Abstract
COVID-19 infection is an ongoing catastrophic global pandemic with significant morbidity and mortality that affects most of the world population. Respiratory manifestations predominate and largely determine patient prognosis, but gastrointestinal (GI) manifestations also frequently contribute to patient morbidity and occasionally affect mortality. GI bleeding is usually noted after hospital admission and is often one aspect of this multisystem infectious disease. Although the theoretical risk of contracting COVID-19 from GI endoscopy performed on COVID-19-infected patients remains, the actual risk does not seem to be high. The introduction of PPE and widespread vaccination gradually increased the safety and frequency of performing GI endoscopy in COVID-19-infected patients. Three important aspects of GI bleeding in COVID-19-infected patients are (1) GI bleeding is often from mucosal erosions from mucosal infalammation that causes mild GI bleeding; (2) severe upper GI bleeding is often from PUD or stress gastritis from COVID-19 pneumonia; and (3) lower GI bleeding frequently arises from ischemic colitis associated with thromboses and hypercoagulopathy from COVID-19 infection. The literature concerning GI bleeding in COVID-19 patients is presently reviewed.
Keywords: COVID-19, Pandemic, SARS, Gastrointestinal bleeding, Esophagogastroduodenoscopy, Colonoscopy, Therapeutic endoscopy, Endoscopy safety
Key points
-
•
Gastrointestinal bleeding occurs in about 1.5% to 3.0% of patients hospitalized with COVID-19 infection.
-
•
GI bleeding is generally mild-to-moderate with findings of mild-to-moderate mucosal inflammation, but the bleeding is occasionally severe and life-threatening, especially when from peptic ulcer disease or stress gastritis associated with severe COVID-19 pneumonia.
-
•
Lower GI bleeding associated with COVID-19 infection is frequently due to ischemic colitis, associated with thromboembolism and a hypercoagulable state, that is in turn associated with COVID-19 infection.
-
•
GI bleeding can occasionally contribute to morbidity in COVID-19 infected patients, but rarely contribute to mortality in patients hospitalized with COVID-19 infection.
-
•
Although at the pandemic onset, GI bleeding was rarely evaluated by GI endoscopy in COVID-19-infected patients, patients with moderate GI bleeding from COVID-19 infection are increasingly undergoing semi-elective GI endoscopy.
-
•
Patients with COVID-19 infection were initally intubated before undergoing EGD, but now patients undergoing EGD are generally intubated only for pneumonia and respiratory decompensation from COVID-19 infection.
Introduction
COVID-19 (coronavirus disease of 2019) infection caused by the highly contagious severe acute respiratory virus coronavirus 2 (SARS-CoV-2 virus) is responsible for the current global pandemic that has caused about 600 million proven acute infections and about 6 million deaths worldwide as of September 2022, including about 1 million deaths in the United States.1 It predominantly causes respiratory disease, most prominently pneumonia that can cause respiratory failure. The initial phase of the pandemic in the United States (March-May 2020) caused considerable mortality, especially in those with risk factors of obesity, diabetes mellitus, immunosuppression, other significant comorbidities, and old age. Subsequent phases were characterized by decreased mortality, but increased contagiousness associated with newly emerging viral strains from genetic mutations. Vaccines are now available in much of the developed world that are moderately effective in preventing infection as are specific medications, such as nirmatrelvir (Paxlovid) and ritonavir, that can decrease infection severity and shorten disease duration. Public health measures, such as facemasks and patient isolation techniques, that vary regionally, likely blunted the pandemic spread. Nonetheless, COVID-19 infection remains an ongoing global crisis with notable morbidity and mortality.
Respiratory and systemic manifestations of COVID-19 infection, including dyspnea, cough, rhinorrhea, pyrexia, and malaise, predominate, but extrapulmonary manifestations occur frequently, including gastrointestinal (GI) and hepatic manifestations, such as anorexia, nausea, emesis, diarrhea, abdominal pain, and elevated transaminase levels. GI manifestations can produce morbidity and occasionally contribute to mortality.2
GI bleeding in COVID-19 subjects is uncommon. A meta-analysis of symptoms among greater than 25,000 COVID-19 subjects noted anorexia in 20%, diarrhea in 13%, and hematemesis in 9%.3 However, the incidence of GI bleeding in this study is much higher than that reported in most clinical studies at 1.5% to 3.0%. GI bleeding is rarely the initial manifestation of COVID-19 infection4 and most commonly manifests after hospitalization for COVID-19 infection.5, 6, 7 GI bleeding usually arises from the upper GI tract, and manifests as anemia, melena, or hematemesis and less commonly arises from the lower GI tract and manifests as hematochezia, or occasionally melena. GI bleeding occurs more frequently in older patients, especially men, and especially in those with comorbidities.8
In a large meta-analysis, about 27% of infected subjects shed COVID-19 RNA in their stool.3 The SARS-CoV-2 angiotensin-converting enzyme-2 receptor is highly expressed in the gut and this receptor provides a portal of entry for the virus, especially in the stomach and small intestine, which can cause local GI infection and clinical manifestations, including GI inflammation and GI bleeding.9 The host immune response, including potential cytokine storm, multiorgan viral involvement, and side reactions of administered medications, such as corticosteroids or anticoagulants, may contribute to gut inflammation and exacerbate the GI bleeding.10 GI bleeding from COVID-19 is usually self-limited even when caused by a “double-hit” of an initial viral cytopathic effect and secondary immune-mediated or medication-induced side effects.11 Patients with severe COVID-19 infection may occasionally develop massive GI bleeding from physiologic stress, caused by sepsis, severe pneumonia, and respiratory or multiorgan failure, akin to the historically important phenomena of Cushing ulcer, related to increased intracranial pressure, and Curling ulcer, related to severe and extensive burns.12 Stress gastritis and peptic ulcer disease (PUD) are common in patients with pneumonia and respiratory failure from COVID-19 infection, which constituted a common outcome in the early pandemic.13 The propensity of the GI tract to bleed is somewhat related to the disparate effects of hypercoagulopathy in COVID-19-infected patients (perhaps reducing their bleeding tendency), administration of prophylactic anticoagulation to counteract this hypercoagulopathy that potentially promotes GI bleeding, and occurrence of thromboses potentially causing mucosal ischemia or necrosis with GI bleeding from sloughing of GI mucosa or necrotic mucosal ulcers.14 In one case report a patient with COVID-19 infection and sickle cell disease had GI bleeding with nonerosive gastroduodenitis secondary to microthrombi.15
Methods
The literature was reviewed using two independent computer search engines, PubMed and Ovid. The literature review was continuously updated until nearly submitting this review to the Journal in September 2022. This work constitutes a semiquantitative review as defined in the article “The Impact of COVID-19 Infection on Miscellaneous Inflammatory Disorders in the Gastrointestinal Tract,” elsewhere in this issue. This semiquantitative review differs from a systematic review only by not listing articles identified by the computerized search, which were excluded from this review, and by not compiling their reasons for exclusion. This computerized literature review included the following search terms, key words, or phrases (with listing in parentheses identifying the number of articles found by PubMed per search term): esophagogastroduodenoscopy (EGD) and COVID (465), colonoscopy and COVID (220), flexible sigmoidoscopy and COVID (11), therapeutic GI endoscopy and COVID (139), enteroscopy and COVID (2), capsule endoscopy and COVID (29), balloon enteroscopy (endoscopy) and COVID (5), nasal endoscopy and COVID (135), endoscopic retrograde cholangiopancreatography and COVID (64), disposable endoscopes and COVID (39), computed tomography colonography and COVID (10), GI bleeding scan and COVID (3), mesenteric angiography and GI bleeding and COVID (5), therapeutic angiography and GI bleeding and COVID (3), hematemesis and COVID (25), GI bleeding and COVID (182), melena and COVID (35), hematochezia and COVID (148), fecal occult blood and COVID (84), fecal immunologic testing and COVID (610), iron deficiency anemia and COVID (40), colonoscopy screening and COVID (156), colonoscopy surveillance and COVID (104), colonic pseudo-obstruction and COVID (5), colonoscopy and decompression and COVID (2), peroral endoscopic myotomy and COVID (9), long COVID and GI endoscopy (8), GI endoscopy complications and COVID (33), GI endoscopy guidelines and COVID (33), and GI endoscopy and COVID transmission (193).
Specific GI disorders/diseases in the literature review included (with number of cited articles per search term listed in parentheses) included: Mallory-Weiss tear and COVID (2), esophageal toxicity and medications and COVID (2), nasogastric tube erosions and COVID (2), Kaposi sarcoma and COVID (24), peptic ulcer and COVID (41), gastric ulcers and COVID (21), duodenal ulcers and COVID (16), hemorrhagic esophagitis and COVID (34), Barrett esophagus and COVID (13), gastroesophageal reflux disease and COVID (38), gastrointestinal stromal tumor and COVID (10), gastric antral vascular ectasia and COVID (1), gastric leiomyoma and COVID (1), colon and adenomas and COVID (12), colon cancer screening and colonoscopy and COVID (32), mucosal associated lymphoid tissue-OMA and COVID (9), Cameron lesion and COVID (1), Dieulafoy lesion and COVID (0), gastric lymphoma and COVID (0), esophageal adenocarcinoma and COVID (12), esophagus squamous cell cancer and COVID (4), gastric adenocarcinoma and COVID (22), linitis plastica and COVID (0), paraesophageal hernia and COVID (7), hemobilia and COVID (1), hemosuccus pancreaticus and COVID (0), marginal or anastomotic ulcer and COVID (35), Billroth II and COVID (6), radiation proctitis and COVID (0), radiation-associated vascular ectasia and COVID (0), gastric varices and COVID (10), bleeding esophageal varices and COVID (7), portal hypertensive gastropathy and COVID (1), rectal ulcer and COVID (11), bleeding hemorrhoids and COVID (10), and rectal fissure and COVID (0).
