Abstract
Aims
To investigate the impact of patiromer on the serum potassium level and its ability to enable specified target doses of renin–angiotensin–aldosterone system inhibitor (RAASi) use in patients with heart failure and reduced ejection fraction (HFrEF).
Methods and results
A total of 1642 patients with HFrEF and current or a history of RAASi-related hyperkalemia were screened and 1195 were enrolled in the run-in phase with patiromer and optimization of the RAASi therapy [≥50% recommended dose of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, and 50 mg of mineralocorticoid receptor antagonist (MRA) spironolactone or eplerenone]. Specified target doses of the RAASi therapy were achieved in 878 (84.6%) patients; 439 were randomized to patiromer and 439 to placebo. All patients, physicians, and outcome assessors were blinded to treatment assignment. The primary endpoint was between-group difference in the adjusted mean change in serum potassium. Five hierarchical secondary endpoints were assessed. At the end of treatment, the median (interquartile range) duration of follow-up was 27 (13–43) weeks, the adjusted mean change in potassium was +0.03 mmol/l in the patiromer group and +0.13 mmol/l in the placebo group [difference in the adjusted mean change between patiromer and placebo: −0.10 mmol/l (95% confidence interval, CI −0.13, 0.07); P < 0.001]. Risk of hyperkalemia >5.5 mmol/l [hazard ratio (HR) 0.63; 95% CI 0.45, 0.87; P = 0.006), reduction of MRA dose (HR 0.62; 95% CI 0.45, 0.87; P = 0.006), and total adjusted hyperkalemia events/100 person-years (77.7 vs. 118.2; HR 0.66; 95% CI 0.53, 0.81; P < 0.001) were lower with patiromer. Hyperkalemia-related morbidity-adjusted events (win ratio 1.53, P < 0.001) and total RAASi use score (win ratio 1.25, P = 0.048) favored the patiromer arm. Adverse events were similar between groups.
Conclusion
Concurrent use of patiromer and high-dose MRAs reduces the risk of recurrent hyperkalemia (ClinicalTrials.gov: NCT03888066).
Keywords: Heart failure with reduced ejection fraction, Renin–angiotensin–aldosterone system inhibitor (RAASi), Hyperkalemia, Patiromer, Potassium-binding polymer
Structured Graphical Abstract
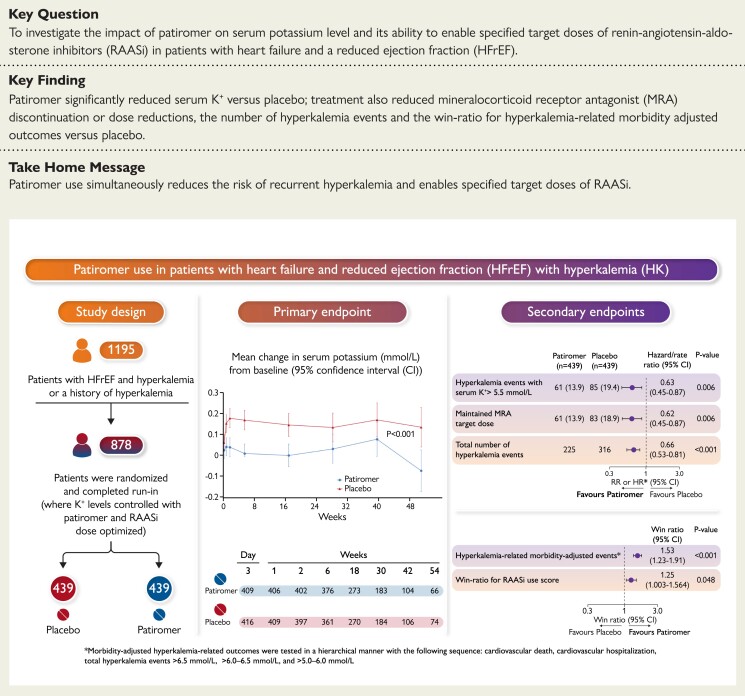
Structured Graphical Abstract.
Study design, primary and secondary endpoints of the DIAMOND trial.
See the editorial comment for this article ‘Potassium binders for patients with heart failure? The real enlightenment of the DIAMOND trial’, by M. Packer, https://doi.org/10.1093/eurheartj/ehac399.
Introduction
Hyperkalemia is associated with an increased risk of arrhythmias and mortality.1 Renin–angiotensin–aldosterone system inhibitors (RAASi) improve symptoms and reduce hospitalizations for heart failure and cardiovascular mortality for patients with heart failure and reduced ejection fraction (HFrEF), but they increase the risk of hyperkalemia,2–4 especially for those with concomitant chronic kidney disease and/or diabetes mellitus.4–6 Hyperkalemia, or the fear of inducing it, often leads to suboptimal use and dose of RAASi,2,4,7 especially mineralocorticoid receptor antagonists (MRAs), placing patients at an increased risk for adverse outcomes.5,6
Patiromer is a novel potassium-binder that exchanges potassium for calcium in the gastrointestinal tract that can be used to improve control of serum potassium.8 Previous trials of patiromer have been limited in terms of duration of follow-up and sample size. The DIAMOND (Patiromer for the Management of Hyperkalemia in Participants Receiving RAASi Medications for the Treatment of Heart Failure) trial was designed to assess the longer-term ability of patiromer to control serum potassium, prevent hyperkalemia events, and improve outcomes and the proportion of patients achieving guideline-recommended doses of RAASi in patients with HFrEF with hyperkalemia related to RAASi use or a history thereof. Due to slow enrollment rates, changing hospitalization patterns, lower than expected event rates, the uncertainty of the course of the pandemic as a consequence of COVID-19, the primary endpoint was revised during the study from time to first occurrence of cardiovascular death or cardiovascular hospitalization, to changes in serum potassium levels from the baseline.
Methods
Study design
The DIAMOND trial was a prospective Phase 3, multicenter, double-blind, randomized withdrawal, placebo-controlled study done at 389 sites in the USA, South America, Europe, and Russia. The study design has been previously described.9 An independent ethics committee at each center approved the trial. The executive committee whose members included academic investigators and representatives of Vifor Pharma developed and amended the protocol and the statistical plan, and supervised enrollment and follow-up. The trial is conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice, and local and national guidelines. All authors approved the manuscript and its submission for publication, take full responsibility for completeness and accuracy of the analyses, and attest to adherence of the trial protocol (see Supplementary material online, Appendix). Vifor Pharma provided funding for the study, supported the study design, data collection, and statistician support for the publication. The corresponding authors had unrestricted access to all data and prepared the draft of the manuscript, which was reviewed and edited by all authors.
