Abstract
Describe the use of tofacitinib in severe and critical coronavirus disease-2019 (COVID-19), and explore the association of drug initiation time with survival. A retrospective study of inpatients with severe or critical COVID-19 at a tertiary care hospital, who were prescribed generic tofacitinib for at least 48 hours, was conducted. Baseline demographics, comorbidities, illness severity, treatment, adverse effects and outcomes were analyzed. Patients were grouped based on median duration of symptomatic illness prior to tofacitinib administration, as early or late initiation groups. Forty-one patients ([85.4% males], mean age 52.9 ± 12.5 years), were studied. 65.9% (n = 27) had severe COVID-19, while 34.1% (n = 14) were critically ill. Death occurred in 36.6% patients (n = 15). The median time to prescription of tofacitinib was 13 (9.50, 16.0) days of symptom onset. Tofacitinib was initiated early (8–13 days) in 56.1% of patients (n = 23), while the remaining received it beyond day 14 of symptom onset (late initiation group). Multivariate logistic regression adjusted for age, presence of diabetes mellitus and illness duration prior to hospitalization demonstrated higher odds of survival (adjusted odds ratio 19.3, 95% confidence interval 2.57, 145.2) in the early initiation group, compared to the late initiation group. Early initiation of tofacitinib in severe and critical COVID-19 has potential to improve survival odds.
Keywords: COVID-19, early initiation, JAK inhibitor, mortality, SARS CoV-2, tofacitinib
1. Introduction
Coronavirus disease (COVID-19) caused by SARS CoV-2, has infected nearly 209.87 million people and led to around 4.40 million deaths, as of 15th August, 2021.[1] Although more than 80% of infections are asymptomatic or mild, about 15% could progress to severe or critical illness.[2] The backbone of treatment includes corticosteroids, oxygen therapy, and anticoagulants with or without antiviral agents for hospitalized patients. Several biologicals and small molecules (Janus kinase inhibitors) have been tested through clinical trials, in an attempt to reduce the intensity of cytokine storm and thereby improve outcomes.[3–5] The complexity of immunopathogenesis in COVID-19 has demonstrated that multi-targeted therapies such as glucocorticoids rather than a single target, are likely to retard the progression of severe- critical illness.[6,7]
Janus kinase inhibitors (Jakinibs) inhibit the Janus kinase/signal transducers and activators of transcription signaling pathway, and may play an important role in mitigating the hyperinflammatory state.[4,8] This has been investigated through several observational studies and subsequent clinical trials involving many different Jakinibs, such as tofacitinib, ruxolitinib, and baricitinib.[3,9–12] In a recent meta-analysis of these studies, it was shown that when compared to the controls, Jakinibs reduced day 28 all-cause mortality (odds ratio [OR], 0.57; 95% confidence interval [CI], 0.36–0.92), resulted in higher clinical recovery rates (OR, 1.45; 95% CI, 1.09–1.93), demonstrated shorter time to recovery (mean difference, −2.84; 95% CI, −5.56 to −0.12) and required invasive ventilation in fewer instances.[13] Concurrently, adverse events were not higher (OR, 0.92; 95% CI, 0.64–1.34). Tofacitinib along with standard of care (SOC) was recently incorporated in Infectious Diseases Society of America guidelines in patients with oxygen saturation <94% at room air and was on supplemental oxygen therapy.[14]
Tofacitinib is a JAK1,3 inhibitor with partial selectivity to JAK2, resulting in diminished levels of multiple cytokines such as tumour necrotic factor-α, interleukins-17, interleukins-6, interferon-γ, and several others.[15] Generic formulations of tofacitinib are locally manufactured, economically viable and easily obtainable options in India. We report our experience of generic tofacitinib administration in hospitalized, severe to critically ill COVID-19 patients, during the peak of the 2nd wave of the SARS CoV-2 pandemic in an Indian tertiary hospital setting. During this period, the scarcity of oxygen and hospital beds escalated the mortality rate for COVID-19. We hypothesized that tofacitinib may contribute to mortality reduction when initiated early in the course of treatment of patients with severe-critical COVID-19.
