PURPOSE
Liquid biopsies in metastatic renal cell carcinoma (mRCC) provide a unique approach to understand the molecular basis of treatment response and resistance. This is particularly important in the context of immunotherapies, which target key immune-tumor interactions. Unlike metastatic tissue biopsies, serial real-time profiling of mRCC is feasible with our noninvasive circulating tumor cell (CTC) approach.
METHODS
We collected 457 longitudinal liquid biopsies from 104 patients with mRCC enrolled in one of two studies, either a prospective cohort or a phase II multicenter adaptive immunotherapy trial. Using a novel CTC capture and automated microscopy platform, we profiled CTC enumeration and expression of HLA I and programmed cell death-ligand 1 (PD-L1). Given their diametric immunological roles, we focused on the HLA I to PD-L1 ratio (HP ratio).
RESULTS
Patients with radiographic responses showed significantly lower CTC abundances throughout treatment. Furthermore, patients whose CTC enumeration trajectory was in the highest quartile (> 0.12 CTCs/mL annually) had shorter overall survival (median 17.0 months v 21.1 months, P < .001). Throughout treatment, the HP ratio decreased in patients receiving immunotherapy but not in patients receiving tyrosine kinase inhibitors. Patients with an HP ratio trajectory in the highest quartile (≥ 0.0012 annually) displayed significantly shorter overall survival (median 18.4 months v 21.2 months, P = .003).
CONCLUSION
In the first large longitudinal CTC study in mRCC to date to our knowledge, we identified the prognostic importance of CTC enumeration and the change over time of both CTC enumeration and the HP ratio. These insights into changes in both tumor burden and the molecular profile of tumor cells in response to different treatments provide potential biomarkers to predict and monitor response to immunotherapy in mRCC.
INTRODUCTION
Kidney cancer is a common cancer among both men and women with approximately 400,000 new cases and 175,000 deaths globally each year.1 Renal cell carcinoma (RCC) encompasses a large heterogeneous group of cancers, of which clear cell RCC (ccRCC) is the most common, distinct in its immunogenicity and dependence on angiogenesis. Advances in our understanding of the pathogenesis of ccRCC have resulted in an expansion of treatment options for patients with advanced disease, which now includes immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) targeting agents.2
CONTEXT
Key Objective
Although several US Food and Drug Administration–approved regimens exist for metastatic renal cell carcinoma (mRCC), our understanding of the molecular features associated with treatment response and resistance is limited. Using a circulating tumor cell (CTC) capture and automated immunofluorescence platform with sensitivity and specificity for mRCC CTCs, we provide the first insights into the molecular evolution of mRCC to treatment.
Knowledge Generated
By monitoring real-time shifts in CTC enumeration and HLA I and programmed cell death-ligand 1 protein expression, with a focus on the longitudinal trajectory of their ratio, we were able to capture new insights into renal cell carcinoma evolution to tyrosine kinase inhibitors and immune checkpoint blockers and demonstrate their prognostic importance.
Relevance
We demonstrate how liquid biomarkers can be used to monitor patients over time, rather than just as a single snapshot. This work forms the foundation for serial, blood-based molecular profiling in renal cell carcinoma and potentially has implications for immunotherapy in other tumor types.
Historically, patients with advanced disease received cytokine-based systemic therapy designed to provoke immune effectors. Only a subset of highly selected patients, however, experienced a response, but at the cost of substantial treatment-related toxicity.3 Subsequently, VEGF tyrosine kinase inhibitors (TKIs), designed to target angiogenesis, entered the clinic giving improved responses and progression-free survival for patients. Despite the initial efficacy, resistance proved to be inevitable and nearly all patients developed disease progression.4 The advent of immune checkpoint blockers (ICBs) targeting programmed cell death protein 1, programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated protein-4 has dramatically changed the treatment paradigm for metastatic ccRCC. Immune checkpoint blockade in combination (ICB-ICB) or paired with a VEGF-TKI (ICB-VEGF) has now become the standard first-line therapy for most patients with metastatic RCC (mRCC). Combination therapy results in substantial responses in approximately 40%-60% of patients with 8%-10% of individuals experiencing a complete response5-7; nevertheless, acquired resistance continues to pose a clinical challenge.8
Although several US Food and Drug Administration–approved regimens exist for mRCC, our understanding of the molecular features associated with treatment response and resistance is limited. The expression of PD-L1 is the most investigated biomarker for ICB therapy. Multiple studies have demonstrated that PD-L1 expression is a negative prognostic factor in advanced RCC9,10; however, the benefit of ICB-based therapy is independent of PD-L1 status.5-7,11 HLA class I (HLA I) expression is less studied in mRCC but is a prognostic factor in TKI-treated patients, but not those treated with immunotherapies.12 Given the opposing effects of HLA I, an antigen presentation molecule responsible for stimulating an immune response, and PD-L1, an immune checkpoint protein responsible for inhibition of an immune response, with respect to immune evasion by tumors, we hypothesized that the interplay of these two key immune modulators would provide an informative metric to characterize the dynamic changes of the immune repertoire in real time. To our knowledge, this relationship has never been studied in the context of RCC or ICB.