Patients
Eligible participants were men or women, aged ≥18 years with New York Heart Association (NYHA) Class II–IV heart failure and a left ventricular ejection fraction ≤40%. The protocol required patients to have hyperkalemia at screening (defined as two serum potassium values of >5.0 mmol/l) while receiving an angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB), angiotensin receptor-neprilysin inhibitor (ARNi), and/or MRA therapy. Patients were also eligible if they were normokalemic at screening but had a history of dose reduction or discontinuation of the RAASi therapy due to hyperkalemia in the previous 12 months, which was ascertained via investigator reporting/medical records. Patients were excluded if they had an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2, systolic blood pressure <90 mmHg or symptomatic hypotension, or any significant comorbidity that could change their clinical course independent of heart failure. Complete inclusion and exclusion criteria are listed in Supplementary material online, Appendix. Written informed consent was obtained from all patients before any study-related procedures were done.
Randomization and masking
Eligible patients were enrolled into a single-blind run-in phase with weekly visits. Following the run-in phase, eligible patients underwent double-blind randomization in a 1:1 ratio, using a secure, central, interactive, web-based response system to receive continued patiromer or switch to placebo (patiromer withdrawal). Randomization was performed by using a permuted block design and was stratified by the geographic region. Patiromer and placebo were supplied to the study sites in masked kits after randomization. Both patiromer and the placebo were powder for oral suspension with identical appearances and could not be visually distinguished. All patients, physicians, and outcome assessors were masked to treatment assignment.
Procedure
The run-in phase could last up to 12 weeks and was designed to control potassium with patiromer (titrated up to maximum three packs/day; 8.4 g/pack) while concurrently optimizing the RAASi therapy, including MRAs titrated to 50 mg/day based on previous clinical trial maximum dose,2 and ≥50% of recommended doses of other RAASi drugs. Following the run-in phase, patients who were randomized to patiromer continued the established number of packets of study drug. In both groups, the RAASi agents and doses that were administered at the end of the run-in phase were continued after randomization and were maintained or adjusted at investigator discretion throughout the trial.
Prior to initiation of assigned patiromer/placebo, potassium concentration was measured at baseline. Thereafter, participants were evaluated at every visit, starting from Day 3, and then at Weeks 1, 2, 6, 18, and every 3 months thereafter until the end of study for serum potassium, adverse events, and occurrence of outcomes. Patients who prematurely discontinued investigational drug remained in the study for collection of event data and received usual care.
Outcomes
Due to the slow enrollment, changing hospitalization patterns, lower than expected event rates, the uncertainty of the course of the pandemic, and the risks associated with disrupted supply of investigational products and laboratory testing due to the COVID-19 pandemic, the sponsor, with recommendations from the Executive Steering Committee, changed the study objectives, and the primary and secondary endpoints. The original trial primary outcome was the time to first occurrence of cardiovascular death or cardiovascular hospitalization and secondary outcomes included proportion of subjects on ≥50% of target dose of ACEi, ARB, or ARNi and ≥50% of target dose of MRA at the end of study visit, total heart failure hospitalizations (or equivalent in outpatient clinic) and change from randomization in the clinical summary score of Kansas City Cardiomyopathy Questionnaire at 8 months. The decision was made to maximize the scientific value of the data already collected in the trial and at the same time ensure the safety of patients.
The revised primary endpoint was the adjusted mean change in serum potassium from baseline. The cut-off for data was the end of study date of 24 June 2021, all efficacy results are analyses up to the cut-off date, safety results include all data collected. Five secondary endpoints were tested in a hierarchical manner: (i) time to the first event of hyperkalemia of >5.5 mmol/l; (ii) lack of durable enablement of MRA at target dose, i.e. time to discontinuation or reduction of target MRA dose for at least 14 days or until end of the study; (iii) all investigator-reported adverse events of hyperkalemia (first and recurrent); (iv) a win ratio for morbidity and mortality-adjusted hyperkalemia-related outcomes with the following sequence: cardiovascular death, cardiovascular hospitalization, total hyperkalemia events >6.5, >6.0–6.5, and >5.0–6.0 mmol/l; and (v) a win ratio of novel RAASi use score (range 0–8) based on the sequence of all-cause mortality, cardiovascular hospitalization, and one or two points each for the use of >0% to ≤50% or >50% of target doses of ACEi/ARB/ARNi, MRA, and beta-blocker (see Supplementary material online, Figure S1). There are dependencies between the secondary endpoints, e.g. the secondary endpoint hyperkalemia-related outcomes, includes hyperkalemia events, which is also a secondary endpoint. Furthermore, the RAASi use score includes MRA at target dose, which is also a secondary endpoint. A clinical events committee adjudicated events in a blinded manner. An independent data monitoring committee reviewed safety data periodically. Safety assessments included the occurrence of adverse events (according to the Medical Dictionary for Regulatory Activities), evaluation of blood test results and vital signs.
Statistical analysis
The sample size required to compare two means was calculated using the t-test method and Nquery software (Version 8.6.10, Statistical Solutions Ltd, USA). A total of 820 patients (410 per treatment group) was required to detect a mean between-group difference of 0.116 with a power of 90% and two-sided alpha of 0.05. Further details are provided in the statistical analysis plan.
The differences between the placebo and patiromer groups for the primary endpoint were assessed for statistical significance using a mixed model for repeated measures with adjustment for pre-specified baseline covariate of geographic region, sex, diabetes, serum potassium, and eGFR. Least squares mean changes from baseline were reported for both treatment groups with 95% confidence intervals (CIs), as well as the difference between the least squares group means with 95% CI and P-value testing the null hypothesis of no treatment effect. Secondary endpoints were analyzed in a hierarchical manner through calculations of point estimates by treatment group along with 95% CI for the treatment differences, including (i) time to the first event of hyperkalemia of >5.5 mmol/l, analyzed using a Cox proportional hazards regression model; (ii) time to the event of a discontinuation or reduction of MRA dose to below target, analyzed using a Cox proportional hazards regression model; (iii) investigator-reported adverse events of hyperkalemia (first and recurrent), analyzed using a negative binomial regression with the logarithm of the individual follow-up time as offset, (iv) hyperkalemia-related outcomes adjusted for morbid events, assessed with an unmatched win ratio approach, and (v) comprehensive RAASi use score, compared using an unmatched win ratio approach. All endpoints were tested for statistical significance for a two-side alpha of <0.05. An independent statistician replicated and verified the analyses. This study is registered with ClinicalTrials.gov, NCT03888066.