Discussion
Prevalence
The current literature review reveals a low rate of GI bleeding in COVID-19 subjects, with most studies reporting a rate of 1.5% to 3%, with notable statistical outliers and considerable study variability. Moreover, much GI bleeding in COVID-19-infected patients is subclinical and decidedly less significant than the typical pulmonary manifestations of COVID-19. A global compilation of COVID-19 endoscopies noted up to nearly half of subjects had GI mucosal abnormalities, such as mucosal erosions.16 A large, COVID-19 cohort, mostly from Egypt, reported a high rate of GI bleeding from hematemesis in 9%, melena in 5.3%, hematochezia in 0.6%, and fecal-occult positive stools in 5%.3 Contrariwise, systematic review of 12 studies reported a prevalence of GI bleeding of only 0.06%.17 After excluding two outlier studies, another meta-analysis of 91,887 COVID-19 subjects noted a 2.0% pooled prevalence of GI bleeding of whom 77% had upper GI bleeding.18 A European cohort of 4,128 COVID-19 subjects noted a 1.8% prevalence of GI bleeding.19 An Italian cohort of 4,871 COVID-19-infected patients noted a 0.5% prevalence of upper GI bleeding.20 A New York cohort of 11,158 COVID-19 subjects reported a prevalence of 3% of GI bleeding,21 whereas another New York cohort of 1,206 COVID-19-infected patients reported a 3.1% prevalence of GI bleeding.22 A Spanish cohort of 74,814 COVID-19-infected patients had a prevalence of GI bleeding of 1.1%,23 whereas a Chinese cohort of 36,358 COVID-19 subjects reported a 2% prevalence of GI bleeding.24 These disparate results reflect differences in criteria for GI bleeding, different patient demographics, different countries, different rates of COVID-19 infection, and different study designs (letters to the editor,11 case reports, case series,4 , 12 case-controlled studies, retrospective studies, prospective studies, systematic reviews,17 and meta-analyses3 , 18). The literature on GI bleeding associated with COVID-19 infection has been sparse until recently relative to the overall number of COVID-19-related publications and much less than that reported for several other GI manifestations. In particular, few studies have reported on GI endoscopic findings because of the scarcity of performing EGD for GI bleeding in patients with COVID-19 infection.
Evaluation of Gastrointestinal Bleeders Before Endoscopy
Gastroenterologists should promptly obtain a complete but directed medical history with a focus on GI disease in any patient with acute, overt GI bleeding, but particularly when associated with severe COVID-19 infection. The GI history should focus on prior GI bleeding and its cause, prior GI endoscopies and their findings, and endoscopic therapies because patients often bleed from the same GI lesions that they had previously bled from.25 Patients should be asked about prior GI surgery and prior GI radiologic findings. The patient should be specifically asked about medicines administered for GI diseases, including histamine-2 receptor antagonists; proton pump inhibitors (PPIs); medications for inflammatory bowel disease; chemotherapy for GI cancers; gastrotoxic medications that increase the risk of GI bleeding, such as aspirin; cyclooxygenase-II inhibitors; nonsteroidal anti-inflammatory drugs; esophagotoxic medications (bisphosphonates, doxycycline); and anticoagulants (including coumadin, unfractionated heparin, low molecular heparin, clopidogrel, and rivaroxaban).
The medical history provides clues to the cause of the GI bleed. A history of alcoholism, jaundice, or liver disease increases the likelihood of cirrhosis, portal hypertension, and bleeding from esophageal varices or portal hypertensive gastropathy.25 A history of smoking cigarettes increases the risk of duodenal ulcers. The patient should be asked about prior Helicobacter pylori infection and its therapy.
The patient with acute and overt GI bleeding should undergo a focused physical examination that includes vital signs to help assess the need for fluid resuscitation or blood transfusions; careful abdominal examination including the presence, location, intensity, and pitch of bowel sounds; abdominal tenderness; presence of rebound tenderness; and presence of splenomegaly. Stigmata of chronic liver disease should be searched for, including hepatomegaly, spider angiomas, palmar erythema, caput medusa, Dupuytren contracture, jaundice, asterixis, and ascites because such signs suggest GI bleeding from portal hypertension or cirrhosis. Abdominal tenderness is uncommon with upper GI bleeding, except occasionally from PUD. Severe abdominal pain suggests bowel ischemia, GI obstruction, or GI perforation. Severe direct abdominal tenderness, rebound tenderness, or involuntary guarding suggests a possible acute (surgical) abdomen, which must be excluded before performing EGD for GI bleeding. Air under the diaphragm (free intraperitoneal air) on abdominal roentgenogram or on abdominal computed tomography strongly suggests an acute abdomen. Rectal examination should be carefully performed to assess the patient for the presence, type, and quantity of rectal bleeding and check for anal masses, hemorrhoids, anal fissures, and anal stenoses. Nasogastric tube aspiration can help check for gross blood in the upper GI tract and clear the stomach of residual blood for unobstructed gastric visualization at EGD.
The presentation and appearance of the blood offers clues to the site of GI bleeding, its acuity, and its severity. Melena, recognized as black and tarry stools, mostly (90%) arises from an upper GI lesion because of degradation of blood with GI transit, but occasionally (10%) arises from small intestinal or right colonic lesions in the presence of slowed intestinal transit. Hematemesis indicates bleeding proximal to the ligament of Treitz.25 Hemorrhoidal bleeding classically presents with a clinical triad of bright red blood per rectum caused by arterialized blood from the hemorrhoidal plexus, blood coating rather than admixed with stools, and postdefecatory bleeding caused by traumatic stool evacuation. Hypotension, orthostatic hypotension, tachycardia at rest, mental confusion, orthostatic dizziness, cold and clammy extremities, angina, and severe palpitations may suggest hemodynamic compromise from severe GI bleeding.25 Cutaneous ecchymoses or petechia are signs of a coagulopathy.
Laboratory Evaluation for the Gastrointestinal Bleeder
Patients should have blood samples determined for routine electrolytes, chemistries, blood sugar, blood urea nitrogen (BUN), creatinine, liver function tests, lipase, and other standard biochemical parameters. Patients should have a complete blood count, including hematocrit, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, platelet count, leukocyte count, and leukocyte differential. The international normalized ratio of the prothrombin time should be determined. Patients with upper GI bleeding typically have an elevated BUN level with a BUN/creatinine ratio greater than 20:1 because of the combined effects of prerenal azotemia from hypovolemia and BUN elevation from GI resorption of degraded blood during intestinal transit.25 Iron level, ferritin level, and percent iron saturation are useful to demonstrate iron deficiency from chronic GI blood loss. The medical history, physical examination, and initial laboratory values are important to assess resuscitation requirements for intravenous fluids or blood transfusions, triage to regular hospital beds versus monitored beds versus an intensive care unit (ICU), and timing of endoscopy including emergency or urgent endoscopy versus delayed or deferred endoscopy. Other consultative services may be urgently required for the GI bleeder, including surgery, radiology, and the intensivist.
Patient Stabilization Before Gastrointestinal Endoscopy
All patients with acute GI bleeding require hemodynamic evaluation and stabilization of vital signs before evaluation for potential GI endoscopy. Evaluation and stabilization is particularly important in patients with significant COVID-19 infection who may suffer from multiorgan failure including respiratory compromise from severe pneumonia, acute kidney injury or chronic kidney disease with chronically depressed glomerular filtration rate, and coagulation disorders. Respiratory compromise from COVID-19 pneumonia may not necessarily present with overt hypoxia but may be subtly suggested by other abnormal vital signs, unexplained acidosis, restlessness, or unexplained severe pyrexia or sepsis. Prerenal azotemia contributed by GI bleeding may exacerbate acute kidney injury. Hematocrit levels should be followed serially with time. A serial hematocrit decline may signify significant GI blood loss because of hemodilution to compensate for acute blood loss, but a hematocrit decline is a lagging indicator of blood loss.
Patients with overt GI bleeding should have two secure, large-bore, intravenous lines placed to ensure adequate fluid infusion for volume resuscitation. Patients with overt bleeding should be typed and crossed for potential transfusions of packed erythrocytes. All patients with acute upper GI bleeding should initially receive PPI therapy continuously and intravenously. This is most important for PUD or hemorrhagic esophagitis because these disorders are related to gastric acidity, but PPI therapy is also recommended for upper GI bleeding of any cause before performing EGD because the cause of the GI bleeding is usually uncertain before EGD and maintaining the intraluminal gastric pH greater than five stabilizes a gastric clot and prevents rebleeding of GI lesions.
Patients with potential hemodynamic instability manifested by systemic hypotension, orthostatic hypotension, or significant orthostatic changes (orthostasis) with evident active GI bleeding should be evaluated for an ICU bed. Admission to an ICU bed for blood transfusions is even more important in patients with concomitant COVID-19 pneumonia because of potential respiratory decompensation from fluid overload. In patients with active GI bleeding the preferred therapy for hypovolemia is transfusion of packed erythrocytes rather than intravenous colloid infusion or normal saline. Despite a current shortage of blood products attributed to the effects of the omicron surge during the pandemic,26 blood transfusions for acute GI bleeders are generally considered a medical emergency.
Conservative Management with Deferral of Gastrointestinal Endoscopy
COVID-19 is usually transmitted from infected individuals via fine respiratory droplets. Aerosolization promotes spread with some potential for viral transmission when droplets land on surfaces. GI endoscopy, especially EGD, is a moderately high-risk procedure for COVID-19 transmission to the endoscopist and endoscopy staff because of aerosolization of droplets expired by COVID-19-infected patients. This transmission risk and the diversion of medical resources to counter the COVID-19 pandemic underlies the reluctance or deferral of performing GI endoscopy for hospitalized patients for nonurgent indications. This approach was historically reflected in publications noting the “dilemma” of performing endoscopy and the “less is more” attitude regarding performing GI endoscopy for GI bleeding in patients with COVID-19 infection.27, 28, 29 A higher threshold for performing endoscopy for GI bleeding was proposed in COVID-19-infected patients, reflected semiquantitatively by higher Glasgow-Blatchford scores of GI bleeding severity.30 , 31 Guidelines promulgated by different national professional gastroenterology societies throughout the world reflect a conservative approach to GI endoscopy in COVID-19-infected patients, including deferral of GI endoscopy if possible.32, 33, 34 In the early pandemic (Spring 2020) rates of GI endoscopy plummeted by 10-fold from the prepandemic baseline rate.35 Most GI bleeding is subclinical and therefore does not require GI endoscopy, but even overt GI bleeding was sometimes approached conservatively without GI endoscopy in COVID-19-infected patients. The pandemic generally diminished endoscopic volume for COVID-19 and non-COVID-19 patients, but this diminished volume did not adversely affect patient outcomes in a Canadian cohort study.36 Nonetheless, COVID-19-infected patients with hemodynamically significant bleeding should be strongly considered for EGD after patient stabilization including volume resuscitation or packed erythrocyte transfusions and stabilization of the respiratory status.37
COVID-19-infected patients with GI bleeding are more likely to have a longer hospital stay, and less frequently undergo GI endoscopy than GI bleeders without COVID-19 infection.38 Furthermore, upper GI hemorrhage in COVID-19 patients is positively associated with ICU admission and mechanical ventilation.39 Longer time to GI endoscopy in COVID-19 subjects does not negatively impact their clinical outcomes.20 , 40 GI bleeding in COVID-19 subjects is often subclinical or at least self-limited.41 A conservative approach may be justified. A small cohort of COVID-19 subjects with GI hemorrhage did well with conservative management and the author suggested a 24-hour waiting period before evaluating GI bleeders for GI endoscopy, a position endorsed by a journal editorial.42 , 43 The general principles of managing bleeders with COVID-19 infection are summarized in Box 1 , with an emphasis on differences between COVID-19-infected versus noninfected patients.
