Objective
Epilepsy causes physical and mental damage to patients. As well known, microRNAs (miRNAs) provide therapeutic target potentials for patients with epilepsy. miR-128-3p was previously reported to be downregulated in temporal lobe epilepsy (TLE) patients, however, its detailed function in epilepsy is unknown.
Methods
Astrocytes function in epilepsy, penicillin-induced astrocytes can be used as a model for seizures in vitro. Currently, the expression levels of mitogen-activated protein kinase 6 (MAPK6), interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) were determined by western blot and reverse transcription-quantitative PCR analyses (RT-qPCR). The expression level of miR-128-3p was evaluated by RT-qPCR. TargetScan 7.1 and dual luciferase reporter assay were used for prediction and verification of interaction between miR-128-3p and MAPK6 3′ untranslated region (UTR). Cell viability was detected by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay.
Results
We found that penicillin-induced decrease in cell viability, and increase of TNF-α/IL-1β in primary astrocytes. There were lower miR-128-3p and higher MAPK6 in penicillin-treated primary astrocytes. miR-128-3p overexpression rescued penicillin-induced reduction of cell viability, and upregulation of TNF-α/IL-1β, which was partially abolished by MAPK6 overexpression.
Conclusion
Altogether, miR-128-3p attenuates penicillin-induced cell injury and inflammation in astrocytes by targeting MAPK6, thus providing a protective role in epilepsy.
Keywords: epilepsy, inflammation, miR-128-3p, MAPK6
Introduction
Epilepsy is a chronic neurological disorder caused by abnormal neuronal activity in the brain, which predisposes the patients to epileptic seizures throughout the lifetime and leads to social discrimination [1–3]. Epileptic seizure causes severe physical and mental damage to patients, with the potential pathogenesis of structural and functional injuries to the hippocampus and the limbic system, which are resulted from pathological changes including mossy fiber sprouting, neuronal apoptosis and synaptic plasticity [4–6]. The lifetime prevalence of epilepsy is approximately 1% globally [1]. Brain inflammation is conducive to the determination of the seizure threshold from the susceptible regions in the brain, and functions in the precipitation and recurrence of seizures [7,8]. Unfortunately, the current anti-epileptic drugs are effective in only <35% of patients [9]. Up to now, there have not been specific anti-epileptic drugs for patients with epilepsy.
As acknowledged, microRNAs (miRNAs) belong to a family of non-coding RNAs which reduce protein expression by inhibiting mRNA stability and translation, thus providing essential biomarkers and therapeutic target potentials for patients with epilepsy [10,11]. Downregulation of miR-128 in epilepsy has been reported in several studies, for instance, miR-128-2 is decreased in mice postnatal neurons, which upregulates motor activity and fatal epilepsy in mice [12], and miR-128-3p is downregulated in temporal lobe epilepsy (TLE) patients and all phases of TLE development in rats [13], 2R, 4R-APDC prevents hippocampal cells from seizure-induced apoptosis by upregulating miR-128-3p [14]. However, the detailed function of miR-128-3p in epilepsy has not been clarified.
The genes which are involved in inflammation and can be targeted by miR-128-3p attract our attention. By searching articles, the mitogen-activated protein kinase (MAPK) signaling pathway that takes part in numerous cellular processes including inflammation [15], comes into our sight. Among these, MAPK6 is dysregulated in numerous diseases, and can be targeted by various miRNAs. For instance, let7f-5p inhibits inflammation by targeting MAPK6 in pneumonia [16]; MAPK6 promotes the production of IL-8 and chemotaxis in breast cancer [17]; miR-26a-5p negatively regulates neuropathic pain by targeting MAPK6 [18]. However, the role of MAPK6 in epilepsy as well as the relationship between miR-128-3p and MAPK6 in epilepsy has not been investigated yet. The current study aimed to explore whether the function of miR-128-3p in epilepsy can be realized by regulation of MAPK6.
Materials and methods
Cell culture
Briefly, the neonatal (1–5 day postnatal) Sprague–Dawley male rats (n = 8) were subjected to euthanasia with cervical dislocation by pinching and disrupting the spinal cord in the high cervical region. Primary astrocytes were extracted from the cerebral cortices of the rats, then seeded into flasks with the culture medium (DMEM/F12, 15% FBS, L-glutamine and 500 ng/ml insulin), and incubated till confluence, as a previous study described [19]. The current study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated with Shandong First Medical University.
