Abstract
Etretinate, an acitretin metabolite, has a long retention duration in adipose tissues with a teratogenic potential. FDA advises a contraceptive period of at least three years after discontinuing acitretin. However, the effect of accumulated etretinate in adipose tissues on fetus is unknown. Although the teratogenic threshold for serum concentration of etretinate has been presented as higher than 2 ng/mL, that of acitretin is unknown. To examine factors affecting body retention of acitretin and etretinate, effects of acitretin dosage, acitretin-taking duration, elapsed time after stopping acitretin, age, sex, concomitant alcohol consumption, and foods and supplements rich in vitamin A intake on serum concentrations of acitretin and etretinate were analyzed in 14 acitretin-taken patients and 58 controls without taking acitretin or etretinate. Serum concentrations of acitretin, but not etretinate, tended to be inversely related to the discontinuation duration. They were also related to old age. Different from a published result that alcohol consumption could promote the metabolism of acitretin into etretinate, alcohol intake did not affect serum concentrations of etretinate. Unexpectedly, more frequent intake of vitamin A or provitamin A-rich food and supplements was associated with higher serum acitretin, whereas less frequent intake of vitamin A or provitamin A-rich food and supplements was associated with higher serum levels of etretinate in acitretin-taken patients. Despite preliminary data, inter-individual variations in serum retention of etretinate suggest the necessity of further research before applying the same guidelines to everyone to minimize unnecessary contraception.
Keywords: Acitretin/etretinate, Teratogenicity, Detectable serum concentration, Discontinuation duration, Age, Vitamin A- or provitamin A-rich food and supplements
INTRODUCTION
Acitretin and etretinate have been used to treat various skin diseases such as psoriasis and other hyperkeratotic disorders (Dunn et al., 2011; Lynde et al., 2011; Sarkar et al., 2013; Guenther et al., 2017). Etretinate has a half-life of approximately 120 days. It accumulates in adipose tissues up to three years after drug discontinuation (Larsen et al., 1988, 1991; Bouvy et al., 1992; Sturkenboom et al., 1994). Compared to etretinate, acitretin has a shorter half-life of approximately 60 h without accumulation in adipose tissues (Bouvy et al., 1992; Meyer et al., 1993). Due to its shorter elimination period, acitretin has replaced etretinate in the United States (US) and Europe. Although acitretin is a metabolite of etretinate, reverse metabolism of acitretin to etretinate occurs, which can be increased by alcohol consumption (Supplementary Fig. 1) (Bouvy et al., 1992; Larsen et al., 1993, 2000). Because of fetal teratogenic potential of both acitretin and etretinate, the Food and Drug Administration (FDA) prohibits their administration to individuals of childbearing age. The duration of contraception has been determined based on the detection of these drugs in adipose tissues, extending the period to avoid pregnancy post therapy to two years in Europe and three years in the US (Larsen et al., 2000). Based on the FDA guidelines, the Korea Food Drug Administration (KFDA) also recommends contraception up to three years after discontinuation of these drugs (Lynde et al., 2011).
Safety is absolutely crucial; however, contraception for three years may be a long duration for women of childbearing age. The contraception period has been determined based on the duration of drug accumulation in adipose tissues. Etretinate monitoring in subcutaneous fat even after stopping acitretin has been proposed based on the detection of higher concentrations of etretinate in subcutaneous fat in patients without detectable etretinate in their sera (Sturkenboom et al., 1994). However, it is not clear how accumulated etretinate and acitretin in adipose tissues can affect the fetus. Although lipophilic etretinate may be released from body fat due to weight loss, similar to other lipophilic agents stored in body fat (DiGiovanna et al., 1989), no correlation between excess body weight and the rate of etretinate biotransformation has been explained (Pilkington and Brogden, 1992; Maier and Hönigsmann, 2001; Kara Polat et al., 2021). Moreover, there are no teratogenic data available for etretinate at blood levels of less than 2 ng/mL. A high risk of spontaneous abortion or congenital malformation has been reported in humans after administration of etretinate or acitretin during the first trimester of pregnancy (Grote et al., 1985; Geiger et al., 1994; de Die-Smulders et al., 1995; Barbero et al., 2004). A 25-year retrospective analysis showed delivery of a normal baby one year after etretinate discontinuation (Katugampola and Finlay, 2006).