2. Methods
2.1. Study design and data accrual
This study was a retrospective chart review from COVID-19 isolation wards in a tertiary care referral hospital, during May to June 2021. The baseline demographics, comorbidities, severity of illness, administered treatment and outcomes were retrieved using a structured case record form from inpatient records, pharmacy dispensing inventory, and discharge and mortality records. This study was approved by the St. John’s Medical College Institutional Ethics Committee, via IERB no-291/2021. Waiver of consent was obtained as this is a retrospective study.
2.2. Study population
We included hospitalized, severe or critical adult COVID-19 patients, who were prescribed generic tofacitinib for at least 48 hours. Patients with preexisting malignancies or autoimmune rheumatic disease, or those who were on prior tofacitinib therapy, were excluded. All patients received COVID-19 treatment as per SOC guidelines instituted by the Ministry of Health and Family Welfare, Government of India, and in-house COVID-19 treatment protocol which included oxygen therapy, dexamethasone, low molecular weight heparin and other supportive therapy as appropriate.[16–18] The decision to add generic tofacitinib was as per the physician’s discretion, typically based on non-response that is, worsening of oxygenation and deterioration of clinical status while on SOC. The dose of tofacitinib employed was 10 mg twice a day.
Severity of COVID-19 was categorized in line with the World Health Organisation classification. Patients with severe pneumonia requiring either invasive or non invasive ventilation were categorized as critical and patients with oxygen saturation <90% at room air and with impending clinical signs of respiratory distress were categorized as severe.[19] Further, patients were grouped based on the timing of generic tofacitinib prescription as early or late initiation groups, by considering the median duration of symptomatic illness before drug administration.
2.3. Outcome
Our primary outcome measure was mortality at 28 days, as captured in the in-patient records, discharge, and death summaries. Information on infections and other complications were retrieved from the hospital laboratory reporting system, in addition to inpatient records. Bacterial infections were determined as per microbiologic and biochemical results.[20] Fungal infections were categorized based on microbiologic results, serologic assays, and radiologic assessment. We categorized and compared the outcomes according to the severity of COVID-19, and the timing of tofacitinib administration. Follow-up outcomes on day 28 were also recorded.
2.4. Statistics
The assumption of normality was ascertained using the Kolmogorov–Smirnov test. Numbers and percentages were reported for categorical data. Normally distributed continuous data were reported as mean with standard deviation, while non-parametric data were reported as median with 25th and 75th percentiles. The clinical characteristics, comorbidities, duration of symptomatic illness, biochemical parameters, and outcomes were compared between early and late tofacitinib treatment categories using chi-square test, independent t test, or Mann–Whitney U test as appropriate. Variables that had P value less than .10 in the univariate analysis were considered for multivariable analysis. Multivariable analysis was performed using Logistic regression analysis to assess the association of early and late tofacitinib administration with mortality, adjusted for age, presence of diabetes, duration of illness before hospitalization in model 1 and disease severity with model 1 as model 2. In model 3, glycated hemoglobin (HbA1c) was considered replacing the presence of diabetes along with other covariates. Due to small size, various models were explored to get the estimation. Kaplan–Meier survival curve used to depict overall survival. P value less than 5% was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 25.0 (IBM SPSS Statistics for Windows, Version 25.0, IBM Corp, Armonk, NY).
