PURPOSE
Lorlatinib significantly improved progression-free survival (PFS) versus crizotinib and showed robust intracranial activity in patients with previously untreated advanced ALK-positive non–small-cell lung cancer (NSCLC) in the phase III CROWN trial. Here, we report post hoc efficacy outcomes in patients with and without brain metastases at baseline, and present data on the incidence and management of CNS adverse events (AEs) in CROWN.
METHODS
Eligible patients were randomly assigned 1:1 to first-line lorlatinib (100 mg once daily) or crizotinib (250 mg twice a day); no crossover between treatment arms was permitted. Tumor assessments, including CNS magnetic resonance imaging, were performed at screening and then at 8-week intervals. Regular assessments of patient-reported outcomes were conducted.
RESULTS
PFS by blinded independent central review was improved with lorlatinib versus crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% v 22% and 78% v 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression versus crizotinib in patients with (7% v 72%) and without (1% v 18%) brain metastases at baseline. In total, 35% of patients had CNS AEs with lorlatinib, most of grade 1 severity. Occurrence of CNS AEs did not result in a clinically meaningful difference in patient-reported quality of life. At analysis, 56% of CNS AEs had resolved (33% without intervention; 17% with lorlatinib dose modification), and 38% were unresolved; most required no intervention. Lorlatinib dose modification did not notably influence PFS.
CONCLUSION
First-line lorlatinib improved PFS outcomes and reduced CNS progression versus crizotinib in patients with advanced ALK-positive non–small-cell lung cancer with or without brain metastases at baseline. Half of all CNS AEs resolved without intervention or with lorlatinib dose modification.
INTRODUCTION
Lorlatinib is a potent, third-generation inhibitor of anaplastic lymphoma kinase (ALK) indicated for the first-line treatment of adult patients with ALK-positive metastatic non–small-cell lung cancer (NSCLC).1,2 In the phase III CROWN study (ClinicalTrials.gov identifier: NCT03052608), independently assessed progression-free survival (PFS) was significantly improved with lorlatinib compared with crizotinib (hazard ratio [HR] for disease progression or death, 0.28; 95% CI, 0.19 to 0.41; P < .001) in this patient population.2
CONTEXT
Key Objective
Brain metastases develop in more than half of patients with ALK-positive non–small-cell lung cancer (NSCLC); these are associated with poor prognosis, high symptom burden, and decreased quality of life. We report a post hoc exploratory analysis of efficacy from the phase III CROWN study of lorlatinib versus crizotinib in patients with advanced treatment-naïve ALK-positive NSCLC by the presence/absence of brain metastases at baseline, and the safety/management of CNS-related adverse events.
Knowledge Generated
Lorlatinib improved progression-free survival and reduced CNS progression versus crizotinib regardless of the presence/absence of brain metastases at baseline. For many patients, CNS adverse events were managed either without any intervention or through dose modifications (with/without concomitant medication). Dose modifications had no detectable effect on lorlatinib efficacy on the basis of ad hoc progression-free survival analysis.
Relevance
Our data support lorlatinib as first-line treatment in patients with advanced ALK-positive NSCLC with/without brain metastases.
Approximately 29%-40% of patients with ALK-positive NSCLC have brain metastases at initial evaluation, and more than half will develop brain metastases.3-8 Development of brain metastases in patients with NSCLC is associated with significant morbidity, reduction in quality of life,9-11 and increased health care utilization with substantial financial burden because of increased inpatient, outpatient, and pharmacy costs.12,13 Although three ALK tyrosine kinase inhibitors (alectinib,14,15 brigatinib,16 and ensartinib8) have demonstrated superior efficacy to crizotinib in patients with brain metastases, prognosis among patients with NSCLC and brain metastases remains poor.9,17
Lorlatinib treatment in CROWN was associated with intracranial activity resulting in higher complete and partial intracranial response rates than with crizotinib in patients with brain metastases.2 Among the 78 patients with baseline measurable or nonmeasurable brain metastases, the intracranial objective response rate (ORR) as assessed by blinded independent central review (BICR) was 66% with lorlatinib and 20% with crizotinib.2 Complete intracranial responses were seen in 61% and 15% of patients, respectively.2 The safety profile of lorlatinib in CROWN2 was consistent with that described in the preceding phase I/II study (ClinicalTrials.gov identifier: NCT01970865).18,19 The potential clinical impact of CNS adverse events (AEs), such as cognitive and mood effects, following lorlatinib treatment has been debated.20,21 It has been reported that most AEs with lorlatinib can be effectively managed by dose modification and concomitant medication,2,18,19,22 with low rates of permanent treatment discontinuation.2,18,19
Here, we present post hoc exploratory efficacy and safety outcomes from the CROWN study, including subgroup analyses in patients with and without brain metastases at baseline, and data on the incidence and management of CNS-related AEs.
METHODS
Data Source and Patients
CROWN (ClinicalTrials.gov identifier: NCT03052608) is an ongoing randomized phase III trial of patients with locally advanced or metastatic ALK-positive (determined by Ventana ALK [D5F3] companion diagnostic immunohistochemical assay) NSCLC. Details of study design, inclusion and exclusion criteria, and primary data have been previously reported.2 In brief, patients were randomly assigned 1:1 to either oral lorlatinib 100 mg once daily or oral crizotinib 250 mg twice a day, and stratified by the presence of brain metastases (yes/no) and ethnicity (Asian/non-Asian). Patients with asymptomatic treated or untreated brain metastases were eligible. Dose levels for lorlatinib could be reduced to either 75 mg once daily or 50 mg once daily, depending on AE type and severity.
The Protocol (online only) was approved by the institutional review board or independent ethics committee; all patients provided written informed consent before participating.
Procedures
Tumor assessments (including magnetic resonance imaging) were performed at screening, and then every 8 weeks (±1 week) starting from random assignment until disease progression (on the basis of BICR). Intracranial disease response was assessed by a modified version of Response Evaluation Criteria in Solid Tumors v1.1, which included up to five intracranial target lesions ≥ 5 mm in diameter assessed by gadolinium-enhanced magnetic resonance imaging with slices of 1 mm for lesions of 5 mm to < 10 mm.23 Patient-reported outcomes (PROs) were assessed on day 1 of each cycle using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30).
End Points
PFS by BICR, cumulative incidence of CNS progression and non-CNS progression as first progression event, intracranial complete response rate, duration of response (DOR), and safety were assessed by patient subgroup (with or without brain metastases at baseline). PFS by BICR was also assessed in subgroups of patients with brain metastases with or without prior brain radiotherapy. PROs were assessed in patients with and without CNS AEs.
