Abstract
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is related to Helicobacter pylori infection. Specifically, it has been pointed out that pathogenesis of MALT lymphoma involves the 60-kDa heat shock protein (hsp60). To investigate humoral immune responses to the H. pylori hsp60 in patients with gastroduodenal diseases and patients with MALT lymphoma, the hsp60 of H. pylori was expressed with a glutathione S-transferase fusion protein and was purified (recombinant hsp60). Sera were obtained from H. pylori-positive patients with gastroduodenal diseases (MALT lymphoma, n = 13; gastric ulcer, n = 20; duodenal ulcer, n = 20; gastritis, n = 20) and from H. pylori-negative healthy volunteers (n = 9). Sera from patients with MALT lymphoma were also obtained at two times: before and after eradication therapy. Antibodies to hsp60 and H. pylori were assessed by enzyme-linked immunosorbent assay. The levels of immunoglobulin G (IgG) antibodies to the hsp60 of H. pylori-positive patients with gastroduodenal diseases were significantly elevated compared to those in the controls. The levels of IgG1 antibodies to hsp60 were elevated and correlated with the levels of anti-H. pylori antibodies in patients with MALT lymphoma. Humarol immunity against hsp60 may be important and relevant to gastroduodenal diseases induced by H. pylori infection.
Helicobacter pylori is now recognized as a cause of gastritis, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (9, 19). Several H. pylori pathogenic factors, including CagA and VacA, may contribute to the gastric mucosal damage (6, 7). In recent years, it has also been accepted that host immune reactions play an important role in the pathogenesis of H. pylori infection. Although H. pylori is a noninvasive bacterium and is restricted to gastric epithelial cells, infection with H. pylori induces humoral and cellular immune responses in the gastric mucosa (16, 22). Bacterial attachment causes induction of interleukin-8 (IL-8) release, and Cag proteins evoke activation of nuclear factor-κB and release of IL-8 that may support leukocyte attachment during inflammation (8, 17, 21). However, the bacterial antigens associated with inflammation are still not clear.
Heat shock proteins (hsp's) are immunodominant antigens in various diseases including H. pylori infection (4, 26). Our previous studies have shown that the 60-kDa hsp (hsp60) is expressed in the follicular dendritic cells of the gastric mucosa in patients with gastric MALT lymphoma (15) and that antibodies to human hsp60 can be detected in MALT lymphoma patients (14). These results indicate that hsp60 may be associated with the host immune reaction in H. pylori infection, specifically, the pathogensis of gastric MALT lymphoma.
In the present study, H. pylori hsp60 recombinant protein was expressed, and the levels of immunoglobulin G (IgG), IgG1, IgG2, IgM, and IgA antibodies to hsp60 in sera were measured in patients with gastric ulcer, duodenal ulcer, gastritis, and gastric MALT lymphoma to demonstrate the immunological role of hsp60 in H. pylori-infected patients.
MATERIALS AND METHODS
Patients.
Sera were obtained from 73 H. pylori-positive patients, including 13 patients with gastric MALT lymphoma (5 women and 8 men; mean age, 56.6 years), 20 patients with gastric ulcer (3 women and 17 men; mean age, 46.3 years), 20 patients with duodenal ulcer (10 women and 10 men; mean age, 30.4 years), and 20 patients with gastritis (6 women and 14 men; mean age, 41.5 years). Sera were also obtained from nine H. pylori-negative volunteers (six women and three men; mean age, 37.9 years old). Informed consent was obtained from each patient and healthy volunteer. In nine patients with gastric MALT lymphoma who were treated with eradication therapy (a 1-week course of omeprazole at 40 mg/day, amoxicillin at 1,500 mg/day, and clarithromycin at 400 mg/day), endoscopic findings and histopathology were assessed 6 months after therapy and serum samples were collected. All sera were collected at the Hokkaido University Hospital and were stored at −80°C.
Diagnosis.