3. Results
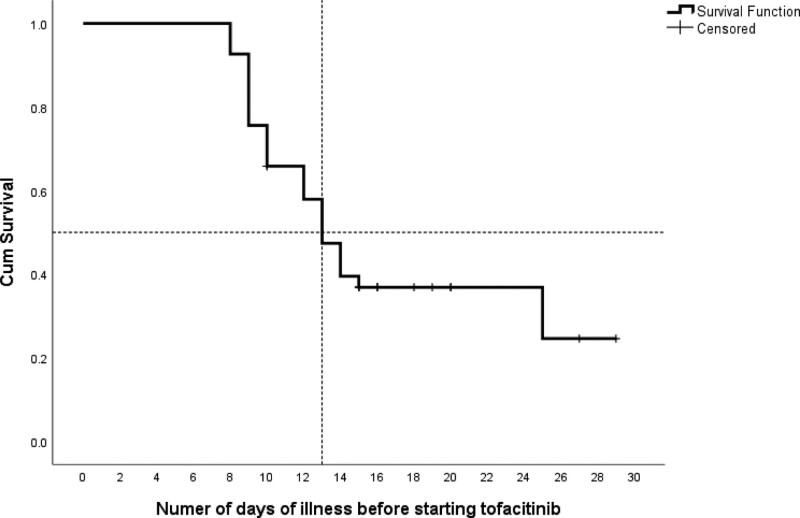
Forty-one patients (85.4% males, n = 35) with a mean age of 52.9 ± 12.5 years were included. 65.9% (n = 27) of the patients were categorized as severe COVID-19 patients, while the rest belonged to the critical category. Overall, 36.6% (n = 15) patients died - 12/14 (85%) from the critical group, and 3/27 (11.1%) from the severe group. The median time-to-initiation of tofacitinib was 13 days (interquartile range, 9.5–16) as presented in Figure 1. We categorized tofacitinib recipients into an “early initiation” group (8–13 days) and “late initiation group” (≥14 days) from symptom onset, based on this median. There were 56.1% (n = 23) and 43.9% (n = 18) patients in the early and late initiation groups respectively.
Figure 1.
Kaplan–Meier showing the cumulative survival as per number of days of symptomatic illness before starting tofacitinib.
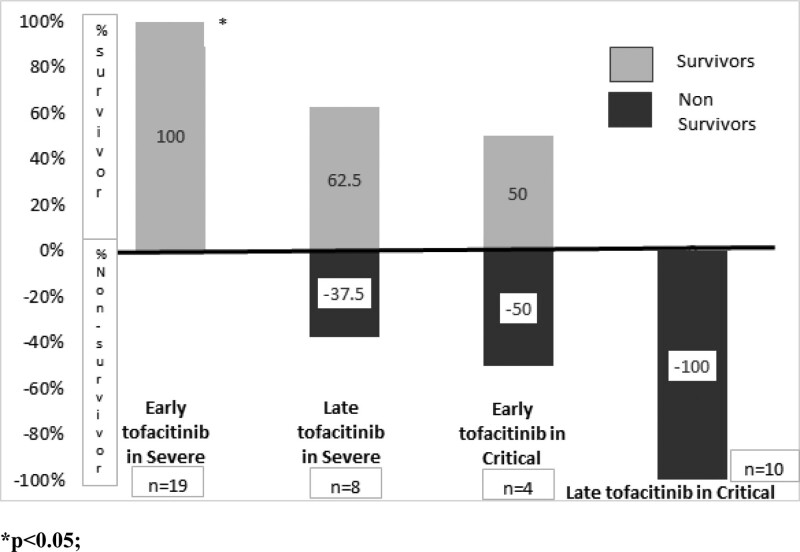
A comparison of clinical characteristics between early and late tofacitinib initiation groups is presented in Table 1. The baseline demographics, comorbidities, and biochemical parameters were not significantly different between the two treatment initiation groups. The mean HbA1c level was 9.1 (interquartile range, 6.9–12.9), and was higher in the early initiation group (P = .08). The association of the proportion of survivors by early and late initiation groups and by disease severity, is presented in Figure 2. The proportion of survivors was significantly higher in the early initiation group (21/23, 91.3%) compared to the late initiation group (5/18, 27.8%) (P < .0001). When stratified by disease severity, severe COVID-19 patients demonstrated survival rates of 100% and 62.5%, for the early and late initiation groups respectively (P < .01). From the critical COVID-19 patients, 50% of the early initiation group were alive on day 28, while in-hospital mortality occurred for all patients from the late initiation group (P = .06).
