Abstract
Aims
Sodium–glucose co-transporter 2 inhibition reduces the risk of hospitalization for heart failure and for death in patients with symptomatic heart failure. However, trials investigating the effects of this drug class in patients following acute myocardial infarction are lacking.
Methods and results
In this academic, multicentre, double-blind trial, patients (n = 476) with acute myocardial infarction accompanied by a large creatine kinase elevation (>800 IU/L) were randomly assigned to empagliflozin 10 mg or matching placebo once daily within 72 h of percutaneous coronary intervention. The primary outcome was the N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP) change over 26 weeks. Secondary outcomes included changes in echocardiographic parameters. Baseline median (interquartile range) NT-proBNP was 1294 (757–2246) pg/mL. NT-proBNP reduction was significantly greater in the empagliflozin group, compared with placebo, being 15% lower [95% confidence interval (CI) −4.4% to −23.6%] after adjusting for baseline NT-proBNP, sex, and diabetes status (P = 0.026). Absolute left-ventricular ejection fraction improvement was significantly greater (1.5%, 95% CI 0.2–2.9%, P = 0.029), mean E/e′ reduction was 6.8% (95% CI 1.3–11.3%, P = 0.015) greater, and left-ventricular end-systolic and end-diastolic volumes were lower by 7.5 mL (95% CI 3.4–11.5 mL, P = 0.0003) and 9.7 mL (95% CI 3.7–15.7 mL, P = 0.0015), respectively, in the empagliflozin group, compared with placebo. Seven patients were hospitalized for heart failure (three in the empagliflozin group). Other predefined serious adverse events were rare and did not differ significantly between groups.
Conclusion
In patients with a recent myocardial infarction, empagliflozin was associated with a significantly greater NT-proBNP reduction over 26 weeks, accompanied by a significant improvement in echocardiographic functional and structural parameters.
ClinicalTrials.gov registration
Keywords: Clinical trial, Randomised controlled trial, Empagliflozin, Myocardial infarction, Heart failure, NT-proBNP
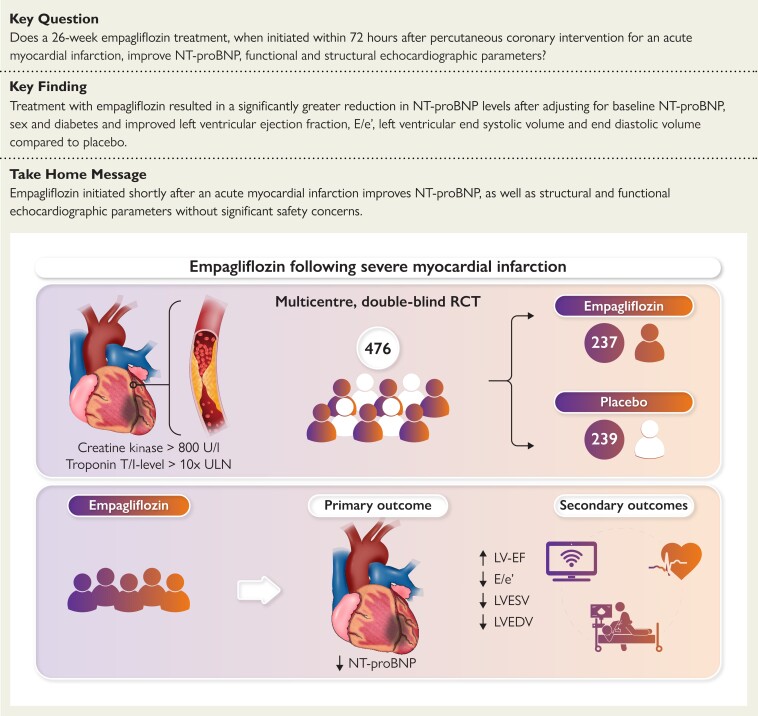
Structured Graphical Abstract
Structured graphical abstract.
A total of 476 people with acute myocardial infarction were randomized to either empagliflozin 10 mg or matching placebo once daily within 72 h of acute percutaneous coronary intervention. The change in NT-proBNP concentrations as well as echocardiographic functional and structural parameters over 26 weeks of treatment was evaluated. LVEF, left-ventricular ejection fraction; LVEDV, left-ventricular end-diastolic volume; LVESV, left-ventricular end-systolic volume; NT-proBNP, N-terminal pro-hormone of brain natriuretic peptide.
See the editorial comment for this article ‘Putting the puzzle together: SGLT2 inhibitors from prevention to treatment of heart failure’, by Josephine Harrington et al., https://doi.org/10.1093/eurheartj/ehac483.
Introduction
In chronic heart failure with reduced ejection fraction (HFrEF), sodium–glucose co-transporter 2 inhibitors (SGLT2i) have been shown to reduce the risk of hospitalization for heart failure (HHF) as well as all-cause mortality and cardiovascular mortality.1–4 Recent evidence also indicates beneficial effects of initiating treatment after acute heart failure.5 In addition, empagliflozin was the first drug shown prospectively in the EMPEROR-Preserved trial to improve the primary outcome of HHF and cardiovascular death in heart failure patients with mildly reduced (HFmrEF) or preserved ejection fraction (HFpEF).6 The use of SGLT2i for HFrEF was recently recommended in the European and American heart failure guidelines as part of first-line therapy,7,8 with the more recent American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of America (HFSA) guidelines also advocating SGLT2i use in patients with HFmrEF and HFpEF.8
Sodium–glucose co-transporter inhibitors appear to exhibit cardioprotective effects attributable to metabolic9 and anti-inflammatory10 mechanisms, as well as modification of myocardial signal transduction by inhibition of Na+/H+ exchanger.11,12 Strikingly, onset of the beneficial cardiovascular effects observed in cardiovascular outcome trials (CVOTs) emerged within a few weeks of treatment initiation and have been shown to be independent of glycaemic status.2,6 The question as to whether early SGLT2i initiation following myocardial infarction (MI) is effective and safe is of key importance, since MI is a major cause of incident heart failure with a 15% event rate (symptomatic heart failure and/or reduced ejection fraction) within 12 months.13,14 Accordingly, we designed the EMpagliflozin in patients with acute MYocardial infarction (EMMY) trial to investigate whether empagliflozin treatment given in addition to guideline-recommended post-MI therapy,15 and initiated within 72 h after percutaneous coronary intervention (PCI) in people with a large acute MI, with or without diabetes, would result in a larger decline in N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP) and larger improvement in ejection fraction.
