time concomitantly with the serological response (Figure 1). In HCWs, the booster dose favored a significant increase of the IFN-γ-S-specific response compared with T2 (P-value = 0.0089) (Figure 6a and Table 3). In contrast, in patients with RA, the T-cell response did not show significant modulations (Figure 6b), but it persisted over time, retaining significantly lower IFN-γ levels than HCWs (P <0.0001) even after the booster dose. These results were consistent across all treatments. Indeed, in stratifying patients with RA, the IFN-γ-S-specific response was stable from T2 to T3 independently of therapy (Figure 6c). Moreover, equal or similar proportions of T cell responders were observed over time in both cohorts (Table 3).
Figure 6.
The IFN-γ T cell response detected by IGRA remains stable in HCWs and patients with RA after COVID-19 vaccine booster dose.
T cell response was evaluated by IGRA in the HCWs (a) and patients with RA (b) followed over time (n = 25 for each group). Blood sampling were performed after 5 weeks (T1) and 6 months (T2) from the first vaccine dose and after 4-6 weeks from the booster dose (T3). (c) T cell response was reported by stratifying patients with RA according to therapy: TNF-α inhibitors (n = 4), IL-6 inhibitors with or without DMARDs/CSs (n = 6), CTLA-4-Ig with or without DMARDs/CSs (n = 9) and DMARDs with or without CSs (n = 6). IFN-γ levels were measured by automatic enzyme-linked immunoassay and reported after subtracting the unstimulated control value. The cut-off was set at 16 pg/ml (dashed lines). Statistical analysis was performed using Friedman test adjusted with Dunn's multiple comparisons test. A P-value <0.05 was considered significant.
Abs, antibodies; CSs, corticosteroids; CTLA-4, cytotoxic T-lymphocyte antigen 4; DMARDs, disease-modifying anti-rheumatic drugs; HCWs, health care workers; IFN, interferon; Ig, immunoglobulin; IGRA, IFN-γ-release assay; IL, interleukin; RA, rheumatoid arthritis; RBD, receptor-binding domain; TNF, tumor necrosis factor.
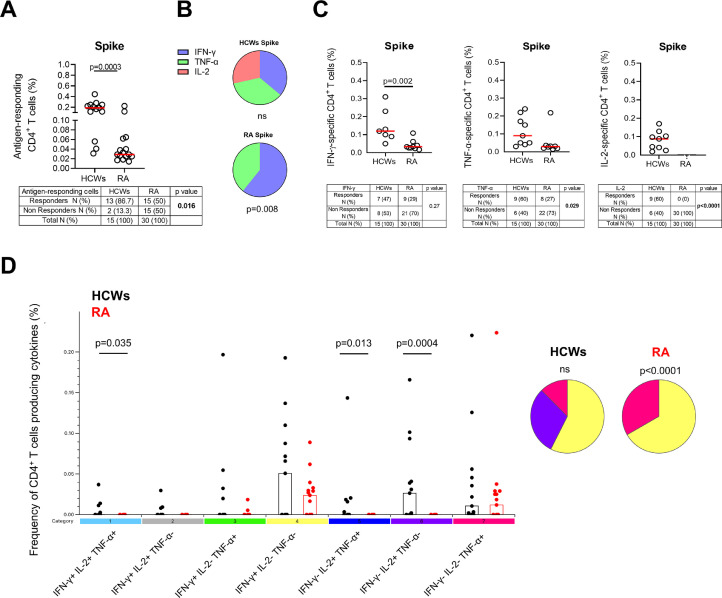
Multi-functional cytokine production in patients with RA is impaired compared with controls
At T3, the response to SARS-CoV-2 vaccination was evaluated by also investigating the frequency of clusters of differentiation (CD)4+ and CD8+ T cells producing IFN-γ, IL-2, or TNF-α in response to S protein. HCWs and patients with RA significantly differed in the antigen-specific CD4 T-cell response rate (P-value = 0.016) (Figure 7a ). Among responders, patients with RA showed a significantly lower frequency of the total S-specific response than HCWs (P-value = 0.0003) (Figure 7a). The response of patients with RA was characterized mainly by IFN-γ and TNF-α (P-value = 0.008), whereas HCWs produced all IFN-γ, TNF-α, and IL-2 (Figure 7b). In particular, CD4+ T cells of patients with RA failed to produce IL-2 in response to S and showed a significantly lower frequency of IFN-γ-specific CD4+ T cells (P-value = 0.002) and a trend of a lower frequency of TNF-α-specific CD4+ T cells compared with HCWs (Figure 7c).
