Abstract
Objective:
To determine whether fluctuations in functional T-cell subsets can explain why multiple sclerosis (MS) relapses decline during pregnancy and increase in the postpartum period.
Design:
Case-control study.
Setting:
Kaiser Permanente Northern California and Stanford University.
Participants:
Twenty-six pregnant women with MS and 24 age-matched, pregnant controls.
Intervention:
We prospectively followed up the pregnant women with MS and the age-matched, pregnant controls; conducted structured interviews; and collected peripheral blood mononuclear cells during each trimester and 2, 4, 6, 9, and 12 months post partum.
Main Outcome Measures:
Sixteen functional cell types, including interferon-γ(IFN-γ)–and tumor necrosis factor–producing T-cell subsets, were measured using multicolor flow cytometry. Since these cell types may also fluctuate with pregnancy, lactational amenorrhea, or MS treatment, the data were analyzed taking into account these factors.
Results:
Fifteen women with MS (58%) had relapses during the postpartum year. CD4+IFN-γ–producing cells fluctuated with MS relapses, declining during pregnancy in women with MS (P<.001) and continuing to decline after parturition in women with relapses (P=.001), yet rising or remaining stable in women with nonrelapsing MS or healthy pregnant women. Lactational amenorrhea was associated with a rise in CD4+IFN-γ–producing cells in women with MS (P=.009). In contrast, CD4+ tumor necrosis factor–producing cells decreased during lactational amenorrhea in all groups of women and, once this was taken into account, obscured any relationship to MS relapses. CD8+IFN-γ–producing cells were elevated in women with MS throughout the study (P<.001) but did not fluctuate with relapses.
Conclusions:
Our findings suggest that a decline in circulating CD4+IFN-γ–producing cells leads to postpartum MS relapses. Our findings also suggest that the decline in these cells may begin during late pregnancy and that lactational amenorrhea induced by exclusive breastfeeding may be able to interrupt this process.
Multiple Sclerosis (MS) is a chronic T cell–mediated inflammatory illness that usually begins in young adulthood. Clinically, MS is characterized by relapses and, over time, variable degrees of neurological disability. It is well known that relatively few women with MS have relapses during pregnancy and a large proportion of women with MS experience relapses in the early postpartum period.1 This pattern of pregnancy-associated remission and postpartum flares is shared with other chronic T cell–mediated inflammatory diseases2 (rheumatoid arthritis, Hashimoto thyroiditis), yet the immunological basis is unclear.
One popular hypothesis is that suppression of the principal proinflammatory T cells in MS and rheumatoid arthritis (CD4+ cells that produce interferon-γ [IFN-γ], tumor necrosis factor [TNF], or interleukin 2 [IL-2]; helper T subtype 1 [TH1] cells) occurs during pregnancy through augmentation of their mutual inhibitors: CD4 cells that produce IL-10 or IL-4.2 Support for this hypothesis is largely based on data from healthy women3–6 and only 4 studies have examined changes in cytokines during pregnancy and the postpartum period in women with MS.7–10 These studies have significant methodological limitations, most importantly that none examined the relationship of immunological changes to postpartum relapses or other factors.7–10 In addition, these studies did not identify which T-cell subset was producing the cytokines of interest. Thus, it is unclear if the immunological changes they observed are best explained by pregnancy, MS, MS disease activity, or other factors, such as lactational amenorrhea.
Based on work we have done previously in the animal model of MS,11 we hypothesized that pregnancy-related relapse protection would be due to suppression of TH1 cells, presumably due to a circulating suppressive serum factor. Although in our previous study the animal model did not reflect an increased risk of postpartum flares, we hypothesized that postpartum flares would be accompanied by a rebound in the levels of circulating TH1 cells preceding or around the time of relapses.
We recently reported that prolonged lactational amenorrhea resulting from exclusive breastfeeding is associated with a significantly decreased risk of postpartum MS relapses.12 While the immunological mechanism is unknown, other studies have shown fluctuations in TNF with menstrual status and sex.13
Thus, our goal for this study was to determine whether changes in T-cell subsets that secrete IFN-γ, TNF, IL-2, IL-4, or IL-10 could account for pregnancy-associated relapse protection, postpartum flares of MS, or the protective effects of exclusive breastfeeding. To accomplish this goal, it was essential that we consider the possibility that fluctuations in these cell types might be better explained by pregnancy state or lactational amenorrhea alone. Our study fills a significant gap in knowledge about the relationship between various cytokine-producing T-cell subsets and postpartum MS relapses, pregnancy, and lactational amenorrhea and may also shed light on MS relapse pathophysiology in general.
METHODS
STUDY SUBJECTS
We recruited 26 women with clinically definite MS and 24 healthy pregnant controls matched on age and parity from Kaiser Permanente Northern California and Stanford University between 2003 and 2005. Subjects were eligible if they were less than 35 weeks’ pregnant or planning to become pregnant. Details of the study and testing procedures were explained to each subject, and a written informed consent was obtained. This study was approved by the institutional review boards at Stanford University and Kaiser Foundation Research Institute.
STUDY DESIGN
Study subjects donated blood and completed a structured, interviewer-administered questionnaire within 24 hours on entry into the study and during the remaining trimesters of pregnancy as well as at 2, 4, 6, 9, and 12 months post partum. The study entry questionnaire captured detailed medical and reproductive history and the follow-up questionnaires collected information about changes in neurological, reproductive, and/or medical history; medication use; breastfeeding; and menstrual status. Blood draws and follow-up questionnaires were obtained outside of the regularly scheduled visits if women had a relapse or prior to restarting immunomodulatory medications.
