Abstract
Objective
Increases in stress, anxiety, and depression among women pregnant during the COVID-19 pandemic have been reported internationally. Yet rigorous comparison of the prevalence of maternal mental health problems across countries is lacking. Moreover, whether stress is a common predictor of maternal mental health during the pandemic across countries is unknown.
Methods
8148 pregnant women from Germany, Israel, Italy, Poland, Spain, Switzerland, and the United States were enrolled in the International COVID-19 Pregnancy Experiences (I-COPE) Study between April 17 and May 31, 2020. Sociodemographic characteristics, pandemic-related stress, pregnancy-specific stress, anxiety, and depression were assessed with well-validated instruments. The magnitude of stress and mood disturbances was compared across countries. A path model predicting clinically significant levels of anxiety and depression from maternal characteristics and stress was tested for all study participants and then examined separately in each country with >200 participants.
Results
Countries differed significantly in magnitude of pandemic-related pregnancy stress and pandemic-unrelated pregnancy-specific stress, and in prevalence of clinically significant anxiety and depression levels. A well-fitting common path model for the entire sample indicated that mood and anxiety disturbances were strongly predicted by pandemic-related and pregnancy-specific stress after accounting for maternal characteristics. The model was replicated in individual countries.
Conclusions
Although pregnant women in high-income Western countries experienced different levels of stress resulting from the COVID-19 pandemic, stress is a strong, common predictor of anxiety and depressive symptoms in these individuals. The common model can be used to inform research and clinical interventions to protect against adverse consequences of prenatal maternal stress, anxiety, and depression for mothers and infants.
Keywords: Anxiety, Depression, Pregnancy, Maternal stress, COVID-19 global pandemic, Women's health
1. Introduction
The COVID-19 pandemic has had unprecedented impact on public health, as well as social, psychological, and economic effects on people around the globe. The onset of the pandemic was especially stressful for pregnant women 2 because of unknown effects of the SARS-CoV-2 virus on pregnancy and fetuses, and modifications to prenatal care and labor and delivery practices that were enacted in many countries to reduce viral transmission (Chivers et al., 2020; Pope et al., 2022; Preis et al., 2020b). Such COVID-19 associated stressors likely compounded pregnancy-specific stress, a type of stress that originates from the changes and uncertainty that women typically undergo during pregnancy and which has been observed in pregnant women cross-culturally (Alderdice et al., 2012; Ibrahim and Lobel, 2020; Lobel and Dunkel Schetter, 2016; Lynn et al., 2011). The extent to which pandemic-related stress and pregnancy-specific stress have affected mental health in childbearing women during the COVID-19 pandemic is largely unknown. However, prior evidence and theory implicate stress in poorer maternal mental health (Ahmed et al., 2019; Norhayati et al., 2015; Viswasam et al., 2019). Maternal stress and perinatal mood and anxiety disorders are risk factors for adverse maternal, fetal, infant, and child outcomes (Bussières et al., 2015; Grigoriadis et al., 2018; Lobel and Dunkel Schetter, 2016), reinforcing the importance of investigating maternal psychological status during a global crisis. The purpose of this study was to test the applicability of a theoretically guided, common model of maternal anxiety and depressive symptoms predicted by pandemic-related and pregnancy-specific stress in pregnant women across seven high-income Western countries -- Germany, Israel, Italy, Poland, Spain, Switzerland, and the United States -- and to document the magnitude of stress and mood disturbance in this population. We hypothesized that pandemic-related stress and pregnancy-specific stress would contribute to greater anxiety and depressive symptoms among the childbearing women of these countries despite possible differences in the degree of stress or poor mental health between countries.
1.1. Pre-pandemic prevalence of prenatal maternal stress, poor mental health, and their association
Pregnancy-specific stress is defined by conditions, perceptions, and emotions that arise from women's experiences of physical changes and symptoms, concerns about fetal and infant health, fears of impending labor and delivery, and changes to interpersonal relationships and roles associated with becoming a mother (Alderdice et al., 2012; Bussières et al., 2015; Dunkel Schetter et al., 2016; Ibrahim and Lobel, 2020). This type of stress, which has been shown to be more deleterious than general or generic stress experienced by women during pregnancy (Bussières et al., 2015; Lobel et al., 2008), reflects the interaction of stressful pregnancy-relevant conditions, appraisals, and stress responses described by transactional theories (Lazarus and Folkman, 1984; Lazarus and Launier, 1978; Moos and Swindle, 1990). Consistent with this theoretical approach, pregnancy-specific stress can be exacerbated by circumstances including natural disasters and communal crises such as the COVID-19 pandemic (Lobel and Dunkel Schetter, 2016; Schoenmakers et al., 2022).
Variability in the magnitude of pregnancy-specific stress internationally is evident from studies conducted prior to the pandemic, although the conditions, perceptions, and emotions comprising this type of stress appear to be similar cross-culturally (Ibrahim and Lobel, 2020). Studies conducted in Western countries, for example, indicate that women in the United States (e.g., Simon et al., 2016) experience higher levels of pregnancy-specific stress than women in Spain, Germany, Australia, and New Zealand (Caparros-Gonzalez et al., 2019; Richter et al., 2012; Staneva et al., 2016). Irrespective of their geographic locale, investigators have also identified maternal characteristics that are associated with greater pregnancy-specific stress. Most consistently, these characteristics are younger age, nulliparity, and high-risk pregnancy (Auerbach et al., 2014; Schoch-Ruppen et al., 2018; Staneva et al., 2016).
Estimates of the prevalence of prenatal mood disorders from pre-pandemic research also vary widely, based on sample composition, methodology, and geographic location. For example, pre-pandemic rates of maternal depression have been reported to range from 7.0 to 15.0% in high income countries and 19.0–25.0% in lower- and middle-income countries (Gelaye et al., 2016; Woody et al., 2017). Recent meta-analyses estimate the overall pre-pandemic prevalence of prenatal depression to be 11.9%–17.0% (Underwood et al., 2016; Woody et al., 2017), and 15.2%–20.7% for any type of clinically diagnosed anxiety disorder (Dennis et al., 2017; Fawcett et al., 2019). However, estimates of stress and mental health utilizing metanalytic techniques must be interpreted cautiously because of the frequent use of different assessment tools across studies, methodological differences, and varying measurement timeframes. As evidence, in a meta-regression predicting prenatal depression, instrument type was a significant predictor of symptom prevalence (Woody et al., 2017).
Prior research utilizing various operationalizations of prenatal maternal stress has found that it is strongly linked to greater risk for mood and anxiety problems during the prenatal (Biaggi et al., 2016; Field, 2017) and postpartum periods (Caparros-Gonzalez et al., 2017; Norhayati et al., 2015) and up to five years following birth (Ahmed et al., 2019). Prenatal stress also heightens risk for persistent maternal stress in the years following birth (Monk et al., 2020), which may in turn increase the risk for symptom relapse in women with pre-existing mental health conditions (Biaggi et al., 2016). Furthermore, prenatal maternal stress is a well-documented contributor to adverse birth outcomes such as low birth weight and preterm delivery (Bussières et al., 2015; Lobel and Dunkel Schetter, 2016). Prenatal stress is associated with enduring risk for physical and mental health problems for offspring, including asthma, autism, and obesity (Douros et al., 2017; Tate et al., 2015; Varcin et al., 2017), both internalizing and externalizing problems in childhood (Hentges et al., 2019; Kingston et al., 2018), and poorer emotional and behavioral functioning into adolescence and adulthood (Betts et al., 2015; Korhonen et al., 2014). These effects underscore the importance of documenting the global impact of stress on mental health in women pregnant during the COVID-19 pandemic.