Figure 7.
Multi-functional cytokine profile of CD4+ T cells in response to spike stimulation at T3: in patients with RA the response is impaired for IL-2 and reduced for IFN-γ and TNF-α compared with HCWs.
(a) Graph shows the total cytokine response in HCWs (n = 13) and RA (n = 15) responders. Responders and nonresponders for each group are reported in the tables. (b) Pie charts show the proportion of the CD4+ T cells producing cytokines (IFN-γ, TNF-α, IL-2) within HCWs or RA responders. (c) Frequency of CD4+ IFN-γ+ T cells, CD4+ TNF-α+ T cells and CD4+ IL-2+ T cells in HCWs and RA responders. Differences between responders and nonresponders for each group and for each cytokine are reported in tables below the corresponding graphs. (d) Cytokine profile of CD4+ T cells was evaluated only in the responders using Boolean gate combination; graph shows the frequency of the different subsets of CD4+ T cells producing cytokines in HCWs and RA. Pie charts show the proportion of cytokine-producing subsets within the different groups. Background cytokine production (unstimulated condition) was subtracted from each stimulated condition. Statistical analysis was performed using Mann-Whitney U-test for data reported in a, c, and d with a P-value <0.05 considered significant. Friedman test was used for pie charts (b and d) to compare the median frequency of the antigen-responding T cells expressing IFN-γ, IL-2, or TNF-α within the HCW or patients with RA group; the chi-square test was used for contingency tables. Median is represented by red lines, and each dot represents a different HCW or patient with RA.
CD4, cluster of differentiation 4; HCWs, health care workers; IFN, interferon; IL, interleukin; ns, not significant; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
Only the proportions of TNF-α- (P-value = 0.029) and IL-2-specific CD4+ T cell responders (P <0.0001) were significantly reduced in patients with RA compared with HCWs (Figure 7c).
Regarding the total Tc1-specific CD8 cytokine response, a lower number of responders was found compared with the CD4+ T cell subset in both cohorts (Supplementary Figure 4a). Moreover, no differences were found in terms of magnitude or response rate between patients with RA and controls (Supplementary Figure 4a). In both cohorts of responders, CD8+ T cells produced only IFN-γ in response to S (Supplementary Figure 4b, c). Based on this result and due to the reduced number of CD8+ T cell responders, no further analyses were done within the CD8+ T cell compartment.
We also assessed if the low number of total CD4 responders was due to the lymphocyte counts, but no correlation was found (Supplementary Figure 3e).
Almost all enrolled individuals responded to SEB stimulus in terms of total and single cytokine response (Supplementary Figure 5a-f). In HCWs, either CD4+ or CD8+ T cells produced the three cytokines (Supplementary Figure 5b, c, e, f). In contrast, the CD4+ T cell compartment of patients with RA produced mainly IFN-γ and TNF-α, whereas only a few patients produced IL-2 (Supplementary Figure 5b,c). Interestingly, in patients with RA, a higher number of IL-2 responders was found in the CD8 compartment (Supplementary Figure 5e, f).
Characterization of the spike-specific cytokine profile in HCW and patients with RA
A Boolean gating analysis (Roederer et al., 2011) was performed to identify the triple, double, or single cytokine-producing populations (IFN-γ and/or TNF-α and/or IL-2) in response to S (Figure 7d) and the specificity of the response was evaluated by comparing it with that to SEB (Supplementary Figure 5g).