The histology of MALT lymphoma was assessed according to the Wotherspoon classification. All the lymphomas were diagnosed as low-grade MALT lymphoma. Diagnosis of the other diseases was determined according to the symptoms and by endoscopy. Infection with H. pylori was confirmed by culture, the rapid urease test, and histology of gastric biopsy specimens and by the presence of serum antibodies to H. pylori as detected by enzyme-linked immunosorbent assay (ELISA) (14). H. pylori was detected in the gastric mucosae of all patients whose sera were positive for anti-H. pylori antibodies. In the healthy control volunteers, negativity for H. pylori infection was defined by serology.
Cloning, expression, and purification of recombinant protein.
The hsp60 protein of H. pylori was expressed as a glutathione S-transferase fusion protein by PCR cloning. PCR primers were designed on the basis of sequences published in GenBank. To facilitate cloning, restriction endnuclease cleavage sites were included in the PCR primer sequence. The PCR primer pair of GGAGGATCCCCATGGCAAAAGAAATCAAATTT and GGGGTCGACCATCATGCCGCCCATGCCTCC was used to amplify the open reading frame from purified H. pylori ATCC 43504 genomic DNA.
The PCR was performed in a 20-μl volume containing Extaq polymerase (Takara), 5 nM sense and antisense oligonucleotide primers, and 500 ng of H. pylori genomic DNA. The cycling conditions were 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The amplicon was cut with BamHI and SalI restriction endonucleases and cloned into the vector pGX-5X3 (Amersham Pharmacia) by standard molecular biology techniques, and the resultant plasmid was transformed into Escherichia coli DH-5α. Recombinant protein was expressed at 25°C in Luria-Bertani broth containing 2% glucose and ampicillin (100 μg/ml). At an optical density (OD) at 540 nm of 0.6 to 0.8, the culture was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside and was incubated at 37°C for 3 h. The cells were harvested by centrifugation at 7,000 × g for 10 min and resuspended in ice-cold phosphate-buffered saline (PBS). The bacterial suspension was frozen at −70°C and thawed, and the cells were disrupted by sonication on ice for 2 min with a probe sonicator (Astron) set to full power. Soluble fusion protein expressed by the glutathione S-transferase–hsp clone (hsp60) was purified by glutathione-Sepharose 4B (Amersham Pharmacia) affinity chromatography according to the manufacturer's instructions.
ELISA for detection of antibodies to hsp60 and H. pylori.
Serum antibodies to hsp60 and H. pylori were measured by ELISA. Ninety-six-well microtiter plates were coated with hsp60 fusion protein (10 μg/ml) or H. pylori lysate (50 μg/ml) in 100 μl of 0.1 M carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. After the wells were blocked with PBS containing 10% skim milk, the plates were incubated with sera, at a 1:100 dilution for hsp60 antibody or 1:1,000 for H. pylori antibody, for 2 h and washed with PBS containing 0.05% Tween 20. Peroxidase-labeled rabbit anti-human IgA, IgM, or IgG antibody (DAKO Japan) was then added, and the plates was incubated for 2 h. After the plate was washed, the wells were reacted with 1 mg of o-phenylenediamine (Wako Pure Chemical) per ml in citrate buffer (pH 5.5). The ODs were measured at 490 nm on an ELISA plate reader (Bio-Rad). Cutoff ODs were determined as the mean plus 2 times the standard deviation for the sera obtained from negative controls.
For the detection of IgG1 or IgG2 antibodies, the contents of the wells of the microtiter plates were reacted with human sera as described above. Anti-human IgG1 or IgG2 monoclonal antibodies (Pharmingen) were then added, and the plates were incubated for 2 h, followed by washing. The wells were continuously reacted with anti-mouse antibody conjugated with horseradish peroxidase (DAKO Japan). After the plates were washed, the enzyme reaction described above was used for final color development.
Statistical analysis.