1.2. Prenatal maternal stress and mental health during the COVID-19 pandemic
Shortly after the pandemic outbreak, high prenatal anxiety rates were reported in individual studies around the world (Lebel et al., 2020; Preis et al., 2020a; Saccone et al., 2020; Taubman-Ben-Ari et al., 2020) and in meta-analyses assessing perinatal mood and anxiety symptoms (Hessami et al., 2020; Tomfohr-Madsen et al., 2021; Yan et al., 2020). Yan et al. concluded that cumulatively, pregnant women were 1.65 times likelier to experience anxiety compared to the pre-pandemic period (Yan et al., 2020). In addition, there was an increase in prenatal depressive symptoms in some countries, but no increase in depressive symptoms in pooled estimates (Yan et al., 2020). Substantial heterogeneity exists among studies, with prevalence for anxiety ranging from 3.0% to 82.0% and for depressive symptoms, from 5.0% to 64.0%. Some of these differences may be attributable to geographic conditions (e.g., different rates of infection and measures implemented to mitigate infection) and to cultural norms, but as with studies conducted prior to the pandemic, this variation may also reflect the use of dissimilar assessment tools (Woody et al., 2017) and study populations (e.g., non-clinical vs. clinical).
Existing evidence implicates pandemic-related conditions (e.g., death of relative from COVID-19, disruptions to prenatal care, restricted access to outdoors, employment loss), pregnancy-specific stress, and maternal characteristics such as youth and nulliparity as contributors to prenatal anxiety and depression during the COVID-19 pandemic within individual countries (Hessami et al., 2020; Preis et al., 2020b; Tomfohr-Madsen et al., 2021; Yan et al., 2020). However, whether these are common risk factors for poor mental health during the pandemic in childbearing women from different countries remains unknown. Rigorous inter-national comparisons and modeling to predict prenatal mood and anxiety problems have not been undertaken. A study conducted in June–July 2020 of 3907 pregnant women from five European countries reported differences in the prevalence of anxiety, general stress, and depression, but prediction of maternal mental health, including the likely contribution of general stress, was not investigated in this study (Ceulemans et al., 2021). Nor were pregnancy-specific or pandemic-related types of stress examined.
1.3. The current study
There is a critical need to identify common processes leading to poorer mental health in pregnancy irrespective of geography. This need has been made more compelling by growing evidence internationally that the COVID-19 pandemic exacerbated maternal stress and increased risk for mood and anxiety disorders, with concerns about the potential long-term harm to women and children (Abdoli et al., 2020; McDonald et al., 2021). Robust investigation to test a common model of maternal stress and mental health requires a consistent methodological protocol across data collection sites and comparisons conducted with the same, validated and reliable psychological assessment tools, administered within a focused timeframe. Incorporating these methodological features in the present study, we examined the reproducibility across seven high-income Western countries of a hypothesized multivariate path model predicting clinically relevant maternal anxiety and depressive symptoms from pregnancy-specific and pandemic-related stress and maternal characteristics, despite likely differences in the prevalence of stress and anxiety and depressive symptoms from country to country.
2. Methods
2.1. Overview
This report is from the first timepoint of the International COVID-19 Pregnancy Experiences (I-COPE) Study. The I-COPE Study is a collaboration among experts from Germany, Israel, Italy, Poland, Spain, Switzerland, and the United States, originating from the Stony Brook COVID-19 Pregnancy Experiences (SB-COPE) Study conducted in the U.S. (Preis et al., 2020b). Shortly after the COVID-19 pandemic onset was declared on March 11, 2020 by the World Health Organization, collaborators in each participating country agreed to use the same study instruments and protocol (with minor variations) and obtained ethical approval from their home institutions to conduct the research. All of the countries except for Spain and Italy recruited participants online via social media (Spain and Italy recruited patients from prenatal care clinics). Eligibility to participate in the I-COPE Study included being pregnant at the time of questionnaire completion and able to read the language of the study questionnaire. Recruitment began on April 17, 2020 and ended by mid-late May in Germany, Israel, and Poland; recruitment in Italy, Spain, Switzerland, and the U.S. continued but is not part of the current report. To enhance comparability across countries, in the current report we included only participants who were recruited before May 31, 2020.
2.2. Transparency and openness
We followed STROBE guidelines for observational studies, including reporting of eligibility criteria, wording of research materials, and description of statistical analysis. The data that support the findings of this report are available upon request from the corresponding author and are not publicly available in order to maintain the privacy of research participants in the ongoing I-COPE Study.
3. Measures
3.1. Maternal characteristics
We assessed Age (in years), Gestational Age (in weeks), Parity (Nulliparous vs. Multiparous), self-reported High-Risk Pregnancy status (No vs. Yes or Unsure), and Access to Outdoors (Yes, whenever I want vs. Sometimes or Rarely). These factors have been shown to predict pregnancy-specific stress, pandemic-related prenatal stress, and/or prenatal mental health in numerous studies.
3.2. Stress
Pandemic-related stress was assessed using the Pandemic-Related Pregnancy Stress Scale (PREPS) which was developed for the I-COPE Study and was translated and rigorously validated by each participating I-COPE Study country (Garcia-Silva et al., 2021, Ilska et al., 2021, Penengo et al., 2021, Preis et al., 2020, Schaal et al., 2021, Yirmiya et al., 2021). The PREPS includes two stress subscales. Pandemic-Related Preparedness Stress (PREPS-Preparedness) assesses the extent to which women experience stress about being unprepared for birth or postpartum due to the pandemic with seven items such as “I am worried that the pandemic could ruin my birth plans”. The Perinatal Infection Stress subscale (PREPS-Infection) assesses stress from concerns about COVID-19 infection to oneself or the fetus/baby and is comprised of five items such as “I am worried that my baby could get COVID-19 at the hospital after birth”. Women rated their agreement with each PREPS statement on a scale from 1 (Very Little) to 5 (Very Much). Scores were derived by calculating the average item response for each subscale. Both PREPS subscales were internally consistent in all participating countries with Cronbach's alphas ranging from 0.65 to 0.88 for PREPS-Preparedness and 0.60–0.88 for PREPS-Infection.
Pregnancy-Specific Stress was assessed using the Revised Prenatal Distress Questionnaire (NuPDQ), which has been translated and validated in numerous countries (Ibrahim and Lobel, 2020). Women rate the extent to which they are “feeling bothered, upset, or worried” about 17 pregnancy-relevant stressors such as “physical symptoms of pregnancy”, “what will happen during labor and delivery”, and “whether you might have an unhealthy baby” on a scale from 0 (Not at All) to 2 (Very Much). The NuPDQ was not administered to participants in Spain (instead, its predecessor, the Prenatal Distress Questionnaire, was used, and is not analyzed here). Cronbach's alphas ranged from 0.55 to 0.79 among the remaining I-COPE Study countries. Average NuPDQ item response was analyzed.