Statistical Analyses
The statistical methodology has been previously described.2 To assess the impact of brain metastases and prior brain radiotherapy on efficacy, post hoc exploratory PFS analyses were conducted. The probability of the first event being CNS progression, non-CNS progression, or death was evaluated with a competing risk approach by estimating cumulative incidence functions. To assess the effect of lorlatinib dose modifications on efficacy, measured as relative dose intensity (RDI) or dose reduction, a post hoc PFS landmark analysis was performed. The landmark point of 16 weeks was chosen to allow for early assessment while providing sufficient time for potential dose modifications. P values were one-sided without adjustment for multiple comparisons.
A longitudinal random-intercept, random-slope, mixed-effect model was used to assess EORTC QLQ-C30 score change from baseline up to, but not including, end of treatment. The model had an intercept term, treatment, time (continuous variable), treatment by time, baseline, and randomization stratification factors as covariates. A ≥ 10-point minimally important difference from baseline in EORTC QLQ-C30 has been established as correlative with clinically meaningful change in disease symptoms and functioning.24 P values were two-sided without adjustment for multiple comparisons. PRO changes from baseline included all postbaseline assessments; the results were presented up to cycle 18 to ensure a meaningful sample size.
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03, and the grading of CNS AEs is provided in the Data Supplement (online only). CNS AEs were grouped according to Medical Dictionary for Regulatory Activities (MedDRA) v23.0 group terms into four categories: COGNITIVE EFFECTS (any event from high-level group terms [HLGT]: Cognitive and attention disorders and disturbances, Deliria [including confusion], or Mental impairment disorders); MOOD EFFECTS (any event from HLGT Anxiety disorders and symptoms, Depressed mood disorders and disturbances, Manic and bipolar mood disorders and disturbances, Mood disorders and disturbances not elsewhere classified, or Personality disorders and disturbances in behavior); SPEECH EFFECTS (any event from high-level term Speech and language abnormalities); and PSYCHOTIC EFFECTS (any event from Standardised MedDRA Queries narrow Psychosis and psychotic disorders or preferred term of Psychotic symptom).
RESULTS
Analyses are based on a data cutoff for the primary analysis of March 20, 2020. A CONSORT diagram of study flow and patient baseline characteristics have been previously published.2 Among the 149 patients in the lorlatinib arm, 38 (26%) had baseline brain metastases according to BICR assessment and of these, eight (21%) had received prior brain radiotherapy. Among the 147 patients in the crizotinib treatment arm, 40 (27%) had baseline brain metastases according to BICR assessment, of whom 10 (25%) had received prior brain radiotherapy.
PFS
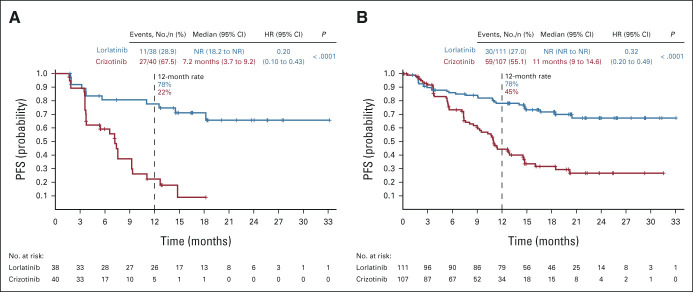
In patients with brain metastases at baseline (n = 78), PFS by BICR was improved with lorlatinib (n = 38) compared with crizotinib (n = 40; median PFS not reached [NR] v 7.2 months; 12-month PFS rate 78% [95% CI, 60 to 88] v 22% [95% CI, 9 to 39]; HR, 0.20; 95% CI, 0.10 to 0.43; P < .0001; Fig 1A). Similarly, patients without brain metastases at baseline (n = 218) showed a significant improvement in PFS with lorlatinib (n = 111) versus crizotinib (n = 107; median PFS NR v 11.0 months; 12-month PFS rate 78% [95% CI, 69 to 85] v 45% [95% CI, 34 to 55]; HR, 0.32; 95% CI, 0.20 to 0.49; P < .0001; Fig 1B). In patients with brain metastases at baseline who received lorlatinib and had prior brain radiotherapy (n = 8), the 12-month PFS rate was 88% (95% CI, 39 to 98). In patients with baseline brain metastases who received lorlatinib without prior brain radiotherapy (n = 30), the 12-month PFS rate was 75% (95% CI, 55 to 87; Appendix Fig A1, online only). Median PFS was NR in either prior brain radiotherapy subgroup.
FIG 1.
Kaplan-Meier plot of PFS in patients from the intent-to-treat population (A) with and (B) without brain metastases at baseline per blinded independent central review. HR, hazard ratio; NR, not reached; PFS, progression-free survival.
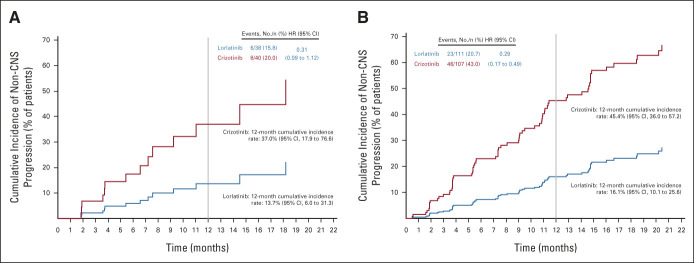
Cumulative Incidence of CNS and Non-CNS Progression
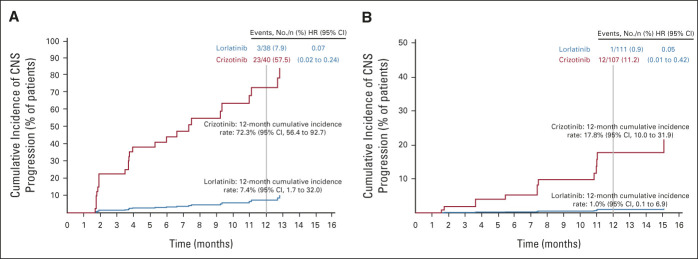
The cumulative incidence of CNS progression without prior non-CNS progression or death was lower with lorlatinib than with crizotinib in patients with and without brain metastases at baseline (Figs 2A and 2B). Twelve-month cumulative incidence rates of CNS progression were 7% with lorlatinib and 72% with crizotinib in patients with baseline brain metastases (HR, 0.07; 95% CI, 0.02 to 0.24) and 1% with lorlatinib and 18% with crizotinib in patients without baseline brain metastases (HR, 0.05; 95% CI, 0.01 to 0.42). Cumulative incidence of non-CNS progression was lower with lorlatinib than with crizotinib in patients with and without baseline brain metastases (Appendix Fig A2, online only).
FIG 2.