It may not be reasonable to apply the same regulations to all women because considerable individual variations in etretinate elimination have been suggested (DiGiovanna et al., 1989; Larsen et al., 1993, 2000). Drug information provided by the KFDA recommends the administration of acitretin once a day at a dose of 25-50 mg/day, with a maximum dose of 75 mg/day, which may be suitable for Western population. However, optimal dosing of acitretin has been proposed previously through a dose-escalation strategy with initiation at low doses (10 to 25 mg/day) due to individual variations in both efficacy and safety (Ling, 1999). Koreans usually take less than one-third dose, such as 10-20 mg/day, to reduce severe dryness of the lips and skin, which is a common acitretin-induced adverse drug reaction (Pilkington and Brogden, 1992; Lee and Koo, 2005; Kara Polat et al., 2021). Thus, this study examined several factors that influence the retention of acitretin and etretinate in the body, particularly in the serum.
MATERIALS AND METHODS
Participants
Fourteen patients aged more than 15 years who had received acitretin at least once between January 2016 and April 2020 at the Department of Dermatology, Dongguk University Ilsan Hospital (Goyang, Korea) were included. Data such as age, sex, alcohol intake, daily dosage and duration of acitretin, and discontinuation duration of acitretin were collected using patients’ electronic medical records. Fifty-seven subjects aged over 18 years who had never received acitretin and one of the 14 patients before receiving acitretin were included as controls. All participants (either patients or controls) filled out a questionnaire regarding the intake of vitamin A- and provitamin A-rich foods or supplements (Supplementary Table 1). The Institutional Review Board (IRB) of Dongguk University Ilsan Hospital approved this study (approval number: DUIH 2019-08-026). This study was conducted after obtaining written informed consent of all participants according to the principles of the Declaration of Helsinki.
Food questionnaire
All participants answered the questionnaire, which consisted of questions about the frequency of intake of vitamin A- or provitamin A-rich foods or vitamin A supplements (Supplementary Table 1). Foods rich in vitamin A include cow liver, bluefin tuna, butter, cream cheese, salmon, trout, and boiled eggs. Foods rich in provitamin A include cooked sweet potato, cooked carrot, cooked kale, spinach, Romaine lettuce, Swiss chard, and melon. The frequency was assessed as every day, every two days, semi-weekly, weekly, or less than once a week.
Detection of acitretin or etretinate in serum
Detection of acitretin or etretinate in serum was done for a total of 72 sera samples from 14 patients who had received acitretin, one of the 14 patients before receiving acitretin, and 57 controls who had never taken acitretin. One of the 14 patients was also examined for the presence of these drugs in adipose tissues. All samples were frozen and transferred to the College of Pharmacy, Seoul National University (Seoul, Korea).
All frozen serum samples were thawed in ice-cold water. Five μL of isotretinoin (IS) stock solution (20 μg/mL isotretinoin) was added to an aliquot of 95 μL serum sample through uniform vortexing. Acetonitrile (100 μL) was then added to each sample and vortexed for 1 min. Next, 800 μL of methyl tert-butyl ether was added, and the mixture was vortexed for 1 min. The samples were then centrifuged at 13,000×g for 30 min at 4°C, and supernatants were collected and evaporated under a stream of nitrogen gas (15 psi) at ambient temperature. Each dried sample was reconstituted with 50 μL of methanol. Subsequently, 5 μL of each sample was introduced into high performance liquid chromatography-triple quadrupole mass spectrometry (HPLC-QqQ-MS) system (Supplementary Fig. 2).
Agilent 1260 HPLC coupled with Agilent 6460 QqQ-MS was used for the analysis (Kumar et al., 2011; Sharma et al., 2012). Chromatographic separation was achieved using Agilent Eclipse Plus C18 column (4.6 mm×150 mm, 5 μm). The temperature of the column oven was maintained at 30°C during analysis. The mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B), at a flow rate of 1.0 mL/min. The gradient elution was programmed as follows: 0-1 min, 75% B; 1-6 min, 75-100% B; and 6-8 min, 100% B. Source conditions of electrospray ionization (ESI) were as follows: gas temperature=325°C, gas flow=11 L/min, nebulizer=25 psi, capillary voltage=3,500 V, and nozzle voltage=1,000 V. For detection purposes, positive ESI mode was used with multiple reaction monitoring (MRM) mode to determine targets in samples (Supplementary Table 2). Optimized m/z ion transitions were monitored at 327→159 for acitretin, 355→309 for etretinate, and 301→123 for IS (Fig. 1). This method for the detection of acitretin or etretinate has been reported to be accurate and reliable (Kumar et al., 2011; Sharma et al., 2012), without showing any detection of other vitamin A, including alitretinoin, as shown in controls (Table 1).
Fig. 1.