Box 1. General principles of GI bleeding in COVID-19-infected patients.
-
1.
Literature on GI bleeding in COVID-19-infected patients is sparse relative to respiratory manifestations or many other GI disorders. This may relate to relative infrequency of performing GI endoscopy because of perceived risks to patients and endoscopy staff from the infection.
-
2.
Higher threshold and less urgency for performing GI endoscopy on COVID-19-infected patients. May relate to perceived risks to patients and to endoscopy staff during the pandemic.
-
3.
Often GI bleeding in COVID-19-infected patients is clinically mild and does not require GI endoscopy.
-
4.
GI endoscopy may be deferred in acutely ill patients with mild-to-moderate GI bleeding because such patients often have multiple acute medical problems, especially respiratory failure.
-
5.
Concern about transmission of COVID-19 to endoscopic staff during GI endoscopy. However, the risk to GI endoscopic staff of contracting COVID-19 infection during GI endoscopy is apparently not high if strict universal precautions are applied to all GI endoscopies performed during the pandemic.
-
6.
Concern about prophylactic endotracheal intubation for EGD. In the initial stages of the pandemic (March-April 2020), endotracheal intubation was nearly universally performed for EGD, but subsequently endotracheal intubation is rarely and selectively performed for EGD.
-
7.
Most critically ill COVID-19-infected patients receive anticoagulation, which must usually be withheld periprocedurally.
-
8.
Modest need for endoscopic hemostasis during GI endoscopy in COVID-19-infected patients because the bleeding is often not severe. Endoscopic therapy may not be feasible in patients receiving anticoagulation (see point 7).
-
9.
Need for percutaneous endoscopic gastrostomy in intubated and ventilated patients with COVID-19 infection but fortunately rarely causes bleeding complications.
-
10.
Therapies often used in patients with COVID-19 infection, including corticosteroids, anticoagulation, and tocilizumab, negatively impact the GI tract and enhance GI bleeding.
In a retrospective study, 24 (1.8%) of 1342 patients with COVID-19 presented with GI bleeding of whom 23 patients had upper GI bleeding, including 22 (91.6%) with evident cirrhosis and 21 presented with upper GI bleeding from esophageal varices. Two patients without cirrhosis were presumed to have nonvariceal bleeding. Medical therapy for esophageal variceal bleeding included vasoconstrictors, either somatostatin in 17 (73.9%) or terlipressin in four (17.4%). All patients with upper GI bleeding received PPIs and antibiotics. Fourteen patients (60.9%) were transfused packed erythrocytes, with a median transfusion of one unit. Initial control of upper GI bleeding was achieved in all 23 patients and none required emergency GI endoscopy. At 5-day follow-up, none rebled or died. Two patients later rebled; one from intermittently bleeding gastric antral vascular ectasia, and another rebled 19 days after hospital discharge. Three (12.5%) patients with cirrhosis succumbed to acute hypoxemic respiratory failure during the hospitalization. This study suggests that conservative management strategies including pharmacotherapy, and restrictive transfusions with close hemodynamic monitoring can successfully manage GI bleeding in COVID-19 patients and reduce their need for urgent GI endoscopy. The decision to proceed to GI endoscopy should be performed by a multidisciplinary team emphasizing limited use of GI endoscopy.44
Concerns regarding endoscopists contracting COVID-19 infection while performing conventional (tube) GI endoscopy have promoted alternative tests for diagnosis and treatment of GI bleeding, such as disposable endoscopes,45 experimental robotic endoscopes,46 telemetric sensor capsules,47 small intestinal capsules for screening before GI enteroscopy,48 and colonic capsules for screening before colonoscopy49 to reduce the endoscopist’s risks. A novel barrier device may minimize viral transmission from infected patients to endoscopists.50 For example, positioning the endoscopist’s head away from the patient’s mouth during EGD decreased the endoscopist’s facial exposure to visible droplets from 87% at 70 cm distance to 0% at 100 cm distance (P < 0.001) and thereby theoretically reduced transmission of COVID-19 infection to the endoscopist during EGD. Moreover, a physical barrier device reduced endoscopist’s exposure to droplets to 0% at all distances.50 A European study suggested an interventional radiologic approach to GI bleeding among 11 COVID-19 subjects to reduce transmission of COVID-19 to examining physicians.51 The current supply of intravenous contrast medium is insufficient because of the pandemic.52
Suggested Approach to Gastrointestinal Bleeding in COVID-19 Subjects
The advent of COVID-19 vaccination and personalized protective equipment (PPE) for endoscopic staff minimizes potential COVID-19 transmission during GI endoscopy resulting in greater safety to endoscopic staff. PPE should optimally include an N95 mask or equivalent, a disposable, waterproof (impervious) surgical gown, safety goggles, face shield, hair cap, and protective footwear.53 The endoscopist and the endoscopy staff should be offered such PPE. Endoscopists should be fitted for appropriately sized N95 masks and briefly trained on how to wear them. It is important to use new N95 masks at least daily. The endoscopist should be offered the option of thicker surgical gloves (eg, Microflex NeoPro NPG888 synthetic chloroprene disposable gloves) insead of the standard thin disposable gloves.
The endoscopy suite should be thoroughly cleaned between endoscopic cases with viricidal antiseptic, sterilizing solutions that are highly effective against COVID-19, concentrating on endoscopic equipment, endoscopic accessories, and the endoscopy cart to prevent COVID-19 transmission during GI endoscopy. At the endoscopy center where I worked antiseptic solutions were changed to use antiseptic solutions with proven high efficacy against COVID-19 soon after the pandemic onset throughout all clinical areas, including the sinks of bathrooms.35 With all the aforementioned precautions, COVID-19 transmission to endoscopy staff during endoscopy is reported but highly uncommon.54 GI endoscopy should be performed judiciously and conservatively in COVID-19 subjects, with less urgency and lower priority than that recommended for noninfected patients. Elective or semielective endoscopy in COVID-19-infected patients may be reasonably deferred until after the patient becomes noninfected, as proven by nasal swabs testing for the viral RNA of COVID-19. It may be helpful to perform GI endoscopy on COVID-19-infected patients as the last case on the normal daily endoscopy schedule to permit extra time to disinfect the room after this last case. Endoscopy staff should minimize their time exposed to a COVID-19-infected patient. For example, they should generally avoid being present in the endoscopy suite during intubation and extubation in infected patients.
In the early pandemic (March-May 2020), GI fellows were excused from performing GI endoscopy on their patients and attending GI endoscopists were required to perform GI endoscopy by themselves. This concept was irrational and unsubstantiated by data. GI fellows tend to be young, healthy, and at low risk of serious complications from COVID-19 infection, whereas GI attendings are often old (with about half of them older than 65 years old),55 , 56 less healthy, with a higher incidence of comorbidities, and at high risk of severe COVID-19 complications. This recommendation negatively impacted endoscopic training of GI fellows who performed few GI endoscopies in March-May 2020 and this recommendation was withdrawn by May 2020.35 , 56 Even currently, in 2022, GI fellows report less training in GI endoscopy than fellows trained before the pandemic began because of not performing GI endoscopy during the pandemic onset and the lingering effects of the ongoing pandemic on somewhat restricting GI endoscopy.
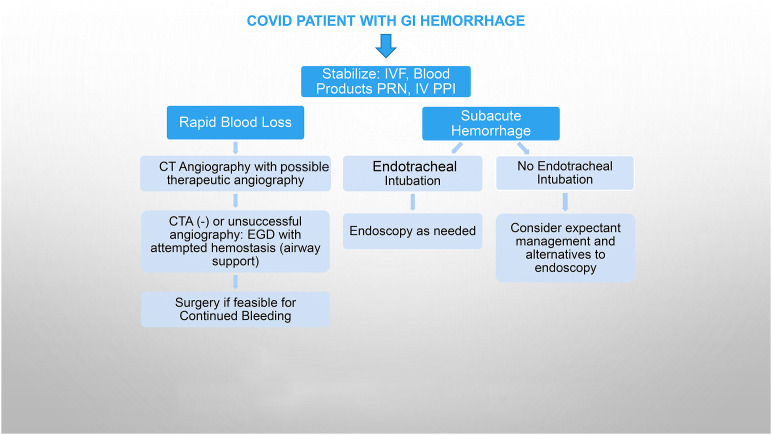
In the beginning of the pandemic, all patients with proven COVID-19 infection were intubated prophylactically before EGD.25 However, universal endotracheal intubation for EGD in COVID-19 patients entails potential difficulty of patient extubation postprocedure, especially for patients with COVID-19 pneumonia and respiratory compromise with consequent deleterious effects on patient prognosis.57 Thus, endotracheal intubation for GI endoscopies should generally be avoided, if possible, when advocated solely for the indication of COVID-19 infection without additional reasons. However, this practice varies according to local endoscopy practice patterns, local prevalence of COVID-19 infection, and institutional policies. Ancillary services, such as interventional radiology, nuclear medicine, or GI surgery, may decrease the need for GI endoscopic intervention (Fig. 1 ).