Table 1.
Characteristics and outcomes as per timing of tofacitinib administration (early [8–13 d], late [>14 d]).
| Total (n = 41) | Early initiation (N = 23) | Late initiation (N = 18) | P value | |
|---|---|---|---|---|
| Age (yr)* | 52.9 ± 12.5 | 51.7 ± 11.9 | 54.3 ± 13.4 | .524 |
| Gender (male) | 35 (85.4%) | 18 (78.3%) | 17 (94.4%) | .205 |
| Smoking | ||||
| Past | 32 (78%) | 20 (87%) | 12 (66.7%) | .214 |
| Current | 5 (12.2%) | 1 (4.3%) | 4 (22.2%) | |
| Never | 4 (9.8%) | 2 (8.7%) | 2 (11.1%) | |
| Obesity | 4 (9.8%) | 1 (4.3%) | 3 (16.7%) | .303 |
| Comorbidities | ||||
| Diabetes mellitus | 21 (51.2%) | 13 (56.5%) | 8 (44.4%) | .536 |
| Hypertension | 15 (36.6%) | 10 (43.5%) | 5 (27.8%) | .300 |
| Chronic heart disease | 3 (7.3%) | 3 (13%) | 0 (0%) | – |
| Chronic lung disease | 2 (4.9%) | 2 (8.7%) | 0 (0%) | – |
| HIV | 1 (2.4%) | 0 (0%) | 1 (5.6%) | – |
| Laboratory parameters | ||||
| NLR† | 8.09 (6.06, 12.6) | 6.79 (4.38, 13.9) | 8.97 (6.68, 10.1) | .979 |
| CRP (mg/L)† | 80.5 (32.3, 147.5) | 71.3 (27.8, 139.7) | 94.7 (38.5, 169.9) | .454 |
| Ferritin (ng/mL)† | 709.9 (533, 1272.3) | 601 (345, 1136) | 893 (619, 1509) | .052 |
| LDH (IU/L)† | 452 (368, 579) | 424 (349, 579) | 462 (434, 580) | .462 |
| HbA1C† | 8.00 (6.17, 10.9) | 9.1 (6.9, 12.9) | 6.80 (5.9, 9.80) | .082 |
| D-Dimer† | 437 (290, 669.5) | 366 (262,1136) | 504 (359, 724) | .109 |
| Clinical course, treatment and outcomes | ||||
| Symptom duration prior to hospitalization* | 7.51 ± 2.74 | 6.30 ± 2.53 | 9.06 ± 2.23 | .001 |
| Duration of Tofacitinib use* | 7.39 ± 2.71 | 7.87 ± 2.83 | 6.78 ± 2.48 | .204 |
| Duration of O2 therapy* | 10.86 ± 6.04 | 12.18 ± 5.79 | 8.79 ± 6.04 | .101 |
| Duration of Hospital stay* | 17.9 ± 7.27 | 17.2 ± 8.32 | 18.7 ± 5.79 | .530 |
| Severe COVID-19 | 27 (65.9%) | 19 (82.6%) | 8 (44.4%) | .011 |
| Critical COVID-19 | 14 (34.1%) | 4 (17.4%) | 10 (55.6%) | |
| Non-survivors | 15 (36.6%) | 2 (8.7%) | 13 (72.2%) | <.001 |
| Survivors | 26 (63.4%) | 21 (91.3%) | 5 (27.8%) | |
| Supplemental O2 on day 28 | 7 (26.9%) | 6 (28.6%) | 1 (20.0%) | .698 |
Reported as number within parentheses %.
CRP = C reactive protein, HbA1C = glycated hemoglobin, HIV = human immunodeficiency virus, LDH = lactate dehydrogenase, NLR = neutrophil-lymphocyte ratio, O2 = oxygen.