Capturing the molecular heterogeneity and complexity of the immune system and metastatic cancer burden through in vitro and in vivo model systems is challenging; thus, human studies are required. However, this presents its own challenges as tissue biopsies for molecular profiling of mRCC are invasive, logistically challenging to scale, and carry the risk of procedural complications. Therefore, many of the landmark studies in mRCC13-17 have relied at least in part on archival nephrectomy specimens, which may not accurately reflect changes in the current tumor state because of time or treatment-induced alterations. Even when metastatic biopsies are available, the serial sampling required to understand real-time tumor evolution is not feasible and almost never performed. Liquid biopsies that assess circulating tumor cells (CTCs) represent a noninvasive alternative.
Past CTC studies in mRCC focused primarily on enumeration and demonstrated reduced overall survival (OS) with increased CTC abundance.18,19 Herein, we profiled 457 longitudinal blood samples from 104 patients from two cohorts, a prospective institutional cohort and the phase II OMNIVORE (OM) immunotherapy trial.20 We used an institutional CTC capture and immunofluorescence platform21 to assess enumeration and HLA I and PD-L1 protein expression, with a focus on the longitudinal trajectory of their ratio. By monitoring the real-time shifts in enumeration and the molecular profile of CTCs, we were able to capture new insights into RCC evolution to ICB therapy and demonstrate their prognostic importance.
METHODS
Two cohorts of patients with RCC were assessed in parallel: a phase II multicenter, adaptive immunotherapy trial (OM)20 and a prospective institutional cohort from the University of Wisconsin (UW). CTCs were captured using an institutional platform as previously described.21-23 Neither of the cohorts included patients with co-occurring malignancies. CTC enumeration and fluorescence were evaluated using a novel automated quantitative microscopy approach. Normalized CTC abundance was calculated as the number of CTCs divided by the volume of blood analyzed. The interplay between HLA I and PD-L1 has been investigated in other tumor types,24-29 but not in the context of RCC or ICB. Given their diametric roles within the immune system, we sought to contextualize the expression of each by analyzing the log-ratio between the two, hereafter referred to as the HP ratio. To ensure that measures of expression were not biased by CTC abundance, we compared HLA I, PD-L1, and log10(HLA I/PD-L1) expressions relative to normalized CTC abundance within samples (Data Supplement, online only). The controlled treatment of patients in the OM cohort allowed us to determine the value of several CTC-specific variables in predicting OS. The rate of change in abundance and the HP ratio were estimated for each patient. Treatment response was assessed by local radiology in OM. In UW, response was initially determined by the treating physician and then independently confirmed by a study investigator.
Ethics Approval and Consent to Participate
Patients consented under the institutional review board–approved protocol from either Dana-Farber Cancer Institute (2017-1567) or the UW-Madison Carbone Cancer Center (2014-2014); all patients provided written informed consent before study enrollment, and all methodologies conformed to the standards of the Declaration of Helsinki.
Statistical Methods
Plotting and statistical tests were performed in R v4.1.0. Statistical tests performed were all two-sided. Cox regression was used for survival analyses. A Wilcoxon signed-rank test was used to compare continuous variables between groups and the paired pre/postinduction nivolumab samples. Boxplots represent quartiles, and whiskers are equal to 1.5× interquartile range. All analyses were also reproduced in only the patients with ccRCC, excluding the small number of non-ccRCC patients (Data Supplement). Additional details on these cohorts, such as sample collection, processing, and analysis, can be found in the Data Supplement.
RESULTS
CTC Enumeration Is Associated With Clinical Outcomes
We collected a total of 457 blood samples for CTC analysis from 104 patients. The OM cohort contained 305 samples from 60 patients while the UW cohort contained 152 samples from 44 patients. Baseline characteristics including timing/frequency of blood collection for the two cohorts are shown in the Data Supplement. CTC enumeration at baseline is prognostic for OS in patients with mRCC treated with TKI therapy.18,19 We sought to evaluate the importance of CTC enumeration in the setting of the modern ICB-containing treatment paradigm and determine if its utility could extend to longitudinal monitoring. Normalized CTC abundance ranged from 0 to 718.4 CTCs/mL in the OM cohort and 0.7 to 91.3 CTCs/mL in the UW cohort. In total, only two samples from two patients in the OM cohort had no detectable CTCs, and both were at baseline.
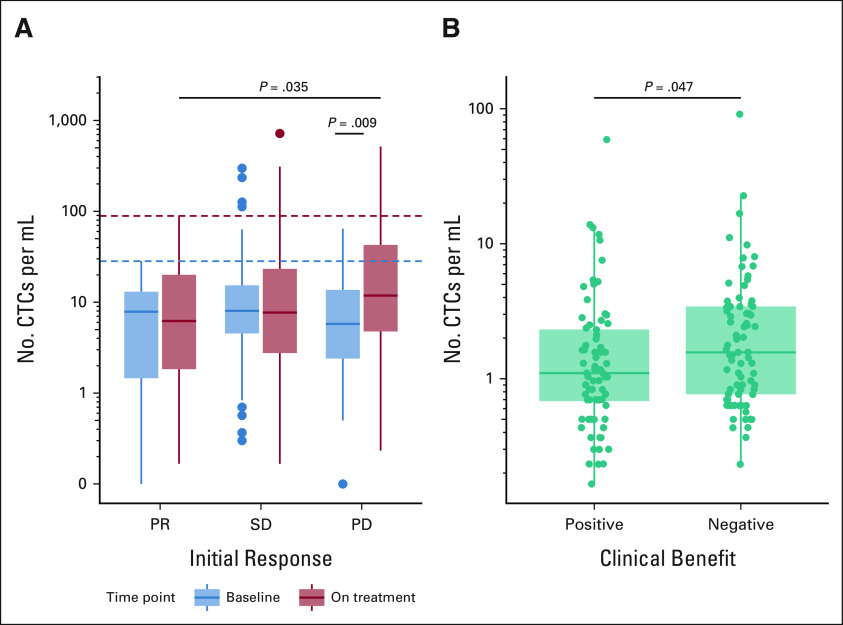
Patients with confirmed progressive disease (PD) to induction nivolumab displayed a significantly higher abundance of CTCs while on treatment than at baseline (Wilcoxon P = .009, Fig 1A); these patients also displayed significantly higher on-treatment CTC abundances than patients with partial response (PR) who were on treatment (P = .035). Furthermore, patients with a PR exhibited generally lower baseline CTC abundances, never exceeding 28.3 CTCs/mL, whereas several patients with stable disease (SD; n = 7, 17%) or PD (n = 3, 7%) exceeded this value. When extended to the entire follow-up period, we observed a similar pattern, with PR patients never exceeding 89 CTCs/mL throughout the course of their treatment, while 12 patients with SD (7%) and 9 patients with PD (8%) surpassed this value. We next sought to confirm these findings in the UW cohort, where response specifically at the time of CTC collection was available. Given the smaller numbers within each response category, samples were compared between those associated with (complete response/PR/SD) and without (mixed response/PD/untreated) a clinical benefit. Significantly higher CTC abundance (Wilcoxon P = .047, Fig 1B) was observed in the UW collections associated with a negative clinical benefit at the time of collection when compared with collections associated with a positive clinical benefit, consistent with our findings from OM.