Penicillin was commonly used for the establishment of experimental epilepsy in animals [20,21]. The amount of penicillin is crucial in epilepsy models, eg, in the intracortical penicillin rat model, the higher the penicillin dose, the higher the number of hippocampal pyramidal neuronal loss [22]. The same was in neuronal cell culture, 100–5 000 μM penicillin is required to block GABA [23], while GABAergic action is pivotal in astrocyte cell death [24]. In the current study, primary astrocytes were stimulated by penicillin 2 500 µM (500 IU) for 12 hours for the establishment of an in vitro model of seizure (astrocyte death) as previously described [25]. Above all, the results are related to epilepsy.
Cell transfection
miR-128-3p mimic, miR-negative control (NC) mimic, pcDNA3.1 and pcDNA3.1-MAPK6 were obtained from GenePharma (Shanghai, China). Cell transfection of miR-128-3p mimic (50 nM) and pcDNA3.1-MAPK6 (100 ng) was performed by Lipofectamine 2000 (Invitrogen). At 48 hours after cell transfection, cells were harvested for the subsequent experiments.
RNA extraction and reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR)
Total RNA was isolated from cells by TRIzol (Invitrogen). cDNA was synthesized from the above products by PrimeScript RT kit (Takara Bio, Inc.). After that, RT-qPCR was carried out with SYBR Premix Ex Taq (Takara Bio, Inc.) by the Bio-Rad CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc.). Expression of interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), MAPK6 and miR-128-3p were referred to as GAPDH and U6, respectively. The primer sequences were as listed: IL-1β, forward 5′-TGAAATGCCACCTTTTGACAG-3′ and reverse 5′-CCACAGCCACAATGAGTGATAC-3′; TNF-α, forward 5′-CCTGTCTCTTCCT
ACCCAACC-3′ and reverse 5′-GCAGGAGTGTCCGTGTCTTC-3′; MAPK6 forward 5′- TAAAGCCATTGACATGTGGG-3′ and reverse 5′- TCGTGCACAACAGGGAT
AGA -3′; GAPDH, forward 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse 5′-GTAGAGGCAGGGATGATGTTCT-3′. miR-128-3p forward 5′-TCACAGTGAACCGGTCTCTTT-3′ and reverse 5′-AAAGAGACCGGTTCACTGTGA-3′; U6 forward 5′-GCTTGCTTCGGCAGCACATATAC-3′ and reverse 5′- TGCATGTCATCCTTGCTCAGGG-3′. The cycling conditions were as listed: 95°C for 5 minutes, followed by 40 cycles of 95°C for 10 seconds, 60°C for 30 seconds and 72°C for 1 second.
Western blot
Total protein was isolated from cells by RIPA (Sigma-Aldrich) on ice. The supernatants were centrifuged at 12 000g for 10 minutes at 4°C before the collection of the supernatant proteins. The concentration of protein in each sample was measured by the BCA kit (Sigma-Aldrich). Each protein sample (15 µg) was separated by the SDS-PAGE and transferred onto a PVDF membrane. Then the PVDF membrane was blocked by 5% fat-free milk at 37°C for 1 hours, incubated with primary antibodies against IL-1β (ab200478; RRID:AB_2888939; 1:1,000; Abcam), TNF-α (ab205587; RRID:AB_2889389; 1:1,000; Abcam), MAPK6 (ab53277; RRID:AB_2140288; 1:1,000; Abcam) and GAPDH (ab181602; RRID:AB_2630358; 1:1,000; Abcam) at 4°C overnight; and HRP-conjugated goat anti-rabbit secondary antibody (ab7097; RRID:AB_955411; 1:3,000; Abcam) at 37°C for 1 hours, successively. At last, the protein bands were visualized by ECL Kit (Pierce). Immunodetection was analyzed by Image Lab version 3.0 (Bio-Rad Laboratories, Inc.). IL-1β, TNF-α, and MAPK6 were referred to as GAPDH.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay
Cell viability was measured by MTT assay. Cells (5 × 103 cells/well) were seeded in 96-well plates. After incubation for 24 hours at 37°C, each well was added with MTT (20 µl of 5 mg/ml) and incubated for another 4 hours at 37°C. Thereafter, each well was added with DMSO (150 µl). To dissolve the dye thoroughly, the microtitre plate was shaken by a shaker. Finally, the absorbance at 570 nm was recorded by a Bio-Rad iMark plate reader (Bio-Rad Laboratories, Inc.).