Fig. 1.
Approach to GI bleed in COVID patient. Expectant management: monitor, transfuse PRN, reversal anticoagulation, and antiplatelet drugs if feasible. Alternative methods: nuclear bleeding scan, CTA, capsule endoscopy. CT, computed tomography; CTA, computed tomography angiography; IV, intravenous; IVF, intravenous fluids.
Cause of Upper Gastrointestinal Tract Bleeding Proximal to Ligament of Treitz
PUD, including erosive gastritis and duodenitis, is the most common cause of upper GI bleeding in COVID-19-infected patients.16 , 18, 19, 20 , 58 A small series of COVID-19 subjects noted a poor prognosis from gastric ulcers.59 , 60 However, generalized mucosal inflammation and friability is a common leitmotif of GI infection with COVID-19 that causes minor GI blood loss.16 A multicenter study of 87 upper GI endoscopies reported upper GI ulcers in one-quarter of patients, with gastroduodenitis in 16%, and petechial/hemorrhagic gastropathy in 9%.16 In a New York cohort of 41 endoscopies in COVID-19-infected patients, EGD commonly revealed gastric/duodenal ulcers.60 An Italian cohort of 38 COVID-19 subjects undergoing EGD reported 37% had esophagitis, erosive gastritis, or peptic ulcers.61 Gastric erosions/ulcers in COVID-19 subjects may preferentially occur in the proximal stomach.62 Reflux esophagitis was a common cause of GI bleeding but was significantly less common than PUD.16 , 61 Esophageal variceal hemorrhage was also common in patients with COVID-19 infection,16 , 44 , 62 , 63 likely reflecting that liver disease is a risk factor for severe COVID-19 infection. One patient hospitalized with hematemesis had an esophageal ulcer at endoscopy with pathologic demonstration of local COVID-19 involvement.64 Case reports of GI hemorrhage include aortoenteric fistula,65 hemobilia,66 and hemorrhagic ulcerative duodenitis.67 One patient had Cameron lesions associated with acute COVID-19 infection. The Cameron lesion (ulcer) was caused by a large paraesophageal hernia that progressed to cause acute gastric volvulus because of the patient’s poor nutritional status from chronic COVID-19 infection.68 The reported causes of upper GI bleeding in COVID-19-infected patients, including rare causes, are listed in Table 1 .16 , 18, 19, 20 , 44 , 58 , 59 , 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73
Table 1.
Reported causes of upper GI bleeding proximal to the ligament of Treitz in patients with COVID-19 infection
| Lesion | Putative Mechanisms | Selected References |
|---|---|---|
| Angiodysplasia | 1 case reported in a large series of 87 EGDs performed mostly for overt GI bleeding | Vanella et al,16 2021 |
| Aortoenteric fistula | Case report | González-Sagredo et al,65 2021 |
| Dieulafoy lesion | 1 case reported in a large series of 87 EGDs mostly performed for overt GI bleeding | Vanella et al,16 2021 |
| Cameron lesion associated with acute COVID-19 infection | Case report Cameron lesion/ulcer was caused by a large paraesophageal hernia that progressed to cause acute gastric volvulus because of patient’s poor nutritional status from COVID-19 infection |
Deliwala et al,68 2021 |
| Esophageal candidiasis (without bleeding) | 2 cases among 87 patients undergoing EGD | Vanella et al,16 2021 |
| Esophageal ulcer | 1 case caused by primary COVID-19 infection 5 cases reported in a large endoscopic series of 87 EGDs performed mostly for overt GI bleeding |
Vanella et al,16 2021, Bisseling et al,64 2021 |
| Esophageal variceal hemorrhage | Increased reporting of esophageal variceal hemorrhage in COVID-19 infection may result from increased susceptibility of patients with cirrhosis, chronic liver disease, or advanced alcoholic liver disease to contracting COVID-19 infection | 16,44,62,63 |
| Esophagitis moderately frequent | Hemorrhagic esophagitis from gastroesophageal reflux disease is moderately common but less frequently reported in COVID-19 patients than peptic ulcer disease | Vanella et al,16 2021, Kuftinec et al,61 2021 |
| Gastric leiomyoma/inflammatory fibroid polyp | 1 case report of a patient with COVID-19-associated severe pneumonia requiring extracorporeal membrane oxygenation who had upper GI bleeding from a gastric inflammatory polyp successfully treated with endoscopic hemostasis | Mur-Murota et al,70 2021 |
| Gastrointestinal stromal tumor | Case report | Aguayo et al,71 2020 |
| Gastropathy- petechial/hemorrhagic | Common reported finding (occurring in 9.2%) in a large study of COVID-19 patients undergoing EGD | Vanella et al,16 2021 |
| Hemobilia | 2 case reports of hemobilia | Koc and Çiçek,66 2021 |
| Marginal (anastomotic) ulcer | Case report | Galvez et al,72 2020 |
| Portal hypertensive duodenopathy | 1 case reported in a large endoscopic series of 87 endoscopies mostly performed for overt GI bleeding | Vanella et al,16 2021 |
| Portal hypertensive gastropathy | Case report | Philips et al,73 2020 |
Diagnostic and Therapeutic Esophagogastroduodenoscopy
Limited data exist regarding upper GI endoscopy for COVID-19-related GI bleeding.58 EGD is often performed with respiratory support in critically ill patients, even rarely including extracorporeal membrane oxygenation.74 Principles of GI endoscopy in COVID-19-infected patients are summarized in Table 2 .
Table 2.
Gastrointestinal endoscopy for GI bleeding in patients with COVID-19 infection
| General Principles/Topic | Clinical Applications | Reasons |
|---|---|---|
| PCR testing for COVID-19 infection. | Many institutions with high institutional prevalence of COVID-19 infection standardly screen all patients scheduled for GI endoscopy by PCR testing for COVID-19 infection. Alternatively, institutions screen all patients with planned GI endoscopy for history of exposure to someone with known COVID-19 within <14 d, and for symptoms suspicious of COVID-19 (eg, cough, dyspnea, or diarrhea). Patients who have at least 1 such exposure or symptom should undergo PCR testing for SARS-CoV-2. |
Reduce COVID-19 exposure of endoscopy staff. |
| EGD is theoretically a high-risk procedure for transmitting COVID-19 infection from a patient with COVID-19 to endoscopy staff because of the presence of aerosolized infective droplets. However, the actual risk of transmission seems to be manageable. Risk to endoscopy staff may be higher in an infected patient with active overt hematemesis. | Endoscopy staff should strongly consider using PPE, including wearing an N95 face mask during endoscopy performed on COVID-19-infected patients. | Reduce personnel exposure to COVID-19 infection from infected patients. |
| Much less performance of EGD for elective indications in patients with active COVID-19 infection as compared with noninfected patients. | Generally, defer EGD for elective indications until patient recovers from acute COVID-19 infection. | Some risks to endoscopy staff from exposure to patient with COVID-19 infection during GI endoscopy. Patient with complicated acute COVID-19 infection may not tolerate EGD. Some patients with COVID-19 infection (eg, patients with severe pneumonia) may require prophylactic endotracheal intubation for EGD. |
| Similar frequency of performing EGD for emergency/urgent indications in patients with acute COVID-19 infection versus noninfected patients. | Perform EGD for overt life-threatening GI bleeding, when therapeutic EGD is likely needed, and when EGD is needed before contemplated GI surgery. | Cannot wait for patient to recover from acute COVID-19 infection when EGD is required emergently or urgently. Maximize patient hemodynamic stability and respiratory status before performing EGD. |
| Deferral of elective GI endoscopy in a COVID-19-infected patient. | Wait a few weeks after the acute infection until the patient tests negative by PCR on a new COVID-19 test. | Reduce risk to endoscopy staff and reduce risks of endoscopy in a patient with active COVID-19 infection. |
| Prophylactic intubation for EGD in COVID-19-infected patients. | In the initial pandemic surge (March-May 2020) patients were generally intubated before EGD. From June 2020 onward only selected patients underwent prophylactic intubation for specific reasons. | Reason for current selective policy for endotracheal intubation before EGD is difficulty in extubating patients with respiratory compromise (especially from COVID-19 pneumonia). |
| Precautions during EGD in COVID-19-infected patients. | Endoscopy staff should exercise universal precautions when performing EGD on all patients during the pandemic, but especially in performing EGD on COVID-19-infected patients. | EGD properly performed with precautions seems to result in a low risk of COVID-19 transmission to endoscopy staff. |
| Routine screening and surveillance colonoscopy often deferred in patients with active COVID-19 infection until after the patient clears the virus as proven by nasal swab. | No reason to subject patient to increased risks of elective colonoscopy when the patient has active COVID-19 infection. No reason to subject the endoscopy staff to the risks of contracting the virus from infected patients. | |
| Patients with GI bleeding associated with COVID-19 infection may have higher risks of morbidity and mortality than patients with GI bleeding without COVID-19 infection. | Patients with GI bleeding associated with severe COVID-19 pneumonia, respiratory compromise, and other serious complications of COVID-19 should generally be followed by an intensivist in an ICU. | Patients with severe pneumonia, respiratory compromise, and other serious complications of COVID-19 are at higher risk of mortality. |
| Protecting endoscopy personnel during EGD performed on a COVID-19-infected patient. | Endoscopy personnel in the endoscopy suite should be minimized during intubation and extubation of patients with COVID-19 infection. | Reduce exposure of endoscopy staff to COVID-19 infection. |
| Management of anticoagulation in COVID-19 patients with GI bleeding: these patients seem to have higher rates of thrombotic complications. Anticoagulation can complicate the management of GI bleeding. This may be particularly important in planning endoscopic therapy for overt, active GI bleed, which may require withholding anticoagulation just before and after therapeutic endoscopy. | Contemplated endoscopic therapy for overt, active GI bleed may require withholding anticoagulation just before and just after therapeutic endoscopy. | COVID-19 patients likely have higher rates of thrombotic complications. Anticoagulation can complicate the management of GI bleeding. This may be particularly important in planning endoscopic therapy for overt, active GI bleed, which may require withholding anticoagulation just before and after therapeutic endoscopy. |
| Telemedicine. | May be considered as an alternative to ambulatory physical patient visits for follow-up after GI endoscopy. | Reduce hospital staff exposure to COVID-19-infected patients. |
Abbreviation: PCR, polymerase chain reaction.