FIG 1.
CTCs per milliliter of blood, grouped by treatment response. (A) All OM collections, grouped by their collection timing (baseline in blue and on-treatment in red) and confirmed response during nivolumab induction (PR, SD, PD). Dashed lines represent the maximum CTCs per milliliter exhibited by partial responders at baseline (28.3 CTCs/mL, blue) and during treatment (89 CTCs/mL, red). (B) All UW collections, grouped by the clinical benefit associated with that collection. CTC, circulating tumor cell; OM, OMNIVORE; PD, progressive disease; PR, partial response; SD, stable disease; UW, University of Wisconsin.
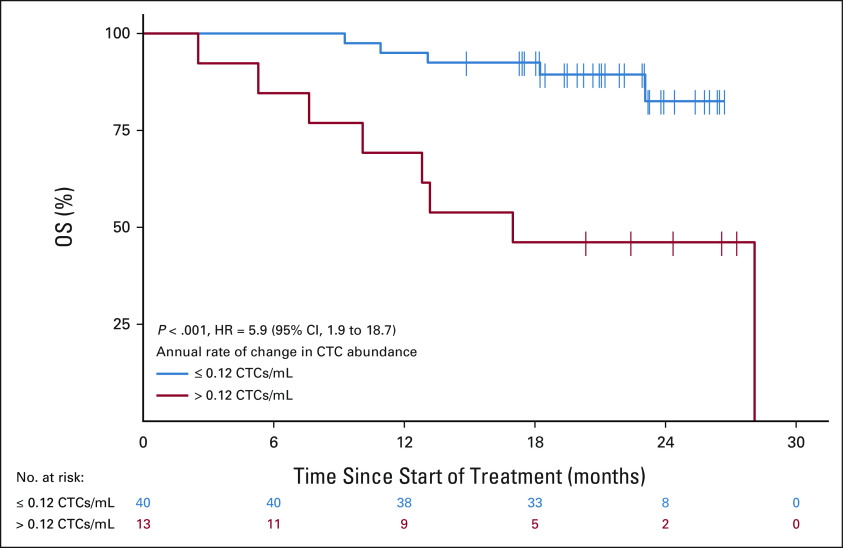
Next, we sought to investigate trends in CTC enumeration over time, as we hypothesized that changes in CTC number could be prognostic for OS. We first performed patient-specific linear regressions, across all OM CTC collections, to quantify the slope of change in their CTC numbers over time. Patients whose annual rate of change in CTC abundance was in the top quartile for the cohort (> 0.12 CTCs/mL annually) had significantly shorter OS relative to patients with a rate of change in the bottom three quartiles (≤ 0.12 CTCs/mL; median 17.2 months v 21.1 months; HR = 5.9; 95% CI, 1.9 to 18.7; P < .001; Fig 2). These results confirm the importance of CTC enumeration and newly demonstrate that the change in CTC enumeration over time is an important prognostic variable in mRCC.
FIG 2.
Kaplan-Meier curve showing association between the rate of change in CTC abundance and patient OS in the OMNIVORE cohort. Patients are grouped by their annual rate of change in CTC abundance, determined from patient-specific linear models fit to sample CTC abundances throughout treatment, with patients in the red group belonging to the top quartile (> 0.12 CTCs/mL annually) versus bottom three quartiles in black group (≤ 0.12 CTCs/mL annually). CTC, circulating tumor cell; HR, hazard ratio; OS, overall survival.
HP Ratio Decreases Over Time With ICB
As no trend was observed between HLA I or PD-L1 expression and CTC abundance (Data Supplement), we next examined the log-ratio of HLA I to PD-L1 protein expression (HP ratio). We hypothesized that a ratio capturing both PD-L1 and HLA I expression would potentially be an informative metric to characterize the dynamic changes in the immune repertoire in real time. Specifically, we were interested in how this HP ratio changed over time (using the slope of a global linear model across patients) under the selective pressure of ICB, and if this carried any clinical importance.