Results
Patient characteristics and disposition
Between 24 April 2019 and 24 June 2021, a total of 1642 patients were screened for eligibility, and 1195 patients were enrolled in the run-in phase at 389 centers in 21 countries (see Supplementary material online, Figure S2). The reasons for screening failure are described in Supplementary material online, Table S1. A total of 878 patients successfully completed the run-in-phase and were randomly assigned to continue patiromer (439 patients) or switch to placebo (439 patients) (see Supplementary material online, Figure S2). The baseline characteristics in the two treatment groups were similar (Table 1). Most patients were men and were enrolled in Europe. Overall, 372 (42.4%) patients had Stage 3 chronic kidney disease, and 356 (40.5%) had diabetes. Mean ± standard deviation (SD) serum potassium at the baseline was 4.6 ± 0.3 mmol/l. At screening, 354 (40.3%) patients were hyperkalemic and 524 (59.7%) had a normal serum potassium with a history of hyperkalemia leading to previous dose reduction or discontinuation of RAASi.
Table 1.
Characteristics of patients (n = 878) prior to randomization
| Characteristic | Patiromer (n = 439) | Placebo (n = 439) |
|---|---|---|
| Age (years) | 66.6 ± 10.0 | 67.1 ± 9.9 |
| Women, n(%) | 112 (25.5) | 126 (28.7) |
| Region, n (%) | ||
| USA/Canada | 31 (7.1) | 32 (7.3) |
| Latin America | 28 (6.4) | 30 (6.8) |
| Western Europe and Other | 30 (6.8) | 28 (6.4) |
| Central/Eastern Europe | 350 (79.7) | 349 (79.5) |
| White race | 433 (98.6) | 427 (97.3) |
| Ethnicity - Not Hispanic or Latino | 381 (86.8) | 379 (86.3) |
| Ethnicity - Hispanic or Latino | 56 (12.8) | 57 (13.0) |
| NYHA functional class—n (%) a | ||
| I | 10 (2.3) | 4 (0.9) |
| II | 221 (50.3) | 251 (57.4) |
| III | 208 (47.4) | 178 (40.7) |
| IV | 0 (0.0) | 4 (0.9) |
| Body mass index (kg/m2)—mean ± SD | 28.9 ± 4.7 | 28.7 ± 4.6 |
| Heart rate (beats/min)—mean ± SD | 71 ± 9 | 71 ± 8 |
| Systolic blood pressure (mmHg)—mean ± SD | 125 ± 12 | 124 ± 13 |
| Left ventricular ejection fraction—mean ± SD | 33.5 ± 5.8 | 33.5 ± 5.7 |
| NT-proBNP, pg/ml—median (Q1, Q3) | 1305 (666, 2591) | 1322 (684, 2797) |
| Ischemic heart failure etiology—n (%) | 317 (72.2) | 310 (70.6) |
| Atrial fibrillation—n (%) | 160 (36.4) | 181 (41.2) |
| Diabetes mellitus—n (%) | 182 (41.5) | 174 (39.6) |
| Hypertension—n (%) | 406 (92.5) | 396 (90.2) |
| eGFR (ml/min/1.73 m2)b—mean ± SD | 62.6 ± 22.6 | 63.5 ± 21.4 |
| Chronic kidney disease—n (%) | ||
| Stage 1 (eGFR ≥90 ml/min/1.73 m2) | 68 (15.5) | 65 (14.8) |
| Stage 2 (eGFR 60–89 ml/min/1.73 m2) | 159 (36.2) | 172 (39.2) |
| Stage 3 (eGFR 30–59 ml/min/1.73 m2) | 182 (41.5) | 190 (43.3) |
| Stage 4 (eGFR 15–29 ml/min/1.73 m2) | 30 (6.8) | 12 (2.7) |
| Serum potassium (mmol/l) | 4.6 ± 0.3 | 4.6 ± 0.3 |
| Hyperkalemia at screening—n (%) | 182 (41.5) | 172 (39.2) |
| Normokalemia at screening—n (%) | 257 (58.5) | 267 (60.8) |
| Medication and device use—n (%) | ||
| Angiotensin-converting enzyme inhibitor | 248 (56.5) | 235 (53.5) |
| Angiotensin receptor blocker | 128 (29.2) | 136 (31.0) |
| Angiotensin receptor-neprilysin inhibitor | 67 (15.3) | 76 (17.3) |
| Any RAASi | 439 (100.0) | 439 (100.0) |
| Beta-blockers | 429 (97.7) | 425 (96.8) |
| SGLT2 inhibitor | 29 (6.6) | 20 (4.6) |
| Mineralocorticoid receptor antagonists | 439 (100.0) | 438 (99.8) |
| Implantable cardioverter-defibrillator | 52 (11.8) | 56 (12.8) |
| Cardiac resynchronization therapy | 17 (3.9) | 22 (5.0) |
| At 100% target dose—n (%) | ||
| Angiotensin-converting enzyme inhibitor | 200 (45.6) | 184 (41.9) |
| Angiotensin receptor blocker | 50 (11.4) | 61 (13.9) |
| Angiotensin receptor-neprilysin inhibitor | 25 (5.7) | 39 (8.9) |
| Any RAASi | 275 (62.6) | 285 (64.9) |
| Mineralocorticoid receptor antagonists | 437 (99.5) | 430 (97.9) |
| At ≥50% target dose—n (%) | ||
| Angiotensin-converting enzyme inhibitor | 246 (56.0) | 232 (52.8) |
| Angiotensin receptor blocker | 125 (28.5) | 133 (30.3) |
| Angiotensin receptor-neprilysin inhibitor | 61 (13.9) | 72 (16.4) |
| Any RAASi | 431 (98.2) | 436 (99.3) |
| Mineralocorticoid receptor antagonists | 439 (100.0) | 437 (99.5) |
| Dual therapy with RAS inhibitor and MRA—n (%) | 439 (100.0) | 438 (99.8) |
| Triple therapy with RAASi and MRA and beta-blocker—n (%) | 429 (97.7) | 424 (96.6) |
Plus–minus values are mean ± SD.