*Mean ± standard deviation.
†Median (25, 75 percentile).
Figure 2.
Association of survivor status by treatment group and disease severity.
Multivariable analysis using logistic regression adjusted covariates are reported in Table 2. Adjusted for age, presence of diabetes mellitus (DM), and duration of illness before hospitalization showed that the patients who were administered tofacitinib earlier in the course of their treatment, had higher odds of survival (adjusted OR 19.3, 95% CI 2.57, 145.2) P < .001, compared to patients who were in the late initiation group. The Association of early and late-initiation groups with the proportion of survivors remained significant, even after adjusting for disease severity in model 2 and HbA1c in model 3 along with the previously mentioned covariates.
Table 2.
Multivariable logistic regression analysis showing the association between administration of tofacitinib timings with survival status.
| Adjusted odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Model 1 | 19.3 | 2.57, 145.2 | .001 |
| Model 2 | 22.7 | 1.18, 435.8 | .038 |
| Model 3 | 33.7 | 3.81, 298.7 | .002 |
Model 1: Association of administration of tofacitinib and survival status adjusted for age, presence of diabetes, duration of illness before hospitalization.
Model 2: Association of administration of tofacitinib and survival status adjusted for age, presence of diabetes, duration of illness before hospitalization and disease severity score.
Model 3: Association of administration of tofacitinib and survival status adjusted for age, glycated hemoglobin levels, duration of illness before hospitalization and disease severity scores.
The majority of patients tolerated tofacitinib well. Overall, 26.8% of the patients (n = 11) had infections, and all except one died. Around 19.5% (n = 8) had secondary bacterial infections and 9.8% (n = 4) patients had fungal infections. One patient had concomitant mucormycosis and aspergillosis, as diagnosed by tissue sampling and automated fungal cultures. All patients with fungal infections had uncontrolled DM with high HbA1c levels. The most common cause of death was a refractory respiratory failure (7/15; 46.6%), with one patient also displaying pulmonary thrombosis as reported in Table 3. All of the discharged patients were alive on day 28, and 26.9% (7/26) patients continued to require oxygen therapy.
Table 3.
Mortality (n = 15) and infections (n = 41).
| Event | N (%) | |
|---|---|---|
| Cause of death | Refractory respiratory failure | 7/15 (46.6) |
| Cerebrovascular event | 1/15 (6.6) | |
| Cardiovascular event | 1/15 (6.6) | |
| Sepsis with septic shock | 6/15 (40) | |
| Infections | Secondary bacterial infections | 8/41 (19.5) |
| Mucormycosis | 2/41 (4.8) | |
| Aspergillosis | 3/41 (7.3) | |
| >1 infection | 5/41 (12.2) | |
| Adverse events attributed to tofacitinib | Pulmonary thrombosis | 1/41 (2.4) |
4. Discussion
We report our experience of generic tofacitinib use in hospitalized COVID-19 patients with severe to critical illness on the background of SOC which includes glucocorticoids and anticoagulants. Tofacitinib administration early (8–13 days) in the course of severe-critical COVID-19 illness resulted in significantly higher odds of survival (adjusted for age, presence of DM, and the number of days of illness from first symptoms [adjusted OR 19.3, 95% CI 2.57, 145.2]). This would indirectly indicate that tofacitinib may be best juxtaposed when the SOC treatment including glucocorticoids is failing. Considering post-COVID sequelae which may result in lung fibrosis and long-term respiratory support requirement, efforts must be directed at nullifying the cytokine storm during its early phase rather than after the cytokine storm has peaked. Ours is the first study that attempts to identify the optimal timing of tofacitinib in the treatment protocols for severe-critical COVID-19. Further, the survival benefit was more impressive in severe COVID-19 than critical illness. The side effects profile of tofacitinib was acceptable in our cohort, as only one out of the fifteen deaths that occurred could be attributed to tofacitinib, where the patient developed pulmonary thrombosis.