Dual luciferase reporter assays
The interaction between miR-128-3p and MAPK6 was predicted by the online tool TargetScan 7.1 (http://www.targetscan.org/vert_71/). Wild type (WT, 5′-GUUAAGUAAAGUGCUCACUGUGU-3′) and mutant (Mut, 5′-GUUAAGUAAAGUGCUCAACGUGU-3′) MAPK6 3′UTR contained pGL3‑luciferase reporter plasmid (Promega Corporation, Madison, WI, USA) were cloned by GenePharma (Shanghai, China). miR-128-3p mimic or miR-NC mimic was co-transfected with WT MAPK6 3′UTR or Mut MAPK6 3′UTR into primary astrocytes by Lipofectamine 2000 (Invitrogen). At 48 hours after cell transfection, the luciferase reporter activity was determined by Dual Luciferase Assay System (Promega Corporation), which was referred to as Renilla luciferase activity.
Statistical analysis
Each experiment was repeated three independent times. Data were analyzed by Graphpad Prism 6 and expressed as mean ± SD. Two groups were compared by unpaired Student’s t test, and three or more groups were compared by one-way ANOVA followed with Newman Keuls analysis. P value < 0.05 indicated statistically significant.
Results
Verification of penicillin-induced injury in primary astrocytes
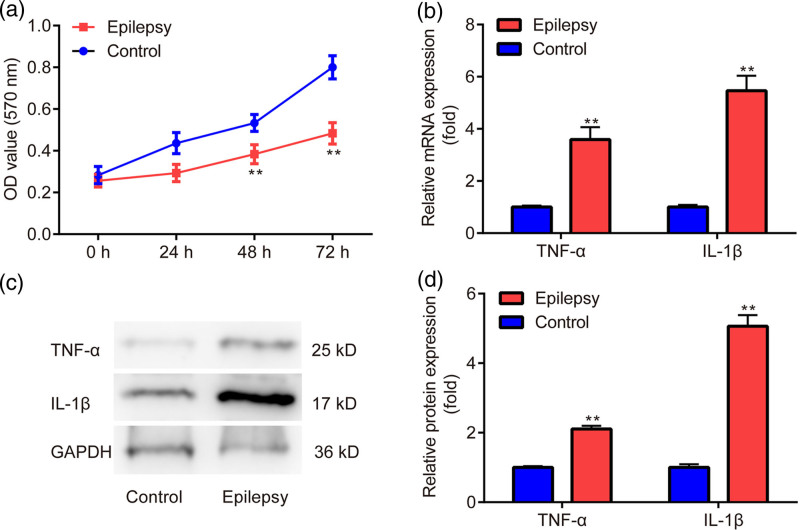
As presented in Fig. 1a, there was decreased cell viability in the epilepsy group when compared to the control group. In addition, there was increased mRNA (Fig. 1b) and protein level (Fig. 1c,d) of TNF-α and IL-1β in the epilepsy group compared with the control group. The results indicated the successful induction of injury and inflammation by penicillin in primary astrocytes.
Fig. 1.
Verification of effects of penicillin in primary astrocytes. (a) MTT assay is used to examine cell viability. (b) RT‑qPCR is used for testing the mRNA level of TNF-α and IL-1β. (c, d) Western blot is applied to detect the protein level of TNF-α and IL-1β. **P < 0.01, epilepsy vs. control. Epilepsy, treated with penicillin; control, without penicillin.
Expression profile of miR-128-3p and MAPK6 in penicillin-treated primary astrocytes
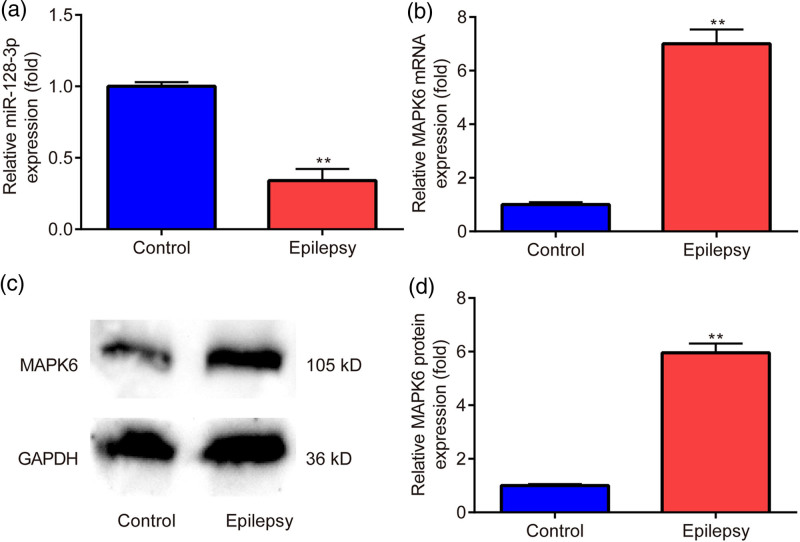
Thereafter, we aimed to examine the expression changes of miR-128-3p and MAPK6 in penicillin-treated primary astrocytes. As presented in Fig. 2a, there was downregulated miR-128-3p expression in the epilepsy group in contrast to the control group. However, there was upregulated mRNA (Fig. 2b) and protein level (Fig. 2c,d) of MAPK6 in the epilepsy group in comparison with the control group.