Methods
Trial design
We conducted a prospective, multicentre, randomized, double-blind, placebo-controlled trial designed to evaluate the effect of empagliflozin 10 mg once daily (p.o.) for 26 weeks on cardiac function and heart failure biomarkers in patients with acute MI from 11 Austrian sites. Study duration was from 11 May 2017 (first patient first visit) to 3 May 2022 (last patient last visit). The detailed trial protocol has been published.16 The study was approved by the relevant regulatory authorities, by the Ethics Committee of the Medical University of Graz, Austria (EK 29–179 ex 16/17; EudraCT 2016-004591-22) and registered on ClinicalTrials.gov (NCT03087773). EMMY was conducted in full conformity with the 1964 Declaration of Helsinki and all subsequent revisions, as well as in accordance with the guidelines laid down by the International Conference on Harmonization for Good Clinical Practice (ICH GCP E6 guidelines). The academic leadership of the trial (see Supplementary material online, Appendix) designed the protocol, identified the participating centres, and supervised the implementation of the protocol. EMMY was managed and led by the Interdisciplinary Metabolic Medicine Trials Unit at the Medical University of Graz, Austria.
Patients aged 18–80 years with a confirmed acute large MI (creatine kinase >800 IU/L), a high-sensitivity Troponin T level (or Troponin I level) >10-fold the upper limit of normal, and an estimated glomerular filtration rate >45 mL/min/1.73 m2 were eligible for inclusion. Those with diabetes mellitus other than Type 2, a blood pH <7.32, haemodynamic instability, acute symptomatic urinary tract infection or genital infection, an ongoing SGLT2i treatment or an SGLT2i treatment within 4 weeks prior to enrolment, were excluded. The detailed inclusion and exclusion criteria are listed in the design paper16 and the Supplementary material online, Section C. Patients were enrolled within 72 h after a PCI for acute MI. Before randomization, patients were required to be haemodynamically stable (defined as no use of haemodynamically active intravenous drugs) and have a blood pressure ≥110/70 mmHg.
Trial procedures
After giving written informed consent, eligible patients were randomized in a 1:1 ratio to oral empagliflozin 10 mg day or matching placebo once daily via Randomizer Software (Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, http://www.randomizer.at), utilizing a randomization schedule provided by an independent statistician. Randomization was stratified by site, presence of Type 2 diabetes (yes/no) and by sex. Follow-up visits were scheduled at 6, 12, and 26 weeks.
NT-proBNP values were measured in local laboratories, but were also assayed centrally at three time-points for the final data analysis at the CIMCL (Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria) using the Elecsys proBNP platform (Roche Diagnostics, Mannheim, Germany) with chemiluminescence technology.
Echocardiography was performed in accordance with the current guidelines of the European Association of Cardiovascular Imaging and the American Society of Echocardiography using locally available ultrasound devices. The protocol included 2D, Doppler echocardiography, and M-mode imaging.17 Studies were archived in DICOM-format and analysed locally.
Outcomes
The primary endpoint was the change in NT-proBNP levels from randomization to Week 26. Secondary endpoints included changes in NT-proBNP levels from randomization to Week 6, and changes in left-ventricular ejection fraction (LVEF) from randomization to Weeks 6 and 26, as well as echocardiographic parameters for diastolic dysfunction, left-ventricular end-systolic (LVESV) and end-diastolic volume (LVEDV), and changes in ketone body and glycated haemoglobin concentrations and body weight. Additional exploratory endpoints were hospitalizations due to heart failure or other causes, duration of hospital stay and all-cause mortality.
Key safety outcomes were the incidence of serious adverse events (SAEs), severe hypoglycaemic events, number of genital infections, number of ketoacidosis events, and acute liver or renal injury. Hospitalizations during the follow up were adjudicated by an independent adjudication committee prior to unblinding.
Sample size
Details of the sample size estimation have been published previously.16 Briefly, based on previous data, NT-proBNP levels were assumed to decrease after acute MI by ∼50% within 6 months.18 To detect a 40% larger relative reduction in NT-proBNP levels in the empagliflozin group, compared with the placebo group, with 80% power and an alpha level of 0.05%, the estimated sample size was 432 participants (216 per group). To allow for a potential 10% dropout rate, the recruitment target was set at 476 participants (238 per group).
Statistical analysis
The statistical analysis plan was finalized prior to database lock. Baseline characteristics were summarized using descriptive statistics with mean and standard deviation for continuous measures and frequency tables for categorical variables. Categorical variables were compared using χ2 or Fisher’s exact tests, and continuous variables using an unpaired t-test or its non-parametric equivalent (Wilcoxon rank-sum test), where the normality assumption was violated. The primary endpoint (change in NT-proBNP from baseline to Week 26) was analysed in the intention-to-treat (ITT) population using a robust linear mixed effect model (LMEM)19 in which the dependent variable was log-transformed NT-proBNP and the fixed effects were treatment, visit, treatment-by-visit interaction, the stratification factors sex and presence/absence of Type 2 diabetes, and baseline NT-proBNP concentration. For the primary analysis, no missing data were imputed. At Week 26, estimated mean values and mean differences between treatment groups were derived from the robust LMEM using marginal means (or least squares means). Their associated P-values and two-sided 95% confidence intervals (CIs) were derived from the robust LMEM using bootstrap techniques.20 To claim superiority of empagliflozin over placebo, the primary efficacy analysis was required to demonstrate a statistically significant treatment at Week 26 at a 5% alpha level with a two-sided test.
Sensitivity analyses
To assess the sensitivity of the primary efficacy analysis to missing data, analyses were also conducted in the ITT population with missing values imputed for all visits using the Multiple Imputation with Chained Equation approach. A total of 33 multiply-imputed data sets were generated. The primary efficacy analysis was repeated for each of the imputed data sets to estimate the treatment effect and the standard error of that estimate. Finally, the set of estimates and standard errors obtained from the multiply-imputed data sets were pooled to produce overall estimates, CIs, and P-values for the treatment effect.
A further sensitivity analysis used the Week 26 NT-proBNP as the primary endpoint. This analysis was performed in the ITT population with no imputation for missing values using a multiple linear regression model where the dependent variable was the Week 26 NT-proBNP and the independent variables were treatment, sex, diabetes status, and baseline NT-proBNP concentration.
Secondary endpoints including LVEF, E/e′, LVESV, or LVEDV were analysed using the same statistical methods as described for the primary efficacy analysis. All statistical analyses were performed using R software version 4.1.0 (https://www.r-project.org). P-values <0.05 were considered statistically significant.