3.3. Anxiety and depressive symptoms
Anxiety Symptoms were assessed using the Generalized Anxiety Disorder-7 (GAD-7), a well-validated 7-item self-report instrument (Spitzer et al., 2006). Respondents report frequency of symptoms over the last two weeks on a scale ranging from 0 (Not at All) to 3 (Nearly Every Day). Individual scores were calculated as a sum of item responses. Recommended clinical cut-offs for anxiety severity are: 0–4 minimal, 5–9 mild, 10–14 moderate, and 15–21 severe (Spitzer et al., 2006). The GAD-7 had strong internal consistency with Cronbach's alphas ranging from 0.87 to 0.91 among the six I-COPE Study countries that administered this instrument (all but Spain).
Depressive Symptoms were assessed using the well-validated two-item Patient Health Questionnaire-2 (PHQ-2) (Kroenke et al., 2003). Respondents report the frequency of depressive symptoms over the last two weeks on a scale ranging from 0 (Not at All) to 3 (Nearly Every Day). Individual scores were calculated as a sum of item responses. The recommended clinical cut-off for likely major depression is ≥ 3 (Löwe et al., 2005). The PHQ-2 had strong internal consistency with Cronbach's alphas ranging from 0.87 to 0.91 among six I-COPE Study countries (all but Spain administered the PHQ-2).
3.4. Analysis
To compare the magnitude of stress and mental health variables among I-COPE Study countries, we conducted one-way Analyses of Variance for continuous variables and Chi-Square tests for categorical variables. We then examined associations among pandemic-related stress, pregnancy-specific stress, anxiety and depressive symptoms, and maternal characteristics (age, gestational age, parity, high-risk pregnancy status, access to outdoors) for all study participants.
We used Structural Equation Modeling (SEM) to test the hypothesized model in which maternal characteristics, pandemic-related stress (PREPS-Preparedness and PREPS-Infection), and pregnancy-specific stress sequentially predicted two mental health outcomes: moderate/severe anxiety symptoms (GAD-7 score ≥10) and likely depression (PHQ-2 score ≥3) among all I-COPE Study participants (including those from Spain without data on some study variables). Thereafter, we tested this model separately for each country that had all study variable data from at least 200 participants: Germany (n = 1179), Israel (n = 1090), Poland (n = 1050), and the United States (n = 4388). Well-accepted criteria (Hu and Bentler, 1999) were used to assess model goodness-of-fit: Comparative Fit Index (CFI) ≥ 0.95, Normed Fit Index (NFI) ≥ 0.95, Root Mean Square Error of Approximation (RMSEA) ≤ 0.06, and Standardized Root Mean Squared Residual (SRMR) ≤ 0.08. Comparisons of study variables were performed using SPSS version 27.0 (IBM Corp, 2020); SEM was performed using AMOS (Arbuckle, 2014).
In each of the participating countries, rates of missing data were minimal and missing completely at random (MCAR) for all but Italy, with ranges and Little's MCAR test values as follows: Germany 0.0%–0.3%, X2 (6) = 7.69, p = 0.26; Israel 0.0%–0.7%, Χ2 (6) = 14.95, p = 0.46; Italy 0.0%–6.7%, Χ2 (27) = 51.97, p = 0.003; Poland 0.0%–0.1%, Χ2 (7) = 6.11, p = 0.53; Spain 0.0% (for all variables assessed); Switzerland 0.0%; United States 0.0%–0.1%, Χ2 (17) = 10.86, p = 0.86. For univariate analyses, we used pairwise deletion to accommodate missing data. For SEM, maximum likelihood estimates were used as these are robust for data missing completely at random or not (Muthén et al., 1987).
4. Results
A total of 8148 pregnant women from Germany, Israel, Italy, Poland, Spain, Switzerland, and the United States were recruited between April 17 and May 31, 2020 as part of the I-COPE Study. Women were on average 31.1 years old (SD = 4.5) and 26.9 weeks pregnant (SD = 8.9) when they completed the study questionnaire. Central tendencies and frequencies for all study variables are displayed in Table 1 ; detailed information about study participants appears in Table 2 . There were differences in maternal characteristics across the seven countries. For example, women in Poland and the U.S. were younger than participants from countries other than Switzerland, a significantly greater proportion of women in Poland reported limited outdoor access, and the sample from Spain was distinctive by being predominantly at high risk.
Table 1.
Study variables and associations across countries.
| M (SD)a | PREPS -Preparedness Stress | PREPS -Infection Stress | Pregnancy-Specific Stress | Anxiety Symptoms | Depressive Symptoms | |
|---|---|---|---|---|---|---|
| Pearson's Correlationsa | ||||||
| PREPS-Preparedness | 3.30 ± 0.93 | – | ||||
| PREPS-Infection | 3.05 ± 1.06 | 0.61*** | – | |||
| Pregnancy-Specific Stress | 0.82 ± 0.34 | 0.57*** | 0.44*** | – | ||
| Anxiety Symptoms | 7.70 ± 5.24 | 0.46*** | 0.39*** | 0.50*** | – | |
| Depressive Symptoms | 1.63 ± 1.63 | 0.35*** | 0.26*** | 0.42*** | 0.71*** | – |
| Age | 31.13 ± 4.55 | −0.16*** | −0.06*** | −0.14*** | −0.11*** | −0.13*** |
| Gestational Age | 26.86 ± 8.85 | 0.03** | −0.03* | −0.07*** | 0.02 | −0.04** |
| t tests and values | ||||||
| Parity | n (%) | t = 11.40*** | t = 2.61** | t = 17.29*** | t = 4.02*** | t = 2.00* |
| Nullipara | 3931 (48.3) | 3.42 ± 0.91 | 3.09 ± 1.05 | 0.89 ± 0.33 | 7.46 ± 5.16 | 1.59 ± 1.60 |
| Multipara | 4206 (51.7) | 3.19 ± 0.93 | 3.03 ± 1.08 | 0.76 ± 0.33 | 7.93 ± 5.31 | 1.67 ± 1.64 |
| Pregnancy Risk | t = 9.53*** | t = 12.05** | t = 12.45*** | t = 12.47*** | t = 2.00* | |
| Low risk | 5533 (67.9) | 3.24 ± 0.94 | 2.96 ± 1.06 | 0.79 ± 0.33 | 7.21 ± 5.05 | 1.54 ± 1.58 |
| High risk or Unsure | 2615 (32.1) | 3.44 ± 0.89 | 3.26 ± 1.04 | 0.89 ± 0.34 | 8.83 ± 5.49 | 2.21 ± 1.78 |
| Access to Outdoors | t = 12.45*** | t = 8.17*** | t = 11.69*** | t = 8.38*** | t = 6.45*** | |
| Yes, whenever I want | 6864 (86.5) | 3.25 ± 0.93 | 3.01 ± 1.06 | 0.80 ± 0.33 | 7.51 ± 5.16 | 1.55 ± 1.56 |
| Sometimes or Rarely | 1075 (13.5) | 3.63 ± 0.90 | 3.30 ± 1.09 | 0.93 ± 0.34 | 9.03 ± 5.60 | 1.81 ± 1.75 |
*p < 0.05.
**p < 0.01.
***p < 0.001.
Sample sizes ranged from 7934 to 8137. PREPS: Pandemic-Related Pregnancy Stress.
Table 2.