Differences in the ODs of the antibodies among the disease groups were evaluated by the Student t test. Correlation between antibodies to H. pylori and hsp60 were evaluated with Pearson's correlation coefficient. P values of <0.05 were considered to represent a significant difference.
RESULTS
IgG, IgM, and IgA antibodies to hsp60.
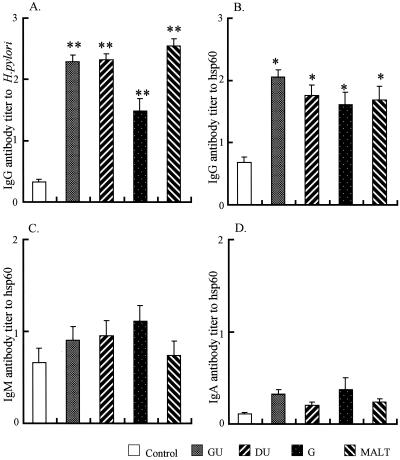
The levels of IgG antibodies to H. pylori whole antigens and hsp60 in the sera of patients with gastroduodenal diseases were significantly higher than those in the sera of healthy control (Fig. 1A and B). The cutoff ODs of the ELISA for anti-whole-antigens and hsp60 antibodies were 0.59 and 1.20 respectively. The specificity and sensitivity of IgG antibodies to the whole antigens were 100 and 91.8%, respectively and the sensitivity and specificity of IgG antibodies to hsp60 were 100 and 69.9% respectively. The levels of IgM antibodies and IgA antibodies were elevated, but they were not significantly different from those in the control group (Fig. 1C and D).
FIG. 1.
Levels of antibodies to H. pylori and hsp60 in patients with gastroduodenal disease. Antibody levels in patients with gastric ulcer (GU), duodenal ulcer (DU), gastritis (G), and gastric MALT lymphoma and in H. pylori-negative healthy volunteer (cont) were measured by ELISA. (A) IgG antibodies to H. pylori; (B) IgG antibodies to hsp60; (C) IgM antibodies to hsp60; (D) IgA antibodies to hsp60. ∗, P < 0.05 versus the control group; ∗∗, P < 0.01 versus the control group.
Correlation coefficient between IgG and IgG1 antibodies to hsp60 and H. pylori.
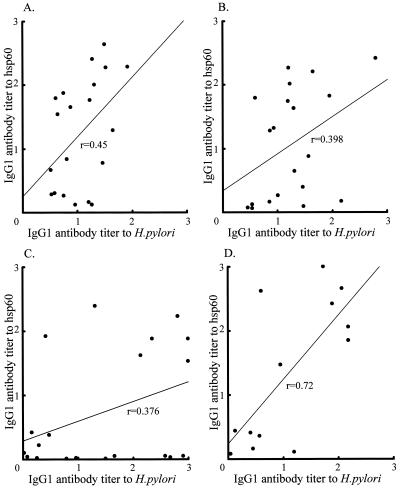
IgG isotype antibodies were measured in patients with gastroduodenal diseases. The levels of IgG1 antibodies to H. pylori and hsp60 were significantly elevated in patients with gastroduodenal diseases compared with the levels in the control group. However, the levels of IgG2 antibodies to both H. pylori whole antigens and hsp60 were increased in patients, but the difference was not statistically significant (data not shown). We statistically analyzed correlations between IgG antibodies to H. pylori and IgG1 antibodies to hsp60 in the patients. The patients with MALT lymphoma had a high correlation coefficient, although patients with other disease had only low correlation coefficients (Fig. 2).
FIG. 2.
Scatter diagrams of anti-H. pylori and anti-hsp60 antibodies. Correlation coefficients for IgG1 antibodies between H. pylori and hsp60 are 0.45 for patients with gastric ulcers (A), 0.398 for patients with duodenal ulcers, (B), 0.376 for patients with gastritis (C), and 0.72 for patients with MALT lymphoma (D).