A relapse was defined as the occurrence, reappearance, or worsening of symptoms of neurological dysfunction that lasted for more than 48 hours. Transient, fever-related worsening of symptoms or fatigue alone were not considered relapses. Symptoms that occurred within 1 month of each other were considered to be part of the same attack. For women who reported worsening MS on questionnaire, the medical records were abstracted for documentation of relapses and progression of disability.
FLOW CYTOMETRY STAINING AND ANALYSIS
Peripheral blood mononuclear cells were isolated and viably frozen. Peripheral blood mononuclear cells were resuspended and stimulated with anti-CD3 and anti-CD28, and surface and intracellular cytokine staining were done according to the manufacturer’s instructions using Cytofix/Cytoperm (BD Biosciences, San Jose, California).
Cells were analyzed on a FACSAria Cell Sorter (BD Biosciences). A minimum of 200 000 events was collected for each sample. Data were compensated, analyzed, and presented using FlowJo software (Tree Star, Inc, Ashland, Oregon). To identify fine T-cell subsets, dead cells (EMA+) and CD14+ cells were removed; subsequently, lymphocytes were selected based on their forward and side scatter profile; then CD19+ B cells were excluded and CD3+ cells were selected; and finally, CD4+ and CD8+ subgroups were identified and analyzed for expression of simultaneous IFN-γ and CD45RA, IL-2 and CD45RA, TNF and CD45RA, IL-4, or IL-10. Several steps were taken to minimize error that could be introduced by variations in flow cytometric staining.
STATISTICAL ANALYSES
The data were analyzed using longitudinal data methods aimed at identifying patterns over time while accounting for interindividual and intraindividual variation. To determine whether lymphocyte subsets changed during pregnancy and the postpartum period or in relationship to the onset of relapse symptoms or return of menses, piecewise linear mixed models were used. The models were hypothesis driven and included each immunologic measurement as the dependent variable and time in days relative to the onset of relapse symptoms (or day of delivery), disease status (MS; yes/no), postpartum relapse (yes/no), concomitant breastfeeding (yes/no), return of menses (yes/no), and/or use of MS treatments within 6 months of the blood draw (corticosteroids, interferon beta, or glatiramer acetate; yes/no) as the independent variables. Immunologic measurements were not normally distributed, so we used square root or log transformations as appropriate. To identify breakpoints in the data, a nonparametric method for estimating local regression (loess) of the immunologic parameters was used.
To graphically depict the differences in absolute and relative values of immunological parameters, we compared the mean (SD) of the normally transformed values at each point between women with MS (with and without relapses within the first 4 months post partum) and healthy pregnant women.
All of these analyses were designed to identify group-level changes. To determine how often the pattern of changes identified in these analyses described what happens on an individual level, we plotted the relative and absolute values for each individual and reported how many subjects displayed the pattern of change identified by the group-level data.
The means and standard deviations of normally distributed variables were compared using 2-sample t tests; for variables with nonparametric distributions, Wilcoxon rank sum test was used; and for binary or categorical variables, χ2 with Fisher exact test. Statistical significance was set at P=.05. To adjust for multiple comparisons, α was set at .01. All statistical analyses were performed using SAS version 9 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
CLINICAL CHARACTERISTICS OF STUDY SUBJECTS
Twenty-six pregnant women with clinically definite relapsing-remitting MS and 24 healthy pregnant women were enrolled in this study. More women with MS joined the study by their second trimester (n=22; 85%) compared with healthy, matched controls (n=12; 50%). All available data were included from the 2 women who dropped out (both with MS; both moved out of the study area after their first postpartum visit). Women with MS were similar in age to healthy women but were less likely to breastfeed (Table). Among women with MS, women who were relapse-free were 50% more likely to have breastfed than women who had postpartum relapses.
Table.
Clinical Characteristics of Study Participants at Onset of Pregnancy, During Pregnancy, and in the Post Partum Period
| No. (%) | |||
|---|---|---|---|
| Women With MS and Postpartum Relapses (n=15) | Women With MS Without Postpartum Relapses (n=11) | Healthy Women (n=24) | |
| Baseline characteristic | |||
| Age, y, median (range) | 33.1 (25.4–41.1) | 32.6 (26.5–37.1) | 32.8 (23.4–39.2) |
| Disease duration, y, median (range) | 5.5 (1.8–20.8) | 4.3 (1.6–16.8) | … |
| Relapses during 2-y period prior to conception | |||
| 0 | 4 (27) | 2 (18) | … |
| 1 | 3 (20) | 4 (36) | … |
| 2 | 6 (40) | 3 (36) | … |
| 3 | 2 (13) | 1 (9) | … |
| Time since last relapse, mo, median (range) | 15.0 (1.2–48.5) | 13.2 (1.4–22.2) | … |
| MS medication use | 11 (73) | 5 (45) | … |
| Pregnancy | |||
| Timing of MS relapses | |||
| First trimester | 2 (13) | 0 | … |
| Second trimester | 0 | 1 (9) | … |
| Third trimester | 0 | 0 | … |
| Postpartum year | |||
| Breastfed | 9 (60.0) | 9 (82) | 23 (93)a |
| MS medication use | 10 (66.7) | 6 (55) | … |
| Timing of MS relapses | |||
| Months 0–4 | 12 (80) | … | … |
| Months 5–8 | 1 (7) | … | … |
| Months 9–12 | 7 (47) | … | … |
Abbreviations: ellipses, not applicable; MS, multiple sclerosis.
P value=.03 between women with MS (regardless of relapse) and healthy women; all other factors were not significantly different between any group (P>.20).