Results
Trial population
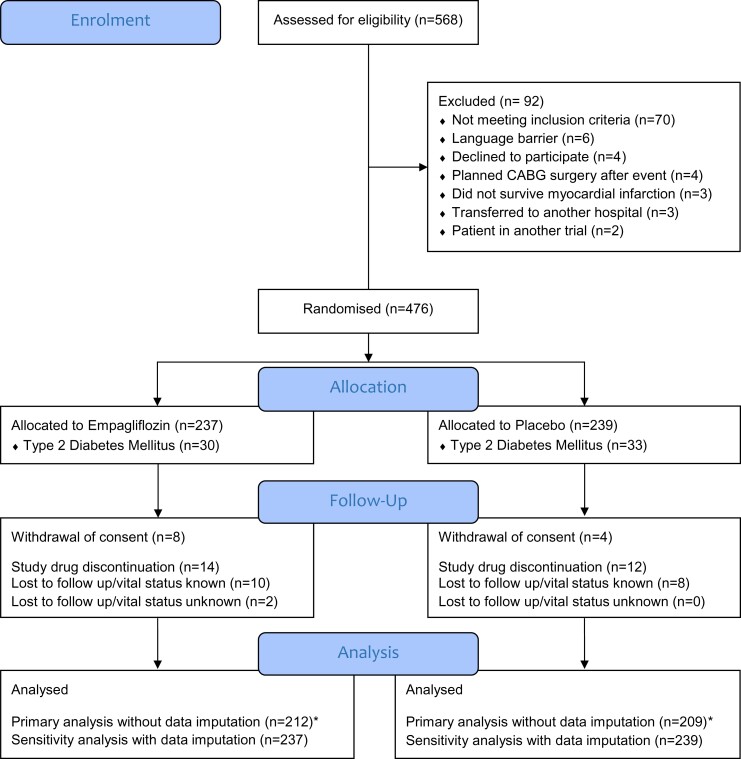
A total of 476 patients were enrolled and randomized to empagliflozin 10 mg/day (n = 237) or matching placebo (n = 239). Twenty-six (5.5%) patients discontinued study medication prematurely (14 empagliflozin, 12 placebo). Twelve (2.5%) participants withdrew informed consent and a total of 20 (4.2%) patients were lost to follow up, with only two patients with unknown vital status at study end (Figure 1). The median age [interquartile range (IQR)] was 57 (52–64) years, body mass index 27.6 kg/m2 (25.1–30.3) with 18% females, and 63 (13%) with established Type 2 diabetes. Baseline characteristics were similar between treatment groups with a median (IQR) baseline creatine kinase of 1673 (1202–2456) IU/L and troponin T of 3039 (2037–4856) ng/L, providing an indirect measure of infarct size (Table 1).
Figure 1.
Patient disposition. *Baseline N-terminal pro-hormone of brain natriuretic peptide and at least one follow-up N-terminal pro-hormone of brain natriuretic peptide measurement at the central laboratory were available.
Table 1.
Participant baseline characteristics
| Characteristic | Overall (n = 476) | Empagliflozin (n = 237) | Placebo (n = 239) | P-valuea |
|---|---|---|---|---|
| Age (years), median (IQR) | 57 (52–64) | 57 (52–64) | 57 (52–65) | 0.78 |
| Male sex, n (%) | 392 (82) | 195 (82) | 197 (82) | 0.97 |
| Body mass index (kg/m2), median (IQR) | 27.6 (25.1–30.3) | 27.7 (25.3–30.3) | 27.2 (24.9–30.2) | 0.20 |
| Systolic blood pressure (mmHg), median (IQR) | 125 (117–131) | 125 (116–131) | 125 (118–131) | 0.21 |
| Diastolic blood pressure (mmHg), median (IQR) | 78 (74–85) | 78 (74–85) | 78 (75–85) | 0.60 |
| Obesity, n (%) | 138 (29) | 68 (29) | 70 (29) | 0.89 |
| Type 2 diabetes, n (%) | 63 (13) | 30 (13) | 33 (14) | 0.71 |
| Hypertension, n (%) | 199 (42) | 92 (39) | 107 (45) | 0.19 |
| Dyslipidaemia, n (%) | 135 (28) | 71 (30) | 64 (27) | 0.44 |
| Smoking (active or former), n (%) | 341 (72) | 171 (72) | 170 (72) | 0.92 |
| Coronary artery disease, n (%) | 53 (11) | 28 (12) | 25 (10) | 0.64 |
| History of stroke, n (%) | 6 (1.3) | 5 (2.1) | 1 (0.4) | 0.12 |
| History of CABG, n (%) | 2 (0.4) | 1 (0.4) | 1 (0.4) | >0.99 |
| History of myocardial infarction, n (%) | 23 (4.8) | 14 (5.9) | 9 (3.8) | 0.28 |
| Depression, n (%) | 24 (5.0) | 15 (6.3) | 9 (3.8) | 0.20 |
| History of carcinoma, n (%) | 24 (5.0) | 11 (4.6) | 13 (5.4) | 0.69 |
| Coronary angiography vessel status | ||||
| 3-vessel disease | 86 (18.1) | 50 (21.1) | 36 (15.0) | 0.08 |
| 2-vessel disease | 162 (34.0) | 82 (34.6) | 80 (33.5) | 0.80 |
| 1-vessel disease | 228 (47.9) | 105 (44.3) | 123 (51.5) | 0.12 |
| Treatment | ||||
| ACE-I/ARB, n (%) | 459 (96) | 228 (96) | 231 (97) | 0.75 |
| ARNI, n (%) | 9 (1.9) | 2 (0.8) | 7 (2.9) | 0.18 |
| Beta-blocker, n (%) | 457 (96) | 223 (94) | 234 (98) | 0.078 |
| MRA, n (%) | 180 (38) | 86 (36) | 94 (39) | 0.54 |
| Loop diuretic, n (%) | 51 (11) | 27 (11) | 24 (10) | 0.61 |
| Statin, n (%) | 462 (97) | 229 (97) | 233 (97) | 0.98 |
| Ezetimibe, n (%) | 59 (12) | 29 (12) | 30 (13) | 0.94 |
| Calcium channel blocker, n (%) | 21 (4.4) | 9 (3.8) | 12 (5.0) | 0.52 |
| Platelet lowering drugs, n (%) | 476 (100) | 237 (100) | 239 (100) | >0.99 |
| Anticoagulation drugs, n (%) | 37 (7.8) | 16 (6.8) | 21 (8.8) | 0.41 |
| Metformin, n (%) | 41 (8.6) | 21 (8.9) | 20 (8.4) | 0.84 |
| DPP4 inhibitor, n (%) | 13 (2.7) | 7 (3.0) | 6 (2.5) | 0.76 |
| Sulfonylurea, n (%) | 4 (0.8) | 2 (0.8) | 2 (0.8) | >0.99 |
| GLP1-RA, n (%) | 4 (0.8) | 2 (0.8) | 2 (0.8) | >0.99 |
| Insulin, n (%) | 11 (2.3) | 5 (2.1) | 6 (2.5) | 0.78 |
| Laboratory parameters | ||||
| NT-proBNP (pg/mL), median (IQR) | 1294 (757–2246) | 1272 (773–2247) | 1373 (754–2217) | 0.91 |