The frequency of S-specific CD4+ T cells IFN-γ+TNF-α+IL-2+ (triple-positive), IFN-γ−TNF-α+IL-2+ (double-positive), or IFN-γ−TNF-α−IL-2+ (single-positive) was significantly reduced in patients with RA compared with HCWs (P-value = 0.035, P-value = 0.013 and P-value = 0.0004, respectively) (Figure 7d).
The antigen-specific response of HCWs was characterized mainly by single cytokine-producing CD4+ T cells (IFN-γ−TNF-α−IL-2+, IFN-γ−TNF-α+IL-2−, IFN-γ+TNF-α−IL-2−). Similarly, in patients with RA, we observed a predominant single cytokine profile based on IFN-γ+TNF-α−IL-2− and IFN-γ−TNF-α+IL-2− CD4+ T cells (P <0.0001) (Figure 7d). Interestingly, unlike patients with RA, HCWs had all the different combinations of cytokine-producing populations in response to SEB, likely due to the stronger stimulus. Within each cohort, significant differences were found in terms of cytokine frequency (P <0.0001 for both) (Supplementary Figure 5g).
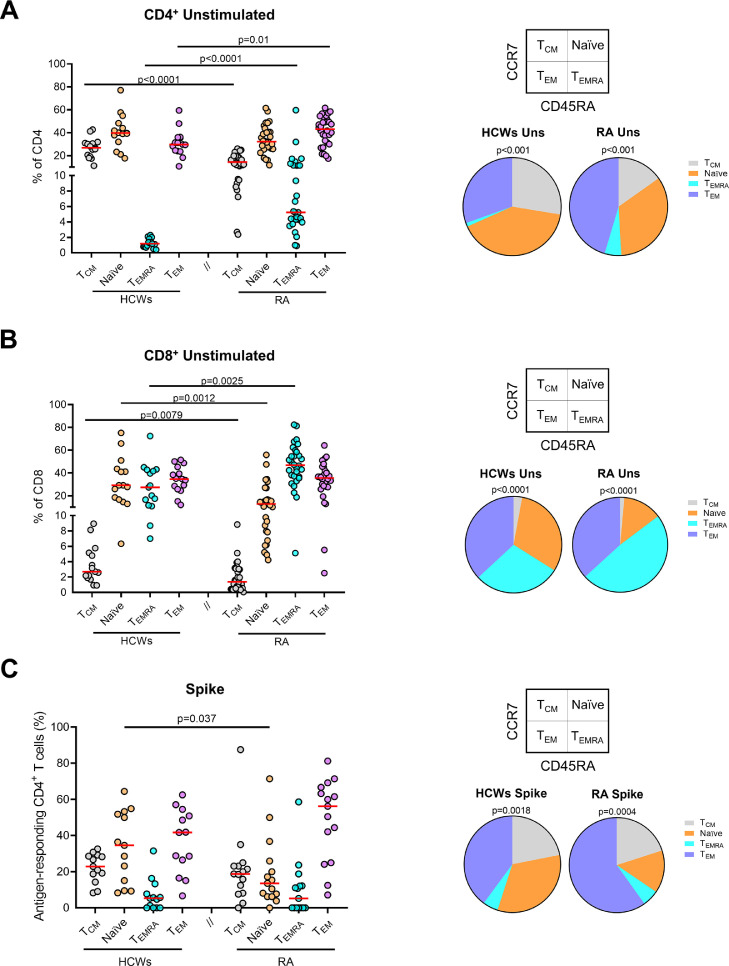
Memory T cell phenotype
We further evaluated whether the booster dose influences T cell phenotype by analyzing the expression of CD45RA and CCR7 in total CD4+and CD8+T cells (Geginat et al., 2003; Tian et al., 2017) arthromyalgia). In the CD4+ T cell subset, central memory cells (TCM) were found to be significantly reduced in patients with RA (P <0.0001), whereas the terminally differentiated effectors (TEMRA) and effector memory (TEM) cells were increased compared with controls (P <0.0001 and P-value = 0.01, respectively) (Figure 8a ). The main difference was found for the TEMRA population. Regarding CD8+ T cells, both cohorts significantly differed for TCM, TEMRA, and naïve populations; TCM and naïve were decreased (P-value = 0.0079 and P-value = 0.0012), whereas TEMRA augmented compared with HCWs (P-value = 0.0025) (Figure 8b).