Cumulative incidence of CNS and non-CNS progression in CROWN: (A) CNS progression in patients with brain metastases at baseline and (B) CNS progression in patients without brain metastases at baseline. HR, hazard ratio.
Intracranial Complete Responses
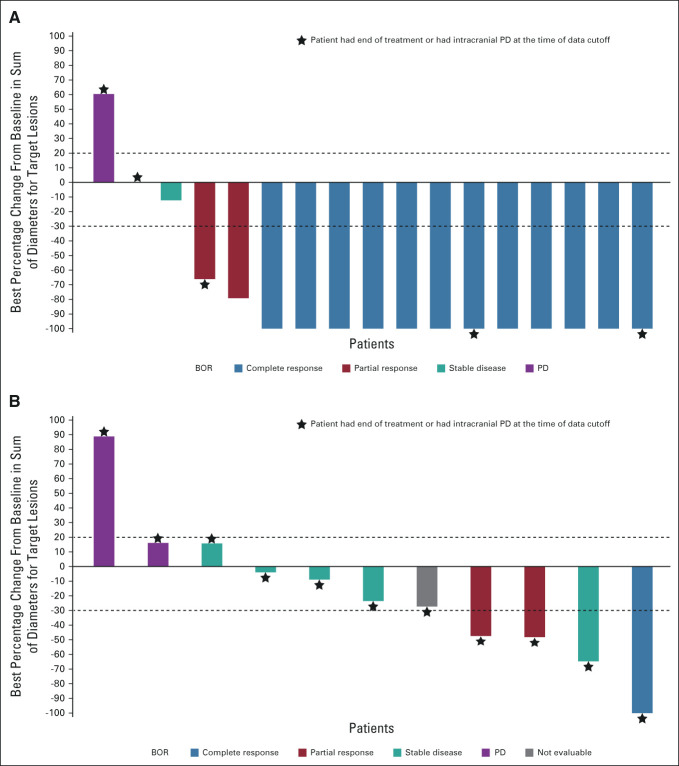
Complete CNS responses with lorlatinib were seen in 23/38 (61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In patients with at least one measurable brain metastasis at baseline, 12/17 (71%) had complete responses with lorlatinib compared with 1/13 (8%) with crizotinib (Fig 3).2 Median DOR with lorlatinib in patients with complete responses and measurable brain lesions at baseline was NR (range, 7.4-31.4 months); 10/12 patients (83%) had a DOR ≥ 12 months and 5/12 patients (42%) had a DOR ≥ 18 months. Most patients with intracranial complete responses were still receiving lorlatinib treatment at data cutoff (Appendix Fig A3, online only).
FIG 3.
Best percentage change in intracranial tumor size per BICR in patients with at least one measurable brain metastases at baseline in the intent-to-treat population following treatments with (A) lorlatinib (n = 17) and (B) crizotinib (n = 11). Only included patients with target lesions at baseline and at least one adequate postbaseline assessment up to the time of PD or new anticancer therapy. Two patients from the crizotinib arm were excluded from the plot as they did not have any postbaseline tumor assessment. BICR, blinded independent central review; BOR, best overall response; PD, progressive disease.
Incidence of CNS AEs Following Treatment
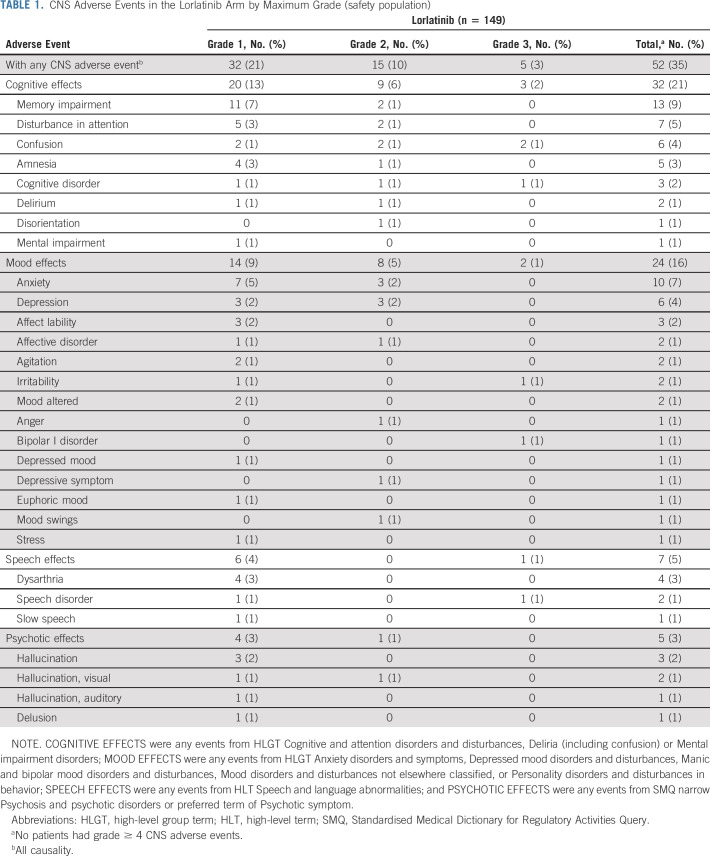
CNS AEs (independent of causality) were reported for 52/149 (35%) patients who received lorlatinib and 15/142 (11%) patients who received crizotinib. The incidence and severity of CNS AEs by cluster term in the lorlatinib arm are summarized in Table 1. Most patients with CNS AEs (32/52 [62%] in the lorlatinib arm and 11/15 [73%] in the crizotinib arm) had a maximum CNS AE severity of grade 1; 15 patients (29%) in the lorlatinib arm and four patients (27%) in the crizotinib arm had maximum severity of grade 2, and five (10%) in the lorlatinib arm had maximum severity of grade 3. There were no grade 4 or 5 CNS AEs. More than one CNS AE was reported in 19/149 (13%) patients in the lorlatinib arm. Further details on managing patients with psychotic effects are presented in the Data Supplement.
TABLE 1.
CNS Adverse Events in the Lorlatinib Arm by Maximum Grade (safety population)
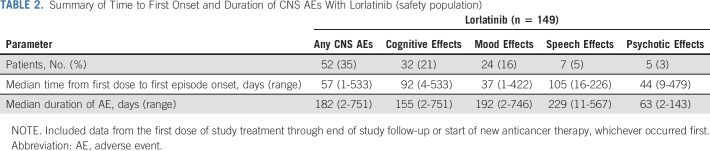
Time to first onset and duration of any CNS AEs and by cluster term with lorlatinib are detailed in Table 2. Of the 38 patients with brain metastases at baseline, 16 (42%) reported CNS AEs versus 36/111 patients (32%) without brain metastases at baseline (Data Supplement). There was no clear association between the location of brain metastases at baseline and type of CNS AE experienced (Data Supplement). The frequency of CNS AEs seemed higher among patients who had prior brain radiotherapy (5/9; 56%) than patients without prior brain radiotherapy (47/140; 34%; Data Supplement); it should be noted, however, that patient numbers were low in the prior brain radiotherapy subgroup.