| eGFR (mL/min/1.73 m2), median (IQR) | 92 (78–102) | 92 (78–101) | 91 (78–102) | 0.89 |
| Haemoglobin A1c (%), median (IQR) | 5.60 (5.40–6.00) | 5.60 (5.40–6.00) | 5.70 (5.40–6.00) | 0.87 |
| Creatine kinase (U/L), median (IQR) | 1673 (1202–2456) | 1668 (1136–2532) | 1701 (1254–2404) | 0.71 |
| Troponin T (ng/L), median (IQR) | 3039 (2037–4856) | 3059 (2082–4775) | 3029 (1980–4856) | 0.56 |
| Total cholesterol (mg/dL), median (IQR) | 188 (162–223) | 188 (163–225) | 188 (162–220) | 0.75 |
| LDL-cholesterol, (mg/dL), median (IQR) | 120 (93–149) | 118 (96–150) | 121 (90–145) | 0.82 |
| HDL-cholesterol (mg/dL), median (IQR) | 44 (36–54) | 44 (36–52) | 43 (36–54) | 0.77 |
| Aspartate aminotransferase (IU/L), median (IQR) | 204 (125–322) | 203 (136–328) | 212 (120–320) | 0.67 |
| Alanine aminotransferase (IU/L), median (IQR) | 50 (37–72) | 50 (37–75) | 50 (38–68) | 0.53 |
| Gamma glutamyltransferase (IU/L), median (IQR) | 31 (21–49) | 29 (21–49) | 32 (21–48) | 0.84 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CABG, coronary artery bypass graft; DPP4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP1-RA, glucagon-like peptide-1 receptor agonist; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-hormone of brain natriuretic peptide.
Wilcoxon rank-sum test; Pearson’s χ2 test; Fisher’s exact test.
At randomization, baseline median (IQR) NT-proBNP was 1294 (757–2246) pg/mL, median systolic blood pressure was 125 (117–131) mmHg. Guideline-recommended post-MI medical therapy was initiated before randomization with >96% of patients receiving angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor, beta-blocker and statins, and ∼40% receiving mineralocorticoid receptor antagonists (Figure 1, Table 1).
Primary efficacy outcome
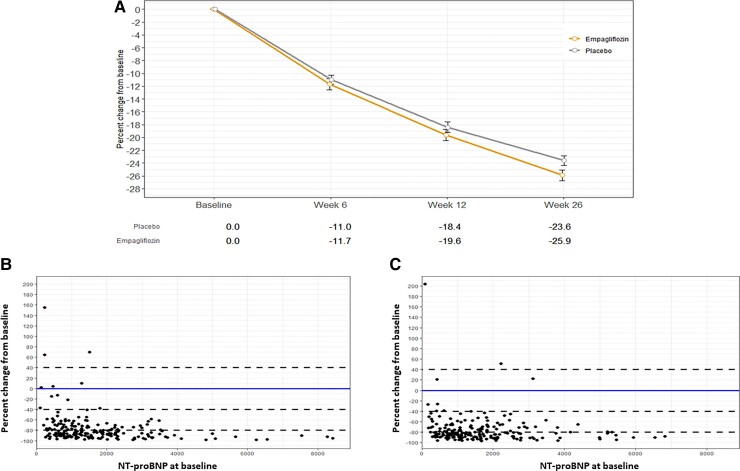
Mean NT-proBNP concentrations decreased in both groups during the study, but to a significantly greater extent in the empagliflozin group compared with placebo. Mean 26-week NT-proBNP was 15% (95% CI −4.4 to −23.6%) lower in the empagliflozin group compared with placebo, after adjusting for baseline NT-proBNP concentration, sex, and diabetes status (P = 0.026). The greater reduction with empagliflozin was already evident by 12 weeks (P = 0.021; Figure 2). The greater NT-proBNP reduction with empagliflozin was confirmed in sensitivity analyses using multiple imputation for missing data (−14.9%; 95% CI −12.5% to −17.3%) with an absolute Week 26 NT-proBNP change of −16.1% (95% CI −2.0% to −28.1%). Figure 2B (empagliflozin group) and 2C (placebo group) demonstrate that the reduction in NT-proBNP is evident across the entire spectrum of baseline NT-proBNP.
Figure 2.
(A) Percentage decline across all visits in N-terminal pro-hormone of brain natriuretic peptide concentration by treatment group (log-transformed data). (B) Percentage decline at Week 26 as function of N-terminal pro-hormone of brain natriuretic peptide at baseline (empagliflozin group). (C) Percentage decline at Week 26 as function of N-terminal pro-hormone of brain natriuretic peptide at baseline (placebo group).
Secondary efficacy and safety outcomes
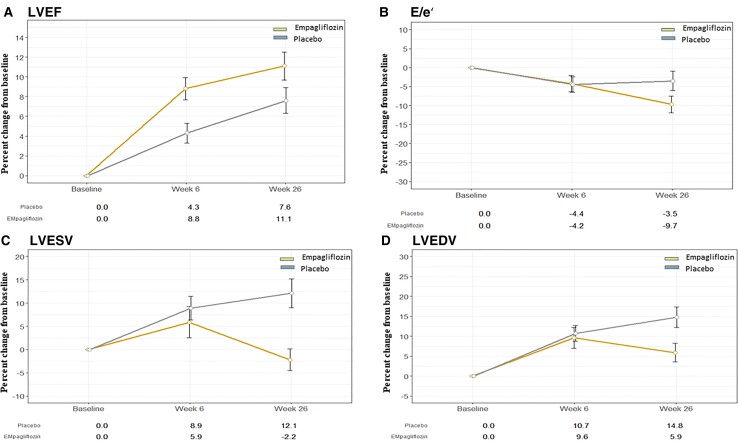
Secondary outcomes are shown in Table 2. Left-ventricular systolic and diastolic function improved in both groups over the course of the trial. Left-ventricular ejection fraction increased by absolute 1.5% (95% CI 0.2–2.9%; P = 0.029) more in the empagliflozin than in the placebo group. The greater increase was already significant by 6 weeks (1.7%, 95% CI 0.35–3.05%; P = 0.014). Left-ventricular diastolic function, as assessed by E/e′, also changed during the trial with significantly greater improvement in the empagliflozin group at 26 weeks, being 6.8% (95% CI 1.3–11.3%, P = 0.015) lower compared with placebo (Figure 3A and B).