Fig. 2.
Expression profile of miR-128-3p and MAPK6 in penicillin-treated primary astrocytes. (a, b) RT‑qPCR is utilized to evaluate the mRNA level of miR-128-3p and MAPK6. (c, d) Western blot is applied to assess the protein level of MAPK6. **P < 0.01, epilepsy vs. control. Epilepsy, treated with penicillin; control, without penicillin.
These findings suggested the potential involvement of miR-128-3p and MAPK6 in the penicillin-induced primary astrocytes. We were eager to identify the detailed functions of miR-128-3p in penicillin-induced primary astrocytes.
Effects of miR-128-3p mimic on MAPK6 expression in penicillin-treated primary astrocytes
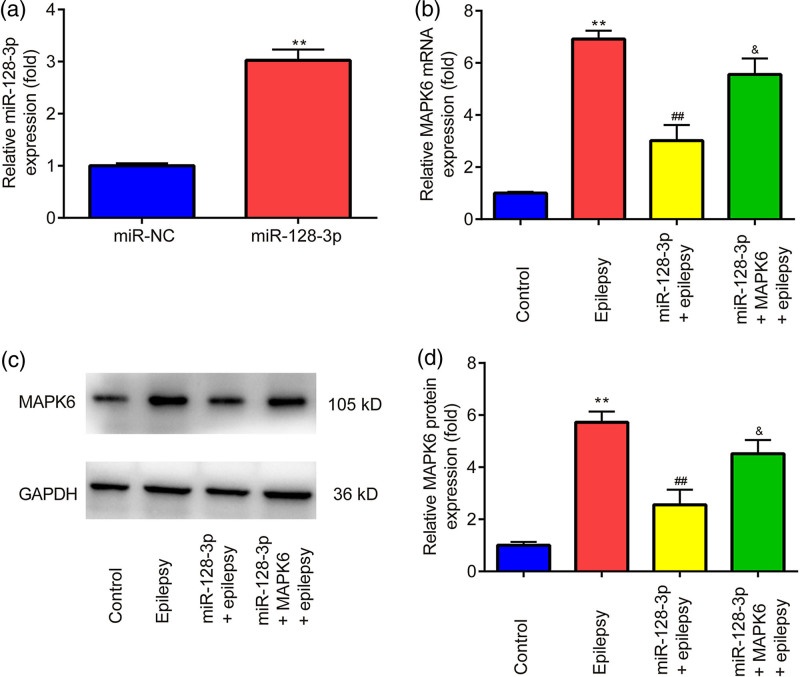
As shown in Fig. 3a, the miR-128-3p level was increased by the miR-128-3p mimic in comparison with the miR-NC mimic. Overexpression of miR-128-3p reversed the upregulation of mRNA (Fig. 3a) and protein level (Fig. 3b,c) of MAPK6 induced by epilepsy, which was partially abolished by the overexpression of MAPK6.
Fig. 3.
Effects of miR-128-3p mimic on MAPK6 expression in penicillin-treated primary astrocytes. (a, b) RT‑qPCR is utilized to evaluate the mRNA level of miR-128-3p and MAPK6. (c, d) Western blot is applied to detect the protein level of MAPK6. **P < 0.01, miR-128-3p vs. miR-NC; epilepsy vs. control. ##P < 0.01, miR-128-3p + epilepsy vs. epilepsy. &P < 0.05, miR-128-3p + MAPK6 + epilepsy vs. miR-128-3p + epilepsy. Control, miR-NC + pcDNA3.1 + without penicillin; miR-NC, miR-NC mimic; miR-128-3p, miR-128-3p mimic; MAPK6, pcDNA3.1-MAPK6.