Two values are missing from the data for the placebo group
Data were derived from central laboratory values. The body mass index is the weight in kilograms divided by the square of the height in meters.
eGFR, estimated glomerular filtration rate; MRA, mineralcorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro B-type natriuretic peptide; RAASi, renin–angiotensin–aldosterone system inhibitor; SGLT2, sodium–glucose cotransporter 2.
Run-in period
Of the 1195 participants who entered the run-in phase, 878 were randomized. Of the 317 patients who were not randomized, 13 were never dosed with patiromer and 46 were stopped by the executive committee during the first wave of COVID-19 when most centers had halted clinical research. In addition, 98 patients in the run-in phase were discontinued after 24 June 2021, when the announcement was made that the trial’s primary endpoint had been changed, and no new patients were to be enrolled. Of the 1038 patients who completed the run-in phase, 878 (84.6%) achieved ≥50% of target dose of the combination RAASi therapy and were randomized. Supplementary material online, Table S2 shows the reasons for the 160 patients in the modified run-in set not being randomized. Patients who discontinued the study during the run-in phase had a lower ejection fraction, blood pressure, and eGFR, and were more likely to have diabetes compared to those who did not (see Supplementary material online, Table S3).
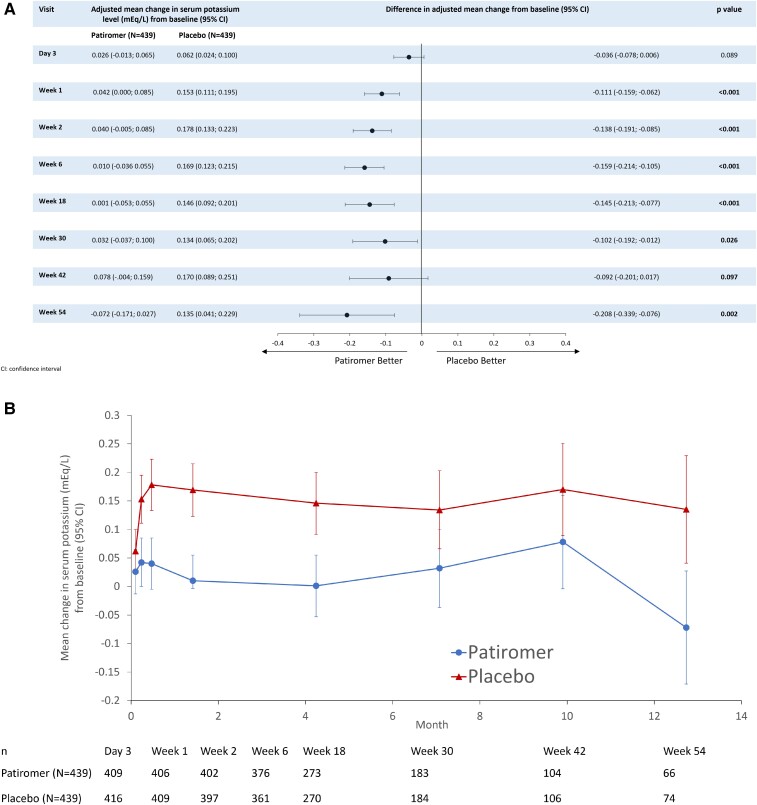
Primary outcome
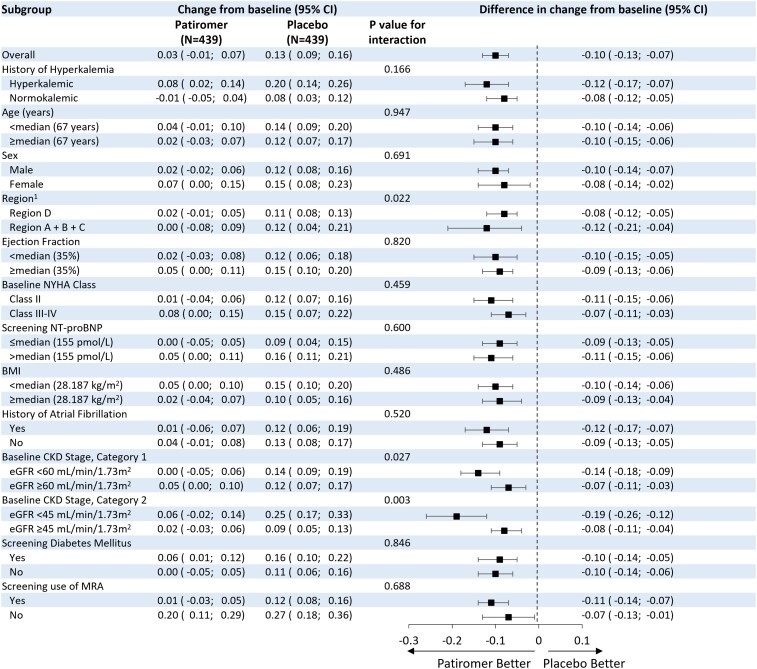
The median (interquartile range) duration of follow-up was 27 (13–43) weeks. The median number of serum potassium assessments for each participant was 5 (4–5). The adjusted mean change in serum potassium from randomization to study end was +0.03 mmol/l (95% CI –0.01, 0.07) in the patiromer group and +0.13 mmol/l (95% CI 0.09, 0.16) in the placebo group, for a between-group difference of –0.10 mmol/l (95% CI –0.13, –0.07; P < 0.001) (Figure 1, Table 2). The results of the primary endpoint were consistent in pre-specified subgroups; however, a significantly greater change from baseline in serum potassium was reported for participants with eGFR <45 ml/min/1.73 m2 [mean change (95% CI) −0.19 (−0.26, −0.12)] compared to participants with eGFR ≥45 ml/min/1.73m2 [mean change (95% CI) −0.08 (−0.11, −0.04)], P = 0.003 (Figure 2).
Figure 1.