Table 2.
Secondary echocardiography outcome parameters
| Empagliflozin | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 26 | Absolute change | Per cent change | Baseline | Week 26 | Absolute change | Per cent change | Difference between empagliflozin and placebo at Week 26a | |
| LVEF (%) | 53 (47–58) | 4.7 (3.6; 5.8) | 11.1 (8.6; 13.6) | 49 (43–54) | 52 (47–57) | 2.8 (1.8; 3.9) | 7.6 (5.2; 9.9) | 1.5% (95% CI: 0.2–2.9%; P = 0.029) | |
| E/e′ | 8 (7–11) | 8 (7–9) | −1.3 (−1.6; −0.9) | −9.7 (−13.1; −6.4) | 9 (8–11) | 8 (7–10) | −0.7 (−1.1, −0.4) | −3.5 (−7.4; 0.4) | −6.8% (95% CI: −11.3% to −1.3%, P = 0.015) |
| LVESV (mL) | 61 (48–76) | 60 (46–73) | −3.6 (−6.3; −1.0) | −2.2 (−6.4; 2.0) | 60 (46–73) | 59 (46–81) | 4.30 (1.2; 7.4) | 12.1 (6.4; 17.7) | −7.5 mL (95% CI: −11.5 to −3.4, P = 0.0003) |
| LVESV/BSA (mL/m2) | 30 (25–37) | 29 (23–38) | −1.1 (−2.5; 0.2) | −0.3 (−4.6; 4.0) | 29 (23–36) | 30 (23–40) | 2.3 (0.7; 3.9) | 13.1 (7.3; 18.9) | −3.2 mL/m2 (95% CI: −5.2 to −1.2, P = 0.002) |
| LVEDV (mL) | 119 (93–139) | 122 (101–145) | 3.4 (−0.7; 7.4) | 5.9 (1.8; 10.1) | 114 (92–134) | 120 (100–154) | 13.5 (8.7; 18.3) | 14.8 (10.2; 19.4) | −9.7 mL (95% CI: −15.7 to −3.7, P = 0.0015) |
| LVEDV/BSA (mL/m2) | 58 (48–68) | 61 (51–73) | 3.0 (0.9; 5.1) | 8.1 (3.8; 12.4) | 56 (48–65) | 61 (51–76) | 7.1 (4.7; 9.5) | 16.0 (11.1; 20.8) | −3.9 mL/m2 (95% CI: −6.9 to −0.8, P = 0.012) |
LVEF, left-ventricular ejection fraction; LVESD, left-ventricular end-systolic volume; LVEDV, left-ventricular end-diastolic volume; BSA, body surface area (square root ((height (cm)×weight (kg))/3600) baseline and Week 26 values are given as median (interquartile range), absolute, and percentage change as mean (95% confidence interval of the mean).
Analysed using linear mixed effect model adjusted for baseline level at visit 1, sex, and diabetes status. The data for E/e′ was log-transformed for reasons of non-normality. The value of delta should be expressed for this marker as a per cent change. For the other markers, it should be expressed as an absolute change.
Figure 3.
Changes in echocardiographic parameters by treatment group: (A) left-ventricular ejection fraction, (B) E/e′, (C) left-ventricular end-systolic volume, and (D) left-ventricular end-diastolic volume.
Echocardiographic parameters reflecting structural cardiac changes were significantly improved in the empagliflozin group compared with the placebo group: LVESV (−7.5 mL; 95% CI −11.5 to −3.4 mL, P = 0.0003) and LVEDV (−9.7 mL; 95% CI −15.7 to −3.7 mL, P = 0.0015) were smaller in the empagliflozin group compared with the placebo group (Figure 3C and D).
Ketone body (beta-hydroxybutyrate) concentrations showed a significantly greater increase in the empagliflozin group, compared with placebo (Δ = 23.4%; 95% CI 5.9–42.4%, P = 0.0066), that was more pronounced at 26 weeks (Δ = 41.9%; 95% CI 21.8–63.8%, P < 0.0001). Body weight decreased more in the empagliflozin group (Δ = −1.76 kg; 95% CI −3.27 to −0.25 kg, P = 0.022). Within the small subgroup of participants with diabetes, there was no significant between-group difference in the degree of haemoglobin A1c lowering at Week 26 (P = 0.11).
Duration of hospital stay due to acute MI did not differ between groups with a median (IQR) duration of 6.0 (3–9) days in the empagliflozin and 6.0 (3–9) days in the placebo group (P = 0.40).
Adverse events
Serious adverse event rates did not differ between the empagliflozin and the placebo groups. There were a total of 72 SAEs with 63 participants hospitalized, out of which seven participants were hospitalized for heart failure (three in the empagliflozin group, four in placebo group). Three deaths occurred during the study, all in the empagliflozin group. Two participants died within 5 days after enrolment in the trial secondary to large MIs and subsequent cardiogenic shock. One participant died 149 days after enrolment due to lung cancer. All three fatalities were considered by the adjudication committee prior to unblinding to be unrelated to study medication. Other safety endpoints such as the number of genital infections also did not differ significantly between the empagliflozin and placebo groups. Moreover, no amputations, no ketoacidosis, and no severe hypoglycaemic episodes were reported throughout the follow up (Table 3).
Table 3.
Adverse events
| Total | Empagliflozin | Placebo | |
|---|---|---|---|
| Serious adverse events | |||
| Death | 3 | 3 | 0 |
| Non-cardiovascular death | 1 | 1 | 0 |
| Death from cardiovascular cause | 2 | 2 | 0 |
| Any hospitalization | 63 (69) | 31 (35) | 32 (34) |
| Hospitalization due to heart failure | 7 (10) | 3 (6) | 4 (4) |
| Hospitalization due to cardiovascular event | 7 (7) | 2 (2) | 5 (5) |
| Adverse events of special interest | |||
| Hepatic injury | 2 | 1 | 1 |
| Renal injury | 0 | 0 | 0 |
| Metabolic acidosis and diabetic ketoacidosis | 0 | 0 | 0 |
| Event involving lower limb amputation | 0 | 0 | 0 |
| Other adverse events | |||
| Urinary tract infection | 18 (26) | 11 (18) | 7 (8) |
| Genital fungal infection | 9 (9) | 7 (7) | 2 (2) |
Given numbers are participants with adverse events (number of events). Renal injury: >two-fold increase creatinine. Hepatic injury: AST/ALT ≥three-fold ULN with elevation of total bilirubin ≥two-fold ULN or AST/ALT elevation ≥five-fold ULN.