Figure 8.
Different modulation of the memory T cell phenotype in CD4+ and CD8+ T cells and in the antigen-responding CD4+ T cells at T3 between patients with RA and HCWs.
T cell phenotype in HCWs (n = 15) and patients with RA (n = 30) characterized in total CD4+ (a) or CD8+ (b) T cells gated according to the expression of CD45RA and CCR7. Pie charts represent the distribution of CD4+ or CD8+ subpopulations (CD45RA+/−CCR7+/−) in HCWs or patients with RA. Colors reported in the graphs correspond to the single population represented in the wedges of the pies. (c) The frequency of CD45RA and CCR7 of antigen-responding CD4+ T cells was evaluated within HCWs (n = 13) and patients with RA (n = 15) responders. Pie charts represent the proportion of the CD45RA+/−CCR7+/− in HCWs or patients with RA. Statistical analysis was performed using Mann-Whitney U-test for graphs with a P-value <0.05 considered significant. Median is represented by red lines, and each dot represents a different HCW or patient with RA. Friedman test was used for pie charts to compare the frequencies within each group.
CD, cluster of differentiation; HCWs, health care workers; RA, rheumatoid arthritis; TCM, central memory; TEM, effector memory; TEMRA, terminally differentiated effector memory; Uns, unstimulated.
Then, we evaluated the phenotype in the S-specific CD4+ T cells producing any cytokine among IFN-γ, TNF-α, or IL-2. We found a significant reduction of naïve T cells in patients with RA compared with HCWs (P-value = 0.037). The other subpopulations, except the TEM, also showed a trend of reduction, although not significant (Figure 8c). In response to SEB, both cohorts showed a memory T cell phenotype similar to the results observed after S stimulation (Supplementary Figure 6).
Discussion
SARS-CoV-2 vaccination is one of the most effective strategies to protect communities by preventing severe outcomes and death from COVID-19 (Istituto Superiore di Sanità (ISS), 2021). Compared with previous studies, this is the first multicenter study that investigates the B and T-cell responses to the vaccine booster dose in patients with RA evaluating the cytokine profile of antigen-specific T cells and the B and T cell memory compartments.
We showed that the antibody response was detected in almost all patients with RA and HCWs independently of the time point. This response significantly decreased from T1 to T2 and increased at T3 in both cohorts, regardless of the immunosuppressive therapy. In contrast, the T-cell response remained stable over time, although the magnitude was significantly lower in patients with RA than HCWs, even after the booster dose. Subjects with no T-cell response at T1 do not acquire it even at subsequent doses (T2 and T3) (Gilboa et al., 2022; Hurme et al., 2022). The lowest magnitude and T-cell response rate were found in CTLA-4-Ig-treated patients, confirming what was previously observed (Farroni et al., 2022; Petrone et al., 2022; Picchianti-Diamanti et al., 2021). Most patients with RA show a “full response” (both humoral and cellular) to the vaccine.
To optimize the balance between vaccine immunogenicity and disease activity, CTLA-4-Ig and methotrexate were interrupted during the first vaccine cycle according to ACR indications (Mikuls et al., 2021), whereas no modification of the ongoing therapy was made for the booster. Our study demonstrates the legitimacy of this strategy both in terms of safety and vaccine immunogenicity. Indeed, no disease flares were observed, and most patients showed an antibody response that further increased after the booster. The only discrepancy arises from T-cell response that did not change after the booster dose. Because the lowest magnitude of response was associated with CTLA-4-Ig therapy, its interruption after each dose may optimize vaccine immunogenicity, as stated by the last ACR indications (Tang et al., 2022; Tedeschi et al., 2022).