Effects of patiromer vs. placebo on adjusted mean change in serum potassium level (mEq/L) from the baseline to the end of the study period (A) difference in adjusted mean change from baseline by visit, and (B) mean change from baseline over time. CI, confidence interval.
Table 2.
Primary and secondary outcomes
| Variable | Patiromer (n = 439) | Placebo (n = 439) | Outcome (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
| Events/100 py | Events/100 py | |||||
| Primary outcome | ||||||
| Adjusted mean change in serum potassium (mmol/l ) (95% CI) | 0.03 (−0.01, 0.07) | − | 0.13 (0.09, 0.16) | − | Difference −0.10 (−0.13, −0.07) | <0.001 |
| Secondary outcomes specified in hierarchical testing procedure—n (%) | ||||||
| Number of patients with hyperkalemia events [serum potassium >5.5 (mmol/l)] n (%) | 61 (13.9) | − | 85 (19·4) | − | Hazard ratio 0.63 (0.45, 0.87) | 0.006 |
| Number of subjects with MRA reduction, n(%) | 61 (13.9) | − | 83 (18.9) | − | Hazard ratio 0.62 (0.45, 0.87) | 0.006 |
| Total number of hyperkalemia events | 225 | 77.7 | 316 | 118.2 | Hazard ratio 0.66 (0.53, 0.81) | <0.001 |
| Hyperkalemia- related outcomes win ratio | − | − | 1.53 (1.23, 1.91) | <0.001 | ||
| RAASi use score win ratio a | − | − | − | − | 1.25 (1.003, 1.564) | 0.048 |
Win ratio of novel RAASi use score (range 0–8) based on the sequence of all-cause mortality, cardiovascular hospitalization, and one or two points each for the use of ≥50% or ≥100% of target doses of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, MRA, and beta-blocker.
MRA, mineralocorticoid receptor antagonist; py, person-years; RAASi, renin–angiotensin–aldosterone system inhibitor.
Figure 2.
Primary endpoint, changes according to pre-specified subgroups. 1Region A (USA and Canada), Region B (Mexico, Argentina, Brazil), Region C (France, Germany, Italy, Netherlands, Spain, UK, Israel, Belgium), Region D (Bulgaria, Czech Republic, Hungary, Poland, Russia, Serbia, Ukraine, Georgia). ARNi, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; MRA, mineralocorticoid receptor agonist; NYHA, New York Heart Association.
Hierarchical secondary outcomes
A total of 61 participants (13.9%) in the patiromer vs. 85 (19.4%) in the placebo group had hyperkalemia events of >5.5 mmol/l [hazard ratio (HR) 0.63; 95% CI 0.45, 0.87; P = 0.006] (see Supplementary material online, Figure S3). A discontinuation or reduction of the target MRA dose occurred in 61 participants (13.9%) in the patiromer and in 83 (18.9%) in the placebo group (HR 0.62; 95% CI 0.45, 0.87; P = 0.006) (see Supplementary material online, Figure S4). In addition, 20 (4.6%) participants in the patiromer and 31 (7.1%) in the placebo group discontinued MRAs during the study (HR 0.64; 95% CI 0.36, 1.12). In further exploratory analyses for patients who were still alive, MRA discontinuation was reported in 12 patients in the patiromer group, compared to 27 in the placebo group [HR (95% CI) 0.44 (0.22, 0.87)]. Total number of adjusted hyperkalemia events/100-person-years were lower with patiromer (77.7 vs. 118.2 with placebo; HR 0.66; 95% CI 0.53, 0.81; P < 0.001) (see Supplementary material online, Figure S5). Both the win ratio for hyperkalemia-related morbidity-adjusted outcomes (1.53; 95% CI 1.23, 1.91; P < 0.001), and RAASi use score (1.25; 95% CI 1.003, 1.564; P = 0.048) favor patiromer (Table 2; medication components shown in Supplementary material online, Table S4). The effect of patiromer on time to the first event of hyperkalemia of >5.5 mmol/l was consistent across pre-specified subgroups similar to the overall population (see Supplementary material online, Figure S6).
Other endpoints
There was a total of 18 and 14 cardiovascular deaths and a total of 17 and 20 heart failure hospitalizations in the patiromer and the placebo groups, respectively, at the end of study. Other exploratory endpoints are shown in Table 3 and Supplementary material online, Figure S7.
Table 3.
Other endpoints
| Variable | Patiromer (n = 439) | Placebo (n = 439) | Hazard ratio, proportion difference, rate ratio or win ratio (95% CI) | P-value |
|---|---|---|---|---|
| MRA dose reduction or discontinuation or serum potassium >5.5 mmol/l, n (%) | 95 (21.6) | 117 (26.7) | 0.74 (0.57, 0.97) | 0.030 |
| Proportion of participants on ≥50% of target dose of ACEi, ARB, or ARNi and MRA | 0.92 | 0.87 | 0.05 (0.007, 0.092) | 0.015 |
| Subjects with MRA discontinuation, n (%) | 20 (4.6) | 31 (7.1) | 0.64 (0.36, 1.12) | 0.117 |
| Subjects with ACEi/ARB/ARNi discontinuation, n (%) | 12 (2.7) | 16 (3.6) | 0.74 (0.35, 1.57) | 0.438 |
| Cardiovascular death, n (%) | 18 (4.1) | 14 (3.2) | 1.31 (0.65, 2.63) | 0.453 |
| All-cause death (%) | 22 (5.0) | 16 (3.6) | 1.39 (0.73, 2.66) | 0.312 |
| Time to first cardiovascular hospitalizationa | 24 (5.5) | 18 (4.1) | 1.34 (0.73, 2.47) | 0.347 |
| Total cardiovascular hospitalizations, n | 27 | 23 | 1.15 (0.59, 2.24) | 0.671 |
| Time to first heart failure hospitalizationsa | 16 (3.6) | 15 (3.4) | 1.08 (0.54, 2.19) | 0.821 |
| Total heart failure hospitalizations, n | 17 | 20 | 0.79 (0.36, 1.71) | 0.544 |
| Change in NT-proBNP (pg/ml) at 66 weeks | −753 (639) | −647 (626) | −106 (−1771, 1559) | 0.900 |
Number of subjects with at least one event, n (%).