Discussion
The EMMY trial evaluated for the first time the efficacy and safety of empagliflozin therapy when initiated within 72 h after PCI for a large acute MI. Early initiation of empagliflozin, given in addition to established guideline-recommended post-MI therapy, led to a greater reduction in median NT-proBNP levels compared with placebo without clinically relevant adverse events (Structured Graphical Abstract).
The NT-proBNP is a well-established biomarker of neurohormonal activation, haemodynamic stress, and subsequent cardiovascular events. The substantial decline in NT-proBNP concentrations which occurs over time following large MI21–23 is a robust predictor of subsequent cardiovascular outcomes. NT-proBNP trajectories within days,13,24 weeks,21,25–27 and months22,23 after a MI further increase the prognostic value of baseline NT-proBNP concentrations collected during the index event.
The only data available concerning the effect of SGLT2i on post-MI NT-proBNP concentrations derives from the EMBODY trial which analysed the impact of empagliflozin treatment on post-MI sympathomimetic activity. This trial reported a decline of NT-proBNP concentrations in the empagliflozin and the placebo group. However, no significant between-group difference was reported, potentially due to the rather small number of participants and the later treatment initiation compared with EMMY.28
The effect of SGLT2i on NT-proBNP concentrations in heart failure trials is heterogeneous within different cohorts with reductions,29 a moderate decline,30 or no significant reduction compared with placebo despite significant improvement in left-ventricular mass as observed in the EMPA-HEART trial.31 Absolute NT-proBNP concentrations did not significantly differed in EMPEROR-Preserved when analysed after 52 weeks,6 but a recent analysis depicted 13% significantly lower NT-proBNP concentrations in the empagliflozin group after 52 weeks when adjusted geometric mean NT-proBNP values were calculated.32 This modest NT-proBNP difference, however, was associated with a highly significant improvement in the primary clinical outcome. Only ∼10% of the HHF reduction has been attributed to the NT-proBNP lowering seen with canagliflozin in the CANVAS trial.29 In line with this finding, recent meta-analyses have shown highly significant reductions in HHF for HFrEF33 and for HFpEF34 despite only moderate and non-significant larger NT-proBNP reductions with SGLT2i treatment. Given the beneficial effects on NT-proBNP concentrations in combination with functional (LVEF, diastolic function) and structural (LVESV, LVEDV) improvements seen in the EMMY trial, established SGLT2i clinical benefits might be even more pronounced after a large MI. The EMMY trial was not powered for hard clinical endpoints but there are two large outcome trials currently ongoing (EMPACT-MI and DAPA-MI) which may provide definitive data. In EMMY, the beneficial effect of empagliflozin on NT-proBNP concentrations was accompanied by a greater increase in LVEF, compared with placebo.
The degree of LVEF recovery in the weeks after a MI has been shown to complement and out-perform baseline LVEF alone when providing prognostic information such as risk of sudden cardiac death and all-cause mortality.35,36 LVEF trajectories separated early in the EMMY trial with the mean increase in the empagliflozin group being twice the size compared with the placebo group by 6 weeks (+8.8 vs. +4.3%). The absolute ∼1.5% difference in the 26-week LVEF change seen in the EMMY trial is comparable with a recent analysis of the BEST trial37 which observed an average LVEF of 4.5 units (%) after 12 months. This analysis compared heart failure patients with LVEF improvement ≥5 units to all other patients and described a significantly better outcome in all endpoints analysed ranging from HHF to all-cause mortality for those with greater LVEF recovery. These differences were independent of the treatment group (bucindolol or placebo). Data in post-MI patients reveal comparable or even more favourable outcome in patients with LVEF recovery compared with those with unaltered LVEF at baseline, whereas those patients without LVEF recovery have significantly worse outcomes.38,39 The highly significant prognostic value of LVEF recovery within the first 6 months has been confirmed in a cohort with >10 years of follow up.40 Thus, differences in LVEF changes, as observed in the EMMY trial with empagliflozin, suggest there may well be beneficial effects on clinical outcomes.
Diastolic function also improved in EMMY, in line with data showing SGLT2i to be the first pharmacological treatment to improve prognosis in HFpEF. This finding is further supported by the smaller increases in left-ventricular volumes seen following MI in the empagliflozin group. Thus, biomarker as well as functional and structural outcome data in the EMMY trial point towards a potential positive impact on clinical outcomes.
Increases in circulating ketone levels and ketone oxidation with SGLT2i have been suggested to improve cardiac efficiency and/or the energy supply in energy starved myocytes in heart failure.9,41,42 Beta-hydroxybutyrate, the commonest ketone body, was significantly increased in the empagliflozin, compared with the placebo group, in EMMY after 12 and 26 weeks.
Beta-hydroxybutyrate has been demonstrated to be increased substantially in the very early phase of a MI and to decrease rapidly within the first 24 h.41 Initial high beta-hydroxybutyrate concentrations were not prognostic but increased levels 24 h after a MI seem to be associated with an adverse impact on infarct size and remodelling within the first 24 weeks, which might be attributed to prolonged elevated sympathomimetic activation in these patients.41 In contrast, beta-hydroxybutyrate infusions of 3 h duration significantly increased left-ventricular function.43 Moreover, beta-hydroxybutyrate is an effective blocker of NOD-like receptor protein 3–mediated inflammatory processes.44 This mechanism seems to be centrally involved in HFpEF development which recently has been shown to be ameliorated in various rodent models, including post-MI models, either by direct ketone ester treatment or by empagliflozin therapy.45,46 Hence, in contrast to endogenously increased beta-hydroxybutyrate concentrations seen in the acute ischaemic phase, therapeutic application of beta-hydroxybutyrate might have a role in stress defence mechanism by ameliorating pathologic cardiac remodelling.47 The frequency of blood sampling in EMMY precludes a detailed study of the post-MI role of beta-hydroxybutyrate, but the concentrations observed during the follow up were significantly higher in those treated with empagliflozin. This EMMY observation strengthens the hypothesis regarding the role of SGLT2i in ketone body-dependent improvements in cardiovascular outcome.
The EMMY results extend the evidence base regarding the use of empagliflozin to post-MI populations for which data have not yet been available.
Strengths and limitations of the study
The EMMY is the first trial to present data on early SGLT2i treatment after a large MI, predominately in patients without established diabetes. A smaller previous trial in Japan was limited to patients with diabetes, initiated SGLT2 inhibition after the acute phase, and focussed on sympathetic activity.28 EMMY demonstrates the significant benefit of SGLT2i with respect to heart failure markers as well as left-ventricular functional and structural parameters in the trial population. Empagliflozin was shown to have beneficial effects, despite optimal guideline post-MI treatment with EMMY providing safety data in the cohort of 474 participants out of the 476 randomized (only two participants were lost to follow up without known vital status).