All the therapeutic modalities available in the general population of GI bleeders are available to patients infected with COVID-19 including injection therapy (with epinephrine or sclerosants), ablative therapy (electrocoagulation with bipolar electrocoagulation probe or Gold Probe [Boston Scientific, Marlborough, MA), noncontact methods (argon plasma coagulation), mechanical therapy (band ligation, hemoclips, or detachable snares), or Hemospray (Cook Medical, Bloomington, IN).75 , 76 However, COVID-19-infected patients have special considerations in selecting endoscopic therapy. First, performing therapeutic EGD in patients with active upper GI bleeding may place the endoscopist and the endoscopy staff at higher risk of contracting COVID-19 infection. Exposure should therefore be minimized by performing therapeutic endoscopy by a senior “master” endoscopist with considerable experience in therapeutic endoscopy in patients at high risk to perform the therapy expeditiously.77 Second, patients with active upper GI bleeding should be intubated endotracheally before performing EGD to protect the endoscopy staff from contracting the infection and to protect the patient’s airway from aspiration of blood during EGD. Third, patients undergoing therapeutic EGD who have recently received prophylactic anticoagulation to reduce their risk of thromboembolism associated with COVID-19 infection may be best served by receiving endoscopic therapy providing the most secure and stable hemostasis (even in the face of anticoagulation in the recent past or contemplated in the future), such as endoscopic clips or band ligation.78 Endoscopic band ligation may be performed in COVID-19-infected patients for esophageal variceal hemorrhage.63 Fourth, endoscopic therapeutic modalities can be selected according to ease of performance and speed of therapy, such as favoring argon plasma coagulation, to minimize exposure of the endoscopist and the endoscopy staff to potentially infectious aerosolized blood from patients with active upper GI bleeding. This may be a highly personal professional decision in that endoscopists may feel more comfortable with therapeutic modalities because of personal experience. Fifth, endoscopists may elect not to apply therapeutic endoscopy altogether in patients with borderline indications for endoscopic therapy, such as a peptic ulcer that is slowly oozing blood (Forrest IB lesion).79 Similarly, an endoscopist may elect not to decapitate a nonbleeding adherent clot (by guillotine by snare) and expose an underlying lesion to determine whether an ulcer with stigmata of recent hemorrhage lies underneath the clot80 to prevent active bleeding after clot decapitation to reduce endoscopist exposure to actively bleeding infected patients. Patients with upper GI bleeding with flat spots or clean-based ulcers do not require endoscopic therapy regardless of the presence or absence of COVID-19 infection.80 Sixth, COVID-19-infected patients who fail endoscopic therapy but are good surgical candidates may reasonably be sent for GI surgery or angiographic embolization, rather than persist at an unsuccessful endoscopic therapy or perform repeat endoscopic therapy.
In a highly selective multicenter North American cohort, only about 1% of subjects underwent GI endoscopy for GI bleeding and only two patients (about 0.1%) of the entire group underwent therapeutic endoscopy.61 Ten of 18 subjects who underwent EGD for GI hemorrhage had reported esophagitis, gastritis, or ulcer, but did not receive endoscopic therapy. The authors noted low utilization of GI endoscopy despite prevalent COVID-19 GI symptoms, that inflammatory and erosive mucosal abnormalities were common in COVID-19 patients, and that most of these abnormalities were related to the underlying critical illness and not a local viral cytopathic effect.61 In another New York cohort, 10 patients deemed to have significant bleeding underwent EGD with eight having gastric or duodenal ulcers and four requiring single or combined endoscopic hemostatic therapy to control the bleeding; four patients had recurrent bleeding, but no fatalities occurred because of the GI bleeding.81 Three of four COVID-19 pneumonia patients hospitalized in ICUs required endoscopic hemostasis for bleeding ulcers in one series.37 Three patients with COVID-19 duodenal hemorrhage were successfully treated with endoscopic vacuum therapy.82
Small Intestine Beyond Ligament of Treitz
The vast small intestinal surface area with its high density of angiotensin-converting enzyme-2 receptors renders this organ particularly susceptible to direct COVID-19 infection and injury. Infections usually manifest subclinically as only inflammation and erosions.16 , 17 One patient had documented small and large intestinal mucosal sloughing with infection.83 Another had a Crohn-like presentation with GI bleeding and fistulas.84 Another patient underwent segmental resection for hemorrhagic enteritis, without Crohn disease, attributed directly to COVID-19 infection.85 He required surgery and survived the surgery.
Jejunal and ileal causes of GI bleeding in COVID-19-infected patients are summarized in Table 3 .16 , 48 , 83, 84, 85, 86, 87, 88, 89
Table 3.
Reported causes of jejunal and ileal bleeding in patients with COVID-19 infection
| Lesion | Putative Mechanisms | Selected References |
|---|---|---|
| Jejunal ulcers and multiple proximal jejunojejunal fistulae | Scattered reported cases (1 with only jejunal ulcers) | 16,84,86 |
| Hemorrhagic enteritis | 2 independent case reports Lesions attributed to COVID-19 infection |
Amarapurkar et al,85 2020, Francese et al,88 2022 |
| Small and large intestinal mucosal sloughing | 1 case report | Yamakawa et al,83 2022 |
| Gastrointestinal stromal tumor in jejunum | Case report | Jablońska et al,89 2022 |
| Ileal angiodysplasia | Francese et al,88 2022 |
Capsule Endoscopy and Enteroscopy to Evaluate Small Intestinal Bleeding
Mucosal abnormalities were commonplace in COVID-19 subjects undergoing capsule endoscopy.48 , 86 Capsule endoscopy was touted as a primary diagnostic modality because of hesitancy about performing traditional tube endoscopy in COVID-19-infected patients.87 For example, in one study,48 146 patients with COVID-19 infection first underwent capsule endoscopy as a triage tool to investigate GI bleeding as compared with 72 historical control subjects undergoing standard of care (SOC) evaluation for GI bleeding initially using EGD in the prepandemic era of January 1 to January 31, 2020. Active bleeding or stigmata of recent hemorrhage were observed in 44 (59.5%) patients in the COVID-19-infected group versus 18 (25.0%) in the SOC group (adjusted odds ratio, 5.23; 95% confidence interval, 2.23–12.27). Only 36 patients (48.7%) in the COVID-19-infected group required any invasive procedure during the hospitalization, compared with 70 (97.2%) in the SOC group (adjusted odds ratio, 0.01; 95% confidence interval, 0.001–0.08). The mean (standard deviation) number of invasive procedures was statistically significantly lower in the capsule endoscopy group: 0.59 (0.77) per patient in the COVID-19 group versus 1.18 (0.48) per patient in the SOC group (adjusted difference, −0.54; 95% confidence interval, −0.77 to −0.31). The authors concluded that the number of invasive GI endoscopies could be significantly decreased in COVID-19 patients when capsule endoscopy was performed as the initial test without increasing the rate of rebleeding or other complications.
Only one case report of push enteroscopy has been reported in COVID-19-infected patients: a 34-year-old man suffered sequelae of subacute GI obstruction and failure to thrive 2 months after a prolonged admission for COVID-19 infection.84 He subsequently underwent push enteroscopy, which was performed safely and was diagnostic: it revealed residual jejunal ulcers and multiple proximal jejunojejunal fistulae. The push enteroscopy directly led to laparotomy, which revealed strictures with dense intra-abdominal adhesions, a large jejunojejunal fistula, and evident prior jejunal perforation from severe COVID-19 infection. The patient recovered after small bowel resection with anastomoses and was discharged home, but represented with dyspnea, acute chest pain, and cardiac arrest 6 weeks postoperatively and subsequently died. The death was believed unrelated to the push enteroscopy 6 weeks earlier. A literature review revealed no data on single or double balloon enteroscopy performed in COVID-19-infected patients. Small intestinal bleeding from Crohn disease is reviewed in the accompanying article by Summa and Hanauer elsewhere in this issue.
Cause of Colonic Bleeding
Sparse colonoscopic data report ischemic colitis is a common cause of lower GI bleeding.90 Ischemic or hemorrhagic colitis was noted in 13% to 33% of patients in several colonoscopy series.16 , 61 , 90 , 91 Ischemic colitis has also been reported after tocilizumab therapy,92 and after COVID-19 vaccination.93 Hemorrhagic colitis can occur with COVID-19 infection, with concomitant cytomegalovirus reported in one case.94 , 95 Other colonoscopic studies revealed rectal ulcers,60,82 often caused by indwelling rectal tubes.81 For GI bleeding in COVID-19 patients with inflammatory bowel disease, either ulcerative colitis or Crohn disease, see the accompanying article by Summa and Hanauer elsewhere in this issue. Five COVID-19 patients with GI bleeding underwent colonoscopy in one study with nonspecific findings, including presumptive bleeding from colonic diverticulosis or internal hemorrhoids.16 Clinically manifest lower GI bleeding including hematochezia is uncommon with colonoscopy often showing minor mucosal lesions consistent with the same pattern for upper GI bleeding. Some bleeding may be incidental to COVID-19 infection.16, 17, 18, 19 , 61 Colonoscopy is generally deferred in COVID-19-infected patients, with colon capsule examination proposed as an initial diagnostic alternative to colonoscopy.49 Interventional radiology should be considered for colonic bleeding if resources are available.29 , 51 , 96 Table 4 lists causes of colonic bleeding in COVID-19-infected patients.16 , 61 , 81 , 90 , 92., 93, 94, 95
Table 4.