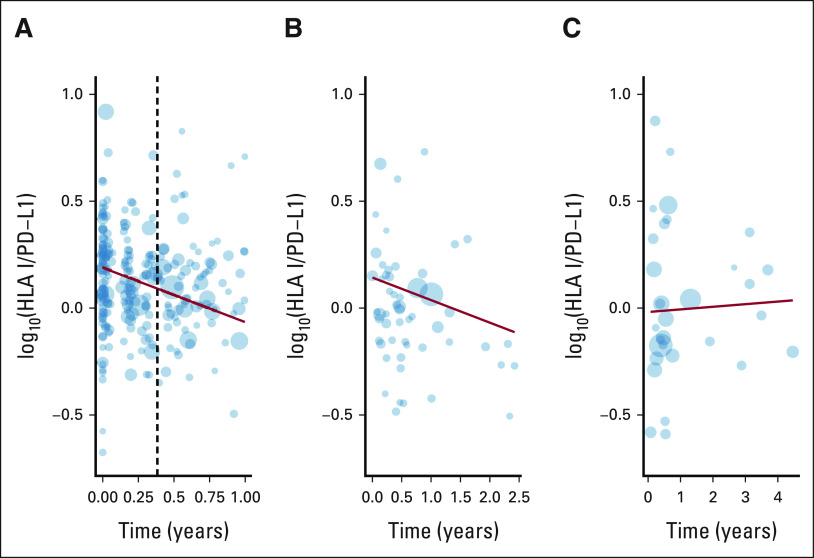
The global linear model of the HP ratio over time revealed a significant decrease in the expression of HLA I relative to PD-L1 within the OM cohort (P < .001, slope = –0.2339, Fig 3A). Intriguingly, although this negative relationship was present in UW samples collected from patients receiving ICB therapy (slope = –0.10548, P = .0671, Fig 3B), it was absent in the samples derived from patients receiving TKI therapy (slope = 0.012, P = .821, Fig 3C). It is important to note that while the rate of change in the HP ratio may not appear large, the log-ratio is base 10, and thus, the difference of one unit indicates a 10-fold change.
FIG 3.
Linear regressions (red), weighted by circulating tumor cells per mL (point size), showing a decrease in measured log10(HLA I/PD-L1) throughout treatment when on ICB therapy in both the (A) OM and (B) UW cohorts, but not (C) TKI therapy (UW only). Vertical dashed line in (A) indicates when the arm assignment is complete. ICB, immune checkpoint blocker; OM, OMNIVORE; PD-L1, programmed cell death-ligand 1; TKI, tyrosine kinase inhibitor; UW, University of Wisconsin.
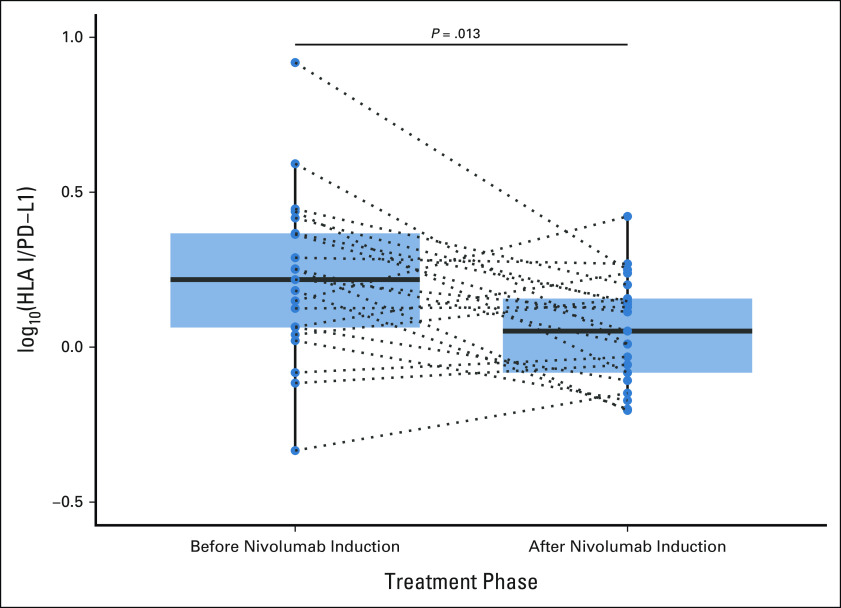
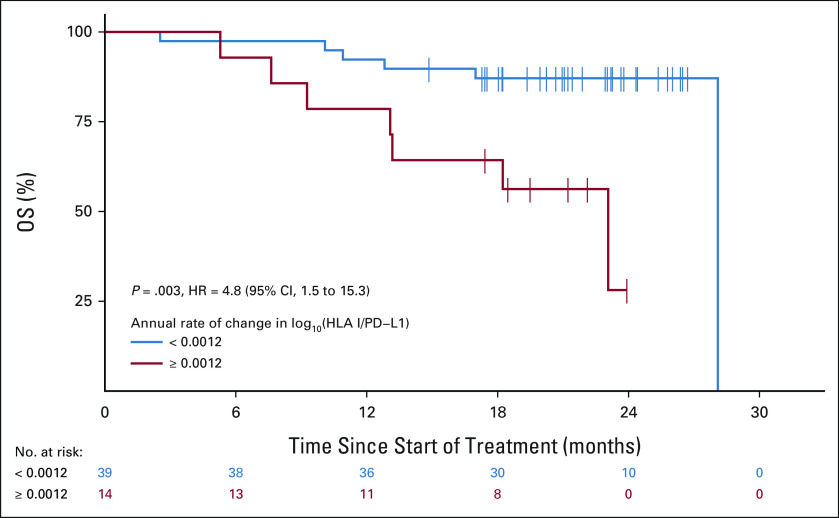
Further analysis showed that this relationship was also present on an individual patient level; a significant decrease in the HP ratio was detected between paired preinduction and postinduction collections within the OM cohort (paired Wilcoxon P = .013, Fig 4), which were separated by a median of 113 days. This shift in the HP ratio over time, in response to ICB therapy, was also prognostic in OM. Patients with an HP ratio trajectory in the top quartile (≥ 0.0012 annually) displayed significantly shorter OS than patients whose trajectory was in the bottom three quartiles (< 0.0012 annually; median 18.35 months v 21.22 months; HR, 4.8; 95% CI, 1.5 to 15.3; P = .003, Fig 5).