Of the 15 women with relapses in the year post partum, the majority (67%) had 1 relapse. Most women experienced their first relapse within 4 months post partum (n=12; median, 44.5 days), while 3 women did not have a relapse until after 9 months post partum (median, 316 days). After delivery, 6 women restarted their medication prior to the onset of relapse symptoms and 4, thereafter. Of the 23 relapses observed in the study period, 10 women were treated with corticosteroids for 10 relapses in the postpartum period. Only 2 women had clinically significant disability prior to conception (defined as an Expanded Disability Status Scale rating ≥4.0) and at 1 year post partum. Most women had no clinically significant disability at study entry or exit (Expanded Disability Status Scale rating≤2.0; n=20).
CIRCULATING IFN-γ–PRODUCING CD4+CD45RA− LYMPHOCYTES DURING PREGNANCY AND THE POSTPARTUM PERIOD
During pregnancy (but not the postpartum period), the absolute proportion of the IFN-γ–producing CD4+CD45RA− lymphocytes was higher in women with MS compared with healthy pregnant women (P=.001) (Figure 1).
Figure 1.
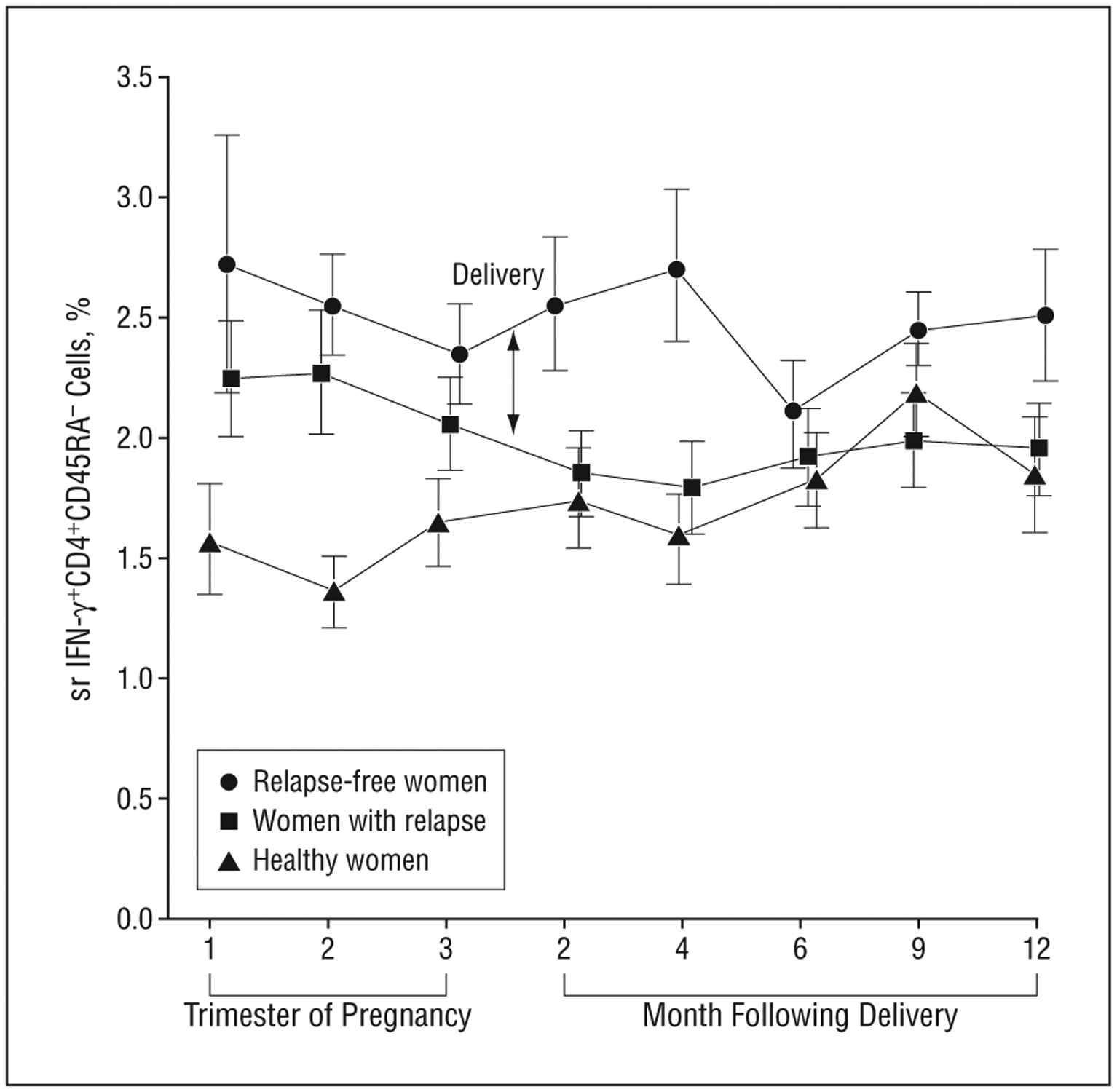
Interferon-γ (IFN-γ)–producing CD4+CD45RA− lymphocytes declined during pregnancy and following parturition in women with postpartum multiple sclerosis (MS) relapses. Depicted is the mean (SD) in the absolute proportion of CD4+CD45RA−IFN-γ+lymphocytes in the normal transformation (square root [sr]) in women with MS who were relapse-free throughout the study (n=11), women with MS who had relapses within the first 4 months post partum (n=12), and healthy women (n=24) during the 3 trimesters of pregnancy and the postpartum period in months (2, 4, 6, 9, and 12). Interferon-γ+CD4+CD45RA− cells declined during pregnancy in women with MS regardless of relapse (P<.001), continued to decline post partum in those women who went on to have relapses (P=.009), and either increased or remained stable in women with relapse-free MS (P=.16). Fewer healthy women had joined the study by the second trimester (n=12) than women with MS (n=22).