However, the sample size in this investigator-initiated trial was insufficient to power it for hard clinical endpoints. Large CVOTs are of particular importance in providing definitive data for patients with acute MI, as for example, positive outcome data in heart failure trials did not necessarily translate into positive outcomes in post-MI trials, as observed in PARADIGM-HF48 and PARADISE-MI,49 although those undergoing PCI during the index event in PARADISE-MI (the population enrolled in EMMY) seemed to benefit from angiotensin receptor–neprilysin inhibition. The role of SGLT2i in acute MI patients will be clarified when the robust outcome data from the two ongoing SGLT2i CVOTs [EMPACT-MI (NCT04509674) and DAPA-MI (NCT04564742)], which are powered for differences in the composite outcome of HHF and CV or all-cause mortality, are reported.
In EMMY, the proportions of female patients and those with diabetes were lower than anticipated. Of note, patients with diabetes more often did not achieve the >800 IU/L creatine kinase threshold. For this analysis, we used locally performed and analysed echocardiography data but loop recordings are available in a substantial subgroup of participants which will be looked at in subsequent analyses.
Conclusion
Among patients who were hospitalized with an acute large MI, early initiation of empagliflozin given in addition to guideline-recommended post-MI treatment resulted in a significantly greater median NT-proBNP reduction than with placebo over 26 weeks. There were no significant differences with regard to safety endpoints such as hospitalization, alterations of glucose metabolism, renal, or liver function.
Multicentre trial performed on following sites: University Hospital Graz; Hospital Klagenfurt am Woerthersee; Paracelsus Medical Private University of Salzburg; Hospital Landstrasse Vienna; Medical University of Vienna/Vienna General Hospital (AKH); Kardinal Schwarzenberg Hospital Schwarzach; Academic Teaching Hospital Feldkirch/Vorarlberg Institute for Vascular Investigation and Treatment; Kepler University Hospital Linz; Brothers of Saint John of God Eisenstadt; Hospital Graz South West, West Location; University Hospital St Pölten.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank all study participants for their dedication and adherence to the trial protocol. R.R.H. is an emeritus National Institute for Health and Care Research (NIHR) Research Senior Investigator. D.M. and P.A. acknowledge the contribution of NÖ Landesgesundheitsagentur, legal entity of University Hospitals in Lower Austria, for providing the organizational framework to conduct this research. H.S. is supported the Austrian Science Fund (FWF) grants KLIF-851-B and KLIF-1076-B. We would like to thank Tatjana Stojakovic and Hubert Scharnagl for their support in analysing the lab samples.
Contributor Information
Dirk von Lewinski, Department of Internal Medicine, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Ewald Kolesnik, Department of Internal Medicine, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Norbert J Tripolt, Department of Internal Medicine, Division of Endocrinology and Diabetology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria; Interdisciplinary Metabolic Medicine Trials Unit, Medical University of Graz, Graz, Austria.
Peter N Pferschy, Department of Internal Medicine, Division of Endocrinology and Diabetology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria; Interdisciplinary Metabolic Medicine Trials Unit, Medical University of Graz, Graz, Austria.
Martin Benedikt, Department of Internal Medicine, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Markus Wallner, Department of Internal Medicine, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Hannes Alber, Department of Cardiology, Public Hospital Klagenfurt am Woerthersee, Klagenfurt am Woerthersee, Austria.
Rudolf Berger, Department of Internal Medicine, Brothers of Saint John of God Eisenstadt, Eisenstadt, Austria.
Michael Lichtenauer, Department of Internal Medicine II, Division of Cardiology and Internal Intensive Care Medicine, Paracelsus Medical Private University Salzburg, Salzburg, Austria.
Christoph H Saely, Vorarlberg Institute for Vascular Investigation and Treatment (VIVIT), Feldkirch, Austria.
Deddo Moertl, Karl Landsteiner University of Health Sciences, 3050 Krems, Austria; Department of Internal Medicine 3, University Hospital St. Poelten, 3100 St. Poelten, Austria.
Pia Auersperg, Karl Landsteiner University of Health Sciences, 3050 Krems, Austria; Department of Internal Medicine 3, University Hospital St. Poelten, 3100 St. Poelten, Austria.
Christian Reiter, Department of Cardiology and Intensive Care Medicine, Kepler University Hospital Linz, Linz, Austria.
Thomas Rieder, Department of Medicine, Kardinal Schwarzenberg Hospital Schwarzach, Schwarzach, Austria.
Jolanta M Siller-Matula, Department of Cardiology, Medical University of Vienna, Vienna, Austria.
Gloria M Gager, Department of Cardiology, Medical University of Vienna, Vienna, Austria.
Matthias Hasun, 2nd Medical Department with Cardiology and Intensive Care Medicine, Hospital Landstrasse, Vienna, Austria.
Franz Weidinger, 2nd Medical Department with Cardiology and Intensive Care Medicine, Hospital Landstrasse, Vienna, Austria.
Thomas R Pieber, Department of Internal Medicine, Division of Endocrinology and Diabetology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Peter M Zechner, Department of Cardiology and Intensive Care Medicine, Hospital Graz South West, West Location, Graz, Austria.
Markus Herrmann, Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria.
Andreas Zirlik, Department of Internal Medicine, Division of Cardiology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria.
Rury R Holman, Radcliffe Department of Medicine, University of Oxford, Oxford, UK.
Abderrahim Oulhaj, Department of Epidemiology and Population Health, College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, UAE; Research and Data Intelligence Support Center, Khalifa University, Abu Dhabi, UAE.
Harald Sourij, Department of Internal Medicine, Division of Endocrinology and Diabetology, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria; Interdisciplinary Metabolic Medicine Trials Unit, Medical University of Graz, Graz, Austria.