FIG 4.
Boxplots showing the distribution of the measured log10(HLA I/PD-L1) before nivolumab induction and again after a mean of 113 days on treatment (28-day cycles) for 30 patients with paired collections in the OMNIVORE cohort. Paired measures are connected by dotted lines. PD-L1, programmed cell death-ligand 1.
FIG 5.
Kaplan-Meier curve showing association between the rate of change in log10(HLA I/PD-L1) and patient OS in the OMNIVORE cohort. Patients are grouped by annual rate of change in log10(HLA I/PD-L1), determined from patient-specific linear models fit to sample abundances across treatment, with patients in the red group belonging to the top quartile. HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death-ligand 1.
Radiation Therapy
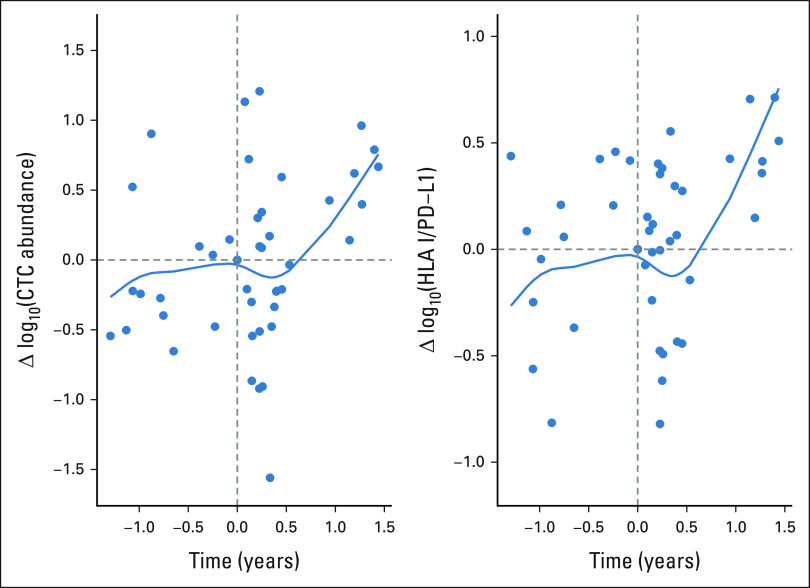
Radiation therapy has been hypothesized to potentially synergize with ICB30,31 and lead to systemic responses from local radiation treatment.32 In an exploratory analysis, we sought to examine how CTC abundance and the HP ratio fluctuated after palliative radiotherapy. Although radiotherapy was not allowed in the OM protocol, 12 patients from the UW cohort did receive palliative radiation during their treatment course. For these patients, we calculated the pairwise differences in CTC abundance and HP ratio between an individual's sample collected directly before radiotherapy and all other collections. We then plotted these differences relative to their time from receiving radiotherapy (Fig 6). Interestingly, when we fit polynomial spline curves to the abundance and ratio, we found that although patients initially displayed a trend of increasing CTC abundance and HP ratio before radiotherapy (a negative prognostic sign), this trend reversed for approximately 6 months upon receiving radiotherapy with both the abundance and ratio decreasing, both of which were found to associate with a positive prognosis above. Ultimately, however, this change did not last and both trends continued to increase, which is not surprising as palliative radiation is typically only given to patients with a poor response. Nevertheless, our data suggest that radiotherapy is having an impact on the expression of immunomodulatory molecules on mRCC tumor cells, through which a positive response, albeit short-term and variable, is elicited. Immunological changes in response to radiation have been previously observed,31,33 which warrants further study. The fact that we do observe transient biomarker changes associated with better prognoses may suggest the potential for combining radiotherapy and immunotherapy, a concept that is being tested in prospective clinical trials.
FIG 6.
Modeled influence of radiotherapy on CTC measures for 12 patients in University of Wisconsin cohort. Polynomial spline models fit to the difference between measures from a patient's CTC collection taken directly before radiotherapy (time = 0) and CTC collections taken throughout treatment (time = years between collections). Differences are calculated for (A) log10(CTCs/mL) and (B) log10(HLA I/PD-L1). CTC, circulating tumor cell; PD-L1, programmed cell death-ligand 1.
DISCUSSION
Herein, we present the largest CTC study to our knowledge in mRCC, profiling 457 blood samples from 104 patients from a prospective institutional cohort and the phase II OM immunotherapy trial. Using an advanced microfluidic platform that has shown high sensitivity and specificity for ccRCC CTC capture,21 we obtained longitudinal collections which allowed us to monitor changes in CTC enumeration and the log-HP ratio in response to treatment. We identified that the change in CTC abundance over time and the change in the HP ratio were both prognostic. These results provide insights into potential biomarkers for predicting patient response to ICB and assisting in prognostication for mRCC.