However, the pattern of change in the absolute proportion of IFN-γ–producing CD4+CD45RA− lymphocytes from the second trimester of pregnancy to 4 months post partum (Figure 2) differed significantly in women with MS relapses as compared with the other 2 groups (P=.004 and .001 for absolute differences in the slope of women with relapsing MS as compared with women with nonrelapsing MS or healthy pregnant women, respectively), even after adjusting for postpartum treatment and breastfeeding. In women with MS, IFN-γ–producing CD4+CD45RA− cells declined during pregnancy (P<.001) regardless of whether the women went on to have postpartum relapses. However, these cells continued to decline up to 4 months post partum in those women who went on to have relapses (P=.009). In contrast, relapse-free women with MS demonstrated a non-significant rise in IFN-γ–producing CD4+CD45RA− cells after parturition (P=.16) while these lymphocytes did not change in healthy pregnant women (P=.70, third trimester to 4 months post partum). Inspection of individual plots showed that this pattern of postpartum decline in the proportion of IFN-γ+–producing CD4+CD45RA− lymphocytes was found in 80% (12 of 15) of the women with early postpartum relapses and only 18% (2 of 11) of the women who were relapse-free throughout the study.
Figure 2.
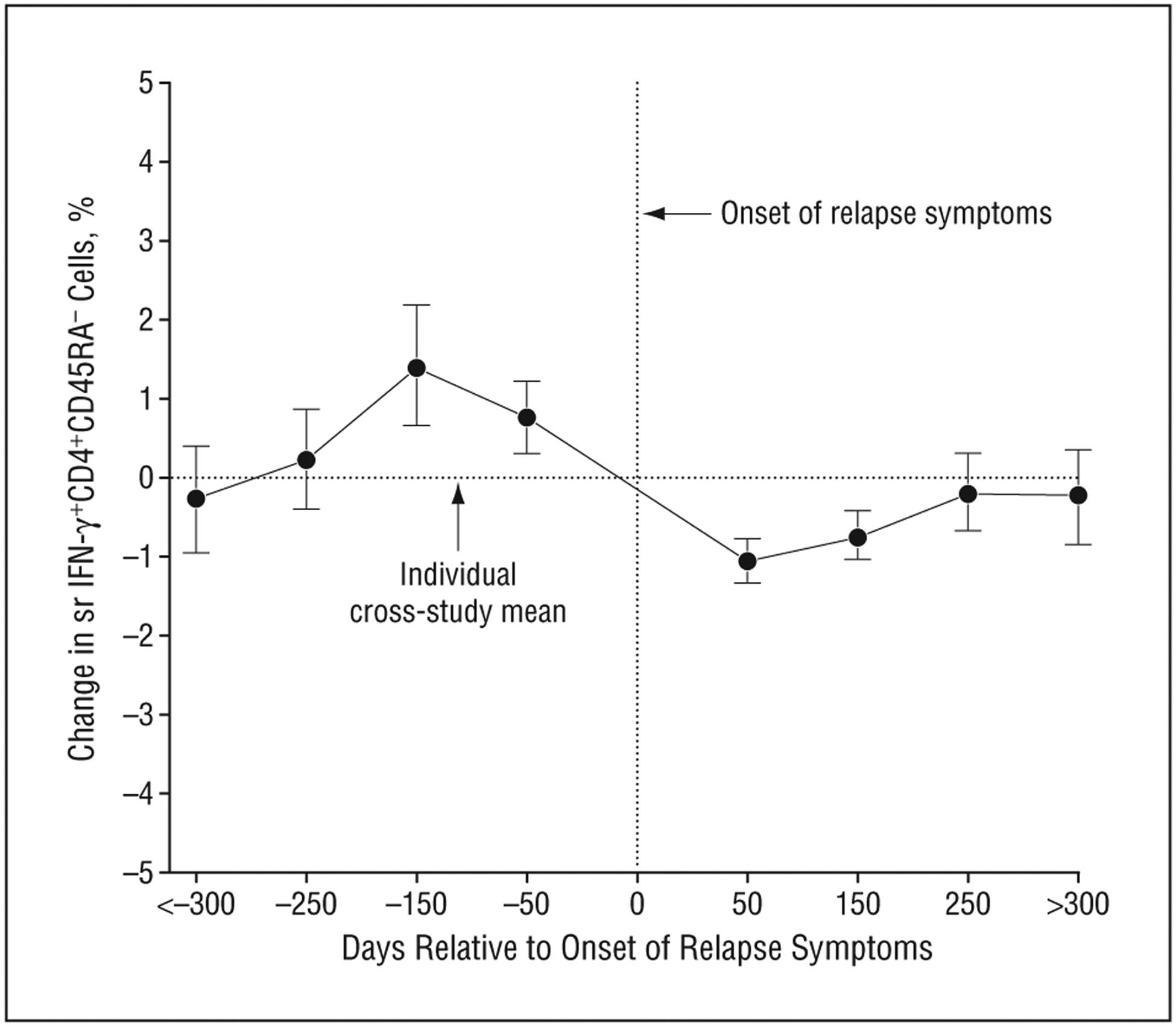
Interferon-γ (IFN-γ)–producing CD4+CD45RA− lymphocytes declined prior to the onset of multiple sclerosis relapse symptoms. The x-axis represents time in days relative to the onset of relapse symptoms (t=0). The day indicated represents the midpoint of that category (eg, 50 days encompasses values from days 1–100). For women who had more than 1 relapse, this represents the first relapse. The y-axis represents the change in the proportion of IFN-γ–producing CD4+CD45RA− cells relative to each individual’s cross-study mean in the normal transformation (square root [sr]). Mean (SD) is depicted at each point. Only values from women who had relapses during the study period are included (n=15). The decline in IFN-γ–producing CD4+CD45RA−cells about 100 days prior to the onset of relapse symptoms is highly significant (P=.001 by repeated-measures linear mixed model).