Our results highlight the benefit of a vaccine booster in patients with RA as it guarantees the maintenance of a high seroconversion rate and induces a 2-fold quantitative increase of the humoral response compared with the first vaccination cycle. Interestingly, although antibody levels continued to be lower, the increase in patients with RA was higher than that observed in HCWs, likely because HCWs might have already achieved a plateau of the response with the two vaccine doses. In contrast, in patients with RA, the initial lower response allows a significant antibody expansion after the booster. A similar trend was observed in larger cohorts of rheumatologic patients (Corradini et al., 2022; Syversen et al., 2022), who likely need longer time to achieve a quantitatively higher humoral response, suggesting the potential benefits of additional booster doses. Moreover, an epidemiological study showed the increased efficacy of mRNA vaccines in preventing COVID-19 hospitalization from the second to the third dose in both immunocompetent (82% vs 97%, respectively) and immunocompromised individuals (69% vs 88%, respectively) (Tenforde et al., 2022).
In contrast, in patients with RA, the T-cell response did not change over time in terms of both magnitude and response rate, whereas a significant increase was observed in HCWs after the booster dose. This discrepancy might be caused by the detrimental effect of immunosuppressive therapies on T cells in patients with RA (Kosmaczewska et al., 2014; Sauzullo et al., 2018).
As previously demonstrated (Farroni et al., 2022), we show that the S response is mainly driven by CD4+ T cells in both cohorts. Analyzing the T cell-specific response, patients with RA are characterized by an antigen-specific CD4 T-cell response associated with an IL-2 reduced production compared with HCWs. The high response to SEB observed in RA suggests that these patients have the ability to respond to a strong and nonspecific stimulus, although with a lower magnitude than HCWs. Moreover, we found a higher number of IL-2 responders within the CD8+ T cells in patients with RA. This result requires further investigation. The lower cytokine response observed in patients with RA independently of the stimulus used (SEB or S) suggests its association with the rheumatologic condition per se and/or the ongoing therapy.
Our results agree with Dayam et al. (2022) reporting, in a different experimental setting, that the IL-2 production is reduced in SARS-CoV-2-vaccinated IMID patients compared with controls. Another study shows that in unvaccinated patients with RA antigen-responding CD4+ T cells mostly produce IFN-γ; the IL-2 production is reduced, and only few double-positive IFN-γ/IL-2 cells are present in the periphery (Ponchel et al., 2012). Moreover, it has been reported that CTLA-4-Ig therapy reduces the bioavailability of soluble IL-2 receptors by decreasing IL-2 levels (Weisman et al., 2006). It is well known that IL-2 is involved in lymphocyte activation, differentiation, survival, and maintenance of the T-regulatory cell compartment (Kosmaczewska et al., 2014; Zhang et al., 2022).
Here, we also show that the S-specific T-cell response is characterized by the induction of a similar memory cell phenotype in both cohorts; this agrees with another setting of patients vaccinated for influenza (Uchtenhagen et al., 2016). In contrast, in both total CD4+ and CD8+ T cells of patients with RA, we observed a higher frequency of effector and/or TEMRA memory subpopulations compared with HCWs. Analyzing the B cell phenotype of patients with RA, we found a significant increase of naïve B cells and a decrease of unswitched memory B cells compared with HCWs, which seems to be independent of COVID-19 vaccination and likely associated with the rheumatologic status, as reported (Moura et al., 2010; Wang et al., 2013, 2019).
Some limitations are acknowledged. First, the proportion of responders detected by flow cytometry is lower than in other experimental settings involving samples from SARS-CoV-2-vaccinated subjects (Gao et al., 2022; Tarke et al., 2022). However, the in vitro conditions (SARS-CoV-2 peptides composition and concentration) used are different, making the comparison difficult to perform. This study was conducted using whole blood samples and not peripheral blood mononuclear cells, potentially limiting the detection of the response (Hoffmeister et al., 2003). Second, the small size of the cohorts might limit the robustness of the data and did not allow us to study in detail the impact of the immunosuppressive treatments in patients with RA. Moreover, the lack of a control group of untreated patients with RA does not allow us to verify whether the impairment of the immune response observed might be RA-specific or more associated with the treatment used.
However, the RA cohort is representative of subjects under different therapies and is well followed clinically and immunologically. The two cohorts differed by age, but we previously showed that the reduced immune response is associated with immunosuppressive therapy and not with age (Picchianti-Diamanti et al., 2021).