CTC enumeration has been the focus of prior CTC studies in mRCC and is prognostic for OS in patients with mRCC treated with TKI therapy in one study,18 whereas a second study showed a nonsignificant trend for cancer-specific survival, also in TKI-treated patients.19 In the first examination in the immunotherapy era, we again found that CTC enumeration is prognostic. Specifically, we demonstrated that the direction that CTC enumeration changes during immunotherapy treatment is strongly associated with OS and may complement imaging in monitoring disease and predicting the trajectory of the disease.
In addition, we used an automated immunofluorescence platform to examine PD-L1 and HLA I protein levels in CTCs. To our knowledge, this is the first such study in mRCC. Downregulation of HLA I is a well-known mechanism of immune evasion in cancer, as is PD-L1 upregulation; however, neither has been individually demonstrated as a clear predictor of ICB response in mRCC.5-7,11,12 As the effects of HLA I and PD-L1 are opposite on immune evasion by tumors, we sought to contextualize their coexpression by examining the log-HP ratio. We found that this ratio decreased over time in patients treated with ICB in both the UW and OM cohorts, but not those treated with single-agent TKI. Tumor cells with high HLA I and low PD-L1 are likely preferentially cleared by immunotherapy, and thus over time, the population average would reflect only the survivors, leading to a decreasing ratio over time. This is further supported by our finding that an increasing HP ratio over time portends worse outcomes. If a patient's HP ratio does not drop with ICB, it may reflect the poor efficacy of ICB, which is not affecting the HP ratio of the overall population of tumor cells. The importance of this relationship on tumor stage, prognosis, and T-cell infiltration has been described in non–small-cell lung carcinoma,24,25 intrahepatic cholangiocarcinoma,26 hepatocellular carcinoma,27 head and neck squamous cell carcinoma,28 and bladder cancer.29 However, none of these prior studies in other cancers were in the context of RCC or ICB. Our data suggest that the changes in the HP ratio on mRCC CTCs complement enumeration and reflect shifts in the population of tumor cells toward resistant subclones in response to immunotherapy.
Although our study was large and diverse, there were limitations within each cohort. Our institutional cohort was heterogeneous in treatments, as all institutional cohorts tend to be. As our study only had a small number of patients treated with ICB plus TKI, as well as those with sarcomatoid/rhabdoid features, future studies focused on these populations will be required to extrapolate our results. Nevertheless, this study did allow for a comparison of the HP ratio between ICB and TKI therapy and provided extensive longitudinal data. The OM ICB trial had much more uniform inclusion criteria and treatment, allowing for survival analyses to be performed. However, it was designed as a therapeutic trial, and patients who progressed or had treatment-limiting toxicity went off trial, which meant that liquid biopsies became sparser with longer follow-up. Although each cohort has caveats, the weaknesses of each were balanced by the strengths of the other, allowing for a more complete picture of CTCs in RCC.
In conclusion, we demonstrate the potential clinical utility of monitoring both CTC enumeration and the HP ratio on CTCs. In particular, our study leverages the serial nature of liquid biopsies to demonstrate how biomarkers can be used to monitor patients over time, rather than just as a single snapshot. The trajectory of both CTC enumeration and HLA I/PD-L1 could provide guidance on a range of important treatment decisions such as when to initiate or change systemic therapy, especially in situations where imaging is ambiguous. This work forms the foundation for blood-based molecular profiling in RCC. Further validation of the clinical utility of these biomarkers requires testing in multiple large prospective clinical trials. Therefore, this assay is being implemented as a correlative end point in a large multi-institutional prospective observational mRCC trial, the recently approved randomized NRG SAMURAI trial34 (ICB with or without radiation), and the randomized Alliance RadiCal trial (TKI with or without Radium-223; ClinicalTrials.gov identifier: NCT04071223).
ACKNOWLEDGMENT
We would like to acknowledge the assistance of Katie Kovacich, Laura Ruelle, Hannah Ranous, Lyndsey Deverman, and the University of Wisconsin Carbone Cancer Center Biospecimen collection team. We also thank the staff of the University of Wisconsin Carbone Cancer Center (UWCCC) Circulating Biomarker Core (CBC) shared resource for their valuable contributions to this research.