Identification of acitretin and etretinate by fragmentation patterns in mass spectrometry and elution time on liquid chromatography column. Acitretin (A) and etretinate (B) in samples were identified based on characteristic fragmentation patterns in the collision cell of mass spectrometer. Moreover, acitretin and etretinate in the serum were well separated (4.32 min and 7.61 min) in reversed-phase HPLC column (C).
Table 1.
Demographics in 72 subjects
| Characteristics | Patients (n) | Controls (n) |
|---|---|---|
| Total (n) | 14 | 58 |
| Sex (n) | ||
| Male | 8 (57.1%) | 26 (44.8%) |
| Female | 6 (42.9%) | 32 (55.2%) |
| Age, mean (range) | 50.9 (16-74) | 36.7 (20-57) |
| Diagnosis | ||
| Psoriasis | 9 | - |
| Porokeratosis | 1 | - |
| PPP* | 1 | - |
| Hand eczema | 1 | - |
| PRP** | 1 | - |
| CARP*** | 1 | - |
| Vitamin A- or provitamin A-rich food intake | ||
| Daily | 0 | 5 |
| 3-4/week | 0 | 7 |
| 2/week | 5 | 13 |
| 1/week | 9 | 20 |
| <1/week | 0 | 13 |
| Vitamin A supplements intake | 0 | 7 |
| Other oral retinoid (alitretinoin) medication | 0 | 1 |
PPP*, Palmoplantar pustulosis; PRP**, Pityriasis rubra pilaris; CARP***, Confluent and reticulated papillomatosis.
Data analysis
Effect of acitretin administration on serum concentrations of acitretin and etretinate was analyzed by each factor after subgrouping. The administration dosage was subgrouped to less than one capsule per day and more than one capsule per day. Administration duration was subgrouped to less than one year and more than one year. Discontinuation duration was categorized as currently taking medication and discontinued medication for less than one year and more than one year. Age was subgrouped to less than 60 years and more than 60 years. Alcohol consumption status was categorized based on the frequency as never drinkers and drinkers. The frequency of intake of vitamin A- or provitamin A-rich foods or vitamin A supplements was categorized as less than twice a week and more than twice a week. Mann-Whitney U test or Kruskal-Walls sum test was performed to examine the correlation between serum concentrations of acitretin and etretinate and the above-mentioned factors. The serum concentration of not detected was set to zero. For all tests, statistical significance was set at p less than 0.05.
RESULTS
Demographics
The mean age was 50.9 years (range, 16-74 years) for the 14 patients and 36.7 years (range, 20-57 years) for the 58 controls. The proportion of males was 57.1% (8/14) among patients and 44.8% (26/58) among controls. The most common diagnosis for acitretin use was psoriasis, followed by palmoplantar pustulosis, acanthosis nigricans, hand eczema, pityriasis rubra pilaris, and porokeratosis. All patients and controls consumed vitamin A- or provitamin A-rich foods or supplements. The frequency of such intake was more than twice a week in five (35.7%) patients and 30 (51.7%) controls (Table 1).
Regrading acitretin administration, four (28.5%) patients received one capsule every other day, whereas ten (71.4%) patients received one (10 mg) or more capsules per day. The mean administration duration was 26 months (range, 1-158 months). Four patients were taking the medication during the study period, whereas six and four patients, respectively, had discontinued the medication for less than one year and more than one year. Eight (57.1%) patients drank alcohol during medication use (Table 2).
Table 2.
Information about acitretin administration, alcohol/food/supplements intake, and serum acitretin or etretinate concentrations in 14 patients
| Age | Sex | Acitretin | Alcohol (n/week) | Food or supplements (S) | Serum | Adipose tissue | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Administration (month) | Discontinuation (month) | Food (n/week) | S. | Acitretin (ng/mL) | Etretinate (ng/mL) | Acitretin (ng/g) | Etretinate (ng/g) | ||||||
| Patients | 16 | M | 1C qd | 1 | - | Never | 1 | - | 118.2 ± 17.7 | ND† | NT†† | NT | ||
| 29 | M | 1C qod | 44 | - | 1 | 2 | - | ND | 5.4 ± 1.0 | NT | NT | |||
| 62 | M | 1C qd | 1 | - | Never | 1 | - | 90.6 ± 2.5 | ND | NT | NT | |||
| 67 | F | 1C qd | 42 | - | Never | 1 | - | 120.0 ± 22.4 | ND | NT | NT | |||
| 71 | M | 1C qd | 158 | 2 | 1 | 1 | - | 157.9 ± 2.3 | ND | NT | NT | |||
| 56 | M | 1C qd | 37 | 5 | 2 | 1 | - | ND | ND | NT | NT | |||
| 48 | F | 1C qd | 24 | 7 | 1/2 | 2 | - | ND | 5.9 ± 1.5 | NT | NT | |||
| 63 | M | 1C qd | 5 | 8 | 1 | 2 | - | ND | 0.6 ± 0.7 | NT | NT | |||
| 30 | F | 1C qod | 6 | 8 | 1 | 1 | - | ND | ND | ND | ND | |||
| 45 | F | 1C qod | 16 | 10 | Never | 2 | - | ND | ND | NT | NT | |||
| 50 | M | 1C qod | 26 | 17 | 1/2 | 1 | - | ND | ND | NT | NT | |||
| 51 | F | 1C qd | 1 | 24 | Never | 2 | - | ND | 5.0 ± 0.4 | NT | NT | |||
| 74 | M | 1C bid | 3 | 25 | Never | 1 | - | ND | ND | NT | NT | |||
| 50 | F | 1C qd | 4 | 36 | 2 | 1 | - | ND | ND | NT | NT | |||
ND†, Not detected; NT††, Not tested.