Sample characteristics by country.
| Germany n = 1179 | Israel n = 1090 | Italy n = 120 | Poland n = 1050 | Spain n = 201 | Switzerland n = 120 | United States n = 4388 | F | |
|---|---|---|---|---|---|---|---|---|
| Age M (SD) | 31.9 (4.3)a | 31.9 (4.2)a | 32.2 (4.2)a | 30.5 (4.0)b | 32.2 (4.2)a | 31.1 (4.2)ab | 30.8 (4.7)b | 19.5*** |
| Gestational Age M (SD) | 28.0 (9.2)a | 27.1 (8.4)ab | 26.3 (9.5)ab | 27.0 (9.2)ab | 25.7 (9.5)ab | 27.2 (9.5)ab | 26.5 (8.6)b | 5.0*** |
| Nulliparity n (%) | 598 (50.9)a | 431 (39.7)b | 25 (20.8)c | 455 (43.4)bd | 102 (50.7)abd | 69 (57.5)ad | 2251 (51.3)a | 102.9*** |
| High-risk n (%) | 360 (30.5)a | 283 (26.0)a | 37 (30.8)ab | 168 (16.0)c | 169 (84.1)d | 24 (20.0)ac | 1574 (35.9)b | 431.0*** |
| Limited Access to Outdoors n (%) | 132 (11.2)ab | 166 (15.2)b | 17 (15.0)ab | 307 (29.3)c | – | 12 (10.0)ab | 441 (10.1)a | 276.4*** |
*p < 0.05, **p < 0.01, ***p < 0.001 Values with different superscripts within a row differ significantly.
4.1. Magnitude of prenatal stress and mental health variables by country
As shown in Table 3 , there were significant differences between countries in prenatal stress and mental health (Fs ranging from 27.14 to 95.56, ps < 0.001). Overall, participants in the U.S. and Poland reported higher levels of stress and poorer mental health. The highest levels of PREPS-Preparedness stress were reported by women in Poland, Spain, and the U.S., followed by Germany and Israel, and the lowest levels of this type of stress were reported in Switzerland. The highest levels of PREPS-Infection stress were reported in Spain followed by the U.S., Poland, Germany, and Israel, with lowest levels in Switzerland. The highest levels of pregnancy-specific stress were reported by women in the U.S., followed by Israel and Poland; the lowest levels of pregnancy-specific stress were reported by participants from Germany and Switzerland. The highest frequencies of anxiety symptoms were reported by women in the U.S., followed by Germany and Poland, with lowest frequencies in Israel. Finally, the highest frequency of depressive symptoms was reported by women in Poland, followed by Germany and the U.S., and the lowest frequencies were in Israel and Switzerland.
Table 3.
Stress and mental health variables by country.
| Range | Germany n = 1179 | Israel n = 1090 | Italy n = 120 | Poland n = 1050 | Spain n = 201 | Switzerland n = 120 | United States n = 4388 | F | |
|---|---|---|---|---|---|---|---|---|---|
| PREPS-Preparedness | 1–5 | 2.99 ± 0.93a | 2.94 ± 0.98a | 2.94 ± 0.34ab | 3.46 ± 0.95c | 3.44 ± 0.61c | 2.62 ± 0.94bc | 3.46 ± 0.87c | 95.56*** |
| PREPS-Infection | 1–5 | 2.64 ± 1.04a | 2.76 ± 1.09a | 2.58 ± 0.96a | 2.99 ± 1.15b | 3.40 ± 0.68c | 2.46 ± 0.97a | 3.27 ± 1.00c | 94.35*** |
| Pregnancy Specific Stress | 0–2 | 0.70 ± 0.31a | 0.80 ± 0.32b | 0.60 ± 0.33a | 0.82 ± 0.33b | – | 0.73 ± 0.31ab | 0.86 ± 0.34c | 57.83*** |
| Anxiety Symptoms | 0–21 | 7.03 ± 4.48a | 5.97 ± 4.79b | 5.30 ± 4.35b | 7.11 ± 5.31a | – | 6.09 ± 4.27ab | 8.57 ± 5.39c | 63.90*** |
| Depressive Symptoms | 0–6 | 1.61 ± 1.47a | 1.30 ± 1.42b | 0.96 ± 1.14b | 2.03 ± 1.67c | – | 1.26 ± 1.27ab | 1.65 ± 1.70a | 27.14*** |
*p < 0.05, **p < 0.01, ***p < 0.001 PREPS: Pandemic-Related Pregnancy Stress. Values with different superscripts within a row differ significantly; bold values are the highest stress and mental health levels by row.
Rates of moderate and severe anxiety symptoms (GAD-7 ≥ 10) ranged from 14.2% in Switzerland (n = 17) to 36.0% in the United States (n = 1587) (Poland 28.0% [n = 294], Germany 24.9% [n = 293], Israel 20.2% [n = 210], Italy 19.1% [n = 22]) and the omnibus difference in prevalence was significant (χ2 (5) = 157.1, p < 0.001). Rates of likely depressive disorder (PHQ-2 ≥ 3) ranged from 10.8% (n = 17) in Switzerland to 30.5% (n = 320) in Poland (United States 23.4% [n = 1027], Germany 20.9% [n = 247], Israel 15.6% [n = 170], Italy 12.7% [n = 15]), and the overall difference in prevalence of this mental health variable was also significant (χ2 (5) = 87.7, p < 0.001).
4.2. Common model of prenatal maternal stress and mental health outcomes
As illustrated by Table 1, aggregated across countries, pandemic-related and pregnancy-specific stress were moderately to strongly correlated (rs = 0.44–0.61, p < 0.001). Stress was moderately to strongly correlated with anxiety symptoms and depressive symptoms (rs = 0.26–0.50, ps < 0.001). Age was inversely associated with stress and with frequency of anxiety and depressive symptoms (rs = -0.06 to −0.16, ps < 0.001). Gestational Age was weakly associated with the stress and mental health variables: positively related to PREPS-Preparedness stress and inversely related to PREPS-Infection stress, pregnancy-specific stress, and depressive symptoms (|r|s = 0.03–0.07, ps < 0.05). Nulliparous women reported significantly higher levels of both types of pandemic-related stress and pregnancy-specific stress (ts = 2.61–17.29, ps < 0.01), less frequent anxiety symptoms, and less frequent depressive symptoms (ts = 2.00–4.02, ps < 0.05). Women with a high-risk pregnancy or who were unsure of their pregnancy risk status (ts = 2.00–12.47, ps < 0.05) and women with limited access to the outdoors had significantly higher levels of stress and more frequent anxiety and depressive symptoms (ts = 6.45–12.45, ps < 0.001).
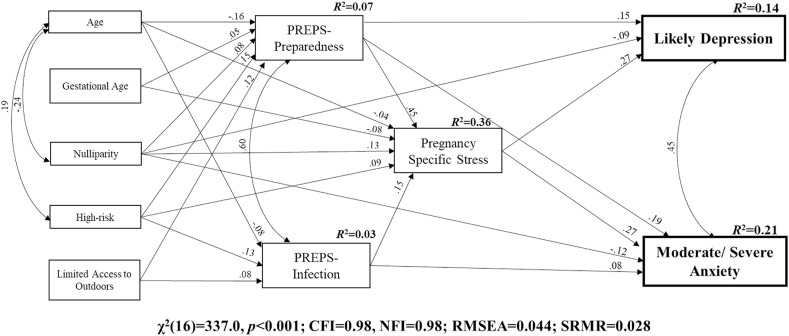
We tested the hypothesized model predicting likely depression (PHQ-2 ≥ 3) and moderate/severe anxiety symptoms (GAD-7 ≥ 10) from maternal characteristics, PREPS-Preparedness stress, PREPS-Infection stress, and pregnancy-specific stress. Based on the bivariate analyses, we included paths representing the correlation of age with nulliparity and risk status, between the two pandemic-related stress variables, and between likely depression and moderate/severe anxiety symptoms. The model, displayed in Fig. 1 , had excellent model fit (CFI = 0.98, NFI = 0.98; RMSEA = 0.044; SRMR = 0.028) (Hu and Bentler, 1999). It explained 21% of variance in moderate/severe anxiety and 14% of variance in likely depression. Both types of pandemic-related stress (PREPS-Preparedness stress and PREPS-Infection stress) indirectly predicted the mental health outcomes via pregnancy-specific stress, which was a strong predictor of likely depression and moderate/severe anxiety (b = 0.27). Likely depression was also directly predicted by PREPS-Preparedness stress (b = 0.15) and inversely by nulliparity (b = −0.09). In addition to its prediction by pregnancy-specific stress, moderate/severe anxiety was predicted inversely by nulliparity (b = −0.12) and by both types of pandemic-related stress (PREPS-Preparedness b = 0.19; PREPS-Infection b = 0.08). The remaining maternal characteristics were predictors of stress variables but were not directly associated with the mental health outcomes.