TABLE 2.
Summary of Time to First Onset and Duration of CNS AEs With Lorlatinib (safety population)
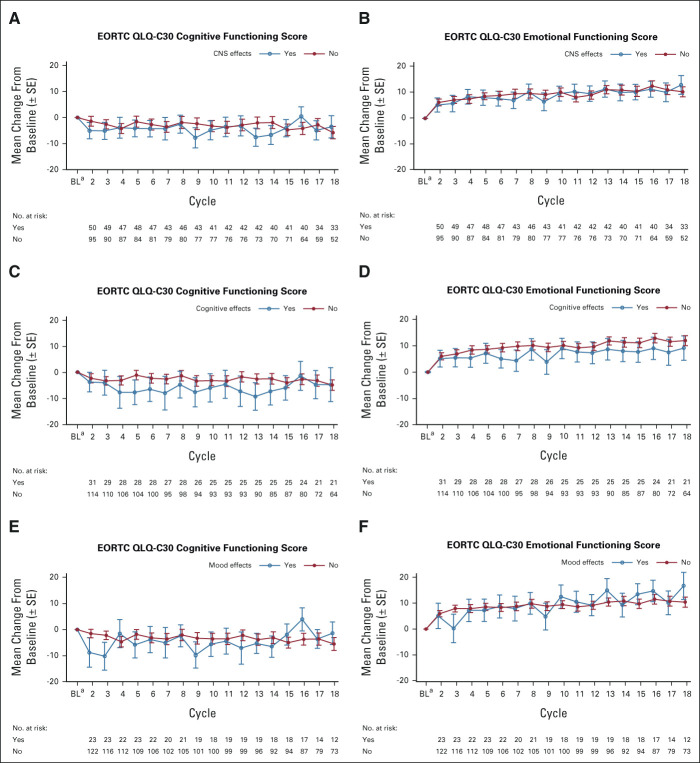
Impact of CNS AEs With Lorlatinib on PROs
The impact of CNS AEs with lorlatinib on PROs over time, as evaluated by the EORTC QLQ-C30 Cognitive and Emotional Functioning domains, is shown in Appendix Fig A4 (online only). Emotional Functioning scores improved over time regardless of the occurrence of CNS AEs, and by cycle 18, clinically meaningful improvements were observed in patients with and without any CNS AEs or mood effects, but not in patients with cognitive effects (Appendix Figs A4B, A4D, and A4F). Although Cognitive Functioning scores generally declined over time (Appendix Figs A4A, A4C, and A4E), there was no clinically meaningful decline at any time point, with the one exception of cycle 3 in patients with mood effects. The estimated mean difference in change from baseline in Cognitive Functioning scores through cycle 38 between patients who experienced CNS AEs compared with those who did not was –4.85 (95% CI, –9.81 to 0.11; P = .06). Those patients who experienced cognitive AEs had a significantly greater decline in Cognitive Functioning scores through cycle 38 compared with patients who did not experience cognitive AEs (estimated mean difference, –6.34; 95% CI, –12.08 to –0.60; P = .03).
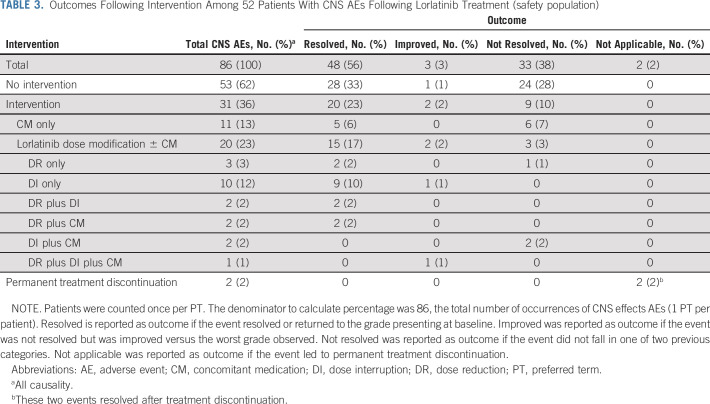
Management of CNS AEs
In total, 86 CNS AEs were reported in 52 patients who received lorlatinib. Of those, 53 (62%) were managed without intervention, and 20 (23%) were managed with lorlatinib dose modification (reduction and/or interruption, with or without concomitant medication; Table 3). In two cases (2%), CNS AEs led to permanent treatment discontinuation (grade 2 confusion, one patient; grade 3 confusion, one patient). Of the 53 CNS AEs managed without intervention, 28 (53%) resolved, 1/53 (2%) improved, and 24/53 (45%) did not resolve. The majority (23/24) of the unresolved AEs were grade 1 in severity. Of the 20 CNS AEs managed with lorlatinib dose modifications, with or without concomitant medication, 15 (75%) resolved; 2/20 (10%) improved, and 3/20 (15%) did not resolve (two of grade 1 severity, and one of grade 2 severity). Lorlatinib dose modification alone was used for the management of 15 CNS AEs, of which 13 (87%) resolved, 1/15 (7%) improved, and 1/15 (7%) did not resolve (Table 3). Concomitant medication used for the management of CNS AEs and the duration of treatment are shown in the Data Supplement.
TABLE 3.
Outcomes Following Intervention Among 52 Patients With CNS AEs Following Lorlatinib Treatment (safety population)
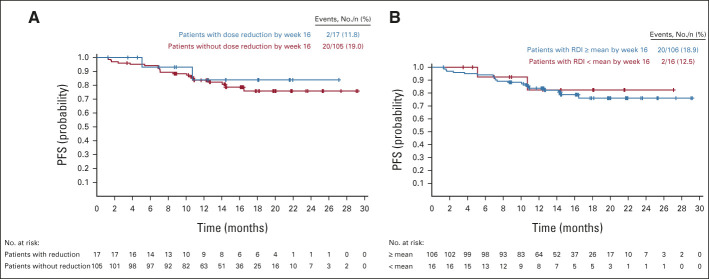
Impact of Lorlatinib Dose Modification on PFS
In total, 41/149 (28%) patients in CROWN had at least one lorlatinib dose reduction because of AEs. Landmark analysis of PFS showed comparable efficacy in patients with or without lorlatinib dose reduction within 16 weeks (12-month PFS rate 84% [95% CI, 49 to 96] with dose reduction [n = 17] v 84% [95% CI, 75 to 90] without dose reduction [n = 105]) and in patients categorized by the mean RDI of 98.6% by week 16 (Appendix Fig A5, online only). The 12-month PFS rate was 84% (95% CI, 75 to 90) in the subgroup whose RDI within 16 weeks was at or above the mean (n = 106), and 83% (95% CI, 45 to 96) in those whose RDI was below the mean (n = 16).