Effects of acitretin dosage, administration duration, and discontinuation duration on serum concentrations of acitretin and etretinate
Acitretin can be reverse metabolized to etretinate (Bouvy et al., 1992). Therefore, effects of acitretin administration on serum concentrations of both acitretin and etretinate were analyzed.
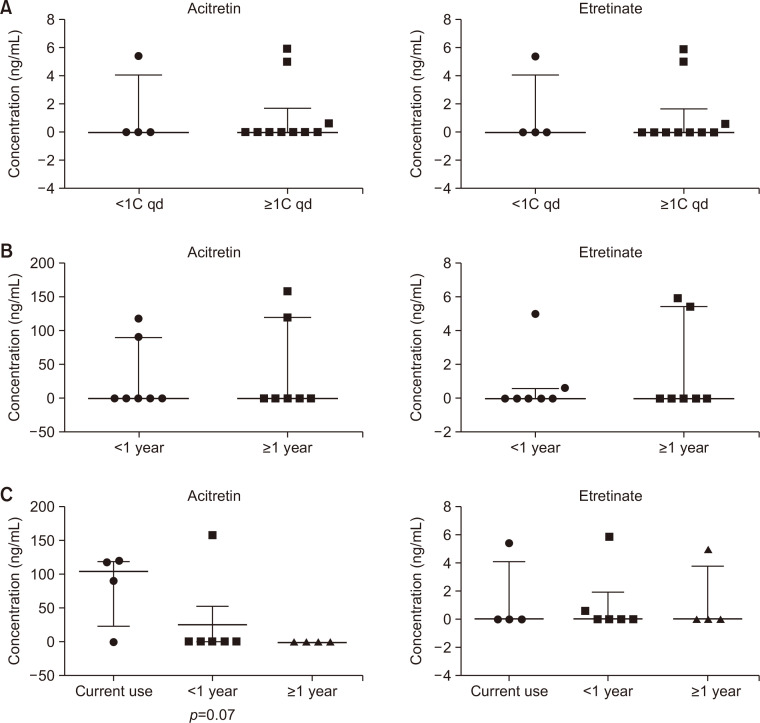
None of the patients had detectable serum concentrations of both acitretin and etretinate (Table 2). Among ten patients who received one or more capsules per day, four and three patients, respectively, had detectable serum concentrations of acitretin and etretinate. Serum etretinate was also detected in one of the four patients who received one capsule every other day (Table 2). Statistical analysis showed no significant association between acitretin dosage and serum concentration of acitretin or etretinate (Fig. 2A).
Fig. 2.
Effects of acitretin dosage, administration duration, and discontinuation duration on serum acitretin and etretinate concentrations. Serum concentrations of acitretin (left side on each graph) and etretinate (right side on each graph) were analyzed in 14 patients who received acitretin according to the dosage, administration duration, and discontinuation duration. (A) Vertical scatter plot for the administration dosage subgrouped as less than one capsule per day and one or more capsules per day. (B) Vertical scatter plot for the administration duration subgrouped as less than one year and more than one year. (C) Vertical scatter plot for the discontinuation duration subgrouped as currently taking, discontinue for less than one year, and discontinued for more than one year. The thicker line of each graph indicates median value, and the thinner one indicates interquartile range.
Two of seven patients who were taking acitretin for less than one year and two of the other seven patients who were taking acitretin for more than one year showed detectable serum acitretin. Similarly, two of the seven patients from each subgroup of administration duration had detectable etretinate (Table 2). The administration duration was also not associated with serum concentration of acitretin or etretinate (Fig. 2B).