The pathophysiological features of severe Covid-19 pneumonia are dominated by diffuse alveolar damage, inflammatory infiltrates, microvascular thrombosis, and an abundance of inflammatory cytokines.[21,22] It is postulated that multitarget immunomodulation rather than single cytokine-based therapies may abrogate the need for escalated oxygen therapy, and invasive mechanical ventilation, while also diminishing the chances of mortality, especially when administered just before the onset or early in the course of the cytokine storm.[4–7,21] This implies a value for precise targeting of signal transduction pathway proteins in pathological circumstances, as evident in moderate to severe COVID-19 infected patients with impending cytokine storm, clinically discerned by the requirement for supplemental oxygen therapy and/or noninvasive ventilation.
The first reports of survival advantage of tofacitinib emerged from a retrospective single-center observational study that was conducted early on in the COVID-19 pandemic (March–September 2020), demonstrating a 70% reduction in the odds of dying by the addition of tofacitinib to dexamethasone, compared to dexamethasone administration alone.[9] Subsequently, STOP-COVID trial investigators from Brazil conducted a randomized, double-blind, placebo-controlled trial involving 289 hospitalized patients with Covid-19 pneumonia, and found that tofacitinib was superior to placebo in reducing the incidence of death (hazard ratio, 0.49; 95% CI, 0.15–1.63) or respiratory failure.[10] Furthermore, these effects were consistent regardless of sex, age, duration of symptoms, glucocorticoids administration across different levels of supplemental oxygen use. In a recent retrospective study from Russia involving 30 patients, mortality advantage was demonstrated with tofacitinib (16.6% vs 40.0%, P = .009).[23] This benefit was established over and the above the SOC, which included antiviral, antibacterial, anticoagulant, and dexamethasone treatment. Recent data from an Indian retrospective study including 50 patients, had shown a significantly lower number of intubations by day 7 with tofacitinib as compared with the control group (32 vs 8%, P = .034). Further, they had also demonstrated better oxygenation in the tofacitinib group compared to placebo (1.26 vs 0.72) but these findings did not translate into mortality benefit at day 21.[24]
We have found that the glycaemic control in the early initiation group was worse than that in the late group. Though not statistically significant, HbA1c of 9 in the early initiation group is clinically significant. Studies have demonstrated that poor glycaemic control is associated with higher mortality.[25] Despite worse glycaemic control in the early treatment group, we have found that tofacitinib has been able to lower mortality. This further proves the suggestion that the timing of tofacitinib may have been strategic in improving outcomes.
Currently, locally manufactured generic formulations of tofacitinib are approved for use in rheumatological disorders in India, and are easily accessible at reasonable costs. Ours is the first report (from India) of generic tofacitinib use in COVID-19. We believe that the results of our study could be conducive to achieving reduced long-term mortality as well as respiratory morbidity. Further, it may help to reduce glucocorticoid-related metabolic complications and infections. This factor, however, has not been addressed in our study.
There are a few limitations to our study. This was a non-randomized, retrospective study, with its inherent drawbacks such as small cohort size, lack of baseline comparability, and selection bias. Further, tofacitinib administration relied on physician discretion, and was not protocol-based. We are also unable to comment on the optimum duration of tofacitinib use in COVID-19. Further validation of our results through a randomized placebo-controlled trial focused on identifying the right time of tofacitinib prescription, would consolidate our hypothesis.
5. Conclusion
This is the first report from India in a real-life setting, on the use of generic tofacitinib in COVID-19 to demonstrate potential benefit of early addition of tofacitinib (8–13 days) to the current SOC for severe-critical COVID-19 illness which could result in higher odds of survival.
Author contributions
Conception and design of the study: V.S., R.K.
Analysis and interpretation of data: R.K., V.S., D.K., and S.S.
Data acquisition: R.K., V.S., and S.U.
Drafted the work and revised it critically for important intellectual content: All authors.