DISCUSSION
These additional post hoc analyses of data from the phase III CROWN study show that lorlatinib has potent and durable efficacy in patients with and without brain metastases at baseline, regardless of prior brain radiotherapy use. Furthermore, we demonstrate that, for some patients, CNS AEs can be managed either without any intervention or through appropriate dose modification.
About one quarter of the patients in CROWN had brain metastases, and lorlatinib prolonged PFS in this population compared with crizotinib (HR, 0.20; 95% CI, 0.10 to 0.43). The PFS benefit with lorlatinib was comparable with that seen with other ALK tyrosine kinase inhibitors in NSCLC in patients with baseline brain metastases (HR, 0.37; 95% CI, 0.23 to 0.58 in ALEX for first-line alectinib v crizotinib14; HR, 0.25; 95% CI, 0.14 to 0.46 in ALTA-1L for brigatinib v crizotinib in ALK inhibitor–naïve patients16). In patients without brain metastases at baseline, improvements in PFS seen with lorlatinib versus crizotinib (HR, 0.32; 95% CI, 0.20 to 0.49) appear to be greater than those seen with alectinib versus crizotinib (HR, 0.46; 95% CI, 0.31 to 0.68), or with brigatinib versus crizotinib (HR, 0.65; 95% CI, 0.44 to 0.97).14,16 One factor likely to contribute to the improved PFS with lorlatinib over crizotinib is the high intracranial complete response rate in patients treated with lorlatinib (71% in patients with measurable brain lesions at baseline), which is numerically higher than that observed in the ALEX (38%) and ALTA-1L (28%) trials.4,16
Patients with ALK-positive NSCLC are more likely to develop brain metastases than patients with RET- or ROS1-rearranged NSCLC, with a cumulative incidence of > 60% at 6 years.7 Lorlatinib was highly effective at preventing CNS progression in the majority of patients. In CROWN, the 12-month cumulative incidence rate of CNS progression in patients treated with lorlatinib with or without brain metastases at baseline was 7% and 1%, respectively. Data from the ALEX trial showed 12-month cumulative incidence rates of CNS progression following alectinib treatment of 16% in patients with baseline brain metastases and 5% in patients without.25
No clinically meaningful differences were previously identified between treatment arms in any EORTC QLQ-C30 functioning domain, although improvements favoring crizotinib were observed in the Cognitive Functioning scale.26 The findings reported here show that overall CNS AEs in the lorlatinib arm did not negatively affect EORTC QLQ-C30 Cognitive or Emotional Functioning domain scores over time but did have a negative impact on Cognitive Functioning and Emotional Functioning scores.
CNS AEs occurred in 35% of patients treated with lorlatinib compared with 11% of patients treated with crizotinib. More than half (62%) of the CNS AEs with lorlatinib were managed without intervention; of these, 53% resolved spontaneously. Only 2% led to permanent treatment discontinuation. Dose modification was used to manage 23% of CNS AEs and did not appear to negatively affect lorlatinib efficacy as indicated by a 12-month landmark PFS analysis, although patient numbers were small and the majority of dose-reduced patients were not included in this analysis. This is consistent with the results from a phase I/II trial that found no significant association between lorlatinib plasma exposure and ORR or intracranial ORR.27 In cases where there was no intervention, 45% of CNS AEs did not resolve, indicating these events may have required further management other than use of concomitant medications or were considered mild enough to be manageable without intervention.
We note several limitations to this analysis. CNS AEs can be difficult to assess, therefore some events may be under-reported. Consistent with this, patients with and without reported CNS AEs experienced some decline in Cognitive Functioning scores over time. Moreover, it is not possible to assess the full duration of all CNS AEs, since 38% of events were not resolved during the follow-up period. We also note that more specific neurologic scales may better assess the impact of specific CNS AEs on patient quality of life than the EORTC QLQ-C30. Data regarding the specific type of prior radiotherapy were not collected.
In conclusion, lorlatinib improved PFS outcomes and reduced CNS progression in patients with previously untreated advanced ALK-positive NSCLC with or without brain metastases at baseline. Intracranial responses were durable, and many CNS AEs resolved without intervention, or with lorlatinib dose modification and/or concomitant medication. These data support the use of lorlatinib as first-line treatment in patients with advanced ALK-positive NSCLC.
ACKNOWLEDGMENT
The authors would like to thank the study participants and study-site personnel. The authors would like to acknowledge Laura Iadeluca, PhD (Pfizer), for the analysis on the patient-reported outcomes. Medical writing support was provided by Paul O'Neill, PhD, of CMC AFFINITY, McCann Health Medical Communications, and was funded by Pfizer.
APPENDIX
FIG A1.
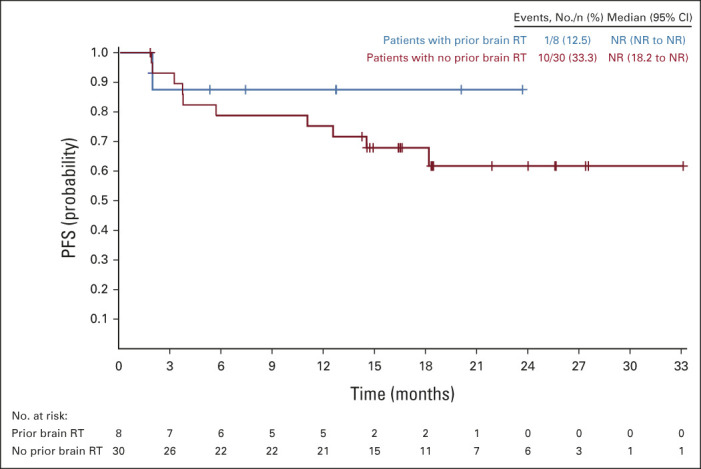
PFS by blinded independent central review assessment in patients with baseline brain metastases who received lorlatinib treatment by prior brain radiotherapy (intent-to-treat population). NR, not reported; PFS, progression-free survival; RT, radiotherapy.
FIG A2.
Cumulative incidence of non-CNS progression in CROWN: (A) non-CNS progression in patients with brain metastases at baseline and (B) non-CNS progression in patients without brain metastases at baseline. HR, hazard ratio.
FIG A3.