Reported causes of lower GI bleeding associated with COVID-19 infection
| Lesion | Putative Mechanisms | References |
|---|---|---|
| Ischemic colitis | Reported in 13%–33% of patients with COVID-19 infection undergoing colonoscopy Association believed caused by COVID-19 infections promoting thrombosis |
16,61,90 |
| Ischemic colitis | Associated with tocilizumab therapy | Forneiro Pérez et al,92 2021 |
| Ischemic colitis | After COVID-19 vaccination | Cui et al,93 2022 |
| Rectal ulcers | Often related to placement of rectal tubes | Martin et al,81 2020 |
| Hemorrhagic colitis | Acute hemorrhagic diarrhea without evident ulcerative colitis or ischemic colitis on colonoscopy including pathologic examination of colonoscopic biopsies. Attributed to "traveler's diarrhea" due to recent visit to Egypt from the United States with negative work-up for bacterial cultures of stool and negative examinations for ova and parasites of stool. | Carvalho et al,94 2020 |
| Hemorrhagic colitis can occur with COVID-19 infection | 1 case associated with concomitant cytomegalovirus infection | Leemans et al,95 2020 |
| Colonic angiodysplasia | 2 cases reported in a review of 27 colonoscopies mostly performed for overt GI bleeding | Vanella et al,16 2021 |
| Bleeding hemorrhoids | 1 case reported in a review of 27 colonoscopies mostly performed for overt GI bleeding | Vanella et al,16 2021 |
| Colonic diverticulosis with bleeding | 4 cases reported in a review of 27 colonoscopies mostly performed for overt GI bleeding | Vanella et al,16 2021 |
Outcomes of Gastrointestinal Bleeding
The spectrum of upper GI bleeding in COVID-19 patients commonly includes presentation with subclinical bleeding related to mucosal friability, anemia and hemoccult positive stools possibly requiring transfusions, but also includes clinically apparent bleeding in critically ill patients with COVID-19 pneumonia. COVID-19 infection may be incidental to the presentation with GI hemorrhage, especially in areas where COVID-19 infection is omnipresent.4 Rarely, GI bleeding in a COVID-19 patient may be massive and fatal.12 , 97 , 98 The literature has somewhat conflicting results concerning predisposing factors and the relationship of GI bleeding to outcome, including mortality. This variability may reflect differences in study design, demographics, and statistical analysis. A consistent finding was decreased use of GI endoscopy for GI bleeding and greater length of stay for patients with GI bleeding during the pandemic than for patients before the pandemic.30 , 99
In an Italian cohort, 78% had GI bleeding associated with thromboprophylaxis with anticoagulants.20 A European study also noted this strong association.19 Other studies have not reported anticoagulation as a significant risk factor.21 , 81 , 100
Mortality secondary to GI bleeding was often not stratified and outcome seemed most related to pulmonary or multisystem failure, including sepsis and thrombosis. GI hemorrhage in COVID-19 subjects in some analyses was not clearly associated with increased overall mortality, although COVID-19-infected patients had greater mortality than non-COVID-19 subjects, at least for all patients presenting with upper GI bleeding.23 , 40 Other studies found GI bleeding was associated with increased COVID-19 mortality.3 , 101
Emerging Trends
COVID-19 infection is a moving and evolving target because of emergent new viral mutations that are likely more infections and less lethal, changes in the biology of the infection caused by improved host defenses from vaccinations and improved antiviral treatments including improved critical care, better management of respiratory compromise, and the introduction of moderately effective antiviral therapy. These changes are reflected in decreasing mortality and likely decreasing morbidity from the virus, which may reduce the burden of GI disease including GI bleeding. Long COVID is being increasingly appreciated, but it does not seem so far to frequently cause significant GI bleeding (See the article by Tindade and colleagues on long COVID elsewhere in this issue.). Although there is a theoretical risk of transmission of COVID-19 infection to uninfected patients, endoscopy staff, and the GI endoscopist from aerosolization of viral particles, particularly in patients with active upper GI bleeding, the risk of spread to these groups seems to be less than initially feared, partly caused by use of PPE, isolation techniques, viricidal cleaning solutions, and universally recommended vaccinations. This change has allowed a gradual increase in the use of GI endoscopy from less than 10% of baseline rate during the pandemic onset to substantial but still incomplete recovery in the use of GI endoscopy toward the prepandemic baseline. Although the data on GI endoscopy were particularly sparse early in the pandemic, these data have been accumulating recently with the recovery of GI endoscopy toward prepandemic levels. This review is best considered a snapshot of the current state of GI bleeding with COVID, with likely future advancements and improvements in clinical therapy, management, and prognosis over time.
Summary
GI symptoms and GI pathology are common in COVID-19 infection, but overt GI bleeding is uncommon. Mucosal abnormalities are frequently noted at GI endoscopy and capsule endoscopy. However, GI hemorrhage associated with COVID-19 is often subclinical. The presentation of GI bleeding in COVID-19 ranges from the bleeding patient incidentally found to have COVID-19 infection to the critically ill COVID-19 subject with pneumonia and multisystem disorders. Use of anticoagulant and antiplatelet drugs and the known association of physiologic “stress” GI bleeding in critically ill patients with respiratory illness may be predisposing factors in this population. Modest endoscopic data suggest that PUD, including erosive gastroduodenitis, are common causes of GI bleeding. Ischemic colitis has a known association with COVID-19, but uncommonly manifests as overt bleeding. The approach to COVID-19 subjects with GI bleeding resembles that of the general population with the cogent exception of often deferring endoscopy for patients with mild GI bleeding and reserving endoscopy for patients with more severe GI bleeding likely requiring therapeutic endoscopy. International and national guidelines reflect this philosophy and practice. Better understanding of COVID-19 pathogenesis and the advent of preventive measures, such as vaccination and improved medications, such as Paxlovid, may ultimately render a conservative approach to GI endoscopy no longer tenable.
The editor of this monograph on GI manifestations and therapy for COVID hopes to contribute to this general advancement on COVID-19 therapy and prognosis by hereby offering the first volume or monograph devoted exclusively to the GI, liver, and pancreatic manifestations of COVID infection. Although COVID-19 is primarily a respiratory disease with prominent respiratory symptoms and frequent respiratory compromise, GI manifestations play an important secondary role in the morbidity and even rarely in the mortality of this infection.
Clinics care points
-
•
COVID-19 has produced >6,000,000 deaths worldwide since March 2020 primarily from pneumonia and respiratory failure.
-
•
GI manifestations of COVID-19 infection, especially diarrhea and GI bleeding, have sometimes produced morbidity and rarely contributed to mortality from this viral infection.
-
•
GI bleeding occurs in 1.5% to 3.0% of patients hospitalized with acute COVID-19 infection.
-
•
GI bleeding is typically mild-to-moderate and associated with mild mucosal erosions/abnormalities but can occassionally cause severe bleeding, especially from peptic ulcer disease or stress gastritis associated with pneumonia or respiratory failure from COVID-19 infection.
-
•
In the pandemic onset, GI bleeding was rarely evaluated by GI endoscopy due to the risks of endoscopy staff contracting the infection from infected patients and the feared risks of infected patients suffering complications from undergoing GI endoscopy.
Acknowledgements
Dr Cappell is employed as a gastroenterologist at the Aleda E. Lutz VA Medical Center at Saginaw Michigan. The Saginaw VA Hospital and the federal government take no position on the opinions expressed in this article. This article is a review article that does not require Internal Review Board approval because there is no original patient data contained in this article.
Footnotes
The two authors are equal first authors.
References
- 1.Cascella M., Rajnik M., Aleem A., et al. StatPearls Publishing; Treasure Island (FL): 2022. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls [Internet]https://www.ncbi.nlm.nih.gov/books/NBK554776/ Accessed May 23, 2022. [PubMed] [Google Scholar]
- 2.Cappell M.S. Moderately severe diarrhea and impaired renal function with COVID-19 infection. Am J Gastroenterol. 2020;115(6):947–948. doi: 10.14309/ajg.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshazli R.M., Kline A., Elgaml A., et al. Gastroenterology manifestations and COVID-19 outcomes: a meta-analysis of 25,252 cohorts among the first and second waves. J Med Virol. 2021;93(5):2740–2768. doi: 10.1002/jmv.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulen M., Satar S. Uncommon presentation of COVID-19: gastrointestinal bleeding. Clin Res Gastroenterol Hepatol. 2020;44(4):e72–e76. doi: 10.1016/j.clinre.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P., Tan X., Li Q., et al. Extra-pulmonary complications of 45 critically ill patients with COVID-19 in Yichang, Hubei province, China: a single-centered, retrospective, observation study. Medicine (Baltimore) 2021;100(9):e24604. doi: 10.1097/MD.0000000000024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L., Jiang X., Zhang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 7.Perisetti A., Gajendran M., Mann R., et al. COVID-19 extrapulmonary illness: special gastrointestinal and hepatic considerations. Dis Mon. 2020;66(9):101064. doi: 10.1016/j.disamonth.2020.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ion D., Panduraru D., Bolocan A., et al. Gastro-intestinal bleeding in COVID-19 patients: is there any causal relation? Chirurgia. 2021;116(6 Suppl):S69–S76. [PubMed] [Google Scholar]
- 9.Zhang J., Garrett S., Sun J. Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis. 2021;8(4):385–400. doi: 10.1016/j.gendis.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Q., Wang B., Zhang T., et al. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiolog Gastrointest Liver Physiol. 2020;319(2):G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dioscoridi L., Giannetti A., Massad M.T., et al. A "double-hit" damage mechanism can explain self-limited GI bleeding in COVID-19 pneumonia. Gastrointest Endosc. 2021;93(5):1192–1193. doi: 10.1016/j.gie.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed M., Nassar M., Nso N., et al. Massive gastrointestinal bleeding in a patient with COVID-19. Arab J Gastroenterol. 2021;22(2):177–179. doi: 10.1016/j.ajg.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook D., Guyatt G. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378(26):2506–2516. doi: 10.1056/NEJMra1605507. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar M., Madabhavi I.V., Quy P.N., et al. COVID-19 and coagulopathy. Clin Resp J. 2021;15(12):1259–1274. doi: 10.1111/crj.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckholz A., Kaplan A., Jessurun J., et al. Microthrombosis associated with GI bleeding in COVID-19. Gastrointest Endosc. 2021;93(1):263–264. doi: 10.1016/j.gie.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanella G., Capurso G., Burti C., et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8(1):e000578. doi: 10.1136/bmjgast-2020-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashktorab H., Russo T., Oskrochi G., et al. Clinical and endoscopic outcomes in coronavirus disease-2019 patients with gastrointestinal bleeding. Gastro Hep Adv. 2022;1(4):487–499. doi: 10.1016/j.gastha.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marasco G., Maida M., Morreale G.C., et al. Gastrointestinal bleeding in COVID-19 patients: a systematic review with meta-analysis. Can J Gastroenterol Hepatol. 2021;2021:2534975. doi: 10.1155/2021/2534975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zellmer S., Hanses F., Muzalyova A., et al. Gastrointestinal bleeding and endoscopic findings in critically and non-critically ill patients with corona virus disease 2019 (COVID-19): results from Lean European Open Survey on SARS-CoV-2 (LEOSS) and COKA registries. United Eur Gastroenterol J. 2021;9(9):1081–1090. doi: 10.1002/ueg2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauro A., De Grazia F., Lenti M.V., et al. Upper gastrointestinal bleeding in COVID-19 inpatients: incidence and management in a multicenter experience from Northern Italy. Clin Res Gastroenterol Hepatol. 2021;45(3):101521. doi: 10.1016/j.clinre.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trindade A.J., Izard S., Coppa K., et al. Gastrointestinal bleeding in hospitalized COVID-19 patients: a propensity score matched cohort study. J Intern Med. 2021;289(6):887–894. doi: 10.1111/joim.13232. [DOI] [PubMed] [Google Scholar]
- 22.Makker J., Mantri N., Patel H.K., et al. The incidence and mortality impact of gastrointestinal bleeding in hospitalized COVID-19 patients. Clin Exp Gastroenterol. 2021;14:405–411. doi: 10.2147/CEG.S318149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González Maker R., Jacob J., Miro O., et al. Incidence, clinical characteristics, risk factors, and outcomes of upper gastrointestinal bleeding in patients with COVID-19: results of the UMC-19-S12. J Clin Gastroenterol. 2022;56(1):e38–e46. doi: 10.1097/MCG.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H., Wu Y., He Y., et al. Age-related risk factors and complications of patients with COVID-19: a population-based retrospective study. Front Med (Lausanne) 2021;8:757459. doi: 10.3389/fmed.2021.757459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappell M.S., Friedel D. Initial management of acute upper gastrointestinal bleeding: from initial evaluation up to gastrointestinal endoscopy. Med Clin North Am. 2008;92(3):491–509. doi: 10.1016/j.mcna.2008.01.005. xi. [DOI] [PubMed] [Google Scholar]
- 26.American Red Cross Red Cross declares first-ever blood crisis amid omicron surge. https://www.redcross.org/about-us/news-andevents/press-release/2022/blood-donors-needed-now-as-omicron-intensifies.html Available at: Accessed September 28, 2022.