These findings implied the potential regulatory relation between miR-128-3p and MAPK6 in penicillin-treated primary astrocytes.
Effects of miR-128-3p on cell viability and inflammation of penicillin-treated primary astrocytes
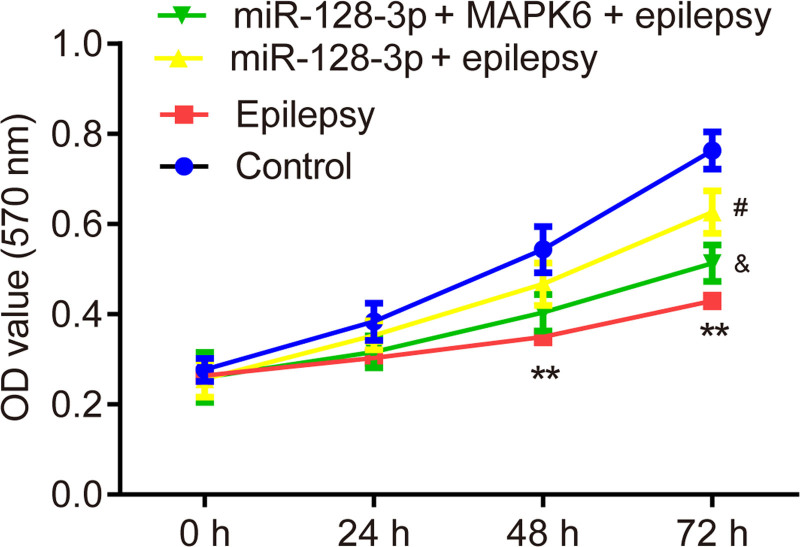
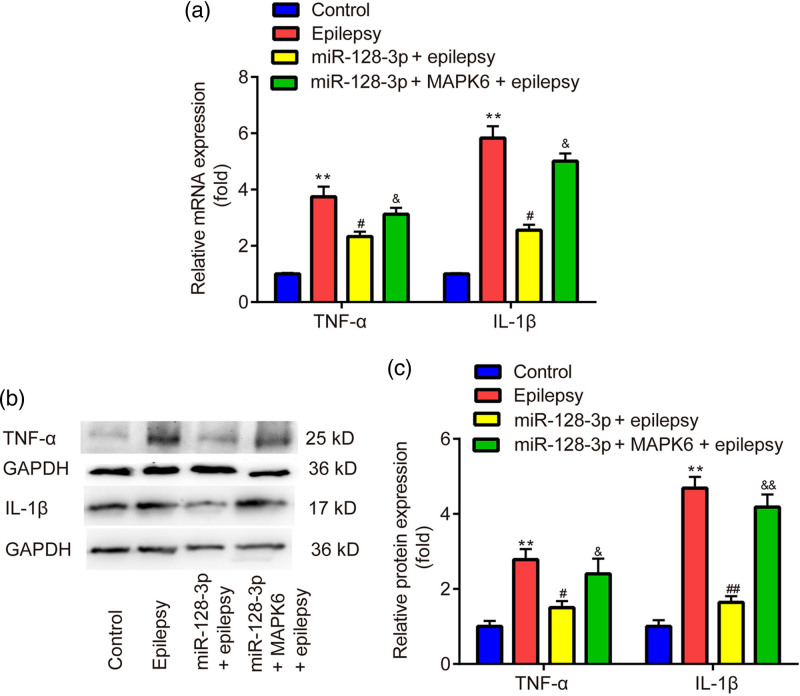
The results of the MTT assay exhibited that, overexpression of miR-128-3p reversed the inhibitory effects of penicillin on cell viability in primary astrocytes, which was partially abolished by the overexpression of MAPK6 (Fig. 4). The results of RT‑qPCR and western blot showed that overexpression of miR-128-3p reversed the upregulation of mRNA (Fig. 5a) and protein level (Fig. 5b,c) of TNF-α and IL-1β induced by epilepsy, which was partially abolished by the overexpression of MAPK6.
Fig. 4.
Effects of miR-128-3p on cell viability of penicillin-treated primary astrocytes. MTT assay is applied for the detection of cell viability. **P < 0.01, epilepsy vs. control. #P < 0.05, miR-128-3p + epilepsy vs. epilepsy. &P < 0.05, miR-128-3p + MAPK6 + epilepsy vs. miR-128-3p + epilepsy. Control, miR-NC + pcDNA3.1 + without penicillin; miR-128-3p, miR-128-3p mimic; MAPK6, pcDNA3.1-MAPK6.