IgG antibodies after eradication therapy in patients with MALT lymphoma.
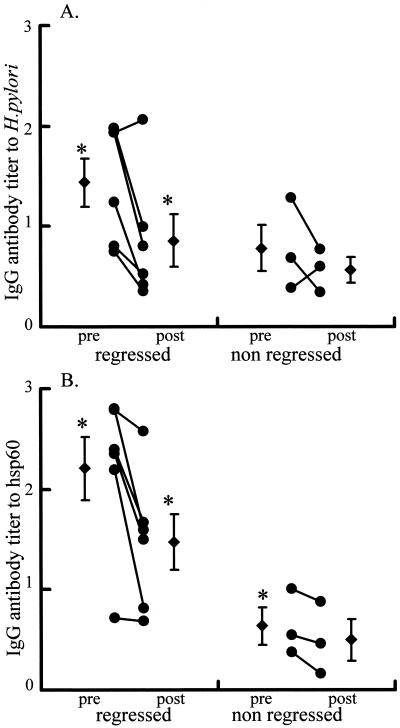
We studied the change in total IgG antibody levels after eradication therapy in nine patients with MALT lymphoma. Eradication therapy resulted in histological and endscopic remission in six MALT lymphoma patients; however, three patients did not have a regression 6 months after eradication therapy. IgG antibody levels to whole H. pylori and hsp60 in the patients with regression were significantly decreased (Fig. 3A and B). One levels of IgG antibodies to hsp60 in patients with or without regressions were decreased following eradication therapy, and at pretreatment levels of IgG antibody to hsp60 in the six patients with regressions were significantly higher than those in the three patients without regressions (Fig. 3B).
FIG. 3.
Antibody levels pre- or posteradication in patients with MALT lymphoma. (A) Anti-H. pylori IgG antibodies at pre- and posteradication therapy in six healed patients (left side of the panel) and in three patients without regression (right side of the panel). (B) Anti-hsp60 IgG antibodies. ∗, P < 0.05 versus antibody levels pretreatment in patients with regression as a result of eradication therapy.
DISCUSSION
We have reported on the measurement of the levels of immunoglobulin antibody against hsp60 in H. pylori-infected patients. Specifically, the IgG1 response against hsp60 is closely associated with MALT lymphoma in H. pylori-infected patients. IgG1 antibodies are produced by B cells activated under the T-helper 2 (Th2) cytokine IL-4. Th1 cells that produce gamma interferon are able to promote the production of IgG2 opsonizing and complement-fixing antibodies. An IgG subclass response to H. pylori in patients with chronic active gastritis and duodenal ulcer was reported by Bontkes et al (5). They indicated that the titers of IgG1 antibodies to the bacteria were raised in patients with H. pylori infection and that the titers of IgG2 antibodies in duodenal ulcer patients were higher than those in gastritis patients. In this study, there was a strong correspondence between total serum anti-H. pylori and anti-hsp60 IgG levels in patients with gastritis; however, the correspondence for IgG1 and IgG2 antibodies individually was weak. The anti-hsp60 IgG1 antibodies of patients with MALT lymphoma had a strong correspondence with anti-H. pylori IgG1 antibodies. The results indicate that a long-standing H. pylori infection induced the Th-2 reaction against hsp60 in patients with gastric MALT lymphoma.
We have reported that autoimmunity through human hsp60 is involved in patients with MALT lymphoma (14, 15) and that it may be caused by a long-term Th-2 reaction against bacterial hsp60. Infection with H. pylori also leads to an IgG response against the bacteria and hsp60 in patients with peptic ulcer and gastritis. We assessed the anti-hsp60 IgG antibody titers in a few patients with peptic ulcers and found that total levels of IgG to hsp60 were decreased by antibacterial eradication therapy (data not shown). However, the responses of Th-1 (IgG2) and/or Th-2 (IgG1) were diverse in patients with gastritis and peptic ulcer; hence, gastritis and ulcer diseases may be characterized by the development of cellular or humoral immune responses at various stages.