- 27.Duan Z., Liu K., Zhou S. The dilemma in the management of suspected upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc. 2020;92(6):1273–1274. doi: 10.1016/j.gie.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards C., Penman I.D., Coleman M. Gastrointestinal endoscopy during COVID-19: when less is more. Frontline Gastroenterol. 2020;11(4):256–257. doi: 10.1136/flgastro-2020-101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzwanger E.A., Bilal M., Stallwood C.G., et al. Acute lower gastrointestinal bleeding during the COVID-19 pandemic: less is more. Endoscopy. 2020;52(9):816–817. doi: 10.1055/a-1194-4864. [DOI] [PubMed] [Google Scholar]
- 30.Laursen S.B., Gralnek I.M., Stanley A.J. Raising the threshold for hospital admission and endoscopy in upper gastrointestinal bleeding during the COVID-19 pandemic. Endoscopy. 2020;52(10):930–931. doi: 10.1055/a-1202-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Z., Zhou S., Niu Z. Use of the Glasgow-Blatchford score during the COVID-19 pandemic needs more rigorous research. Endoscopy. 2021;53(2):209. doi: 10.1055/a-1300-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soetikno R., Teoh A.Y., Kaltenbach T., et al. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176–183. doi: 10.1016/j.gie.2020.03.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gralnek I.M., Hassan C., Beilenhoff U., et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52(6):483–490. doi: 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal U., Patel P.D., Pluskota C.A., et al. Outcomes of acute gastrointestinal bleeding in patients with COVID-19: a case-control study. Gastroenterol Res. 2022;15(1):13–18. doi: 10.14740/gr1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappell M.S. Local COVID-19 epicenter in Detroit metropolitan area causing profound and pervasive reorganization of clinical, educational, research, and financial programs of a large academic gastroenterology division with a GI fellowship and primary medical school affiliation. Dig Dis Sci. 2021;66(11):3635–3658. doi: 10.1007/s10620-021-07192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan R., Saha S., Gimpaya N., et al. Outcomes for upper gastrointestinal bleeding during the first wave of the COVID-19 pandemic in the Toronto area. J Gastroenterol Hepatol. 2022;37(5):878–882. doi: 10.1111/jgh.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan F., Alishahi Y. Gastrointestinal bleeding and endoscopic outcomes in patients with SARS-CoV-2. Clin Endosc. 2021;54(3):428–431. doi: 10.5946/ce.2020.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu P.W.Y., Ng S.C., Inoue H., et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;6:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosevics L., Fossati B.S., Teixeiba S., et al. COVID-19 and digestive endoscopy: emergency endoscopic procedures and risk factors for upper gastrointestinal bleeding. Arq Gastroenterol. 2021;58(3):337–343. doi: 10.1590/S0004-2803.202100000-57. [DOI] [PubMed] [Google Scholar]
- 40.Benites-Goñi H., Pascacio-Fiori M., Monge-Del Valle F., et al. Impact of the COVID-19 pandemic in the time to endoscopy in patients with upper gastrointestinal bleeding. Rev Gastroenterol Peru. 2020;40(3):219–223. [PubMed] [Google Scholar]
- 41.Barrett L.F., Lo K.B., Stanek S.R., et al. Self-limited gastrointestinal bleeding in COVID-19. Clin Res Gastroenterol Hepatol. 2020;44(4):e77–e80. doi: 10.1016/j.clinre.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavaliere K., Levine C., Wander P., et al. Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc. 2020;92(2):454–455. doi: 10.1016/j.gie.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sbeit W., Mari A., Pellicano R., et al. When and whom to scope in case of gastrointestinal bleeding in the COVID-19 era? Minerva Gastroenterol (Torino) 2021;67(4):307–309. doi: 10.23736/S2724-5985.21.02830-0. [DOI] [PubMed] [Google Scholar]
- 44.Shalimar Vaishnav M., Elhence A., Kumar R., et al. Outcome of conservative therapy in coronavirus disease-2019: patients presenting with gastrointestinal bleeding. J Clin Exp Hepatol. 2021;11(3):327–333. doi: 10.1016/j.jceh.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu F., Yang Y., Ding Z. Esophagogastroduodenoscopy procedure using disposable endoscope to detect the cause of melena in a patient with COVID-19. Dig Endosc. 2021;33(1):e1–e2. doi: 10.1111/den.13840. [DOI] [PubMed] [Google Scholar]
- 46.Onaizah O., Koszowska Z., Winters C., et al. Guidelines for robotic flexible endoscopy at the time of COVID-19. Front Robot A. 2021;8:612852. doi: 10.3389/frobt.2021.612852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsayed I., Meier B., Caca K., et al. Potential use of a novel telemetric sensor capsule in patients with suspected gastrointestinal bleeding during the COVID-19 pandemic. Endoscopy. 2021;53(3):337–338. doi: 10.1055/a-1319-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hakimian S., Raines D., Reed G., et al. Assessment of video capsule endoscopy in the management of acute gastrointestinal bleeding during the COVID-19 pandemic. JAMA Netw Open. 2021;4(7):e2118796. doi: 10.1001/jamanetworkopen.2021.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLeod C., Wilson P., Watson A.J.M. Colon capsule endoscopy: an innovative method for detecting colorectal pathology during the COVID-19 pandemic? Colorectal Dis. 2020;22(6):621–624. doi: 10.1111/codi.15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki S., Kusano C., Ikehara H. Simple barrier device to minimize facial exposure of endoscopists during COVID-19 pandemic. Dig Endosc. 2020;32(5):e118–e119. doi: 10.1111/den.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ierardi A.M., Del Giudice C., Coppola A., et al. Gastrointestinal hemorrhages in patients with COVID-19 managed with transarterial embolization. Am J Gastroenterol. 2021;116(4):838–840. doi: 10.14309/ajg.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Hospital Association Shortage of contrast media for CT imaging affecting hospitals and health systems | AHA. https://www.aha.org/advisory/2022-05-12-shortage-contrast-media-ct-imaging-affecting-hospitals-and-health-systems Available at: Accessed June 23, 2022.
- 53.Jayasena H., Abeynayake D., De Silva A., et al. The use of personal protective equipment in endoscopy: what should the endoscopist wear during a pandemic? Exp Rev Gastroenterol Hepatol. 2021;15(12):1349–1359. doi: 10.1080/17474124.2021.2011213. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal A., Chowdhury S.D., Sachdeva S., et al. Low risk of transmission of SARS-CoV2 and effective endotherapy for gastrointestinal bleeding despite challenges supports resuming optimum endoscopic services. Dig Liver Dis. 2021;53(1):4–7. doi: 10.1016/j.dld.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.www.aamc.org/data-reports/workforce/interactive-data/active-physicians-age-and-specialty-2019. Accessed October 10, 2022.