Fig. 5.
Effects of miR-128-3p on inflammation of penicillin-treated primary astrocytes. (a) RT‑qPCR is utilized to evaluate the mRNA level of TNF-α and IL-1β. (b, c) Western blot is applied for detecting the protein level of TNF-α and IL-1β. **P < 0.01, epilepsy vs. control. ##P < 0.01, miR-128-3p + epilepsy vs. epilepsy. &P < 0.05, miR-128-3p + MAPK6 + epilepsy vs. miR-128-3p + epilepsy. Control, miR-NC + pcDNA3.1 + without penicillin; miR-128-3p, miR-128-3p mimic; MAPK6, pcDNA3.1-MAPK6.
The above findings exerted that in primary astrocytes, miR-128-3p rescued penicillin-induced cell injury and inflammation by regulating MAPK6.
Relation between miR-128-3p and MAPK6 in astrocytes
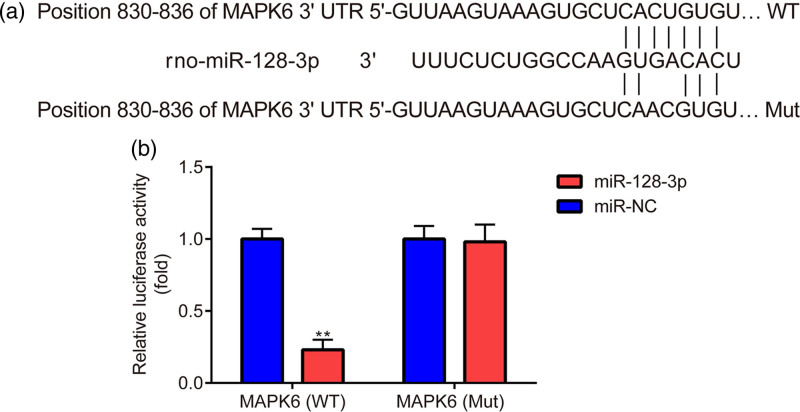
At last, we were eager to clarify the relationship between miR-128-3p and MAPK6 in primary astrocytes. As shown in Fig. 6a, MAPK6 3′UTR was predicted to be complementary to miR-128-3p. In addition, compared to the miR-NC mimic, the miR-128-3p mimic significantly decreased the luciferase activity of primary astrocytes transfected with WT MAPK6; however, the miR-128-3p mimic failed to decrease the luciferase activity of primary astrocytes transfected with Mut MAPK6 (Fig. 6b).
Fig. 6.
Relation between miR-128-3p and MAPK6 in astrocytes. (a) TargetScan 7.1 is used for the prediction of the interaction between MAPK6 and miR-128-3p. (b) Dual luciferase activity is used to verify the interaction between MAPK6 and miR-128-3p. **P < 0.01, miR-128-3p vs. miR-NC. miR-NC, miR-NC mimic; miR-128-3p, miR-128-3p mimic.
The results proposed that in primary astrocytes, miR-128-3p indeed targeted MAPK6 3′UTR.
Discussion
Epilepsy is one of the most frequent neurological disorders [26], which is characterized by epileptic seizures [3]. However, there is a lack of effective treatment strategies for epilepsy.
In the brain, astrocytes provide structural and functional support for neurons, also, they take part in ionic homeostasis and energy metabolism [27], synaptic network formation [28] and synaptic transmission [29], etc. Additionally, astrocytes which arouse and magnify immune-related mechanisms, are correlated with numerous human diseases, including epilepsy [30].
Mounting evidence have suggested the potential participation of inflammation in the injured brain during the progression of epilepsy [8,31]. For instance, an in vitro study has verified the potential of active astrocytes in producing inflammatory cytokines, evidenced by the upregulation of IL-1β and TNF-α in the tissues from the experimental and human epileptogenic brain [32].