Some markers related to the pathogenesis of gastric MALT lymphoma have been investigated. Homozygous p16 tumor suppressor gene deletion was found in the gastric mucosae of patients with high-grade MALT lymphoma but were not found in those of patients with gastritis (18). The cell cycle regulatory gene (cdc2/cdk1) might play an important role in the modulation of cellular death, proliferation, and translation during the evolution of chronic gastritis to MALT lymphoma (3). PCR detection of IgH rearrangement in gastric biopsy specimens could be used to monitor MALT lymphoma regression during treatment (1). These observations about host epithelial cell growth and/or lymphocyte regulations indicated that some bacterial antigens were involved in the progression of the gastric lesion of MALT lymphoma; however, the bacterial antigen was not clearly detected. The present study showed that the levels of IgG antibodies against hsp60 are markedly decreased during eradication therapy and may possibly be used as a marker of the host immune reactions in patients with MALT lymphoma.
Bacterial virulence factors, like CagA and VacA s1, show a strong association with duodenal ulcer disease (2, 20, 23). Serological study of patients indicated that MALT lymphoma is associated with H. pylori strains expressing the CagA protein (10). On the other hand, a large variety of H. pylori strains could be considered responsible for the induction of MALT lymphoma, as judged from the heterogeneity of their antigenic properties and the fact that CagA did not seem to be a virulence marker associated with the disease in Japan (13). H. pylori infection induces class II major histocompatibility complex expression and CD4-positive T-cell activation (11); however, antigens associated with lymphocyte activation were not detected. We have reported that local immunoglobulin against hsp60 is associated with infiltrating cell numbers in the gastric mucosae of patients with gastritis (12) and have also reported that anti-hsp60 antibodies are relevant to gastric inflammation in patients with peptic ulcer during eradication therapy (25). In addition, the level of expression of CD40L on the peripheral blood mononuclear cells (PBMCs) of patients with MALT lymphoma was increased by cytokines (granulocyte-macrophage colony-stimulating factor and IL-4) or by cytokine plus hsp60 stimulation compared to the level of expression on PBMCs of patients with gastritis or healthy volunteers. The production of IL-4 in PBMC cultures was also increased by cytokines and/or hsp60 stimulation in patients with MALT lymphoma. In patients with MALT lymphoma, however, the level of production of gamma interferon was at the low end (24). Hence, hsp60 may be an important antigen for the production of antibodies and T-cell activation in patients with H. pylori infection.
In conclusion, the present study indicated that H. pylori infection induced an IgG1 response (humoral Th-2 immunity) against hsp60 in patients with MALT lymphoma, and that may be a disposition of MALT lymphoma as a host factor. This study also indicated that hsp60 is an immunodominant antigen in H. pylori infection, and analysis of the subclass of IgG antibody to hsp60 may be used to assess the immunological future in patients with H. pylori-associated diseases. This information will provide immunological data that can be used to make diagnoses once further studies have been conducted.