Funding
The study was funded by an unrestricted grant of Boehringer Ingelheim (no. 1245.151). NT-proBNP Elecsys kits were kindly provided by Roche Diagnostics.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. . SGLT2 Inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 4. Gager GM, Gelbenegger G, Jilma B, von Lewinski D, Sourij H, Eyileten C, et al. . Cardiovascular outcome in patients treated with SGLT2 inhibitors for heart failure: a meta-analysis. Front Cardiovasc Med 2021;8:691907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. . The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. . 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263–e421. [DOI] [PubMed] [Google Scholar]
- 9. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 2020;5:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Ropero A, Santos-Gallego CG, Badimon JJ. The anti-inflammatory effects of SGLT inhibitors. Aging (Albany NY) 2019;11:5866–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baartscheer A, Schumacher CA, Wust RC, Fiolet JW, Stienen GJ, Coronel R, et al. . Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 2017;60:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye Y, Jia X, Bajaj M, Birnbaum Y. Dapagliflozin attenuates Na(+)/H(+) exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther 2018;32:553–558. [DOI] [PubMed] [Google Scholar]
- 13. Carvalho LSF, Bogniotti LAC, de Almeida OLR, E Silva JCQ, Nadruz W, Coelho OR, et al. . Change of BNP between admission and discharge after ST-elevation myocardial infarction (Killip I) improves risk prediction of heart failure, death, and recurrent myocardial infarction compared to single isolated measurement in addition to the GRACE score. Eur Heart J Acute Cardiovasc Care 2019;8:643–651. [DOI] [PubMed] [Google Scholar]
- 14. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 15. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2017;39:119–177. [DOI] [PubMed] [Google Scholar]
- 16. Tripolt NJ, Kolesnik E, Pferschy PN, Verheyen N, Ablasser K, Sailer S, et al. . Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction-The EMMY trial. Am Heart J 2020;221:39–47. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 18. Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2009;54:357–364. [DOI] [PubMed] [Google Scholar]
- 19. Koller M. Robustlmm: an R package for robust estimation of linear mixed-effects models. J Stat Softw 2016;75:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mason F, Cantoni E, Ghisletta P. Parametric and semi-parametric bootstrap-based confidence intervals for robust linear mixed models. Methodology 2021;17:271–295. [Google Scholar]
- 21. Olivier A, Girerd N, Michel JB, Ketelslegers JM, Fay R, Vincent J, et al. . Combined baseline and one-month changes in big endothelin-1 and brain natriuretic peptide plasma concentrations predict clinical outcomes in patients with left ventricular dysfunction after acute myocardial infarction: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) study. Int J Cardiol 2017;241:344–350. [DOI] [PubMed] [Google Scholar]
- 22. Lee JW, Choi E, Khanam SS, Son JW, Youn YJ, Ahn MS, et al. . Prognostic value of short-term follow-up B-type natriuretic peptide levels after hospital discharge in patients with acute myocardial infarction. Int J Cardiol 2019;289:19–23. [DOI] [PubMed] [Google Scholar]
- 23. Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, et al. . Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA 2005;294:2866–2871. [DOI] [PubMed] [Google Scholar]
- 24. Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD. Platelet receptor inhibition in ischemic syndrome management I. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004;110:3206–3212. [DOI] [PubMed] [Google Scholar]
- 25. Gustafsson F, Steensgaard-Hansen F, Badskjaer J, Poulsen AH, Corell P, Hildebrandt P. Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. J Card Fail 2005;11:S15–S20. [DOI] [PubMed] [Google Scholar]
- 26. Squire IB, Orn S, Ng LL, Manhenke C, Shipley L, Aarsland T, et al. . Plasma natriuretic peptides up to 2 years after acute myocardial infarction and relation to prognosis: an OPTIMAAL substudy. J Card Fail 2005;11:492–497. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki S, Yoshimura M, Nakayama M, Mizuno Y, Harada E, Ito T, et al. . Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long-term follow-up analysis. Circulation 2004;110:1387–1391. [DOI] [PubMed] [Google Scholar]
- 28. Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, et al. . Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 2020;19:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Januzzi JL J, Xu J, Li J, Shaw W, Oh R, Pfeifer M, et al. . Effects of canagliflozin on amino-terminal pro-B-type natriuretic peptide: implications for cardiovascular risk reduction. J Am Coll Cardiol 2020;76:2076–2085. [DOI] [PubMed] [Google Scholar]
- 30. Januzzi JL Jr, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. . Prognostic importance of NT-proBNP and effect of empagliflozin in the EMPEROR-reduced trial. J Am Coll Cardiol 2021;78:1321–1332. [DOI] [PubMed] [Google Scholar]
- 31. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. . Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes Mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 32. Januzzi JL Jr, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. . Prognostic implications of N-terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin T in EMPEROR-preserved. JACC Heart Fail 2022;10:512–524. [DOI] [PubMed] [Google Scholar]
- 33. Chambergo-Michilot D, Tauma-Arrue A, Loli-Guevara S. Effects and safety of SGLT2 inhibitors compared to placebo in patients with heart failure: a systematic review and meta-analysis. Int J Cardiol Heart Vasc 2021;32:100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou H, Peng W, Li F, Wang Y, Wang B, Ding Y, et al. . Effect of sodium-glucose cotransporter 2 inhibitors for heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized clinical trials. Front Cardiovasc Med 2022;9:875327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chew DS, Wilton SB, Kavanagh K, Southern DA, Tan-Mesiatowsky LE, Exner DV. Left ventricular ejection fraction reassessment post-myocardial infarction: current clinical practice and determinants of adverse remodeling. Am Heart J 2018;198:91–96. [DOI] [PubMed] [Google Scholar]
- 36. Chew DS, Heikki H, Schmidt G, Kavanagh KM, Dommasch M, Bloch Thomsen PE, et al. . Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol 2018;4:672–682. [DOI] [PubMed] [Google Scholar]
- 37. Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail 2016;9:e002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lei Z, Li B, Li B, Peng W. Predictors and prognostic impact of left ventricular ejection fraction trajectories in patients with ST-segment elevation myocardial infarction. Aging Clin Exp Res 2022;34:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otero-García O, Cid-Álvarez AB, Juskova M, Álvarez-Álvarez B, Tasende-Rey P, Gude-Sampedro F, et al. . Prognostic impact of left ventricular ejection fraction recovery in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: analysis of an 11-year all-comers registry. Eur Heart J Acute Cardiovasc Care 2021;10:898–908. [DOI] [PubMed] [Google Scholar]
- 40. Wu WY, Biery DW, Singh A, Divakaran S, Berman AN, Ayuba G, et al. . Recovery of left ventricular systolic function and clinical outcomes in young adults with myocardial infarction. J Am Coll Cardiol 2020;75:2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Koning MLY, Westenbrink BD, Assa S, Garcia E, Connelly MA, van Veldhuisen DJ, et al. . Association of circulating ketone bodies with functional outcomes after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2021;78:1421–1432. [DOI] [PubMed] [Google Scholar]
- 42. Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, et al. . Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res 2021;117:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. . Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. . The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, et al. . Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res 2021;128:232–245. [DOI] [PubMed] [Google Scholar]
- 46. Yurista SR, Matsuura TR, Silljé HHW, Nijholt KT, McDaid KS, Shewale SV, et al. . Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail 2021;14:e007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, et al. . The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 49. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, et al. . Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. N Engl J Med 2021;385:1845–1855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.