Currently, we first demonstrated the reduced cell viability and elevated TNF-α and IL-1β levels in the penicillin-treated primary astrocytes. Afterward, penicillin-treated primary astrocytes were used as an experimental model of epilepsy for the subsequent experimentations.
miR-128-3p plays different roles (protective or deleterious) in different diseases, for instance, miR-128-3p prevents dopamine neurons from apoptosis by targeting AXIN1 in Parkinson’s disease (PD) [33], miR-128-3p accelerates the progression of Alzheimer’s disease (AD) by targeting PPARγ [34], and miR-128-3p worsens doxorubicin-induced liver injury by targeting Sirtuin-1 [35]. Regarding the role of miR-128-3p in epilepsy, it remains controversial. For example, miR-128-3p exerts deleterious function on neuron migration and outgrowth by targeting Phf6 [36]; however, 2R and 4R-APDC prevent hippocampal cells from apoptosis after seizure by increasing the expression of miR-128-3p [4]). Herein, we found decreased miR-128-3p level in penicillin-treated primary astrocytes, additionally, overexpression of miR-128-3p rescued penicillin-induced downregulation of cell viability, and upregulation of IL-1β and TNF-α in primary astrocytes, which suggested the protective role of miR-128-3p in epilepsy, and were consistent with the findings of Feng et al. [4].
As miRNAs can inhibit protein expression by inhibiting mRNA stability and translation [10,11]. The genes which are involved in inflammation and can be targeted by miR-128-3p attracted our attention.
As well-known, the MAPK signaling pathway plays a role in numerous cellular processes including inflammation [15]. Among these, MAPK6 is also found to be dysregulated in inflammation, and can be targeted by various miRNAs. For instance, let7f-5p inhibits inflammation by targeting MAPK6 in pneumonia [16]; MAPK6 regulates the production of IL-8 and chemotaxis in breast cancer [17]. However, the role of MAPK6 as well as the relation between miR-128-3p and MAPK6 in epilepsy has not been reported yet.
Herein, we found increased MAPK6 levels in penicillin-treated primary astrocytes, and overexpression of MAPK6 partially reversed the protective role of miR-128-3p on penicillin-induced primary astrocytes. By searching the online tool TargetScan 7.1 (http://www.targetscan.org/vert_71/), the interaction between miR-128-3p and MAPK6 exhibited a relatively higher context++ score percentile, ie, 90. Moreover, miR-128-3p was identified to target MAPK6 3’UTR, indicating the involvement of MAPK6 in the progression of epilepsy.
Taken together, miR-128-3p reverses penicillin-induced cell injury and inflammation in astrocytes by targeting MAPK6, providing a protective role in epilepsy.
There are two limitations in the current study, (1) only a few measures of cell damage and protection were presented, no functional studies were conducted, which will be investigated in the future study; (2) The penicillin model was used to replicate aspects of astrocytic damage in epilepsy, but it is vetted over 30 years ago, which will be improved in the future study.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Espínola-Nadurille M, Crail-Melendez D, Sánchez-Guzmán MA. Stigma experience of people with epilepsy in Mexico and views of health care providers. Epilepsy Behav 2014;32:162–169. [DOI] [PubMed] [Google Scholar]
- 2.Kanner AM, Meador KJ. Remember…there is more to epilepsy than seizures! Neurology 2015;85:1094–1095. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017;88:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cilio MR, Sogawa Y, Cha BH, Liu X, Huang LT, Holmes GL. Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia 2003;44:518–528. [DOI] [PubMed] [Google Scholar]
- 5.Zucchini S, Buzzi A, Barbieri M, Rodi D, Paradiso B, Binaschi A, et al. Fgf-2 overexpression increases excitability and seizure susceptibility but decreases seizure-induced cell loss. J Neurosci 2008;28:13112–13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes FG, Gomes Da Silva S, Cavalheiro EA, Arida RM. Beneficial influence of physical exercise following status epilepticus in the immature brain of rats. Neuroscience 2014;274:69–81. [DOI] [PubMed] [Google Scholar]
- 7.Librizzi L, Noè F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol 2012;72:82–90. [DOI] [PubMed] [Google Scholar]
- 8.Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol 2017;30:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker MC. Pathophysiology of status epilepticus. Neurosci Lett 2018;667:84–91. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y. The Challenge of microRNA as a Biomarker of Epilepsy. Curr Neuropharmacol 2018;16:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reschke CR, Henshall DC. microRNA and Epilepsy. Adv Exp Med Biol 2015;888:41–70. [DOI] [PubMed] [Google Scholar]