Fig. 1.
Structural equation model predicting prenatal mental health outcomes in seven countries during the COVID-19 pandemic onset. Note: All paths are significant at p < 0.001. Path weights are standardized regression coefficients. Data are from I-COPE Study participants in all seven countries, including those without some study variables (i.e., Spain).
We also tested the model shown in Fig. 1 with continuous anxiety symptoms and depressive symptoms instead of dichotomized mental health variables. This model had excellent fit, as well. Variance explained in continuous anxiety and depressive symptoms was greater (32% and 21%, respectively) than for dichotomized variables, with minor differences in the magnitude of path coefficients. Full results of this model are reported in Supplemental Materials.
4.3. Evaluating the common model of prenatal maternal stress and mental health outcomes by country
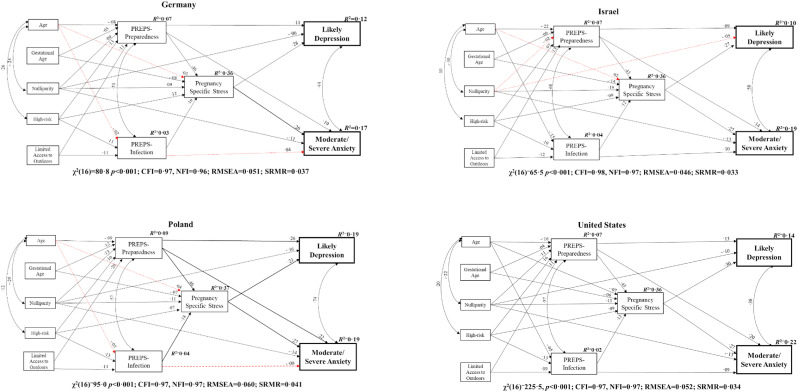
The model shown in Fig. 1 was tested separately for each country with full study variable data from at least 200 participants: Germany, Israel, Poland, and the United States. As displayed in Fig. 2 , the model had excellent fit in each of the four countries (CFIs = 0.97–0.98, NFIs = 0.96–0.97; RMSEAs = 0.046–0.060; SRMRs = 0.033–0.041) (Hu and Bentler, 1999). In each of these countries, all paths from the pandemic-related stress variables to pregnancy-specific stress, from pregnancy-specific stress to the mental health outcomes, and from PREPS-Preparedness stress to the mental health outcomes remained significant. However, PREPS-Infection stress was not a significant predictor of moderate/severe anxiety when examined only among participants in Germany or Poland. Some of the paths from maternal characteristics were also no longer significant when examined by individual country, including associations of age with PREPS-Infection stress and pregnancy-specific stress in Germany and Poland, and of age and nulliparity respectively with pregnancy-specific stress, PREPS-Preparedness stress, and likely depression among participants in Israel.
Fig. 2.
Structural equation model predicting prenatal mental health outcomes in four countries during the COVID-19 pandemic onset. Note: Black (solid) paths are significant at p < 0.05. Red (dotted) paths are not significant. Path weights are standardized regression coefficients.
5. Discussion
Study findings suggest that during the pandemic onset, pregnant women across the Western world experienced substantial pandemic-related and pregnancy-specific stress. These types of stress were associated with more frequent anxiety and depressive symptoms, including levels of symptoms above clinically defined thresholds for poor mental health. Whereas the magnitude of stress and mood disturbances varied among the seven high-income Western countries that were part of the I-COPE Study, a common model predicting anxiety and depression from maternal characteristics and stress was supported.
Among the seven countries, rates of moderate and severe anxiety symptoms ranged from 14.2% to 36.0% and rates of likely depressive disorder ranged from 10.8% to 30.5%. Rates of both types of mood disturbance among women in Germany, Poland, and the U.S. exceeded global ranges reported in meta-analyses conducted prior to the pandemic (Dennis et al., 2017; Fawcett et al., 2019; Underwood et al., 2016; Woody et al., 2017). Differences in the prevalence of high anxiety and likely depression found across the seven Western countries examined here indicate that while the COVID-19 pandemic is a global event, its impacts on maternal mood disturbance have varied from country to country. The dissimilarities in anxiety and depressive symptom magnitude are likely attributable to cultural differences and to differences in pandemic conditions. Pre-pandemic studies attribute differences in prenatal stress, mood, and anxiety across countries to corresponding differences in factors such as family structure, healthcare, childcare policies, and the status of women (Woody et al., 2017). Correspondingly, it is likely that the differences we observed across countries in associations of maternal age and parity with stress and mood disturbance are due to the different resources and supports that are provided to pregnant women in each nation. Additionally, pandemic-related conditions such as rates of infection, hospitalization, and deaths also differed across the countries where we conducted this research. Reliable analysis controlling for such variables was not possible because of a lack of rigorous data: several of the participating countries offered limited SARS-CoV-2 testing and did not collect or report public health data systematically during the time period of this study. Nonetheless, we speculate that differences in pandemic-related conditions help explain why, for example, pregnant women from the U.S. reported greatest levels of pandemic-related stress. That is, even considering the limited reliability of comparison data, the U.S. appears to have had the highest numbers of confirmed cases and deaths per capita during the study period (Appel et al., 2021).
Despite differences in the magnitude of stress and mood disturbance, and a few differences in predictors of these, substantial similarity across countries was found in contributors to these psychological and emotional variables. Study findings implicate maternal characteristics, namely maternal age, gestational age, nulliparity, and high-risk pregnancy, restricted outdoor access, and most importantly, pandemic-related and pregnancy-specific stress, as common components in prediction of anxiety and depression. This common model highlights the deleterious impact of various types of stress on the mental health of women pregnant during a public health crisis. Both types of stress precipitated by the current pandemic -- stress related to feeling unprepared for birth because of pandemic restrictions, and stress associated with fearing perinatal COVID-19 infection -- predicted higher pregnancy-specific stress, a well-documented risk factor for pregnant women (Bussières et al., 2015; Ibrahim and Lobel, 2020). Together, these types of stress explained a significant portion of variance in anxiety and depression experienced by women during the COVID-19 pandemic onset. This pattern of findings bolsters the conclusion that varying levels of stress across the seven participating countries were culprits in their heterogeneous mood disturbance prevalence. The often-unmeasured impact of stress in previous studies of maternal anxiety and depression prevalence -- conducted both prior to and during the pandemic -- may help explain the wide range of estimates produced by those studies. Applied beyond the seven countries studied here, and in conjunction with other research investigating the impact of prenatal maternal stress, study findings suggest that the impact of stress on pregnant women's mental health is a universal phenomenon, especially during times of peril.