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor-neprilysin inhibitor; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
Safety
During the blinded treatment phase and including assessments recorded after the end of study, the proportion of patients with any adverse events was similar in the patiromer (72.9%) and placebo (74.0%) groups (Table 4). Diarrhea, constipation, and nausea were reported for 19 (4.3%), 11 (2.5%), and 4 (0.9%) patients in the patiromer group and 15 (3.4%), 5 (1.1%), and 4 (0.9%) patients in the placebo group, respectively. The proportion of patients that discontinued the study drug due to adverse events was similar in the patiromer (2.7%) and placebo (2.5%) groups. More patients treated with patiromer experienced hypokalemia [n = 66 (15.0%)] compared with those in the placebo group [n = 47 (10.7%)]. The majority of hypokalemic events were mild [57 (13.0%) in the patiromer group, and 42 (9.6%) in the placebo group]. Severe hypokalemic events were reported in one patient (0.2%) in each group.
Table 4.
Patients experiencing adverse events during the randomized phase
| Variable | Patiromer (n = 439) | Placebo (n = 439) |
|---|---|---|
| Any adverse events, n (%) | 320 (72.9) | 325 (74.0) |
| Hypokalemia | 66 (15.0) | 47 (10.7) |
| Mild | 57 (13.0) | 42 (9.6) |
| Moderate | 8 (1.8) | 4 (0.9) |
| Severe | 1 (0.2) | 1 (0.2) |
| Hypomagnesemia | 19 (4.3) | 22 (5.0) |
| Diarrhea | 19 (4.3) | 15 (3.4) |
| Constipation | 11 (2.5) | 5 (1.1) |
| Nausea | 4 (0.9) | 4 (0.9) |
| Adverse events leading to withdrawal, n (%) | 12 (2.7) | 11 (2.5) |
| Any serious adverse event, n (%) | 54 (12.3) | 58 (13.2) |
Discussion
There were several notable findings in this trial. The run-in phase shows that most patients (84.6%) with HFrEF and RAASi-related hyperkalemia could achieve specified target doses of the RAASi therapy,10 including an MRA, when treated with patiromer while maintaining normal serum potassium. This is important as failure to provide guideline-recommended RAASi therapy (i.e. ARNi/ACEi/ARB and MRA, increased to target dose as tolerated) is associated with an increased risk of heart failure hospitalizations and death in these patients.2–4,10–13 The randomized phase showed that discontinuation of patiromer was associated with a rise in serum potassium, an increased incidence of hyperkalemia events and fewer patients being maintained on MRA at target doses. Moreover, treatment with patiromer led to a 35% relative risk reduction in the total number of hyperkalemia events. The win ratio for hyperkalemia-related morbidity-adjusted outcomes and the RAASi use score were both significantly higher with patiromer treatment. During the randomized phase, fewer patients discontinued MRAs in the patiromer group vs. placebo (Structured Graphical Abstract). Although this result was not statistically significant, the difference would be expected to produce a clinically meaningful effect and is consistent with the other results and the totality of data. This difference is further highlighted when considering the patients with MRA discontinuation still alive in each group [patiromer n = 12, placebo n = 27; HR 0.44 (95% CI 0.22, 0.87)].
Triple therapy with a renin–angiotensin system inhibitor, an MRA, and a beta-blocker form the cornerstone of evidence-based HFrEF care, more recently with the addition of sodium–glucose cotransporter 2 inhibitors.14 However, their use in clinical practice remains suboptimal. Contemporary data from the CHAMP-HF (Change the Management of Patients with Heart Failure) registry showed that <25% of patients simultaneously received any dose of all three medications and fewer than 5% were on guideline-recommended doses of all three. These patterns, particularly low use of MRAs, are consistent across multiple health care setting and geographic regions.15,16 In a large study of new MRA users, nearly 20% experienced hyperkalemia within a year; among these, 47% discontinued MRA use; and among these, 75% were not reintroduced to MRA therapy within the following year.17 Even in a clinical trial setting, their use is suboptimal, e.g. in the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial, only 59.7% of patients were receiving triple therapy.18 In this regard, it is important to note that in the DIAMOND trial, 84.6% of the patients at the end of run-in phase were able to achieve ≥50% of target dose of the combination RAASi therapy and 97% were able to take the triple therapy at some dose. This underscores that increasing the proportion of patients achieving specified target doses of therapy for HFrEF is feasible.1 Furthermore, the target doses of MRA in this study, i.e. 50 mg/day of eplerenone/spironolactone, were selected as the maximum doses in the RALES and EMPHASIS-HF trials.3,19 The pre-RALES trials found that 50 mg spironolactone produced the highest reduction in N-terminal pro-atrial natriuretic peptide,20 which is associated with heart failure prognosis, and whilst it is not an established biomarker of response to therapy, the investigators felt that due to its prognostic association it would be of interest to target 50 mg spironolactone. In RALES, due to the risk of hyperkalemia, the starting dose was 25 mg/day spironolactone (considered to be therapeutically equivalent1,2 to 50 mg eplerenone), which was increased if heart failure progressed to a maximum of 50 mg/day.19 In EMPHASIS-HF, the target dose of eplerenone/placebo was stratified at randomization according to eGFR (50 mg/day if eGFR ≥50 ml/min/1.73 m2 and ≤25 mg/day if eGFR 30–49 ml/min/1.73 m2).3
Patients with HFrEF in whom hyperkalemia develops during the RAASi therapy usually have other risk factors, e.g. diabetes and chronic kidney disease.4–6 The effects of patiromer on the primary endpoint were consistent across all pre-specified subgroups, including patients with and without diabetes and or chronic kidney disease, providing evidence for the potential of RAASi enablement across risk factors with the use of patiromer. While the difference in serum potassium between the two groups was modest, they represent the cumulative data despite down-titration or discontinuation of the RAASi therapy.