- 12.Tan CL, Plotkin JL, Venø MT, von Schimmelmann M, Feinberg P, Mann S, et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 2013;342:1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsharafi W, Xiao B. Dynamic expression of MicroRNAs (183, 135a, 125b, 128, 30c and 27a) in the rat pilocarpine model and temporal lobe epilepsy patients. CNS Neurol Disord Drug Targets 2015;14:1096–1102. [DOI] [PubMed] [Google Scholar]
- 14.Feng YB, Lin YT, Han YX, Pang YJ, Xu JJ, Xue Y, Yao H. 2R,4R-APDC, a Metabotropic glutamate receptor agonist, reduced neuronal apoptosis by upregulating microRNA-128 in a rat model after seizures. Neurochem Res 2018;43:591–599. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001;81:807–869. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Song Q, Ouyang Z, Zhang X, Zhang C. let7f‑5p attenuates inflammatory injury in in vitro pneumonia models by targeting MAPK6. Mol Med Rep 2021;23:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogucka K, Pompaiah M, Marini F, Binder H, Harms G, Kaulich M, et al. ERK3/MAPK6 controls IL-8 production and chemotaxis. Elife 2020;9:e52511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Su Z, Liu HL, Li L, Wei M, Ge DJ, Zhang ZJ. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed Pharmacother 2018;107:644–649. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Chen Y, Du Y, Wan Q, Xu Y, Wu J. Astragaloside IV attenuates penicillin-induced epilepsy via inhibiting activation of the MAPK signaling pathway. Mol Med Rep 2018;17:643–647. [DOI] [PubMed] [Google Scholar]
- 20.Taskiran M, Tasdemir A, Ayyildiz N, Ayyildiz M, Agar E. The effect of serotonin on penicillin-induced epileptiform activity. Int J Neurosci 2019;129:687–697. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RS. Animal models of the epilepsies. Brain Res Brain Res Rev 1989;14:245–278. [DOI] [PubMed] [Google Scholar]
- 22.Akdogan I, Adiguzel E, Yilmaz I, Ozdemir MB, Sahiner M, Tufan AC. Penicillin-induced epilepsy model in rats: dose-dependant effect on hippocampal volume and neuron number. Brain Res Bull 2008;77:172–177. [DOI] [PubMed] [Google Scholar]
- 23.Twyman RE, Green RM, MacDonald RL. Kinetics of open channel block by penicillin of single GABAA receptor channels from mouse spinal cord neurones in culture. J Physiol 1992;445:97–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arzimanoglou A, Hirsch E, Nehlig A, Castelnau P, Gressens P, Pereira de Vasconcelos A. Epilepsy and neuroprotection: an illustrated review. Epileptic Disord 2002;4:173–182. [PubMed] [Google Scholar]
- 25.Özdemir MB, Akça H, Erdoğan Ç, Tokgün O, Demiray A, Semin F, Becerir C. Protective effect of insulin and glucose at different concentrations on penicillin-induced astrocyte death on the primer astroglial cell line. Neural Regen Res 2012;7:1895–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsk R. How common are the ‘common’ neurologic disorders?. Neurol 2007;68:326–337. [DOI] [PubMed] [Google Scholar]
- 27.Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol 2012;814:3–7. [DOI] [PubMed] [Google Scholar]
- 28.Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last). Trends Neurosci 2003;26:520–522. [DOI] [PubMed] [Google Scholar]
- 29.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, et al. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci 2015;18:219–226. [DOI] [PubMed] [Google Scholar]
- 30.Seifert G, Carmignoto G, Steinhäuser C. Astrocyte dysfunction in epilepsy. Brain Res Rev 2010;63:212–221. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Nordli DR, Jr, Alden TD, DiPatri A, Jr, Laux L, Kelley K, et al. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J Neuroinflammation 2009;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronica E, Crino PB. Inflammation in epilepsy: Clinical observations. Epilepsia 2011;52(Suppl 3):S26–S32. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Yang L, Li YJ, Mei R, Yu HL, Gong Y, et al. MicroRNA-128 protects dopamine neurons from apoptosis and upregulates the expression of excitatory amino acid transporter 4 in Parkinson’s disease by binding to AXIN1. Cell Physiol Biochem 2018;51:2275–2289. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhang Y, Liu P, Bai H, Li X, Xiao J, et al. MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. Eur J Pharmacol 2019;843:134–144. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Jin Y, Li L, Xu L, Tang Z, Qi Y, et al. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol Res 2019;146:104276. [DOI] [PubMed] [Google Scholar]
- 36.Franzoni E, Booker SA, Parthasarathy S, Rehfeld F, Grosser S, Srivatsa S, et al. miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf6. Elife 2015;4:e04263. [DOI] [PMC free article] [PubMed] [Google Scholar]