Regarding discontinuation duration, acitretin and etretinate, respectively, were detected in three and one of the four patients who were currently taking acitretin. One and two of the six patients who had discontinued the medication for less than one year had detectable serum acitretin and etretinate, respectively. Etretinate, but not acitretin, was detected in one of four patients who had discontinued medication for more than one year (Table 2). A possible correlation was observed between discontinuation duration and serum concentration of acitretin, but not etretinate (Fig. 2C).
Effects of age and sex on serum concentrations of acitretin and etretinate
Pharmacokinetic and pharmacodynamic changes occur with increasing age. Sex differences are also observed in pharmacokinetics and pharmacodynamics of drugs (Schwartz, 2007), particularly in lipophilic drugs including acitretin and etretinate because of higher percentage of body fat in the elderly and women (Shi and Klotz, 2011). Therefore, correlation of age and sex with serum acitretin and etretinate concentrations was examined.
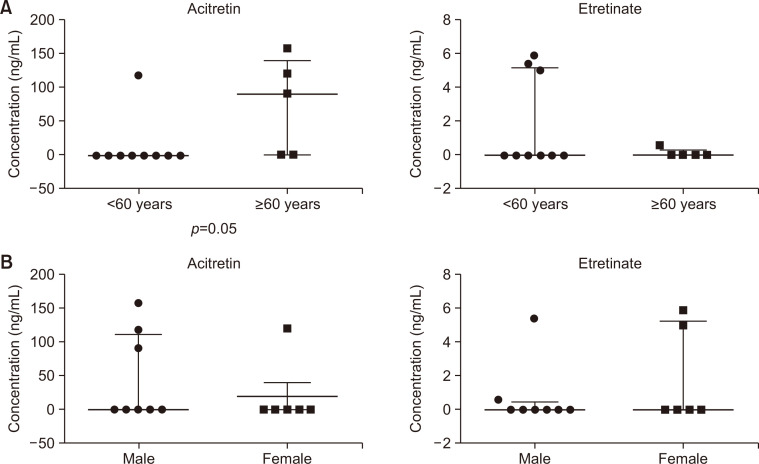
One of nine patients who were younger than 60 years and three of five patients who were older than 60 years had detectable serum acitretin, whereas three and one patients from the above respective subgroups showed detectable serum etretinate (Table 2). Serum concentration of acitretin, but not etretinate, was significantly higher in patients who were older than 60 years than that in patients who were younger than 60 years (Fig. 3A).
Fig. 3.
Effects of age and sex on serum acitretin and etretinate concentrations. Serum concentrations of acitretin (left side on each graph) and etretinate (right side on each graph) were analyzed in 14 patients who received acitretin with respect to age and sex difference. (A) Vertical scatter plot for the age subgrouped as younger than 60 years and older than 60 years. (B) Vertical scatter plot for the sex subgrouped as males and females. The thicker line of each graph indicates median value, and the thinner one indicates interquartile range.
Three of eight males and one of six females showed detectable acitretin, whereas two males and two females had detectable etretinate (Table 2). Sex difference did not affect serum concentration of acitretin or etretinate (Fig. 3B).
Effect of alcohol intake on serum concentrations of acitretin and etretinate
Metabolism of acitretin to etretinate is increased by alcohol consumption (Bouvy et al., 1992; Larsen et al., 1993, 2000). Thus, serum levels of etretinate were examined according to the frequency of concomitant alcohol intake. Three of six drinkers and one of eight who never drank had detectable acitretin, whereas one and three patients from the above respective subgroups showed detectable concentrations of etretinate (Table 2). Although ethanol can stimulate etretinate formation from acitretin (Bouvy et al., 1992; Larsen et al., 1993, 2000), the association between concomitant alcohol consumption and serum concentration of etretinate was not identified (Fig. 4A).
Fig. 4.
Effects of alcohol intake and vitamin A- or provitamin A-rich foods and supplements consumption on serum acitretin and etretinate concentrations. Serum concentrations of acitretin (left side on each graph) and etretinate (right side on each graph) were analyzed in 14 patients who received acitretin with respect to alcohol consumption and intake of vitamin A- or provitamin A-rich foods and supplements. (A) Vertical scatter plot for the alcohol consumption status subgrouped as never drinkers and drinkers. (B) Vertical scatter plot for the intake of vitamin A- or provitamin A-rich foods and supplements subgrouped as less than twice a week and more than twice a week. The thicker line of each graph indicates median value, and the thinner one indicates interquartile range.