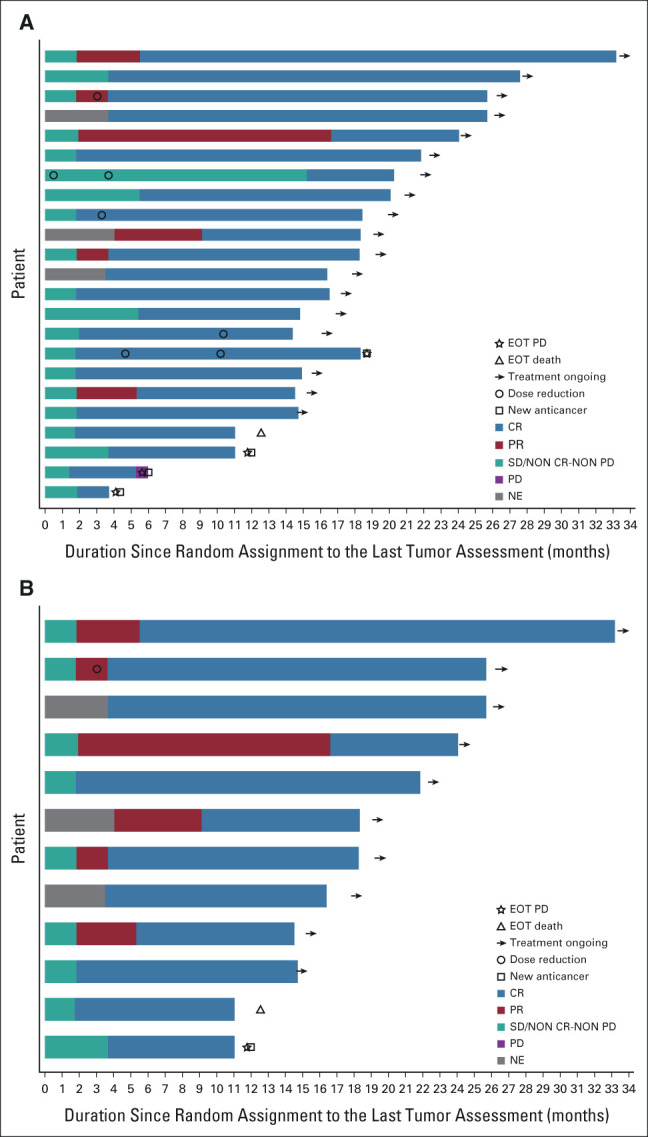
Swimmer plots of intracranial complete responses in the intent-to-treat population with lorlatinib over time (A) in patients with measurable or nonmeasurable brain metastases (n = 23) and (B) in patients with at least one measurable brain metastasis (n = 12). CR, complete response; EOT, end of treatment; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
FIG A4.
Changes from baseline in EORTC QLQ-C30 Cognitive and Emotional Functioning scores with lorlatinib by (A and B) CNS adverse events; (C and D) cognitive effects; and (E and F) mood effects cluster terms (PRO population). The PRO population consisted of all randomly assigned patients who completed a baseline assessment and at least one postbaseline assessment. COGNITIVE EFFECTS were any events from HLGT Cognitive and attention disorders and disturbances, Deliria (including confusion) or Mental impairment disorders; MOOD EFFECTS were any events from HLGT Anxiety disorders and symptoms, Depressed mood disorders and disturbances, Manic and bipolar mood disorders and disturbances, Mood disorders and disturbances not elsewhere classified, or Personality disorders and disturbances in behavior. aBL was defined as the last assessment performed on or before the date of the first dose of study treatment. CNS effects defined as any event from the cognitive effects, mood effects, speech effects, or psychotic effects cluster terms. BL, baseline; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HLGT, high-level group terms; PRO, patient-reported outcome.
FIG A5.
Landmark analysis of progression-free survival per BICR assessment (A) by first lorlatinib dose reduction within 16 weeks and (B) by mean relative lorlatinib dose intensity within 16 weeks (intent-to-treat population). Patients with PFS time ≤ 16 weeks were excluded. For patients included in the analysis, PFS time is recalculated starting at the landmark time. BICR, blinded independent central review; PFS, progression-free survival; RDI, relative dose intensity.
Benjamin J. Solomon
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche/Genentech, Pfizer, Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer (Inst), Roche/Genentech, Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Novartis (Inst), Janssen
Research Funding: Pfizer (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Veristrat (Biodesix), UpToDate
Todd M. Bauer
Employment: Tennessee Oncology
Consulting or Advisory Role: Loxo, Pfizer, Bayer, AstraZeneca
Speakers' Bureau: Bayer, Bristol Myers Squibb, Lilly
Research Funding: Daiichi Sankyo (Inst), Incyte (Inst), Mirati Therapeutics (Inst), MedImmune (Inst), AbbVie (Inst), AstraZeneca (Inst), MabVax (Inst), Merck (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Genentech/Roche (Inst), Immunogen (Inst), Immunocore (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Top Alliance BioScience (Inst), Loxo (Inst), Janssen (Inst), Takeda (Inst), Onyx (Inst), Foundation Medicine (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pfizer
Sai-Hong Ignatius Ou
Stock and Other Ownership Interests: Turning Point Therapeutics, Elevation Oncology
Honoraria: Pfizer, ARIAD/Takeda, BeiGene, Lilly, Daiichi Sankyo, JNJ/Jassen, DAVA Oncology LP, Caris Life Sciences
Consulting or Advisory Role: Pfizer, Takeda, Janssen, Lilly, Elevation Oncology, Daiichi Sankyo, BeiGene
Speakers' Bureau: Caris Life Sciences
Research Funding: Pfizer (Inst), Roche Pharma AG (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), ARIAD (Inst), Revolution Medicines (Inst), Mirati Therapeutics (Inst), Janssen (Inst)
Geoffrey Liu
Honoraria: Pfizer, Novartis, Merck, AstraZeneca, Takeda, AbbVie, Bayer, Bristol Myers Squibb, Roche Canada, Jazz Pharmaceuticals, Amgen
Consulting or Advisory Role: Pfizer, Novartis, AstraZeneca/MedImmune, Takeda, Roche Canada
Speakers' Bureau: AstraZeneca, Takeda, Pfizer
Research Funding: Roche (Inst), AstraZeneca/MedImmune (Inst), Takeda, Boehringer Ingelheim
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Taiho Pharmaceutical, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono
Consulting or Advisory Role: Lilly, AstraZeneca, Boehringer Ingelheim, Chugai Pharma, Pfizer, Merck Serono, Shanghai HaiHe Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co., Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), Parexel (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Alessandra Bearz
Consulting or Advisory Role: Takeda, Pfizer, Boehringer Ingeheim
Speakers' Bureau: Pfizer, Roche/Genentech, AstraZeneca
Konstantin Penkov
Research Funding: AstraZeneca, Pfizer, Novartis, BeiGene, GlaxoSmithKline, Nektar, Regeneron, H3 Biomedicine, Janssen, Sanofi
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Oscar Arrieta
Honoraria: Pfizer, AstraZeneca, Boehringer Ingelheim, Roche
Consulting or Advisory Role: AstraZeneca, Roche, Lilly, Merck, Bristol Myers Squibb
Research Funding: Roche (Inst), BMS (Inst), Merck (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Lilly
Jacek Jassem
Consulting or Advisory Role: AstraZeneca, Exact Sciences, MSD Oncology
Travel, Accommodations, Expenses: Boehringer Ingelheim
Anna M. Calella
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Gerson Peltz
Employment: Reata Pharmaceuticals, Pfizer
Stock and Other Ownership Interests: Pfizer, Reata Pharmaceuticals
Anna Polli
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Holger Thurm
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Tony Mok
Employment: The Chinese University of Hong Kong
Leadership: AstraZeneca, Aurora Tele-Oncology Platform, Lunit, ACT Genomics-Sanomics Group, HUTCHMED
Stock and Other Ownership Interests: Aurora Tele-Oncology Platform, HUTCHMED, ACT Genomics-Sanomics Group
Honoraria: AstraZeneca, Alpha Biopharma, ACEA Pharmaceutical Research, Amgen, Amoy Diagnostics, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Fishawack Facilitate, InMed, Lilly, Merck Sharp & Dohme, Novartis, Origimed, Pfizer, Prime Oncology, Roche, Sanofi Aventis GmbH, Taiho Pharmaceutical, Takeda, Lucence, Medscape, Permanyer Publications, PeerVoice, Physicans' Education Resource, Research to Practice, Shanghai BeBirds Translation & Consulting, Liangyihui Network Technology Co., Ltd, AbbVie, Berry Oncology, Blueprint Medicines, C4 Therapeutics, CStone Pharmaceuticals, Curio Science, D3, Eisai, Gilead Sciences, Gritstone Bio, Guardant Health, touchIME