The results of the DIAMOND trial are consistent with previous trials showing that patiromer reduces the risk of hyperkalemia in patients taking RAASi. However, most of these earlier trials, such as AMBER and AMETHYST-DN, were of a relatively short duration and most patients did not have heart failure.21,22 The OPAL-HK (Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia) trial showed that 4 weeks of patiromer treatment in 237 patients with chronic kidney disease decreased serum potassium and reduced hyperkalemia recurrence.23 The PEARL-HF (Evaluation of RLY5016 in Heart Failure Patients) trial evaluated the effect of 4 weeks of patiromer compared with placebo in 105 patients with HFrEF and showed a significant effect of patiromer on serum potassium and a higher proportion of patients achieving a spironolactone dose of 50 mg/day.24 The current analysis is the largest randomized experience of any potassium-binder assessing control of serum potassium, hyperkalemia events, and achievement of specified target doses of the RAASi therapy in patients with HFrEF and hyperkalemia. Rates of hypokalemia were comparatively high in both arms in this trial (15.0% patiromer, 10.7% placebo) compared with previous trials, where hypokalemia was present in 0–6%21,22,25 of patients. However, the majority of hypokalemia events were mild, with only two patients (one in each arm) reporting severe hypokalemia. Furthermore, when considering the rate of hypokalemia in the placebo arm, the net difference was 4.3% in the patiromer arm. Although it is important to monitor patients for hypokalemia, this is a side effect that is reversible and readily managed in patiromer patients by reducing the dosage.
To comprehensively assess the impact of patiromer on hyperkalemia events and RAASi treatment, two win ratio endpoints were designed, and both were significantly in favor of patiromer use. The first assessed varying severity of hyperkalemia events considering first mortality and hospitalizations, and the second a comprehensive use of RAASi, both provision and doses, also first considering mortality and hospitalizations. Considering the achievement of the comprehensive RAASi therapy in patients with hyperkalemia and simultaneously a reduction in the risk of hyperkalemia, it can be postulated that over the long term, this strategy may result in clinically meaningful reductions in morbidity and mortality. Studies suggest that hyperkalemia may be a risk marker for MRA non-use;26,27 thereby, in treating hyperkalemia, it would be reasonable to consider that outcomes may be improved. However, the revised DIAMOND trial did not have the power to assess hard endpoints of mortality and hospitalizations.
This study should be interpreted in the context of several limitations. Due to the COVID-19 pandemic, the primary endpoint was changed, and the number of patients and events were fewer than planned. However, this represents the largest and the longest randomized experience in heart failure patients with any potassium-binder. Despite this, the inclusion criteria did not allow for inclusion of patients with an eGFR of <30 ml/min/1.73 m2, systolic blood pressure <90 mmHg or symptomatic hypotension; as such, the broader generalizability of these results may be impacted. Although this study was not powered to demonstrate whether enabling RAASi use translates into reduced cardiovascular death or hospitalizations, the use of ancillary therapy to enable primary risk reducing therapy is accepted (e.g. proton-pump inhibitors to enable platelet inhibition and anticoagulants, and anti-emetics to use chemotherapy). Multiple comparisons were made for the primary outcomes and readers should be careful while interpretating these data. Furthermore, there was rather modest reduction in serum potassium levels with patiromer, short duration of treatment and relatively few potassium measurements during follow-up. Also, there were 42 patients included with eGFR <30 ml/min/1.73 m2 at randomization; these patients had values >30 ml/min/1.73 m2 prior to the run-in phase.
In conclusion, the use of patiromer in patients with HFrEF and RAASi-related hyperkalemia was associated with significantly lower serum potassium, fewer hyperkalemia episodes, concurrent use of high doses of MRAs, and overall higher RAASi use. Patiromer was safe and well tolerated. Further prospective trials will be needed to confirm if using patiromer to enhance MRA use can help to improve outcomes.
Supplementary data
Supplementary data is available at European Heart Journal online.
Funding
This work was supported by Vifor Pharma. Funding to pay the Open Access publication charge for this article was provided by Vifor Pharma.
Supplementary Material
Contributor Information
Javed Butler, Baylor Scott and White Research Institute, Dallas, TX, USA; Department of Medicine, University of Mississippi, Jackson, MS, USA.
Stefan D Anker, Department of Cardiology (CVK), Berlin, Germany; Berlin Institute of Health Center for Regenerative Therapies (BCRT), Berlin, Germany; German Center for Cardiovascular Research (DZHK) partner site Berlin, Berlin, Germany; Charité Universitätsmedizin, Berlin, Germany.
Lars H Lund, Department of Medicine, Unit of Cardiology, Karolinska Institutet, Solna, Stockholm, Sweden; Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden.
Andrew J S Coats, University of Warwick, Warwick, UK.
Gerasimos Filippatos, National and Kapodistrian University of Athens, School of Medicine, Athens University, Athens, Greece; Hospital Attikon, Athens, Greece.
Tariq Jamal Siddiqi, Baylor Scott and White Research Institute, Dallas, TX, USA; Department of Medicine, University of Mississippi, Jackson, MS, USA.
Tim Friede, Department of Internal Medicine, Göttingen, Germany; University Medical Center Göttingen, Göttingen, Germany; DZHK (German Center for Cardiovascular Research), Göttingen partner site, Göttingen, Germany.
Vincent Fabien, Vifor Pharma, Glattbrugg, Switzerland.
Mikhail Kosiborod, Department of Cardiovascular Disease, Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA; University of Missouri-Kansas City, Kansas City, MO, USA.
Marco Metra, Cardiology, ASST Spedali Civili and University, Brescia, Italy.
Ileana L Piña, Central Michigan University College of Medicine, Mount Pleasant, MI, USA.
Fausto Pinto, Santa Maria University Hospital, CAML, CCUL, Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal.
Patrick Rossignol, X Université de Lorraine, INSERM, Centre d’Investigations Cliniques-Plurithématique 1433, INSERM Unit 1116, Centre Hospitalier Régional Universitaire (CHRU) de Nancy, Nancy, France; F-CRIN INI-CRCT, Nancy, France.
Peter van der Meer, Department of Cardiology, University Medical Center Groningen, Groningen, the Netherlands.
Cecilia Bahit, INECO Neurociencias Oroño, Rosario, Santa Fe, NM, USA.
Jan Belohlavek, Clinic of Cardiology and Angiology, General University Hospital Prague, Prague, Czech Republic.
Michael Böhm, Klinik für Innere Medizin III, Saarland University, Homburg/Saar, Germany.
Jasper J Brugts, Erasmus MC University Medical Center, Rotterdam, the Netherlands.
John G F Cleland, Institute of Health & Wellbeing, University of Glasgow, Glasgow, UK.
Justin Ezekowitz, Faculty of Medicine & Dentistry, University of Alberta, Alberta, Canada.
Antoni Bayes-Genis, Cardiology Department, Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona, CIBERCV, Spain.