5.1. Strengths and limitations
The current study's strengths derive from its inclusion of a large sample of pregnant women from seven Western countries that were recruited during a well-defined, narrow time span, in close proximity to the pandemic onset, and the study's use of the same assessment tools for participants. However, findings may not generalize to women residing in non-Western and less well-resourced countries who tend to endure poorer mental health (Gelaye et al., 2016; Woody et al., 2017), although they experience comparable effects of prenatal maternal stress on child outcomes (Buffa et al., 2018). In addition, recruitment through social media, which was necessitated by pandemic-related restrictions and enabled us to reach a large sample size in a short amount of time, is not unbiased and limits the generalizability of study findings (additionally, women in Spain and Italy were not recruited in this way). Because of the extraordinary circumstances of this pandemic, and the unprecedented opportunity to examine prenatal maternal stress and its impact across the Western world, we included all of the countries that participated in this collaborative international project, despite the fact that participants in Spain did not complete the GAD-7 or PHQ-2. Finally, while much research indicates that the GAD-7 and PHQ-2 are valid self-report instruments to assess mood disturbances in pregnancy, we did not conduct structured diagnostic interviews to verify clinical diagnoses.
6. Conclusions
Prenatal maternal stress has been linked to adverse maternal, fetal, infant, and child outcomes in numerous pre-pandemic studies (Glover, 2015; Lobel and Dunkel Schetter, 2016; Manzari et al., 2019), including research conducted after other major natural and human-made disasters such as earthquakes, storms, and terrorist attacks (Buthmann et al., 2019; Lederman et al., 2004). In addition to the evidence from this study demonstrating the impact of stress on maternal mental health during the onset of the COVID-19 pandemic, some evidence suggests that prenatal stress during the pandemic onset also adversely affected perinatal outcomes, including greater likelihood of preterm birth and delivery of a newborn small for gestational age (Preis et al., 2021).
Pregnant women are affected by the same pandemic stressors that have burdened all people, including fear of the virus, deaths and illness of loved ones, social isolation, food instability, and financial loss. Yet pregnant women have also been faced with additional, unique stressors during this period. Our findings indicate that the totality of stress experienced by pregnant women in various global locations led to anxiety and depression at the onset of the COVID-19 pandemic. The magnitude and impact of stress on women pregnant during later periods of the pandemic remain to be determined, with numerous research efforts underway. Nevertheless, the mental health impacts of stress experienced during pregnancy pose longer-term threats to the health and well-being of women and their offspring. Prevention and reduction of maternal stress, especially among vulnerable groups such as first-time mothers and those with a high-risk pregnancy, should be prioritized. Supports to childbearing women including affordable health care, mental health screening and services, childcare, and employment accommodations for pregnancy, childbirth, and infant care are cost-effective and produce a range of benefits to societies at large (Lobel and Ibrahim, 2018). Measures that alleviate the burdens placed upon childbearing women offer a means to improve the health and well-being of women and their families across the world, particularly during global crises when stress and its consequences are heightened.
Author contributions
The I-COPE Study was conceived by ML, HP, and BM. ML is Leader and HP is Co-Leader of the I-COPE Study, providing supervision and consultation to the I-COPE Team within each country. Data within each participating country were gathered, curated, and analyzed and project administration was conducted by the following: Germany (NS); Israel (KY, SA, IR), Italy (MB, CP, CC, MG, LD); Poland (MI, AB-S,AK-Z); Spain (RC-G); Switzerland (RC, PLM-G, HM); United States (HP, BM, ML). The first draft of this report was written by a core writing team led by ML, HP, and BM. All authors contributed to the writing and editing of subsequent drafts, and the conclusions. The report was prepared under the general direction of the I-COPE Study Leader (ML) and Co-Leader (HP), with input from BM throughout the process. NS, KY, MB, MI, RC-G, RC, and HP verified the data in their respective countries. HP verified the data combined from all participating countries.
Footnotes
We use terms such as woman and maternal to refer to an individual who is capable of pregnancy and birth although we recognize that not all individuals use these terms and we do not mean to overlook or slight transgendered, agender, or any other childbearing persons.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.socscimed.2022.115499.
Funding for the SB-COPE Study (United States) was provided by a Stony Brook University Office of the Vice President for Research and Institute for Engineering-Driven Medicine COVID-19 Seed Grant to Heidi Preis, Brittain Mahaffey, and Marci Lobel and a “Supplement for NIH Grants to Add or Expand Research Focused on Maternal Health, Structural Racism and Discrimination, and COVID-19” funded by the NIH Office of the Director, Implementing a Maternal Health and Pregnancy Outcomes Vision for Everyone (IMPROVE) Program and the NIH/National Institute on Drug Abuse (R21DA049827-02S1). No funding was provided to other I-COPE participants. The funding sources had no involvement in the conduct of the research or preparation of this article.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abdoli A., Falahi S., Kenarkoohi A., Shams M., Mir H., Jahromi M.A.M. The COVID-19 pandemic, psychological stress during pregnancy, and risk of neurodevelopmental disorders in offspring: a neglected consequence. J. Psychosom. Obstet. Gynecol. 2020;41(3):247–248. doi: 10.1080/0167482X.2020.1761321. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Bowen A., Feng C.X., Muhajarine N. Trajectories of maternal depressive and anxiety symptoms from pregnancy to five years postpartum and their prenatal predictors. BMC Pregnancy Childbirth. 2019;19(1):1–10. doi: 10.1186/s12884-019-2177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderdice F., Lynn F., Lobel M. A review and psychometric evaluation of pregnancy-specific stress measures. J. Psychosom. Obstet. Gynecol. 2012;33(2):62–77. doi: 10.3109/0167482X.2012.673040. [DOI] [PubMed] [Google Scholar]
- Appel C., Beltekian D., Gavrilov D., Giattino C., Hasell J., Macdonald B., Mathieu E., Ortiz-Ospina E., Ritchie H., Rodés-Guirao L., Roser M. 2021. Data on COVID-19.https://covid.ourworldindata.org/data/owid-covid-data.csv (coronavirus) published online at OurWorldInData.org. Available at: [Google Scholar]
- Arbuckle J.L. IBM SPSS; 2014. Amos. [Computer Program] [Google Scholar]
- Auerbach M.V., Lobel M., Cannella D.T. Psychosocial correlates of health-promoting and health-impairing behaviors in pregnancy. J. Psychosom. Obstet. Gynecol. 2014;35(3):76–83. doi: 10.3109/0167482X.2014.943179. [DOI] [PubMed] [Google Scholar]
- Betts K.S., Williams G.M., Najman J.M., Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress. Anxiety. 2015;32(2):82–90. doi: 10.1002/da.22272. [DOI] [PubMed] [Google Scholar]