Rana R. McKay
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Hamid Emamekhoo
Consulting or Advisory Role: Exelixis, Cardinal Health, Seattle Genetics
Wanling Xie
Consulting or Advisory Role: Convergent Therapeutics
Sabina Signoretti
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Crispr Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Receives royalties from Biogenex
Other Relationship: AACR, NCI
Christos E. Kyriakopoulos
Consulting or Advisory Role: Exelixis, Sanofi, AVEO, EMD Serono, Janssen Oncology
Research Funding: Sanofi
Edwin J. Abel
Consulting or Advisory Role: Argos Therapeutics, PrecisCa, Histosonics
Speakers' Bureau: Pfizer
Kyle T. Helzer
Employment: Epic (I), Proteovista LLC
Hamza Bakhtiar
Employment: Epic Systems, Karim Bakhtiar MDSC (I)
Stock and Other Ownership Interests: Karim Bakhtiar MDSC (I)
Travel, Accommodations, Expenses: Epic Systems
Michael Bassetti
Research Funding: Merck, AstraZeneca, EMD Serono
Menggang Yu
Consulting or Advisory Role: Merck
Toni K. Choueiri
Employment: Dana Farber Cancer Hospital
Leadership: Dana Farber Cancer Hospital, NCCN, KidneyCan, ASCO, ESMO
Stock and Other Ownership Interests: Pionyr, Tempest Therapeutics, Osel, Precede Bio
Honoraria: NCCN, UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, Bristol Myers Squibb, Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Health, Kidney Cancer Association, Exelixis, Prometheus, Lpath, The New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Lilly, Janssen Oncology, IQvia, Aveo, NCI Genitourinary Cancers Steering Committee
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Infinity Pharmaceuticals, Aravive, Tempest Therapeutics, Nuscan Diagnostics, Arcus Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genentech (Inst), Celldex (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Prometheus (Inst), Ipsen (Inst), Corvus Pharmaceuticals (Inst), Cerulean Pharma (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Foundation Medicine (Inst), Roche (Inst), Calithera Biosciences (Inst), Analysis Group (Inst), NCI (Inst), GATEWAY for Cancer Research (Inst), Congressionally Directed Medical Research Programs (DOD) (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy” (Inst), International Patent Application No. PCT/US2018/12209, titled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response” (Inst), ctDNA Technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Joshua M. Lang
Stock and Other Ownership Interests: Salus Discovery
Consulting or Advisory Role: Sanofi, Immunomedics, Janssen, Pfizer/Astellas, 4D Pharma, Gilead Sciences, Arvinas, Pfizer/Myovant, 4D Pharma, AstraZeneca
Research Funding: Medivation (Inst), Agensys (Inst), GlaxoSmithKline (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Janssen (Inst), Gilead Sciences (Inst), Arvinas (Inst)
Patents, Royalties, Other Intellectual Property: I am listed on the patent on a technology for rare cell capture and analysis. This technology has been licensed by Salus Discovery LLC though no commercial products are available
Shuang G. Zhao
Employment: Exact Sciences (I)
Stock and Other Ownership Interests: Exact Sciences (I)
Patents, Royalties, Other Intellectual Property: Patent applications pending licensed to Veracyte
No other potential conflicts of interest were reported.
SUPPORT
Funded by the NIH (DP2 OD030734-0) and the Brian Mullins Renal Cell Cancer Research Award to S.G.Z., and the Prostate Cancer Foundation (Challenge Award) to J.M.L. Shared research services at the UWCCC are supported by Cancer Center Support Grant No. P30 CA014520.
CLINICAL TRIAL INFORMATION
NCT03203473 (OMNIVORE)
M.B., R.R.M., and H.E. contributed equally to this work. T.K.C., J.M.L., and S.G.Z. jointly supervised this work.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00219.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew Bootsma, Rana R. McKay, Hamid Emamekhoo, Jamie Sperger, Sabina Signoretti, Toni K. Choueiri, Joshua M. Lang, Shuang G. Zhao
Financial support: Joshua M. Lang, Shuang G. Zhao
Administrative support: Joshua M. Lang, Shuang G. Zhao
Provision of study materials or patients: Hamid Emamekhoo, Sabina Signoretti, Christos E. Kyriakopoulos, John Floberg, Toni K. Choueiri, Joshua M. Lang, Shuang G. Zhao
Collection and assembly of data: Matthew Bootsma, Rana R. McKay, Hamid Emamekhoo, Rory M. Bade, Jennifer L. Schehr, Matthew C. Mannino, Anupama Singh, Serena K. Wolfe, Zachery D. Schultz, Jamie Sperger, Wanling Xie, Christos E. Kyriakopoulos, John Floberg, Nan Sethakorn, Toni K. Choueiri, Joshua M. Lang, Shuang G. Zhao
Data analysis and interpretation: Matthew Bootsma, Rana R. McKay, Hamid Emamekhoo, Zachery D. Schultz, Christos E. Kyriakopoulos, David Kosoff, Edwin J. Abel, Kyle T. Helzer, Nicholas Rydzewski, Hamza Bakhtiar, Yue Shi, Grace Blitzer, Michael Bassetti, Menggang Yu, Marina Sharifi, Paul M. Harari, Joshua M. Lang, Shuang G. Zhao
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Longitudinal Molecular Profiling of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rana R. McKay
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Hamid Emamekhoo
Consulting or Advisory Role: Exelixis, Cardinal Health, Seattle Genetics
Wanling Xie
Consulting or Advisory Role: Convergent Therapeutics
Sabina Signoretti
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Crispr Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Receives royalties from Biogenex
Other Relationship: AACR, NCI
Christos E. Kyriakopoulos
Consulting or Advisory Role: Exelixis, Sanofi, AVEO, EMD Serono, Janssen Oncology
Research Funding: Sanofi
Edwin J. Abel
Consulting or Advisory Role: Argos Therapeutics, PrecisCa, Histosonics
Speakers' Bureau: Pfizer
Kyle T. Helzer
Employment: Epic (I), Proteovista LLC
Hamza Bakhtiar
Employment: Epic Systems, Karim Bakhtiar MDSC (I)
Stock and Other Ownership Interests: Karim Bakhtiar MDSC (I)
Travel, Accommodations, Expenses: Epic Systems
Michael Bassetti
Research Funding: Merck, AstraZeneca, EMD Serono
Menggang Yu
Consulting or Advisory Role: Merck
Toni K. Choueiri
Employment: Dana Farber Cancer Hospital
Leadership: Dana Farber Cancer Hospital, NCCN, KidneyCan, ASCO, ESMO
Stock and Other Ownership Interests: Pionyr, Tempest Therapeutics, Osel, Precede Bio
Honoraria: NCCN, UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, Bristol Myers Squibb, Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Health, Kidney Cancer Association, Exelixis, Prometheus, Lpath, The New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Lilly, Janssen Oncology, IQvia, Aveo, NCI Genitourinary Cancers Steering Committee