Approved the version to be published: All authors.
Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Conceptualization: Vineeta Shobha.
Data curation: Ramya Kodali, Soumya Umesh.
Formal analysis: Ramya Kodali, Soumya Umesh, Sumithra Selvam, Deepak Kamath, Vineeta Shobha.
Methodology: Ramya Kodali, Soumya Umesh, Vineeta Shobha.
Validation: Vineeta Shobha.
Writing – original draft: Ramya Kodali, Sumithra Selvam.
Writing – review & editing: Soumya Umesh, Deepak Kamath, Vineeta Shobha.
Abbreviations:
- CI =
- confidence interval
- COVID-19 =
- corona virus disease 2019
- DM =
- diabetes mellitus
- HbA1c =
- glycated hemoglobin
- Jakinibs =
- Janus kinase inhibitors
- OR =
- odds ratio
- SOC =
- standard of care
Study is approved by the Institutional Ethics Committee, St. John’s Medical College, St. John’s National Academy of Medical Sciences via IERB no-291/2021. Waiver of consent was obtained as it is a retrospective chart review. The identity of patients has not been disclosed.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Kodali R, Umesh S, Selvam S, Kamath D, Shobha V. Timing of tofacitinib therapy is critical to improving outcomes in severe-critical COVID-19 infection: A retrospective study from a tertiary care hospital. Medicine 2022;101:43(e30975).
Contributor Information
Ramya Kodali, Email: ramyasri.kodali@gmail.com.
Soumya Umesh, Email: drsoumya239@gmail.com.
Sumithra Selvam, Email: sumithrars@sjri.res.in.
Deepak Kamath, Email: kamath.deepak@sjri.res.in.
References
- [1].WHO | WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/. [access date August 15, 2021].
- [2].Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–60. [DOI] [PubMed] [Google Scholar]
- [3].Idda ML, Soru D, Floris M. Overview of the first 6 months of clinical trials for COVID-19 pharmacotherapy: the most studied drugs. Front Public Heal. 2020;8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen C-Y, Chen W-C, Hsu C-K, Chao C-M, Lai C-C. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. 2021;99:108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Babaei F, Mirzababaei M, Nassiri-Asl M, Hosseinzadeh H. Review of registered clinical trials for the treatment of COVID-19. Drug Dev Res. 2021;82:474–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stebbing J, Lauschke VM. JAK inhibitors – more than just glucocorticoids. N Engl J Med. 2021;385:463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hayek ME, Mansour M, Ndetan H, et al. Anti-inflammatory treatment of COVID-19 pneumonia with tofacitinib alone or in combination with dexamethasone is safe and possibly superior to dexamethasone as a single agent in a predominantly African American cohort. Mayo Clin Proc Innov Qual Outcomes. 2021;5:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen C, Wang J, Li H, Yuan L, Gale RP, Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020;71:2298–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:318–28. [PubMed] [Google Scholar]
- [16].MoHFW|Home. Available at: https://www.mohfw.gov.in/. [access date August 15, 2021].
- [17].COVID-19 treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. [access date August 15, 2021].
- [18].IDSA guidelines on the treatment and management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. [access date August 15, 2021].
- [19].COVID-19 Clinical management: living guidance. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. [access date August 15, 2021].
- [20].Vaughn VM, Gandhi TN, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72:e533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. [DOI] [PubMed] [Google Scholar]
- [23].Maslennikov R, Ivashkin V, Vasilieva E, et al. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm Pharmacol Ther. 2021;69:102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh PK, Lalwani LK, Govindagoudar MB, et al. Tofacitinib associated with reduced intubation rates in the management of severe COVID-19 pneumonia: a preliminary experience. Indian J Crit Care Med. 2021;25:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Y, Lu R, Wang J, et al. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in-hospital death in adults with COVID-19. BMC Endocr Disord. 2021;21:56. [DOI] [PMC free article] [PubMed] [Google Scholar]