- Biaggi A., Conroy S., Pawlby S., Pariante C.M. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J. Affect. Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa G., Dahan S., Sinclair I., St-Pierre M., Roofigari N., Mutran D., Rondeau J.-J., Dancause K.N. Prenatal stress and child development: a scoping review of research in low-and middle-income countries. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0207235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussières E.-L., Tarabulsy G.M., Pearson J., Tessier R., Forest J.-C., Giguère Y. Maternal prenatal stress and infant birth weight and gestational age: a meta-analysis of prospective studies. Dev. Rev. 2015;36:179–199. [Google Scholar]
- Buthmann J., Ham J., Davey K., Finik J., Dana K., Pehme P., Zhang W., Glover V., Nomura Y. Infant temperament: repercussions of superstorm sandy-related maternal stress. Child Psychiatr. Hum. Dev. 2019;50(1):150–162. doi: 10.1007/s10578-018-0828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros-Gonzalez R.A., Romero-Gonzalez B., Strivens-Vilchez H., Gonzalez-Perez R., Martinez-Augustin O., Peralta-Ramirez M.I. Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros-Gonzalez R.A., Romero-Gonzalez B., Gonzalez-Perez R., Lucena-Prieto L., Perez-Garcia M., Cruz-Quintana F., Peralta-Ramirez M.I. Maternal and neonatal hair cortisol levels are associated with infant neurodevelopment at six months of age. J. Clin. Med. 2019;8(11):1–14. doi: 10.3390/jcm8112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans M., Foulon V., Ngo E., Panchaud A., Winterfeld U., Pomar L., Lambelet V., Cleary B., O'Shaughnessy F., Passier A., Richardson J.L., Hompes T., Nordeng H. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic — a multinational cross-sectional study. Acta Obstet. Gynecol. Scand. 2021;100(7):1219–1229. doi: 10.1111/aogs.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers B.R., Garad R.M., Boyle J.A., Skouteris H., Teede H.J., Harrison C.L. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J. Med. Internet Res. 2020;22(9) doi: 10.2196/22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C.-L., Falah-Hassani K., Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- Douros K., Moustaki M., Tsabouri S., Papadopoulou A., Papadopoulos M., Priftis K.N. Prenatal maternal stress and the risk of asthma in children. Front. Pediatr. 2017;5:202. doi: 10.3389/fped.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C., Niles A.N., Guardino C.M., Khaled M., Kramer M.S. Demographic, medical, and psychosocial predictors of pregnancy anxiety. Paediatr. Perinat. Epidemiol. 2016;30(5):421–429. doi: 10.1111/ppe.12300. [DOI] [PubMed] [Google Scholar]
- Fawcett E.J., Fairbrother N., Cox M.L., White I.R., Fawcett J.M. The prevalence of anxiety disorders during pregnancy and the postpartum period: a multivariate Bayesian meta-analysis. J. Clin. Psychiatr. 2019;80(4):18r12527. doi: 10.4088/JCP.18r12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Prenatal depression risk factors, developmental effects and interventions: a review. J. Pregnancy Child Health. 2017;4(1):301. doi: 10.4172/2376-127X.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Silva J., Caracuel-Romero A., Lozano-Ruiz A., Alderdice F., Lobel M., Perra O., Caparros-Gonzalez R.A. Pandemic-related pregnancy stress among pregnant women during the COVID-19 pandemic in Spain. Midwifery. 2021;103:103163. doi: 10.1016/j.midw.2021.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaye B., Rondon M.B., Araya R., Williams M.A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatr. 2016;3(10):973–982. doi: 10.1016/S2215-0366(16)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. In: Perinatal Programming of Neurodevelopment. Antonelli M.C., editor. Springer; 2015. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms; pp. 269–283. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S., Graves L., Peer M., Mamisashvili L., Tomlinson G., Vigod S.N., Dennis C.-L., Steiner M., Brown C., Cheung A. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J. Clin. Psychiatr. 2018;79(5):17r12011. doi: 10.4088/JCP.17r12011. [DOI] [PubMed] [Google Scholar]
- Hentges R.F., Graham S.A., Plamondon A., Tough S., Madigan S. A developmental cascade from prenatal stress to child internalizing and externalizing problems. J. Pediatr. Psychol. 2019;44(9):1057–1067. doi: 10.1093/jpepsy/jsz044. [DOI] [PubMed] [Google Scholar]
- Hessami K., Romanelli C., Chiurazzi M., Cozzolino M. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020:1–8. doi: 10.1080/14767058.2020.1843155. Ahead-of-Print. [DOI] [PubMed] [Google Scholar]
- Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Modeling. 1999;6(1):1–55. [Google Scholar]
- Ibrahim S.M., Lobel M. Conceptualization, measurement, and effects of pregnancy-specific stress: review of research using the original and revised Prenatal Distress Questionnaire. J. Behav. Med. 2020;43:16–33. doi: 10.1007/s10865-019-00068-7. [DOI] [PubMed] [Google Scholar]
- Ilska M., Kołodziej-Zaleska A., Brandt-Salmeri A., Preis H., Lobel M. Pandemic-related pregnancy stress assessment: Psychometric properties of the Polish PREPS and its relationship with childbirth fear. Midwifery. 2021;96:102940. doi: 10.1016/j.midw.2021.102940. [DOI] [PubMed] [Google Scholar]
- Kingston D., Kehler H., Austin M.-P., Mughal M.K., Wajid A., Vermeyden L., Benzies K., Brown S., Stuart S., Giallo R. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M., Luoma I., Salmelin R., Tamminen T. Maternal depressive symptoms: associations with adolescents' internalizing and externalizing problems and social competence. Nord. J. Psychiatr. 2014;68(5):323–332. doi: 10.3109/08039488.2013.838804. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W. The patient health questionnaire-2: validity of a two-item depression screener. Med. Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lazarus R.S., Folkman S. Springer; 1984. Stress, Appraisal, and Coping. [Google Scholar]
- Lazarus R.S., Launier R. In: Perspectives in Interactional Psychology. Pervin L.A., Lewis M., editors. Springer; 1978. Stress-related transactions between person and environment; pp. 287–327. [Google Scholar]
- Lebel C., MacKinnon A., Bagshawe M., Tomfohr-Madsen L., Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J. Affect. Disord. 2020;277:5–13. doi: 10.1016/j.jad.2020.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman S.A., Rauh V., Weiss L., Stein J.L., Hoepner L.A., Becker M., Perera F.P. The effects of the World Trade Center event on birth outcomes among term deliveries at three lower Manhattan hospitals. Environ. Health Perspect. 2004;112(17):1772–1778. doi: 10.1289/ehp.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M., Dunkel Schetter C. In: Encyclopedia of Mental Health. Friedman H.S., editor. Academic Press; 2016. Pregnancy and prenatal stress; pp. 318–329. [Google Scholar]
- Lobel M., Ibrahim S. Emotions and mental health during pregnancy and postpartum. Womens Reprod. Health. 2018;5(1):13–19. [Google Scholar]