CIRCULATING IFN-γ–PRODUCING CD4+CD45RA− LYMPHOCYTES AND THE ONSET OF RELAPSE SYMPTOMS
In women with relapses, the relative proportion of IFN-γ+–producing CD4+CD45RA− lymphocytes declined approximately 100 days prior to the onset of relapse symptoms (P=.001) (Figure 2). This presymptomatic decline was not diminished after adjusting for treatment or breastfeeding (P=.002). Inspection of individual plots showed that this pattern of presymptomatic decline in the proportion of IFN-γ+–producing CD4+CD45RA− lymphocytes was found in 80% (12 of 15) of women with relapses 100 to 130 days prior to symptom onset.
CIRCULATING IFN-γ–PRODUCING CD4+CD45RA− LYMPHOCYTES AND LACTATIONAL AMENORRHEA
In women with MS who breastfed, the proportion of circulating IFN-γ–producing CD4+CD45RA− cells following parturition increased during lactational amenorrhea and declined following return of menses, even after taking into account postpartum relapses and MS medication use (multivariate analysis, P=.009 for return of menstruation). Among the women with MS who did not breastfeed (and therefore would not experience lactational amenorrhea), changes in circulating IFN-γ–producing CD4+CD45RA− cells following parturition were associated only with postpartum relapses, as described earlier. In healthy women, breastfeeding had no influence on this cell type.
IFN-γ–PRODUCING CD8+CD45RA− CELLS ARE HIGHER IN WOMEN WITH MS THAN HEALTHY WOMEN
The absolute proportions of IFN-γ+–producing CD8+CD45RA− lymphocytes were higher on average throughout the study period in women with MS, regardless of relapse, compared with healthy women (P<.001) (Figure 3). These cells did not fluctuate with relapse or pregnancy state in either women with MS or healthy pregnant women.
Figure 3.
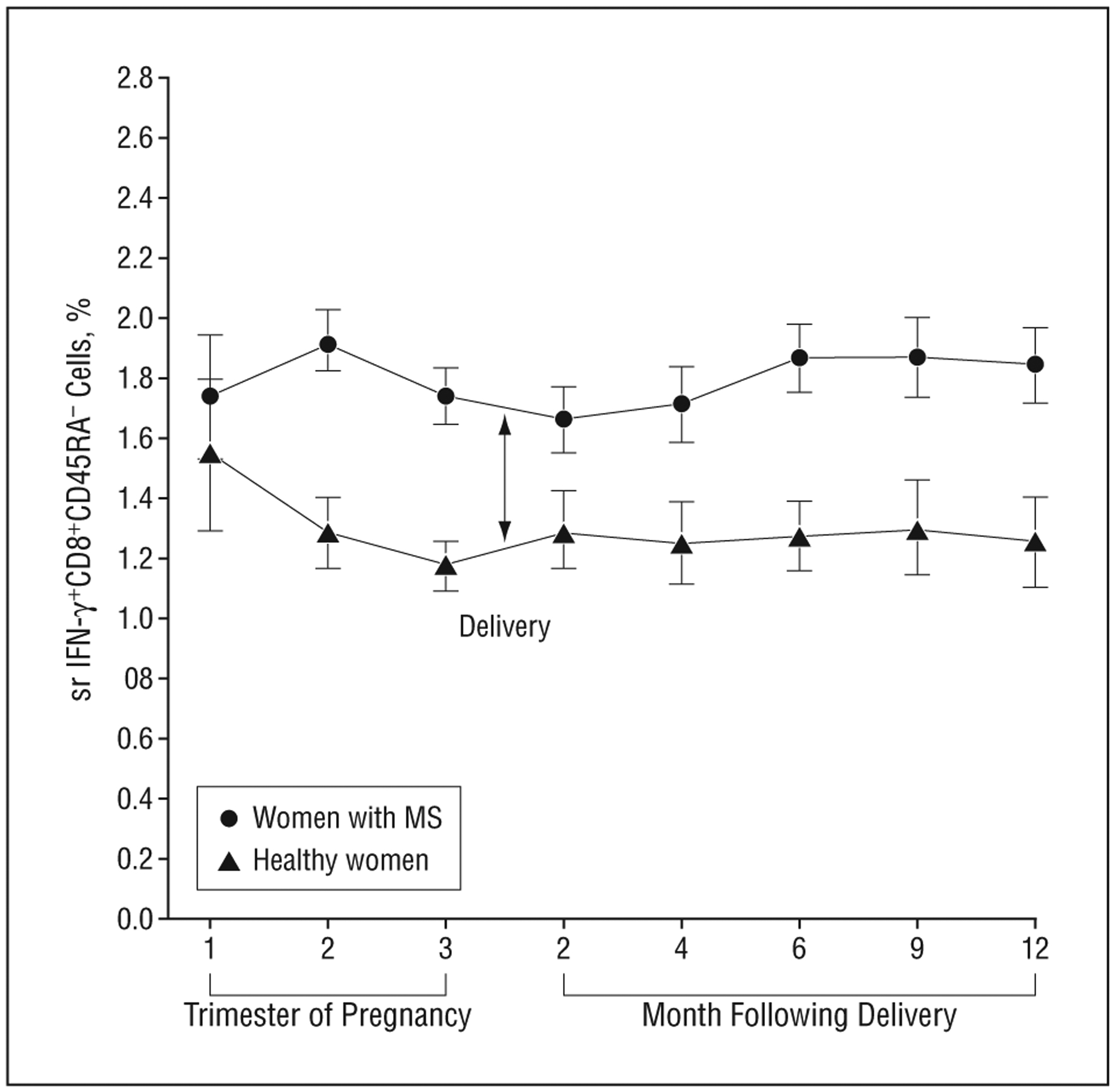
Interferon-γ (IFN-γ)–producing CD8+ cells were higher in women with multiple sclerosis (MS) (regardless of relapse) compared with healthy women. The mean (SD) of the change in absolute proportions of IFN-γ–producing CD8+ lymphocytes in the normal transformation (square root [sr]) was higher in women with MS regardless of relapses (n=26) compared with healthy women (n=24) during the 3 trimesters of pregnancy and the postpartum period in months (2, 4, 6, 9, and 12) (P<.001). No significant fluctuations in these cells were observed during pregnancy or the postpartum period in women with MS or healthy women.