Consulting or Advisory Role: AbbVie, ACEA Pharmaceutical Research, Alpha Biopharma, Amgen, Amoy Diagnostics, AstraZeneca, BeiGene, Berry Oncology, Boehringer Ingelheim, Blueprint Medicines, Bristol Myers Squibb, CStone Pharmaceuticals, Curio Science, Daiichi Sankyo/UCB Japan, Eisai, Fishawack Facilitate, Gritstone Bio, Guardant Health, Hengrui Therapeutics, Ignyta, Incyte, Inivata, IQvia, Lilly, Loxo, Lunit, Merck Serono, Merck Sharp & Dohme, Mirati Therapeutics, Novartis, Pfizer, Puma Biotechnology, Roche, SFJ Pharmaceuticals Group, Takeda, Vertex, Yuhan, Qiming Development (HK) Ltd, D3, C4 Therapeutics, G1 Therapeutics, Gilead Sciences, Janssen, geneDecode
Research Funding: AstraZeneca (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Novartis (Inst), SFJ Pharmaceuticals Group (Inst), Roche (Inst), Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst), Xcovery (Inst), G1 Therapeutics (Inst), Merck Serono (Inst), Takeda (Inst)
No other potential conflicts of interest were reported.
See accompanying editorial on page 3564
SUPPORT
Supported by Pfizer.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin J. Solomon, Todd M. Bauer, Sai-Hong Ignatius Ou, Anna M. Calella, Gerson Peltz, Anna Polli, Holger Thurm, Tony Mok
Provision of study materials or patients: Benjamin J. Solomon, Todd M. Bauer, Sai-Hong Ignatius Ou; Geoffrey Liu, Hidetoshi Hayashi, Alessandra Bearz, Konstantin Penkov, Yi-Long Wu, Oscar Arrieta, Jacek Jassem, Tony Mok
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non–Small-Cell Lung Cancer From the Phase III CROWN Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benjamin J. Solomon
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche/Genentech, Pfizer, Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer (Inst), Roche/Genentech, Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Novartis (Inst), Janssen
Research Funding: Pfizer (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Veristrat (Biodesix), UpToDate
Todd M. Bauer
Employment: Tennessee Oncology
Consulting or Advisory Role: Loxo, Pfizer, Bayer, AstraZeneca
Speakers' Bureau: Bayer, Bristol Myers Squibb, Lilly
Research Funding: Daiichi Sankyo (Inst), Incyte (Inst), Mirati Therapeutics (Inst), MedImmune (Inst), AbbVie (Inst), AstraZeneca (Inst), MabVax (Inst), Merck (Inst), Lilly (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Genentech/Roche (Inst), Immunogen (Inst), Immunocore (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Moderna Therapeutics (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Top Alliance BioScience (Inst), Loxo (Inst), Janssen (Inst), Takeda (Inst), Onyx (Inst), Foundation Medicine (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca, Celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pfizer
Sai-Hong Ignatius Ou
Stock and Other Ownership Interests: Turning Point Therapeutics, Elevation Oncology
Honoraria: Pfizer, ARIAD/Takeda, BeiGene, Lilly, Daiichi Sankyo, JNJ/Jassen, DAVA Oncology LP, Caris Life Sciences
Consulting or Advisory Role: Pfizer, Takeda, Janssen, Lilly, Elevation Oncology, Daiichi Sankyo, BeiGene
Speakers' Bureau: Caris Life Sciences
Research Funding: Pfizer (Inst), Roche Pharma AG (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), ARIAD (Inst), Revolution Medicines (Inst), Mirati Therapeutics (Inst), Janssen (Inst)
Geoffrey Liu
Honoraria: Pfizer, Novartis, Merck, AstraZeneca, Takeda, AbbVie, Bayer, Bristol Myers Squibb, Roche Canada, Jazz Pharmaceuticals, Amgen
Consulting or Advisory Role: Pfizer, Novartis, AstraZeneca/MedImmune, Takeda, Roche Canada
Speakers' Bureau: AstraZeneca, Takeda, Pfizer
Research Funding: Roche (Inst), AstraZeneca/MedImmune (Inst), Takeda, Boehringer Ingelheim
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Taiho Pharmaceutical, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono
Consulting or Advisory Role: Lilly, AstraZeneca, Boehringer Ingelheim, Chugai Pharma, Pfizer, Merck Serono, Shanghai HaiHe Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co., Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), Parexel (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Alessandra Bearz
Consulting or Advisory Role: Takeda, Pfizer, Boehringer Ingeheim
Speakers' Bureau: Pfizer, Roche/Genentech, AstraZeneca
Konstantin Penkov
Research Funding: AstraZeneca, Pfizer, Novartis, BeiGene, GlaxoSmithKline, Nektar, Regeneron, H3 Biomedicine, Janssen, Sanofi
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Oscar Arrieta
Honoraria: Pfizer, AstraZeneca, Boehringer Ingelheim, Roche
Consulting or Advisory Role: AstraZeneca, Roche, Lilly, Merck, Bristol Myers Squibb
Research Funding: Roche (Inst), BMS (Inst), Merck (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Lilly
Jacek Jassem
Consulting or Advisory Role: AstraZeneca, Exact Sciences, MSD Oncology
Travel, Accommodations, Expenses: Boehringer Ingelheim
Anna M. Calella
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Gerson Peltz
Employment: Reata Pharmaceuticals, Pfizer
Stock and Other Ownership Interests: Pfizer, Reata Pharmaceuticals
Anna Polli
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Holger Thurm
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Tony Mok
Employment: The Chinese University of Hong Kong
Leadership: AstraZeneca, Aurora Tele-Oncology Platform, Lunit, ACT Genomics-Sanomics Group, HUTCHMED
Stock and Other Ownership Interests: Aurora Tele-Oncology Platform, HUTCHMED, ACT Genomics-Sanomics Group
Honoraria: AstraZeneca, Alpha Biopharma, ACEA Pharmaceutical Research, Amgen, Amoy Diagnostics, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Fishawack Facilitate, InMed, Lilly, Merck Sharp & Dohme, Novartis, Origimed, Pfizer, Prime Oncology, Roche, Sanofi Aventis GmbH, Taiho Pharmaceutical, Takeda, Lucence, Medscape, Permanyer Publications, PeerVoice, Physicans' Education Resource, Research to Practice, Shanghai BeBirds Translation & Consulting, Liangyihui Network Technology Co., Ltd, AbbVie, Berry Oncology, Blueprint Medicines, C4 Therapeutics, CStone Pharmaceuticals, Curio Science, D3, Eisai, Gilead Sciences, Gritstone Bio, Guardant Health, touchIME