- Lobel M., Cannella D.L., Graham J.E., DeVincent C., Schneider J., Meyer B.A. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27(5):604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Löwe B., Kroenke K., Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J. Psychosom. Res. 2005;58(2):163–171. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Lynn F.A., Alderdice F.A., Crealey G.E., McElnay J.C. Associations between maternal characteristics and pregnancy-related stress among low-risk mothers: an observational cross-sectional study. Int. J. Nurs. Stud. 2011;48(5):620–627. doi: 10.1016/j.ijnurstu.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Manzari N., Matvienko-Sikar K., Baldoni F., O'Keeffe G.W., Khashan A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis. Soc. Psychiatr. Psychiatr. Epidemiol. 2019;54(11):1299–1309. doi: 10.1007/s00127-019-01745-3. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Mew E.J., Hawley N.L., Lowe S.R. Anticipating the long-term neurodevelopmental impact of the COVID-19 pandemic on newborns and infants: a call for research and preventive policy. J. Affect. Disord. Rep. 2021;6 doi: 10.1016/j.jadr.2021.100213. 100213-100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C., Webster R.S., McNeil R.B., Parker C.B., Catov J.M., Greenland P., Merz C.N.B., Silver R.M., Simhan H.N., Ehrenthal D.B. Associations of perceived prenatal stress and adverse pregnancy outcomes with perceived stress years after delivery. Arch. Wom. Ment. Health. 2020;23(3):361–369. doi: 10.1007/s00737-019-00970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R.H., Swindle R.W. Person-environment transactions and the stressor-appraisal-coping process. Psychol. Inq. 1990;1(1):30–32. [Google Scholar]
- Muthén B., Kaplan D., Hollis M. On structural equation modeling with data that are not missing completely at random. Psychometrika. 1987;52(3):431–462. [Google Scholar]
- Norhayati M., Hazlina N.N., Asrenee A., Emilin W.W. Magnitude and risk factors for postpartum symptoms: a literature review. J. Affect. Disord. 2015;175:34–52. doi: 10.1016/j.jad.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Penengo C., Colli C., Garzitto M., Driul L., Sala A., Degano M., Preis H., Lobel M., Balestrieri M. Psychometric properties of the Italian version of the Pandemic-Related Pregnancy Stress Scale (PREPS) and its correlates with anxiety and depression. J. Affect. Dis. 2021;294:48–53. doi: 10.1016/j.jad.2021.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J., Olander E.K., Leitao S., Meaney S., Matvienko-Sikar K. Prenatal stress, health, and health behaviours during the COVID-19 pandemic: an international survey. Women Birth. 2022;35(3):272–279. doi: 10.1016/j.wombi.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis H., Mahaffey B., Heiselman C., Lobel M. Pandemic-related pregnancy stress and anxiety among women pregnant during the COVID-19 pandemic. American Journal of Obstetrics & Gynecology - Maternal-Fetal Medicine. 2020;2(3):1–3. doi: 10.1016/j.ajogmf.2020.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis H., Mahaffey B., Heiselman C., Lobel M. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc. Sci. Med. 2020;266 doi: 10.1016/j.socscimed.2020.113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis H., Mahaffey B., Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS) J. Psychosom. Obstet. Gynaecol. 2020;41(3):191–197. doi: 10.1080/0167482X.2020.1801625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis H., Mahaffey B., Pati S., Heiselman C., Lobel M. Adverse perinatal outcomes predicted by prenatal maternal stress among U.S. women at the COVID-19 pandemic onset. Ann. Behav. Med. 2021;55(3):179–191. doi: 10.1093/abm/kaab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released . IBM Corp; 2020. IBM SPSS Statistics for Windows, Version 27.0. [Google Scholar]
- Richter J., Bittner A., Petrowski K., Junge-Hoffmeister J., Bergmann S., Joraschky P., Weidner K. Effects of an early intervention on perceived stress and diurnal cortisol in pregnant women with elevated stress, anxiety, and depressive symptomatology. J. Psychosom. Obstet. Gynecol. 2012;33(4):162–170. doi: 10.3109/0167482X.2012.729111. [DOI] [PubMed] [Google Scholar]
- Saccone G., Florio A., Aiello F., Venturella R., De Angelis M.C., Locci M., et al. Psychological impact of coronavirus disease 2019 in pregnant women. Am. J. Obstet. Gynecol. 2020;223(2):293–295. doi: 10.1016/j.ajog.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal N.K., La Marca-Ghaemmaghami P., Preis H., Mahaffey B., Lobel M., Castro R.A. The German version of the Pandemic-Related Pregnancy Stress Scale: A validation study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;256:40–45. doi: 10.1016/j.ejogrb.2020.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch-Ruppen J., Ehlert U., Uggowitzer F., Weymerskirch N., Marca-Ghaemmaghami L. Women's word use in pregnancy: associations with maternal characteristics, prenatal stress, and neonatal birth outcome. Front. Psychol. 2018;9:1234. doi: 10.3389/fpsyg.2018.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers S., Verweij E.J.J., Beijers R., Bijma H.H., Been J.V., Steegers-Theunissen R.P.M., Koopmans M.P.G., Reiss I.K.M., Steegers E.A.P. The impact of maternal prenatal stress related to the COVID-19 pandemic during the first 1000 days: a historical perspective. Int. J. Environ. Res. Publ. Health. 2022;19(8):4710. doi: 10.3390/ijerph19084710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C.D., Adam E.K., Holl J.L., Wolfe K.A., Grobman W.A., Borders A.E. Prenatal stress and the cortisol awakening response in African-American and Caucasian women in the third trimester of pregnancy. Matern. Child Health J. 2016;20(10):2142–2149. doi: 10.1007/s10995-016-2060-7. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Staneva A., Morawska A., Bogossian F., Wittkowski A. Pregnancy-specific distress: the role of maternal sense of coherence and antenatal mothering orientations. J. Ment. Health. 2016;25(5):387–394. doi: 10.3109/09638237.2015.1101425. [DOI] [PubMed] [Google Scholar]
- Tate E.B., Wood W., Liao Y., Dunton G.F. Do stressed mothers have heavier children? A meta-analysis on the relationship between maternal stress and child body mass index. Obes. Rev. 2015;16(5):351–361. doi: 10.1111/obr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman-Ben-Ari O., Chasson M., Abu Sharkia S., Weiss E. Distress and anxiety associated with COVID-19 among Jewish and Arab pregnant women in Israel. J. Reprod. Infant Psychol. 2020:1–9. doi: 10.1080/02646838.2020.1786037. [DOI] [PubMed] [Google Scholar]
- Tomfohr-Madsen L.M., Racine N., Giesbrecht G.F., Lebel C., Madigan S. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatr. Res. 2021;300 doi: 10.1016/j.psychres.2021.113912. 113912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood L., Waldie K., D'Souza S., Peterson E.R., Morton S. A review of longitudinal studies on antenatal and postnatal depression. Arch. Wom. Ment. Health. 2016;19(5):711–720. doi: 10.1007/s00737-016-0629-1. 2016/10/01. [DOI] [PubMed] [Google Scholar]
- Varcin K.J., Alvares G.A., Uljarević M., Whitehouse A.J. Prenatal maternal stress events and phenotypic outcomes in Autism Spectrum Disorder. Autism Res. 2017;10(11):1866–1877. doi: 10.1002/aur.1830. [DOI] [PubMed] [Google Scholar]
- Viswasam K., Eslick G.D., Starcevic V. Prevalence, onset and course of anxiety disorders during pregnancy: a systematic review and meta analysis. J. Affect. Disord. 2019;255:27–40. doi: 10.1016/j.jad.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Woody C.A., Ferrari A.J., Siskind D.J., Whiteford H.A., Harris M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Yan H., Ding Y., Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front. Psychol. 2020;11(3324) doi: 10.3389/fpsyg.2020.617001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya K., Yakirevich-Amir N., Preis H., Lotan A., Atzil S., Reuveni I. Women’s depressive symptoms during the COVID-19 pandemic: The role of pregnancy. Int. J. Environ. Res. Public Health. 2021;18(8):4298. doi: 10.3390/ijerph18084298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.