TNF-PRODUCING CD4+ CELLS AND LACTATIONAL AMENORRHEA, MEDICATION USE, AND RELAPSES
Tumor necrosis factor–producing CD4+ cells were most strongly associated with lactational amenorrhea in both women with MS and healthy women. While unadjusted analysis and visual inspection suggested a small apparent increase in TNF-α+CD4+CD45RA− cells around the time of relapse in women with MS (P=.007 unadjusted), this effect disappeared once we adjusted for lactational amenorrhea and medication use (P=.17 after adjustment). In women who breastfed, the proportion of circulating TNF-producing CD4+CD45RA− cells was suppressed during lactational amenorrhea by 76% on average and rose once menses returned in both women with MS and healthy women (P=.004). Among the women with MS who did not breastfeed, both treatment and return of menses influenced the proportion of circulating TNF-producing CD4+CD45RA− cells. When treatment was started before return of menses, TNF-producing CD4+CD45RA− cells increased significantly (P<.001 for treatment), but when treatment was started after return of menses, these cells declined (P<.001 for the interaction term). No significant changes in this cell type were seen during pregnancy or the postpartum period in women with MS or healthy women.
The following lymphocyte subsets showed no significant fluctuations in relationship to pregnancy state or the onset of relapse symptoms nor were the absolute proportion or relative proportions different between the 3 groups of women: CD4+IL-10+, CD4+IL-4+, CD4+CD45RA+IL-2+, CD4+CD45RA−IL-2+, CD4+CD45RA+IFN-γ+, CD8+CD45RA+IFN-γ+, and CD8+CD45RA+IL-2+ cells.
COMMENT
We, like others,1 observed a decrease in relapse frequency during pregnancy and an increase in relapses within the first 4 months post partum. We found that a decline in IFN-γ–producing CD4+ lymphocytes was strongly associated with postpartum MS relapses and that lactational amenorrhea was associated with an increase in these cells. In contrast, CD4+TNF–producing cells were strongly related to lactational amenorrhea but showed no relationship with MS relapse after accounting for return of menses and MS medication use. We found that IFN-γ–producing CD8 cells were higher in women with MS compared with healthy women but did not fluctuate significantly in relationship to pregnancy or relapse, supporting the hypothesis that these cells are associated with disease but perhaps not relapse. We found no significant measurable changes in CD4+IL-2, IL-4, or IL-10–producing subsets in relationship with either pregnancy or MS relapses. Taken together, our study demonstrates that to unravel the relationship between proinflammatory cytokines and MS or MS disease activity it is critical to distinguish between the T-cell subsets producing the cytokine and to examine potential confounders like menstrual status.
That IFN-γ–producing CD4+ lymphocytes are somehow involved in MS pathogenesis is consistent with many previously published works in the animal model of MS, experimental allergic encephalomyelitis, and humans.14–16 However, the relationship between circulating IFN-γ–producing T cells and MS relapses is unclear. Only a few longitudinal studies have examined changes in proinflammatory or anti-inflammatory cytokine secretion associated with relapses, none of which measured cytokine-producing T-cell subsets.17–19 These studies had somewhat conflicting results and reported an increase in IFN-γ18 and TNF 2 weeks before relapse17; no change in IFN-γ 1 month prior to relapse19; an increase in TNF 1 month before relapse19; and a decrease in TNF prior to and during relapse.20 All studies had important methodological limitations, including short periods of observation prior to onset of relapses, and none considered the potential for confounding by other factors, such as sex or menstrual status. Cross-sectional studies comparing pregnant/postpartum subjects with relapsing and nonrelapsing MS have reported differences in immunological biomarkers in the peripheral circulation, including IFN-γ, TNF, IL-4, and IL-10. However, these studies had poorly defined control groups and it is unclear if the results of these studies could be better explained by the well-known interindividual variations in cytokines or could be due to other factors that vary between the groups, such as age, sex, or time since the most recent MS relapse.21–23
In this study, IFN-γ–producing CD4+ cells were elevated during pregnancy in women with MS compared with healthy women. During pregnancy, IFN-γ–producing CD4+ cells declined in all women with MS. But after parturition, this cell type diverged among the women with MS who had a relapse and those who did not. These lymphocytes continued to decline in the group of women who went on to have relapses and increased or remained stable in the group of women who were relapse-free. Among the women who had relapses, this decline in IFN-γ–producing CD4+ cells began during late pregnancy, 2 to 3 months before the onset of relapse symptoms. Among the women with MS who breastfed, prolonged lactational amenorrhea was associated with a rise in IFN-γ–producing CD4+ cells.