Effects of intake of vitamin A- or provitamin A-rich foods and supplements on serum concentrations of acitretin and etretinate
Vitamin A supplementation can increase serum retinol concentration in women (Soares et al., 2019). Pregnant women are generally advised to avoid vitamin A-rich foods despite a critical role of vitamin A in fetal development (Strobel et al., 2007). Therefore, effects of vitamin A- or provitamin A-rich foods and supplements on serum concentrations of acitretin and etretinate were examined. All four patients with detectable acitretin consumed vitamin A- or provitamin A-rich foods and supplements less than twice a week. However, all four patients with detectable etretinate consumed such foods and supplements more than twice a week (Table 2). None of the controls had detectable acitretin or etretinate, although 30 of the 58 controls consumed such foods and supplements more than twice a week (Table 1). Unexpectedly, more frequent consumption of vitamin A- or provitamin A-rich foods and supplements tended to increase serum acitretin concentrations, whereas it significantly reduced serum etretinate concentrations (Fig. 4B).
DISCUSSION
Direct measurement of serum concentrations of acitretin or etretinate could be a reasonable way to confirm whether the FDA guidelines for contraception should be applied equally to everyone. However, this measurement is performed at the laboratory level and not currently available at the hospital level. Therefore, the factors influencing serum concentrations of acitretin and etretinate after acitretin administration were examined in this study.
Discontinuation duration showed an inverse association with serum acitretin concentration (Fig. 2C). Higher dosages in all four patients with detectable acitretin (Table 2) suggests the possibility of a statistically significant result with increase in sample size. However, acitretin dosage and administration duration did not show an association with serum acitretin concentrations (Fig. 2A, 2B). As for etretinate, the serum concentrations were not influenced by any of these factors (Fig. 2A-2C). This result is similar to a previous report showing no association between drug dosage and etretinate elimination (DiGiovanna et al., 1989); this might be due to considerable individual variations in etretinate elimination (DiGiovanna et al., 1989; Larsen et al., 1993, 2000).
There are only a few studies that reported the effect of age and sex on acitretin and etretinate elimination, despite implication of age-related physiological changes that may influence pharmacokinetics and pharmacodynamics of drugs (Schwartz, 2007; Shi and Klotz, 2011). It might be due to the risk of teratogenicity associated with acitretin treatment in women of childbearing age. Acitretin concentration was significantly higher in older patients than that in younger patients. However, these results were not consistent with etretinate concentration (Fig. 3A). Although lipophilic drugs are known to have a prolonged half-life in women (Schwartz, 2007; Shi and Klotz, 2011), no sex difference was observed in serum acitretin or etretinate concentrations (Fig. 3B). Racial difference in body fat such as less body fat in Korean elderly women might have contributed to such result.
Alcohol drinking can extend the retention of body etretinate after acitretin discontinuation (Bouvy et al., 1992; Larsen et al., 1993, 2000). Concomitant alcohol consumption did not increase serum etretinate concentration in this study (Fig. 4A), although the result in adipose tissues might be different. The consumption of vitamin A- or provitamin A-rich foods and supplements did not change serum acitretin or etretinate concentrations in the controls (Table 2). This result indicates that acitretin and etretinate are not metabolites of these foods or supplements, despite complicated pathways of retinoid metabolism (Bouvy et al., 1992). However, more frequent intake of these products was possibly associated with higher serum acitretin concentrations, whereas less frequent intake of these products was associated with higher serum etretinate concentrations in patients (Fig. 4B). This unexpected result suggests that these vitamin A- or provitamin A-rich foods and supplements could affect the metabolism of acitretin and etretinate. Further research is needed to confirm this result.
Acitretin can be reverse metabolized to etretinate (Bouvy et al., 1992). Therefore, etretinate was expected to be detected in some patients with detectable serum acitretin as reported by a previous study (Sturkenboom et al., 1994). However, none of the patients had detectable serum concentrations of both acitretin and etretinate in this study (Table 2). Detectable serum concentrations of acitretin and etretinate were observed in four and the other four patients, respectively. Further studies are needed to clarify this result.
Although teratogenic threshold dose of acitretin and etretinate remains uncertain, teratogenicity of acitretin has been reported to be approximately eight-fold lower than that of etretinate (Kochhar et al., 1989). In addition, acitretin is eliminated faster than etretinate, without accumulation in body fat (Bouvy et al., 1992; Meyer et al., 1993). In case of etretinate, no teratogenic data have been associated with blood level less than 2 ng/mL (Geiger et al., 1994). Based on the above-described facts, three (21.4%) patients satisfied the condition that might cause teratogenicity. In addition, teratogenic data have been associated with medication use during the first trimester of pregnancy (Grote et al., 1985; de Die-Smulders et al., 1995; Barbero et al., 2004). The number of patients who participated in this preliminary study might not be enough to confirm the meaningful factors. None of the participants in this study, except one patient, was willing to donate their subcutaneous fat tissues, suggesting certain difficulties in implementation. Additional clinical data related to their concentrations in fat tissues compared with their concentrations in the serum are needed to determine whether serum acitretin, in particular etretinate concentrations could be sufficient for predicting teratogenicity. Nonetheless, inter-individual variations particularly in serum retention of etretinate suggest the necessity of a system that can examine acitretin and etretinate concentrations at the hospital level with further research before applying the same guidelines to everyone.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HP20C0131).