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Infinity Pharmaceuticals, Aravive, Tempest Therapeutics, Nuscan Diagnostics, Arcus Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genentech (Inst), Celldex (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Prometheus (Inst), Ipsen (Inst), Corvus Pharmaceuticals (Inst), Cerulean Pharma (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Foundation Medicine (Inst), Roche (Inst), Calithera Biosciences (Inst), Analysis Group (Inst), NCI (Inst), GATEWAY for Cancer Research (Inst), Congressionally Directed Medical Research Programs (DOD) (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy” (Inst), International Patent Application No. PCT/US2018/12209, titled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response” (Inst), ctDNA Technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Joshua M. Lang
Stock and Other Ownership Interests: Salus Discovery
Consulting or Advisory Role: Sanofi, Immunomedics, Janssen, Pfizer/Astellas, 4D Pharma, Gilead Sciences, Arvinas, Pfizer/Myovant, 4D Pharma, AstraZeneca
Research Funding: Medivation (Inst), Agensys (Inst), GlaxoSmithKline (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Janssen (Inst), Gilead Sciences (Inst), Arvinas (Inst)
Patents, Royalties, Other Intellectual Property: I am listed on the patent on a technology for rare cell capture and analysis. This technology has been licensed by Salus Discovery LLC though no commercial products are available
Shuang G. Zhao
Employment: Exact Sciences (I)
Stock and Other Ownership Interests: Exact Sciences (I)
Patents, Royalties, Other Intellectual Property: Patent applications pending licensed to Veracyte
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Motzer RJ: Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 376:354-366, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Klapper JA, Downey SG, Smith FO, et al. : High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 113:293-301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722-731, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Penkov K, Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T, Plimack ER, Soulieres D, et al. : Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21:1563-1573, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Figueroa DJ, Fay AP, et al. : Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from COMPARZ, a randomized controlled trial. Clin Cancer Res 21:1071-1077, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, et al. : Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 66:3381-3385, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Liu L, Qu Y, et al. : HLA class I expression predicts prognosis and therapeutic benefits from tyrosine kinase inhibitors in metastatic renal-cell carcinoma patients. Cancer Immunol Immunother 67:79-87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDermott DF, Huseni MA, Atkins MB, et al. : Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Robbins PB, Powles T, et al. : Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med 26:1733-1741, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun DA, Hou Y, Bakouny Z, et al. : Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med 26:909-918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricketts CJ, De Cubas AA, Fan H, et al. : The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 23:313-326.e5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Banchereau R, Hamidi H, et al. : Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 38:803-817.e4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basso U, Facchinetti A, Rossi E, et al. : Prognostic role of circulating tumor cells in metastatic renal cell carcinoma: A large, multicenter, prospective trial. Oncologist 26:740-750, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, Kang YT, Cho YH, et al. : Detection of circulating tumour cells and their potential use as a biomarker for advanced renal cell carcinoma. Can Urol Assoc J 13:E285-E291, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKay RR, McGregor BA, Xie W, et al. : Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: A response-based phase II study (OMNIVORE). J Clin Oncol 38:4240-4248, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bade RM, Schehr JL, Emamekhoo H, et al. : Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma. Mol Oncol 15:2330-2344, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperger JM, Strotman LN, Welsh A, et al. : Integrated analysis of multiple biomarkers from circulating tumor cells enabled by exclusion-based analyte isolation. Clin Cancer Res 23:746-756, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casavant BP, Guckenberger DJ, Beebe DJ, et al. : Efficient sample preparation from complex biological samples using a sliding lid for immobilized droplet extractions. Anal Chem 86:6355-6362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perea F, Sanchez-Palencia A, Gomez-Morales M, et al. : HLA class I loss and PD-L1 expression in lung cancer: Impact on T-cell infiltration and immune escape. Oncotarget 9:4120-4133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai A, Yoneda K, Shimajiri S, et al. : Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg 155:382-392.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Teuber J, Meier S, Aigner K, et al. : Immunohistologic determination of a tumor marker (CEA) in diseases of the gastrointestinal tract [in German]. Immun Infekt 11:91-98, 1983 [PubMed] [Google Scholar]
- 27.Umemoto Y, Okano S, Matsumoto Y, et al. : Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 50:65-75, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Yoo SH, Keam B, Ock CY, et al. : Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci Rep 9:7680, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores-Martin JF, Perea F, Exposito-Ruiz M, et al. : A combination of positive tumor HLA-I and negative PD-L1 expression provides an immune rejection mechanism in bladder cancer. Ann Surg Oncol 26:2631-2639, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Demaria S, Golden EB, Formenti SC: Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 1:1325-1332, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Formenti SC, Demaria S: Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst 105:256-265, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngwa W, Irabor OC, Schoenfeld JD, et al. : Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18:313-322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Felice F, Polimeni A, Valentini V, et al. : Radiotherapy controversies and prospective in head and neck cancer: A literature-based critical review. Neoplasia 20:227-232, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paciotti M, Schmidt AL, Ravi P, et al. : Temporal trends and predictors in the use of stereotactic body radiotherapy for treatment of metastatic renal cell carcinoma in the U.S. Oncologist 26:e905-e906, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00219.