REFERENCES
- 1.Aiello A, Giardini R, Tondini C, Balzarotti M, Diss T, Peng H, Delia D, Pilotti. S S. PCR-based clonality analysis: a reliable method for the diagnosis and follow-up monitoring of conservatively treated gastric B-cell MALT lymphomas? Histopathology. 2000;34:326–330. doi: 10.1046/j.1365-2559.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S K, Weston A P, Zoubine M N, Campbell D R, Cherian R. Expression of Cdc2 and cycline B1 in Helicobacter pylori-associated gastric MALT and MALT lymphoma. Am J Pathol. 2000;156:217–225. doi: 10.1016/S0002-9440(10)64722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton S G, Winrow V R, Rampton D S, Crabtree J E, Beales I L, Calam J. Circulating antibodies to the 60-kD heat shock protein (hsp) family in patients with Helicobacter pylori infection. J Clin Exp Immunol. 1998;112:490–494. doi: 10.1046/j.1365-2249.1998.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bontkes H J, Veenendaal R A, Pena A S, Goedhard J G, van Duijn W, Kuiper J, Meijer J L, Lamers C B. IgG subclass response to Helicobacter pylori in patients with chronic active gastritis and duodenal ulcer. Scand J Gastroenterol. 1992;27:129–133. doi: 10.3109/00365529209165432. [DOI] [PubMed] [Google Scholar]
- 6.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z Y, Figura N, Rappuolli R. Molecular characterization of the 128kDa immunodominant antigen of Helicobacter pylori associated cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;265:10570–10575. [PubMed] [Google Scholar]
- 8.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins S, Perry S, Lindley I J, Rappuoli R. Helicobacter induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eck M, Schmausser B, Hass R, Greiner A, Czub S, Muller-Hermelink H K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 11.Fan X, Crowe S E, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley W K, Ernst P B, Reyes V E. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi S, Sugiyama S, Hisano K, Awakawa T, Kurokawa I, Yachi A, Isogai H, Isogai E, Yokota K, Hirai Y, Oguma K, Fujii N. Quantitative detection of secretory immunoglobulin A to Helicobacter pylori in gastric juice: antibody capture enzyme-linked immunosorbent assay. J Clin Lab Anal. 1996;10:74–77. doi: 10.1002/(SICI)1098-2825(1996)10:2<74::AID-JCLA3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara Y, Yokota K, Mizuno M, Yunoki N, Uetsu T, Okada H, Kobayashi K, Hirai Y, Oguma K, Tsuji T. Antibodies to human gastric epithelial cell and heat shock protein 60 in Helicobacter pylori positive mucosa associated lymphoid tissue lymphoma. Gut. 1999;45:20–23. doi: 10.1136/gut.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi K, Yokota K, Yoshino T, Kawahara Y, Dey A, Hirai Y, Oguma K, Akagi T. Detection of Helicobacter pylori associated antigen and heat shock protein 60 on follicular dendritic cells in the germinal centres of low grade B cell lymphoma of gastric mucosa associated lymphoid tissue (MALT) J Clin Pathol. 1998;51:396–398. doi: 10.1136/jcp.51.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson A, Quiding-Jarbrink M, Hoonroth H, Hamlet A, Ahlistedt I, Svennerholm A-M. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-induced subjects. Infect Immun. 1998;66:2705–2712. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori N, Wada A, Hirayama T, Parks T P, Stratowa C, Yamamoto N. Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-κB in gastric epithelial cancer cells. Infect Immun. 2000;68:1806–1814. doi: 10.1128/iai.68.4.1806-1814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Neumeister P, Hoefler G, Beham-Schmid C, Schmidt H, Apfelbeck U, Schaider H, Linksch W, Sill H. Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas. Gastroenterology. 1997;112:1871–1875. doi: 10.1053/gast.1997.v112.pm9178679. [DOI] [PubMed] [Google Scholar]
- 19.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 20.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle P R, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;36:944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-9 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 22.Sommer F, Faller G, Konturek P, Kirchner T, Hahn E G, Zeus J, Rollinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Doon L J, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki R, Kobayashi K, Yokota K, Takada S, Ishii E, Okada H, Yoshino T, Oguma K, Akagi T. Study of CD40 ligand expression by antigen stimulation with H. pylori-HSP60. Gut. 2000;47(Suppl.):A40–A41. [Google Scholar]
- 25.Yunoki N, Yokota K, Mizuno M, Kawahara Y, Adachi M, Okada M, Hayashi S, Hirai Y, Oguma K, Tsuji T. Antibody to heat shock protein can be used for early serological monitoring of Helicobacter pylori eradication treatment. Clin Diagn Lab Immunol. 2000;7:574–577. doi: 10.1128/cdli.7.4.574-577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zugel U, Kaufmann S H. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]