Footnotes
REFERENCES
- Barbero P., Lotersztein V., Bronberg R., Perez M., Alba L. Acitretin embryopathy: a case report. Birth Defects Res. A Clin. Mol. Teratol. 2004;70:831–833. doi: 10.1002/bdra.20078. [DOI] [PubMed] [Google Scholar]
- Bouvy M. L., Sturkenboom M. C., Cornel M. C., De Jong-Van, den Berg L. T., Stricker B. H., Wesseling H. Acitretin (Neotigason). A review of pharmacokinetics and teratogenicity and hypothesis on metabolic pathways. Pharm. Weekbl. Sci. 1992;14:33–37. doi: 10.1007/BF01980479. [DOI] [PubMed] [Google Scholar]
- de Die-Smulders C. E., Sturkenboom M. C., Veraart J., van Katwijk C., Sastrowijoto P., van der Linden E. Severe limb defects and craniofacial anomalies in a fetus conceived during acitretin therapy. Teratology. 1995;52:215–219. doi: 10.1002/tera.1420520407. [DOI] [PubMed] [Google Scholar]
- DiGiovanna J. J., Zech L. A., Ruddel M. E., Gantt G., Peck G. L. Etretinate. Persistent serum levels after long-term therapy. Arch. Dermatol. 1989;125:246–251. doi: 10.1001/archderm.1989.01670140098019. [DOI] [PubMed] [Google Scholar]
- Dunn L. K., Gaar L. R., Yentzer B. A., O'Neill J. L., Feldman S. R. Acitretin in dermatology: a review. J. Drugs Dermatol. 2011;10:772–782. [PubMed] [Google Scholar]
- Geiger J. M., Baudin M., Saurat J. H. Teratogenic risk with etretinate and acitretin treatment. Dermatology. 1994;189:109–116. doi: 10.1159/000246811. [DOI] [PubMed] [Google Scholar]
- Grote W., Harms D., Jänig U., Kietzmann H., Ravens U., Schwarze I. Malformation of fetus conceived 4 months after termination of maternal etretinate treatment. Lancet. 1985;1:1276. doi: 10.1016/S0140-6736(85)92344-X. [DOI] [PubMed] [Google Scholar]
- Guenther L. C., Kunynetz R., Lynde C. W., Sibbald R. G., Toole J., Vender R., Zip C. Acitretin use in dermatology. J. Cutan. Med. Surg. 2017;21:2S–12S. doi: 10.1177/1203475417733414. [DOI] [PubMed] [Google Scholar]
- Kara Polat A., Oguz Topal I., Aslan Kayıran M., Koku Aksu A. E., Aytekin S., Topaloglu Demir F., Ozkok Akbulut T., Kıvanc Altunay I., Ozkur E., Karadag A. S. Drug survival and safety profile of acitretin monotherapy in patients with psoriasis: a multicenter retrospective study. Dermatol. Ther. 2021;34:e14834. doi: 10.1111/dth.14834. [DOI] [PubMed] [Google Scholar]
- Katugampola R. P., Finlay A. Y. Oral retinoid therapy for disorders of keratinization: single-centre retrospective 25 years' experience on 23 patients. Br. J. Dermatol. 2006;154:267–276. doi: 10.1111/j.1365-2133.2005.06906.x. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D., Minutella L. M. Biotransformation of etretinate and developmental toxicity of etretin and other aromatic retinoids in teratogenesis bioassays. Drug Metab. Dispos. 1989;17:618–624. [PubMed] [Google Scholar]
- Kumar A., Monif T., Khuroo A., Sasmal D., Goswami D., Lahkar V. K. Stability-indicating validation of acitretin and isoacitretin in human plasma by LC-ESI-MS/MS bioanalytical method and its application to pharmacokinetic analysis. Biomed. Chromatogr. 2011;25:680–688. doi: 10.1002/bmc.1503. [DOI] [PubMed] [Google Scholar]
- Larsen F. G., Jakobsen P., Larsen C. G., Kragballe K., Nielsen-Kudsk F. Pharmacokinetics of etretin and etretinate during long-term treatment of psoriasis patients. Pharmacol. Toxicol. 1988;62:159–165. doi: 10.1111/j.1600-0773.1988.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Larsen F. G., Jakobsen P., Eriksen H., Grønhøj J., Kragballe K., Nielsen-Kudsk F. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J. Clin. Pharmacol. 1991;31:477–483. doi: 10.1002/j.1552-4604.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Larsen F. G., Jakobsen P., Knudsen J., Weismann K., Kragballe K., Nielsen-Kudsk F. Conversion of acitretin to etretinate in psoriatic patients is influenced by ethanol. J. Invest. Dermatol. 1993;100:623–627. doi: 10.1111/1523-1747.ep12472293. [DOI] [PubMed] [Google Scholar]