One interpretation of these findings is that a decline in circulating IFN-γ–producing CD4+ cells leads to postpartum MS relapses. This would indicate that the pathophysiological process leading to postpartum relapses is initiated during pregnancy in all women with MS but that factors associated with parturition (such as exclusive breastfeeding) are able to successfully interrupt this process. This interpretation is consistent with our previous report that exclusive breastfeeding for at least the first 2 months (and associated lactational amenorrhea) protects against postpartum relapses.12 This idea that pregnancy may actually be initiating relapses could explain why Achiron et al24 found that prophylactic treatment with intravenous immunoglobulin to prevent postpartum relapses was significantly effective if started during pregnancy but only marginally effective if started shortly after delivery.
In contrast, we found that TNF-producing CD4+ cells appear to fluctuate in response to a combination of factors in unadjusted analyses but once these factors (relapses, lactational amenorrhea, and medication use) were considered in combination, lactational amenorrhea was the most important determinant of variations in this cell type. These findings suggest that TNF-producing CD4+ cells do not have a strong relationship with relapse activity or relapse protection and may help explain why previous unadjusted estimates of the relationship between TNF-producing CD4+ cells and MS produced conflicting results. Future studies of TNF-producing CD4+ cells in MS should include measures of menstrual status, medication use, and breastfeeding and address the role of these factors as potential confounders and effect modifiers.
To our knowledge, this is the first study to examine longitudinal changes in CD8+ cells in relationship to MS relapses. There is emerging evidence that CD8+ lymphocytes play an extensive role in MS immunopathogenesis.25 Studies have shown that more CD8+ than CD4+ lymphocytes are found in MS lesions26; at the site of lesions, CD8+ cells undergo greater clonal expansion than CD4+ cells27; and CD8+ cells may be the principal drivers of axonal damage leading to disability.28,29 Our findings support a role for CD8+ T cells in MS pathophysiology independent of relapse. It is possible that IFN-γ–producing CD8+ cells, while not involved in relapses, are contributing to ongoing axonal damage that may eventually lead to disability.
Our findings are not consistent with the hypothesis2 that suppression of IFN-γ–, TNF-, or IL-2–producing T cells is responsible for pregnancy-associated relapse protection in MS and suggest that pregnancy is influencing MS disease activity through other mechanisms.
Other possible explanations for how pregnancy protects against MS relapses include a direct neuroprotective effect of estrogen30 or progesterone31; decreased permeability of the blood-brain barrier through direct effects of estradiol32; or intralesional suppression of inflammation by one of the many uncharacterized pregnancy-related serum proteins or glycoproteins.11
Alternatively, pregnancy-related protection may be related to other changes in systemic autoimmunity that have been implicated in the experimental allergic encephalomyelitis model, such as enhancement of CD4+CD25+ or other regulatory T cells,33 diminished TH17 cells,34 or impairment of antigen-presenting cells by estrogen or pregnancy-specific glycoproteins.35 In addition, it is possible that the T cells in women with MS do not respond to the pregnancy milieu in the same way as healthy women.
Important limitations of this study include the relatively small sample of women, particularly of relapse-free subjects; the well-recognized error rate of flow cytometry; and the lack of brain magnetic resonance imaging data to examine subclinical disease activity. Some of these could have limited our ability to observe real changes in T-cell subsets, particularly if the magnitude of change was small or the subpopulation was present in extremely low numbers. Thus, our findings should be confirmed in a larger longitudinal study, preferably one that includes magnetic resonance imaging data. Additionally, it is unclear whether our findings can be extrapolated to relapses in nonpregnant/postpartum patients because we did not collect longitudinal data from this group.
Strengths of this study are the importance of the question, the rigorous methods that we used, and the novelty of our findings. We prospectively enrolled a cohort of pregnant women with MS and a control group of matched, healthy pregnant women, studied them longitudinally through their pregnancies and for 1 year post partum, and applied state-of-the-art immunological and statistical methods to generate and analyze data. Our findings that IFN-γ–producing CD4+ cells are strongly associated with MS relapses, yet IFN-γ–producing CD8+ cells are associated only with having MS, and fluctuations in TNF-producing CD4+ cells are best explained by lactational amenorrhea and not MS relapses are novel and have important implications for the design of future MS immunological studies. They highlight the need to carefully define functional T-cell subsets and to examine potential confounders like menstrual status in the quest to understand the complex immunological phenotype of MS.
Finally, our finding that a pregnancy-related decline in IFN-γ–producing CD4+ cells is strongly associated with postpartum relapses and that this decline can be interrupted peripartum by prolonged lactational amenorrhea induced by exclusive breastfeeding and possibly other factors is intriguing and may shed light on the pathogenesis of relapses in both pregnant and nonpregnant patients with MS.
Funding/Support:
During this project, Dr Langer-Gould was supported by a K23 grant (NS43207) from the National Institute of Neurologic Disorders and Stroke of the National Institutes of Health and a Wadsworth Foundation Young Investigator’s Award. Dr Steinman has received support from the National Institutes of Health, the National Multiple Sclerosis Society, and the Phil N. Allen Trust. Dr Nelson has received support from the National Institutes of Health and the National Multiple Sclerosis Society.
Footnotes
Publisher's Disclaimer: Disclaimer: The funding agencies had no role in the study design, conduct, or analysis of the data.
Financial Disclosure:
None reported.