The main strengths of this study are the longitudinal observation of both adaptive immune responses and the immune characterization by flow cytometry of B cells and of the antigen-specific T-cell response to COVID-19 mRNA vaccines in patients with RA and HCWs.
In conclusion, this study demonstrates that the booster dose leads to a gain of the antibodies compared with the levels after the first vaccination cycle. In contrast, T-cell response remains stable over time in patients with RA, as reported in healthy subjects (Hurme et al., 2022), even if it is lower than in HCWs. Despite the reduced IL-2-specific production, the specific memory T-cell response is similar in patients with RA and HCWs, indicating the importance of COVID-19 vaccination in this vulnerable population.
Funding
This work was supported by INMI “Lazzaro Spallanzani” Ricerca Finalizzata COVID-2020-12371675 and Ricerca Corrente on emerging infections, both funded by the Italian Ministry of Health, by the European Union's Horizon 2020 research and innovation program under EVA GLOBAL grant agreement N°871029, and by generous liberal donations funding for COVID-19 research from Camera di Commercio, Industria e Artigianato di Roma (resolution n°395 of May 25, 2021). The funders were not involved in the study design, collection, analysis, and interpretation of data, writing of this article, or the decision to submit it for publication.
Ethical approval
The protocol was approved by the Ethical Committee of INMI-Lazzaro Spallanzani-IRCCS (approval numbers 297/2021, 247/2021, and 318/2021).
Author contributions
DG and EN wrote the project submitted to the ethical committee. DG, EN, APD, BL, CA, and FC conceived and designed the study. Experiments were performed by CF, AA, SM, DL, VV, AMGA, GG, AB, MI, VG, and MM. CF and AA performed the statistical analysis. APD, BL, GC, SV, AM, AD, LN, VP, RDR, SS, GS, PS, VB, MB, and FC enrolled patients and collected clinical data. CF, AA, and DG drafted the article. All authors critically analyzed, discussed, interpreted data, contributed to the article, and approved the submitted version.
Declaration of Competing Interest
APD received fees for educational training or consultancy by Abbvie, Amgen, Novartis, Galapagos, and BMS. EN is a member of the advisory board of Gilead, Lilly, and Roche and received fees for educational training from Gilead, Lilly, and Roche. DG is a member of the advisory board of Biomerieux and Eli-Lilly and received fees for educational training or consultancy by Almirall, Biogen, Cellgene, Diasorin, Janssen, Qiagen, and Quidel. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ackowledgments
The authors gratefully acknowledge the nurses, the study patients, and the collaborators of all the involved centers who helped to conduct this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.10.035.
Appendix. Supplementary materials
References
- Agrati C, Castilletti C, Goletti D, Meschi S, Sacchi A, Matusali G, et al. Coordinate induction of humoral and spike specific T cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. 2021;9:1315. doi: 10.3390/microorganisms9061315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A, Coppola A, Vanini V, Petrone L, Cuzzi G, Salmi A, et al. Accuracy of QuantiFERON SARS-CoV-2 research use only assay and characterization of the CD4+ and CD8+ T cell-SARS-CoV-2 response: comparison with a homemade interferon-γ release assay. Int J Infect Dis. 2022;122:841–849. doi: 10.1016/j.ijid.2022.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A, Grossi A, Meschi S, Meledandri M, Vanini V, Petrone L, et al. Coordinated innate and T cell immune responses in mild COVID-19 patients from household contacts of COVID-19 cases during the first pandemic wave. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.920227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A, Najafi Fard S, Petruccioli E, Petrone L, Vanini V, Farroni C, et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int J Infect Dis. 2021;106:338–347. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa NE, Kupa L de VK, Medeiros-Ribeiro AC, Saad CGS, Yuki EFN, Pasoto SG, et al. Increment of immunogenicity after third dose of a homologous inactivated SARS-CoV-2 vaccine in a large population of patients with autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:1036–1043. doi: 10.1136/annrheumdis-2021-222096. [DOI] [PubMed] [Google Scholar]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- Angyal A, Longet S, Moore SC, Payne RP, Harding A, Tipton T, et al. T cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387:1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benucci M, Damiani A, Gobbi FL, Lari B, Grossi V, Infantino M, et al. Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis. Immunol Res. 2022;70:493–500. doi: 10.1007/s12026-022-09283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.610300. :610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CM, Ruddy JA, Boyarsky BJ, Barbur I, Werbel WA, Geetha D, et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS-CoV-2 messenger RNA vaccination. Arthritis Rheumatol. 2022;74:28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini P, Agrati C, Apolone G, Mantovani A, Giannarelli D, Marasco V, et al. Humoral and T cell immune response after three doses of mRNA SARS-CoV-2 vaccines in fragile patients: the Italian VAX4FRAIL study. Clin Infect Dis. 2022:ciac404. doi: 10.1093/cid/ciac404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayam RM, Law JC, Goetgebuer RL, Chao GY, Abe KT, Sutton M, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune-mediated inflammatory diseases. JCI Insight. 2022;7 doi: 10.1172/jci.insight.159721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni C, Picchianti-Diamanti A, Aiello A, Nicastri E, Laganà B, Agrati C, et al. Kinetics of the B- and T cell immune responses after 6 months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.846753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Cai C, Grifoni A, Müller TR, Niessl J, Olofsson A, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- Gilboa M, Regev-Yochay G, Mandelboim M, Indenbaum V, Asraf K, Fluss R, et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister B, Bunde T, Rudawsky IM, Volk HD, Kern F. Detection of antigen-specific T cells by cytokine flow cytometry: the use of whole blood may underestimate frequencies. Eur J Immunol. 2003;33:3484–3492. doi: 10.1002/eji.200324223. [DOI] [PubMed] [Google Scholar]
- Hurme A, Jalkanen P, Heroum J, Liedes O, Vara S, Melin M, et al. Long-lasting T cell responses in BNT162b2 COVID-19 mRNA vaccinees and COVID-19 convalescent patients. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.869990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena A, Mishra S, Deepak P, Kumar-M P, Sharma A, Patel YI, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21 doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyssum I, Kared H, Tran TT, Tveter AT, Provan SA, Sexton J, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmaczewska A, Swierkot J, Ciszak L, Szteblich A, Chrobak A, Karabon L, et al. patients with the most advanced rheumatoid arthritis remain with Th1 systemic defects after TNF inhibitors treatment despite clinical improvement. Rheumatol Int. 2014;34:243–253. doi: 10.1007/s00296-013-2895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Soyfoo MS, Mekkaoui L, Dahma H, Tant L. Waning humoral immunity of SARS-CoV-2 vaccination in a rheumatoid arthritis cohort and the benefits of a vaccine booster dose. Clin Exp Rheumatol Forthcoming. 2022 doi: 10.55563/clinexprheumatol/ti3tvu. [DOI] [PubMed] [Google Scholar]
- Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusali G, Colavita F, Lapa D, Meschi S, Bordi L, Piselli P, et al. SARS-CoV-2 serum neutralization assay: a traditional tool for a brand-new virus. Viruses. 2021;13:655. doi: 10.3390/v13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology Guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 3. Arthritis Rheumatol. 2021;73:e1–e12. doi: 10.1002/art.41596. [DOI] [PubMed] [Google Scholar]
- Moura RA, Weinmann P, Pereira PA, Caetano-Lopes J, Canhão H, Sousa E, et al. Alterations on peripheral blood B-cell subpopulations in very early arthritis patients. Rheumatology (Oxford) 2010;49:1082–1092. doi: 10.1093/rheumatology/keq029. [DOI] [PubMed] [Google Scholar]
- Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L, Picchianti-Diamanti A, Sebastiani GD, Aiello A, Laganà B, Cuzzi G, et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis. 2022;121:24–30. doi: 10.1016/j.ijid.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchianti-Diamanti A, Aiello A, Laganà B, Agrati C, Castilletti C, Meschi S, et al. ImmunosuppressiveTherapies differently modulate humoral- and T cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchel F, Vital E, Kingsbury SR, El-Sherbiny YM. CD4+ T cell subsets in rheumatoid arthritis. Int J Clin Rheumatol. 2012;7:37–53. [Google Scholar]
- Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzullo I, Scrivo R, Sessa P, Mengoni F, Vullo V, Valesini G, et al. Changes in T cell effector functions over an 8-year period with TNF antagonists in patients with chronic inflammatory rheumatic diseases. Sci Rep. 2018;8:7881. doi: 10.1038/s41598-018-26097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedeberg K, Vuilleumier N, Pagano S, Albrich WC, Ludewig B, von Kempis JV, et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 2022;4:e11–e13. doi: 10.1016/S2665-9913(21)00328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022;310:27–46. doi: 10.1111/imr.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli FR, Favalli EG, Garufi C, Cornalba M, Colafrancesco S, Conti F, et al. Low frequency of disease flare in patients with rheumatic musculoskeletal diseases who received SARS-CoV-2 mRNA vaccine. Arthritis Res Ther. 2022;24:21. doi: 10.1186/s13075-021-02674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syversen SW, Jyssum I, Tveter AT, Tran TT, Sexton J, Provan SA, et al. Immunogenicity and safety of standard and third dose SARS-CoV-2 vaccination in patients recieving immunosuppressive therapy. Arthritis Rheumatol. 2022;74:1321–1332. doi: 10.1002/art.42153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KT, Hsu BC, Chen DY. Immunogenicity, effectiveness, and safety of COVID-19 vaccines in rheumatic patients: an updated systematic review and meta-analysis. Biomedicines. 2022;10:834. doi: 10.3390/biomedicines10040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859. doi: 10.1016/j.cell.2022.01.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi SK, Stratton J, Ellrodt JE, Whelan MG, Hayashi K, Yoshida K, et al. Rheumatoid arthritis disease activity assessed by patient-reported outcomes and flow cytometry before and after an additional dose of COVID-19 vaccine. Ann Rheum Dis. 2022;81:1045–1048. doi: 10.1136/annrheumdis-2022-222232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, Self WH, Gaglani M, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Frosch AE, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Duggal A, Wilson JG, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Botros M, Lauring AS, Shapiro NI, Halasa N, Chappell JD, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Adams K, Surie D, McMorrow ML, Patel MM, Network IVY. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death - United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459–465. doi: 10.15585/mmwr.mm7112e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. 2017;8:1473. doi: 10.1038/s41467-017-01728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchtenhagen H, Rims C, Blahnik G, Chow IT, Kwok WW, Buckner JH, et al. Efficient ex vivo analysis of CD4+ T cell responses using combinatorial HLA class II tetramer staining. Nat Commun. 2016;7:12614. doi: 10.1038/ncomms12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Togt CJT, Ten Cate DF, van den Bemt BJF, Rahamat-Langendoen J, den Broeder N, den Broeder AA. Seroconversion after a third COVID-19 vaccine is affected by rituximab dose but persistence is not in patients with rheumatoid arthritis. Rheumatology (Oxford) 2022:keac486. doi: 10.1093/rheumatology/keac486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma L, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Exp Immunol. 2013;174:212–220. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lloyd KA, Melas I, Zhou D, Thyagarajan R, Lindqvist J, et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci Rep. 2019;9:19995. doi: 10.1038/s41598-019-56279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman MH, Durez P, Hallegua D, Aranda R, Becker JC, Nuamah I, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33:2162–2166. [PubMed] [Google Scholar]
- Zhang X, Miao M, Zhang R, Liu X, Zhao X, Shao M, et al. Efficacy and safety of low-dose interleukin-2 in combination with methotrexate in patients with active rheumatoid arthritis: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2022;7:67. doi: 10.1038/s41392-022-00887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istituto Superiore di Sanità (ISS) Impact of COVID-19 vaccination on the risk of SARS-CoV-2 infection and hospitalization and death in Italy 27.12.2020 - 14.07.2021. Combined analysis of data from the National Vaccination Registry and the COVID-19 Integrated Surveillance System. Istituto Superiore di Sanità (ISS); Roma: 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.