- Larsen F. G., Steinkjer B., Jakobsen P., Hjorter A., Brockhoff P. B., Nielsen-Kudsk F. Acitretin is converted to etretinate only during concomitant alcohol intake. Br. J. Dermatol. 2000;143:1164–1169. doi: 10.1046/j.1365-2133.2000.03883.x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Koo J. A review of acitretin, a systemic retinoid for the treatment of psoriasis. Expert. Opin. Pharmacother. 2005;6:1725–1734. doi: 10.1517/14656566.6.10.1725. [DOI] [PubMed] [Google Scholar]
- Ling M. R. Acitretin: optimal dosing strategies. J. Am. Acad. Dermatol. 1999;41:S13–S17. doi: 10.1016/S0190-9622(99)70360-9. [DOI] [PubMed] [Google Scholar]
- Lynde C. W., Kraft J. N., Lynde C. B. Acitretin revisited. Skin Therapy Lett. 2011;16:1–4. [PubMed] [Google Scholar]
- Maier H., Hönigsmann H. Assessment of acitretin-treated female patients of childbearing age and subsequent risk of teratogenicity. Br. J. Dermatol. 2001;145:1028–1029. doi: 10.1046/j.1365-2133.2001.04424.x. [DOI] [PubMed] [Google Scholar]
- Meyer E., de Bersaques J., Lambert W. E., de Leenheer A. P., Kint A. H. Skin, adipose tissue and plasma levels of acitretin with rare occurrence of esterified acitretin during long-term treatment. Acta Derm. Venereol. 1993;73:113–115. doi: 10.2340/0001555573113115. [DOI] [PubMed] [Google Scholar]
- Pilkington T., Brogden R. N. Acitretin. A review of its pharmacology and therapeutic use. Drugs. 1992;43:597–627. doi: 10.2165/00003495-199243040-00010. [DOI] [PubMed] [Google Scholar]
- Sarkar R., Chugh S., Garg V. K. Acitretin in dermatology. Indian J. Dermatol. Venereol. Leprol. 2013;79:759–771. doi: 10.4103/0378-6323.120721. [DOI] [PubMed] [Google Scholar]
- Schwartz J. B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- Sharma P., Soni K., Guttikar S., Singhal P., Patela D. P., Shrivastav P. S. Separation of all-trans- and 13-cis-isomers of acitretin on a reversed-phase column, optimization of stability conditions and their simultaneous quantitation in human plasma by liquid chromatography-tandem mass spectrometry. Anal. Methods. 2012;4:791–806. doi: 10.1039/c2ay05852a. [DOI] [Google Scholar]
- Shi S., Klotz U. Age-related changes in pharmacokinetics. Curr. Drug Metab. 2011;12:601–610. doi: 10.2174/138920011796504527. [DOI] [PubMed] [Google Scholar]
- Soares M. M., Silva M. A., Garcia P. P. C., da Silva L. S., da Costa G. D., Araújo R. M. A., Cotta R. M. M. Efect of vitamin A suplementation: a systematic review. Cien. Saude Colet. 2019;24:827–838. doi: 10.1590/1413-81232018243.07112017. [DOI] [PubMed] [Google Scholar]
- Strobel M., Tinz J., Biesalski H.-K. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur. J. Nutr. 2007;46 Suppl 1:1–20. doi: 10.1007/s00394-007-1001-z. [DOI] [PubMed] [Google Scholar]
- Sturkenboom M. C., de Jong-Van Den Berg L. T., van Voorst-Vader P. C., Cornel M. C., Stricker B. H., Wesseling H. Inability to detect plasma etretinate and acitretin is a poor predictor of the absence of these teratogens in tissue after stopping acitretin treatment. Br. J. Clin. Pharmacol. 1994;38:229–235. doi: 10.1111/j.1365-2125.1994.tb04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.