REFERENCES
- 1.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339(5):285–291. [DOI] [PubMed] [Google Scholar]
- 2.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. [DOI] [PubMed] [Google Scholar]
- 3.Matthiesen L, Ekerfelt C, Berg G, Ernerudh J. Increased numbers of circulating interferon-gamma- and interleukin-4-secreting cells during normal pregnancy. Am J Reprod Immunol. 1998;39(6):362–367. [DOI] [PubMed] [Google Scholar]
- 4.Russell AS, Johnston C, Chew C, Maksymowych WP. Evidence for reduced Th1 function in normal pregnancy: a hypothesis for the remission of rheumatoid arthritis. J Rheumatol. 1997;24(6):1045–1050. [PubMed] [Google Scholar]
- 5.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridama V, Pacini F, Yang SL, Moawad A, Reilly M, DeGroot LJ. Decreased levels of helper T cells: a possible cause of immunodeficiency in pregnancy. N Engl J Med. 1982;307(6):352–356. [DOI] [PubMed] [Google Scholar]
- 7.Airas L, Saraste M, Rinta S, Elovaara I, Huang YH, Wiendl H; Finnish Multiple Sclerosis and Pregnancy Study Group. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: relevance of natural killer cells. Clin Exp Immunol. 2008;151(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shammri S, Rawoot P, Azizieh F, et al. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J Neurol Sci. 2004;222(1–2):21–27. [DOI] [PubMed] [Google Scholar]
- 9.López C, Comabella M, Tintore M, Sastre-Garriga J, Montalban X. Variations in chemokine receptor and cytokine expression during pregnancy in multiple sclerosis patients. Mult Scler. 2006;12(4):421–427. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore W, Arias M, Stroud N, Stek A, McCarthy KA, Correale J. Preliminary studies of cytokine secretion patterns associated with pregnancy in MS patients. J Neurol Sci. 2004;224(1–2):69–76. [DOI] [PubMed] [Google Scholar]
- 11.Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169(2):1084–1091. [DOI] [PubMed] [Google Scholar]
- 12.Langer-Gould A, Huang SM, Gupta R, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. 2009; 66(8):958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84–90. [DOI] [PubMed] [Google Scholar]
- 14.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25(7):1951–1959. [DOI] [PubMed] [Google Scholar]
- 15.McRae BL, Kennedy MK, Tan LJ, Dal Canto MC, Picha KS, Miller SD. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J Neuroimmunol. 1992;38(3):229–240. [DOI] [PubMed] [Google Scholar]
- 16.Zamvil S, Nelson P, Trotter J, et al. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317 (6035):355–358. [DOI] [PubMed] [Google Scholar]
- 17.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78(4):318–323. [DOI] [PubMed] [Google Scholar]
- 18.Dettke M, Scheidt P, Prange H, Kirchner H. Correlation between interferon production and clinical disease activity in patients with multiple sclerosis. J Clin Immunol. 1997;17(4):293–300. [DOI] [PubMed] [Google Scholar]
- 19.van Oosten BW, Barkhof F, Scholten PE, von Blomberg BM, Ader HJ, Polman CH. Increased production of tumor necrosis factor alpha, and not of interferon gamma, preceding disease activity in patients with multiple sclerosis. Arch Neurol. 1998;55(6):793–798. [DOI] [PubMed] [Google Scholar]
- 20.Schluep M, van Melle G, Henry H, Stadler C, Roth-Wicky B, Magistretti PJ. In vitro cytokine profiles as indicators of relapse activity and clinical course in multiple sclerosis. Mult Scler. 1998;4(3):198–202. [DOI] [PubMed] [Google Scholar]
- 21.van Boxel-Dezaire AH, Hoff SC, van Oosten BW, et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45(6):695–703. [DOI] [PubMed] [Google Scholar]
- 22.Brod SA, Nelson LD, Khan M, Wolinsky JS. Increased in vitro induced CD4+ and CD8+ T cell IFN-gamma and CD4+ T cell IL-10 production in stable relapsing multiple sclerosis. Int J Neurosci. 1997;90(3–4):187–202. [DOI] [PubMed] [Google Scholar]
- 23.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125(2):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achiron A, Kishner I, Dolev M, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004;251(9):1133–1137. [DOI] [PubMed] [Google Scholar]
- 25.Goverman J, Perchellet A, Huseby ES. The role of CD8(+) T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy. 2005;4(2):239–245. [DOI] [PubMed] [Google Scholar]
- 26.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis: identification and characterization of the primary demyelinating lesion. Brain. 1997;120(pt 8):1461–1483. [DOI] [PubMed] [Google Scholar]
- 27.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micro-manipulation and single cell polymerase chain reaction. J Exp Med. 2000;192(3):393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(pt 10):2202–2212. [DOI] [PubMed] [Google Scholar]
- 29.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis: correlation with demyelination and inflammation. Brain. 2000;123(pt 6):1174–1183. [DOI] [PubMed] [Google Scholar]
- 30.Marin R, Guerra B, Alonso R, Ramirez CM, Diaz M. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2(4):287–301. [DOI] [PubMed] [Google Scholar]
- 31.Garay L, Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol. 2007;107(3–5):228–237. [DOI] [PubMed] [Google Scholar]
- 32.Bake S, Sohrabji F. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145(12):5471–5475. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170(1–2):85–92. [DOI] [PubMed] [Google Scholar]
- 34.McClain MA, Gatson NN, Powell ND, et al. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol. 2007;179(12):8146–8152. [DOI] [PubMed] [Google Scholar]
- 35.Bebo BF Jr, Dveksler GS. Evidence that pregnancy specific glycoproteins regulate T-cell function and inflammatory autoimmune disease during pregnancy. Curr Drug Targets Inflamm Allergy. 2005;4(2):231–237. [DOI] [PubMed] [Google Scholar]