Israel Gotsman, Hadassah Medical Centre, Jerusalem, Israel.
Assen Goudev, Department of Emergency Medicine, Medical University of Sofia, Sofia, Bulgaria.
Irakli Khintibidze, Alexandre Aladashvili Clinic, Tbilisi State Medical University, Tbilisi, Georgia.
Joann Lindenfeld, Department of Medicine, Vanderbilt University Medical Centre, Nashville, TN, USA.
Robert J Mentz, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
Bela Merkely, Semmelweis University Heart and Vascular Center, Budapest, Hungary.
Eliodoro Castro Montes, Heart Institute of Queretaro, Santiago de Querétaro, Mexico City, Mexico.
Wilfried Mullens, Ziekenhuis Oost Limburg Genk and University Hasselt, Belgium.
Jose C Nicolau, Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina; Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Aleksandr Parkhomenko, Hospital Oost-Limburg, Genk, Belgium.
Piotr Ponikowski, Insitute of Heart Diseases, Wroclaw Medical University, Wroclaw, Poland.
Petar M Seferovic, Faculty of Medicine Belgrade, Serbia; Serbian Academy of Sciences and Arts, Serbia.
Michele Senni, University of Milano – Bicocca, Cardiovascular Department, Papa Giovanni XXIII Hospital, Bergamo, Italy.
Evgeny Shlyakhto, Almazov Federal Heart, Blood and Endocrinology Centre, Saint-Petersburg, Russia.
Alain Cohen-Solal, Université de Paris, INSERM U942, APHP, Hospital Lariboisiere, Paris, France.
Peter Szecsödy, Vifor Pharma, Glattbrugg, Switzerland.
Klaus Jensen, Vifor Pharma, Glattbrugg, Switzerland.
Fabio Dorigotti, Vifor Pharma, Glattbrugg, Switzerland.
Matthew R Weir, Division of Nephrology, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA.
Bertram Pitt, Division of Cardiology, University of Michigan, Ann Arbor, MI, USA.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with Vifor Pharma's data sharing policy. Enquiries can be made to medinfo@viforpharma.com.
References
- 1. Ferreira JP, Mogensen UM, Jhund PS, Desai AS, Rouleau JL, Zile MR, et al. Serum potassium in the PARADIGM-HF trial. Eur J Heart Fail 2020;22:2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Rossignol P, Duarte K, Girerd N, Karoui M, McMurray JJV, Swedberg K, et al. Cardiovascular risk associated with serum potassium in the context of mineralocorticoid receptor antagonist use in patients with heart failure and left ventricular dysfunction. Eur J Heart Fail 2020;22:1402–1411. [DOI] [PubMed] [Google Scholar]
- 5. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin angiotensin-aldosterone system. N Engl J Med 2004;351:585–592. [DOI] [PubMed] [Google Scholar]
- 6. Trevisan M, Fu EL, Xu Y, Savarese G, Dekker FW, Lund LH, et al. Stopping mineralocorticoid receptor antagonists after hyperkalaemia: trial emulation in data from routine care. Eur J Heart Fail 2021;23:1698–1707. [DOI] [PubMed] [Google Scholar]
- 7. Patel RB, Fonarow GC, Greene SJ, Zhang S, Alhanti B, DeVore AD, et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol 2021;78:330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension 2015;66:731–738. [DOI] [PubMed] [Google Scholar]
- 9. Butler J, Anker SD, Siddiqi TJ, Coats AJS, Dorigotti F, Filippatos G, et al. Patiromer for the management of hyperkalaemia in patients receiving renin–angiotensin–aldosterone system inhibitors for heart failure: design and rationale of the DIAMOND trial. Eur J Heart Fail 2021;24:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–e421. [DOI] [PubMed] [Google Scholar]
- 11. Ouwerkerk W, Teng TK, Tromp J, Tay WT, Cleland JG, van Veldhuisen DJ, et al. Effects of combined renin–angiotensin–aldosterone system inhibitor and beta-blocker treatment on outcomes in heart failure with reduced ejection fraction: insights from BIOSTAT-CHF and ASIAN-HF registries. Eur J Heart Fail 2020;22:1472–1482. [DOI] [PubMed] [Google Scholar]
- 12. Bistola V, Simitsis P, Parissis J, Ouwerkerk W, van Veldhuisen DJ, Cleland JG, et al. Association between up-titration of medical therapy and total hospitalizations and mortality in patients with recent worsening heart failure across the ejection fraction spectrum. Eur J Heart Fail 2021;23:1170–1181. [DOI] [PubMed] [Google Scholar]
- 13. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J 2017;38:1883–1890. [DOI] [PubMed] [Google Scholar]
- 14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 15. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 16. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlström U, et al. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018;20:1326–1334. [DOI] [PubMed] [Google Scholar]
- 17. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail 2018;20:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 19. The RALES Investigators . Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 1996;78:902–907. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B. ACE inhibitor co-therapy in patients with heart failure: rationale for the Randomized Aldactone Evaluation Study (RALES). Eur Heart J 1995;16:107–110. [DOI] [PubMed] [Google Scholar]
- 21. Rossignol P, Williams B, Mayo MR, Warren S, Arthur S, Ackourey G, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): results in the pre-specified subgroup with heart failure. Eur J Heart Fail 2020;22:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN randomized clinical trial. JAMA 2015;314:151–161. [DOI] [PubMed] [Google Scholar]
- 23. Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015;372:211–221. [DOI] [PubMed] [Google Scholar]
- 24. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011;32:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreira JP, Abreu P, McMurray JJV, van Veldhuisen DJ, Swedberg K, Pocock SJ, et al. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. Eur J Heart Fail 2019;21:345–351. [DOI] [PubMed] [Google Scholar]
- 26. Cooper LB, Benson L, Mentz RJ, Savarese G, DeVore AD, Carrero J-J, et al. Association between potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish Heart Failure Registry. Eur J Heart Fail 2020;22:1390–1398. [DOI] [PubMed] [Google Scholar]
- 27. Lund L, Pitt B. Is hyperkalaemia in heart failure a risk factor or a risk marker? Implications for renin-angiotensin-aldosterone system inhibitor use. Eur J Heart Fail 2018;20:931–932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with Vifor Pharma's data sharing policy. Enquiries can be made to medinfo@viforpharma.com.