Consulting or Advisory Role: AbbVie, ACEA Pharmaceutical Research, Alpha Biopharma, Amgen, Amoy Diagnostics, AstraZeneca, BeiGene, Berry Oncology, Boehringer Ingelheim, Blueprint Medicines, Bristol Myers Squibb, CStone Pharmaceuticals, Curio Science, Daiichi Sankyo/UCB Japan, Eisai, Fishawack Facilitate, Gritstone Bio, Guardant Health, Hengrui Therapeutics, Ignyta, Incyte, Inivata, IQvia, Lilly, Loxo, Lunit, Merck Serono, Merck Sharp & Dohme, Mirati Therapeutics, Novartis, Pfizer, Puma Biotechnology, Roche, SFJ Pharmaceuticals Group, Takeda, Vertex, Yuhan, Qiming Development (HK) Ltd, D3, C4 Therapeutics, G1 Therapeutics, Gilead Sciences, Janssen, geneDecode
Research Funding: AstraZeneca (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Novartis (Inst), SFJ Pharmaceuticals Group (Inst), Roche (Inst), Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst), Xcovery (Inst), G1 Therapeutics (Inst), Merck Serono (Inst), Takeda (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pfizer Inc : LORBRENA® (lorlatinib): Prescribing Information. http://labeling.pfizer.com/ShowLabeling.aspx?id=11140 [Google Scholar]
- 2.Shaw AT, Bauer TM, de Marinis F, et al. : First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383:2018-2029, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Rangachari D, Yamaguchi N, VanderLaan PA, et al. : Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88:108-111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, et al. : Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829-838, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Kim HR, Ahn M-J, et al. : Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 379:2027-2039, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Soria J-C, Tan DSW, Chiari R, et al. : First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 389:917-929, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Drilon A, Lin JJ, Filleron T, et al. : Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 13:1595-1601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn L, Wang Z, Wu G, et al. : Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: A randomized clinical trial. JAMA Oncol 7:1617-1625, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roughley A, Damonte E, Taylor-Stokes G, et al. : Impact of brain metastases on quality of life and estimated life expectancy in patients with advanced non-small cell lung cancer. Value Health 17:A650, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Walker MS, Wong W, Ravelo A, et al. : Effect of brain metastasis on patient-reported outcomes in advanced NSCLC treated in real-world community oncology settings. Clin Lung Cancer 19:139-147, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Noh T, Walbert T: Brain metastasis: Clinical manifestations, symptom management, and palliative care. Handb Clin Neurol 149:75-88, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Burudpakdee C, Wong W, Seetasith A, et al. : Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer 119:103-111, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Guérin A, Sasane M, Zhang J, et al. : Brain metastases in patients with ALK+ non-small cell lung cancer: Clinical symptoms, treatment patterns and economic burden. J Med Econ 18:312-322, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Mok T, Camidge DR, Gadgeel SM, et al. : Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 31:1056-1064, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Hida T, Nokihara H, et al. : Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 139:195-199, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Camidge DR, Kim HR, Ahn MJ, et al. : Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. J Clin Oncol 38:3592-3603, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdallah SM-B, Wong A: Brain metastases in non-small-cell lung cancer: Are tyrosine kinase inhibitors and checkpoint inhibitors now viable options? Curr Oncol 25:S103-S114, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon BJ, Besse B, Bauer TM, et al. : Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol 19:1654-1667, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Felip E, Bauer TM, et al. : Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 18:1590-1599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camidge DR: Lorlatinib should not be considered as the preferred first-line option in patients with advanced ALK rearranged NSCLC. J Thorac Oncol 16:528-531, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Nagasaka M, Ou SI: Lorlatinib should be considered as the preferred first-line option in patients with advanced ALK-rearranged NSCLC. J Thorac Oncol 16:532-536, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Bauer TM, Felip E, Solomon BJ, et al. : Clinical management of adverse events associated with lorlatinib. Oncologist 24:1103-1110, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long GV, Trefzer U, Davies MA, et al. : Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol 13:1087-1095, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Osoba D, Rodrigues G, Myles J, et al. : Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139-144, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Gadgeel S, Peters S, Mok T, et al. : Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 29:2214-2222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazieres J, Iadeluca L, Shaw A, et al. : Patient-reported outcomes from the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in ALK+ NSCLC. J Thorac Oncol 16:S175-S176, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Ruiz-Garcia A, James LP, et al. : Lorlatinib exposure-response analyses for safety and efficacy in a phase I/II trial to support benefit-risk assessment in non-small cell lung cancer. Clin Pharmacol Ther 110:1273-1281, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.