- 56.Vignesh S., Butt A.S., Alboraie M., et al. Impact of COVID-19 on endoscopy training: perspectives from a global survey of program directors and endoscopy trainers. Clin Endosc. 2021;54(5):678–687. doi: 10.5946/ce.2021.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melazzini F., Lenti M.V., Mauro A., et al. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115(7):1139–1140. doi: 10.14309/ajg.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deb A., Thongtan T., Costilla V. Gastric ulceration in Covid-19: an ominous sign? BMJ Case Rep. 2021;14(7):e244059. doi: 10.1136/bcr-2021-244059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackett J.W., Kumta N.A., Dixon R.E., et al. Characteristics and outcomes of patients undergoing endoscopy during the COVID-19 pandemic: a multicenter study from New York City. Dig Dis Sci. 2020;60:1–10. doi: 10.1007/s10620-020-06593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuftinec G., Elmunzer B.J., Amin S., et al. The role of endoscopy and findings in COVID-19 patients, an early North American Cohort. BMC Gastroenterol. 2021;21(1):205. doi: 10.1186/s12876-021-01796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papanikolaou I.S., Lazaridis L.D., Rizos E., et al. Untying the knot: acute variceal bleeding in a COVID-19 patient. What should the gastroenterologist keep in mind? Eur J Gastroenterol Hepatol. 2021;33(3):450–451. doi: 10.1097/MEG.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Kassas M., Al Shafie A., Abdel Hameed A.S., et al. Emergency endoscopic variceal band ligation in a COVID-19 patient presented with hematemesis while on mechanical ventilation. Dig Endosc. 2021;32(5):812–815. doi: 10.1111/den.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisseling T.M., van Laarhoven A., Huijbers A., et al. Coronavirus disease-19 presenting as esophageal ulceration. Am J Gastroenterol. 2021;1;116(2):421–424. doi: 10.14309/ajg.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 65.González-Sagredo A., Iborra Ortega E., Herranz Pinilla C., et al. Covid-19 and aorto-enteric fistula. Rev Esp Enferm Dig. 2021;113(12):852–853. doi: 10.17235/reed.2021.8272/2021. [DOI] [PubMed] [Google Scholar]
- 66.Koç E.S., Çiçek B. COVID-19 induced haemobilia: a novel entity. J Gastrointest Liver Dis. 2021;30(4):528–530. doi: 10.15403/jgld-4043. [DOI] [PubMed] [Google Scholar]
- 67.Awwad I., Greuel S., Tacke F., et al. Haemorrhagic ulcerative duodenitis in a patient with COVID-19 infection: clinical improvement following treatment with budesonide. BMJ Open Gastroenterol. 2021;8(1):e000757. doi: 10.1136/bmjgast-2021-000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deliwala S.S., Hussain M.S., Ponnapalli A., et al. Black oesophagus, upside-down stomach and Cameron lesions: cascade effects of a large hiatal hernia. BMJ Case Rep. 2021;14(11):e246496. doi: 10.1136/bcr-2021-246496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez Roldán F., Malik Javed Z., Yagüe Compadre J.L., et al. Gastric ulcers with upper gastrointestinal bleeding in patients with severe SARS-CoV-2. Rev Esp Enferm Dig. 2021;113(2):122–124. doi: 10.17235/reed.2021.7759/2020. [DOI] [PubMed] [Google Scholar]
- 70.Mur-Murota A., Yoshi S., Okuda R., et al. Successful hemostasis of bleeding gastric inflammatory fibroid polyp by endoscopic treatment in a patient with severe COVID-19. Clin J Gastroenterol. 2021;14(4):1008–1013. doi: 10.1007/s12328-021-01402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aguayo W.G., Moyon F.X., Molina G.A., et al. A bleeding GIST in pandemic times, a cooperative approach to a delayed complication, a case report. Int J Surg Case Rep. 2020;77:880–884. doi: 10.1016/j.ijscr.2020.11.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galvez A., King K., El Chaar M., et al. Perforated marginal ulcer in a COVID-19 patient. Laparoscopy in these trying times? Obes Surg. 2020;30(11):4605–4608. doi: 10.1007/s11695-020-04709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philips C.A., Kumbar S., Ahamed R., et al. Managing acute portal hypertensive gastropathy bleed during the time of COVID-19 pandemic: novelty or necessity? Cureus. 2020;12(5):e8333. doi: 10.7759/cureus.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casper M., Lepper P., Danziger G., et al. Gastrointestinal endoscopy during extracorporeal membrane oxygenation (ECMO) for COVID-19. J Gastrointestin Liver Dis. 2020;29(3):471–473. doi: 10.15403/jgld-2826. [DOI] [PubMed] [Google Scholar]
- 75.Cappell M.S. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7(4):214–229. doi: 10.1038/nrgastro.2010.24. [DOI] [PubMed] [Google Scholar]
- 76.Hagel A.F., Albrecht H., Nägel A., et al. The application of Hemospray in gastrointestinal bleeding during emergency endoscopy. Gastroenterol Res Pract. 2017;2017:3083481. doi: 10.1155/2017/3083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cappell M.S. Gastrointestinal endoscopy in high-risk patients. Dig Dis. 1996;14(4):228–244. doi: 10.1159/000171555. [DOI] [PubMed] [Google Scholar]
- 78.Lanas Á. Hemorragia gastrointestinal [gastrointestinal bleeding] Gastroenterol Hepatol. 2015 Sep;38(Suppl 1):56–63. doi: 10.1016/S0210-5705(15)30020-0. Spanish. [DOI] [PubMed] [Google Scholar]
- 79.Jensen D.M., Eklund S., Persson T., et al. Reassessment of rebleeding risk of Forrest IB (Oozing) peptic ulcer bleeding in a large international randomized trial. Am J Gastroenterol. 2017;112(3):441–446. doi: 10.1038/ajg.2016.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laine L., Jensen D.M. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360. doi: 10.1038/ajg.2011.480. [quiz: 361] [DOI] [PubMed] [Google Scholar]
- 81.Martin T.A., Wan D.W., Hajifathalian K., et al. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case-control study. Am J Gastroenterol. 2020;115(10):1609–1616. doi: 10.14309/ajg.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Moura D.T.H., de Moura E.G.H., Hirsch B.S., et al. Modified endoscopic vacuum therapy for duodenal hemorrhage in patients with severe acute respiratory syndrome coronavirus 2. Endoscopy. 2022;54(S 02):E837–E839. doi: 10.1055/a-1803-4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamakawa T., Ishigami K., Takizawa A., et al. Extensive mucosal sloughing of the small intestine and colon in a patient with severe COVID-19. DEN Open. 2022;2(1):e42. doi: 10.1002/deo2.42. Epub 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbassi B., Deb A., Costilla V., et al. A subacute enteritis two months after COVID-19 pneumonia with mucosal bleeding, perforation, and internal fistulas. Surg. 2021 doi: 10.1177/00031348211023461. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amarapurkar A.D., Vichare P., Pandya N., et al. Haemorrhagic enteritis and COVID-19: causality or coincidence. J Clin Pathol. 2020;73(10):686. doi: 10.1136/jclinpath-2020-206743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L., Yang L., Li J., et al. Diagnosis of suspected small bowel bleeding by capsule endoscopy in patients with COVID-19. Inter Med. 2021;60(15):2425–2430. doi: 10.2169/internalmedicine.7235-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAlindon M. COVID-19: impetus to the adoption of capsule endoscopy as a primary diagnostic tool? Frontline Gastroenterol. 2021;12(4):263–264. doi: 10.1136/flgastro-2021-101794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Francese M., Ferrari C., Lotti M., et al. The thin red line: ileal angiodysplasia versus SARS-CoV-2-related haemorrhagic enteritis. J Clin Pathol. 2022 doi: 10.1136/jclinpath-2021-207754. jclinpath-2021-207754. [DOI] [PubMed] [Google Scholar]
- 89.Jablońska B., Szmigiel P., Wosiewicz P., et al. A jejunal gastrointestinal stromal tumor with massive gastrointestinal hemorrhage treated by emergency surgery: a case report. Medicine (Baltimore) 2022;101(35):e30098. doi: 10.1097/MD.0000000000030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paul T., Joy A.R., Alsoub H.A.R.S., et al. Case report: ischemic colitis in severe COVID-19 pneumonia: an unforeseen gastrointestinal complication. Am J Trop Med Hyg. 2021;104(1):63–65. doi: 10.4269/ajtmh.20-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Massironi S., Vigano C., Dioscoridi L., et al. Endoscopic findings in patients infected with 2019 novel coronavirus in Lombardy. Clin Gastroenterol Hepatol. 2020;18:2375–2377. doi: 10.1016/j.cgh.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forneiro Pérez R., Dabán López P., Zurita Saavedra M.S., et al. Tocilizumab as a possible cause of ischemic colitis. Gastroenterol Hepatol. 2021;44(5):373–374. doi: 10.1016/j.gastrohep.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui M.H., Hou X.L., Liu J.Y. Ischemic colitis after receiving the second dose of a COVID-19 inactivated vaccine: a case report. World J Clin Cases. 2022;26;10(12):3866–3871. doi: 10.12998/wjcc.v10.i12.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carvalho A., Alqusairi R., Adams A., et al. SARS-CoV-2 gastrointestinal Infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115(6):942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leemans S., Maillart E., Van Noten H., et al. Cytomegalovirus haemorrhagic colitis complicating COVID-19 in an immunocompetent critically ill patient: a case report. Clin Case Rep. 2020;9(5):e03600. doi: 10.1002/ccr3.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar M.A., Krishnaswamy M., Arul J.N. Post COVID-19 sequelae: venous thromboembolism complicated by lower GI bleed. BMJ Case Rep. 2021;14(1):e241059. doi: 10.1136/bcr-2020-241059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen T., Yang Q., Duan H. A severe coronavirus disease 2019 patient with high-risk predisposing factors died from massive gastrointestinal bleeding: a case report. BMC Gastroenterol. 2020;20(1):318. doi: 10.1186/s12876-020-01458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitrovic M., Tadic B., Jankovic A., et al. Fatal gastrointestinal bleeding associated with acute pancreatitis as a complication of Covid-19: a case report. Int Med. 2022;50(5) doi: 10.1177/03000605221098179. 3000605221098179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J., Doyle J.B., Blackett J.W., et al. Effect of the coronavirus 2019 pandemic on outcomes for patients admitted with gastrointestinal bleeding in New York City. Gastroenterology. 2020;159(3):1155–1157. doi: 10.1053/j.gastro.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rustgi S.D., Yang J.Y., Luther S., et al. Anticoagulation does not increase risk of mortality or ICU admission in hospitalized COVID-19 patients with gastrointestinal bleeding: results from a New York health system. Clin Res Gastroenterol Hepatol. 2021;45(3):101602. doi: 10.1016/j.clinre.2020.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zuin M., Rigatelli G., Fogato L., et al. Higher risk of death in COVID-19 patients complicated by gastrointestinal bleeding events: a meta-analysis. Minerva Gastroenterol (Torino) 2021;67(4):408–410. doi: 10.23736/S2724-5985.21.02863-4. [DOI] [PubMed] [Google Scholar]