Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
In a randomized, open-label, phase III NEJ009 study, gefitinib plus chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) compared with gefitinib-alone in patients with untreated non–small-cell lung cancer harboring mutations in epidermal growth factor receptor. Herein, we report the updated survival outcome and long-term tolerability. Patients were randomly assigned to gefitinib (gefitinib 250 mg orally, once daily) and gefitinib combined with carboplatin plus pemetrexed (GCP in a 3-week cycle for six cycles followed by concurrent gefitinib and pemetrexed maintenance) groups. At the data cutoff (May 22, 2020), GCP demonstrated significantly better PFS2 (hazard ratio, 0.77; 95% CI, 0.62 to 0.97; P = .027) than gefitinib. However, the updated median OS was 38.5 months (95% CI, 31.1 to 47.1) and 49.0 months (95% CI, 41.8 to 56.7) in the gefitinib and GCP groups, respectively (hazard ratio, 0.82; 95% CI, 0.64 to 1.06; P = .127). The OS in both groups was similar for the overall patient population. No severe adverse events occurred since the first report. This updated analysis revealed that the GCP regimen improved PFS and PFS2 with an acceptable safety profile compared with gefitinib-alone. GCP is more efficient than gefitinib monotherapy as a first-line treatment for non–small-cell lung cancer with epidermal growth factor receptor mutations.
INTRODUCTION
NEJ009 (UMIN000006340) was a multicenter, randomized, open-label, phase III study of gefitinib combined with carboplatin plus pemetrexed (GCP) versus gefitinib-alone for patients with non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations.1 Herein, we updated the data for progression-free survival (PFS)2, overall survival (OS), and safety examined over a longer follow-up period and also assessed the impact of subsequent therapy on OS among patients with EGFR-mutated NSCLC.
CONTEXT
Key Objective
The phase III NEJ009 study conducted in Japan showed benefits for combinatorial epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor and platinum-doublet chemotherapy with an acceptable safety profile for patients with non–small-cell lung cancer harboring EGFR mutations. We report updated survival outcomes and long-term tolerability.
Knowledge Generated
Gefitinib combined with carboplatin plus pemetrexed did not improve overall survival although progression-free survival and progression-free survival 2 showed significant improvements as compared with gefitinib-alone. No severe adverse events, including interstitial lung disease, occurred since the first report.
Relevance
A new treatment strategy, gefitinib combined with carboplatin plus pemetrexed regimen, is an effective treatment option for patients with untreated advanced non–small-cell lung cancer harboring EGFR mutations.
PATIENTS AND METHODS
The complete details of the NEJ009 study have been published previously.1 The primary end points included PFS, PFS2, and OS, which were analyzed using a preplanned hierarchical sequential testing method. In this study, preplanned PFS2 was defined as the period from random assignment until both platinum-based chemotherapy and gefitinib were ineffective, that is, not a true comparison of PFS2 in both groups (Appendix Fig A1A, online only). However, as reported previously, preplanned PFS2 was inappropriate because of the influence of the beyond-progressive disease (PD) treatment period that was included only in the gefitinib group.1 Thus, we corrected the definition of PFS2 (corrected PFS2) in the gefitinib group to omit the beyond-PD period—duration since random assignment to PD after second-line therapy or death (Appendix Fig A1B). Notably, the corrected PFS2 was a comparison of PFS1 (GCP) and PFS2 (gefitinib). Moreover, we conducted an additional analysis by comparing the two groups for PFS2 with the same definition; events were the actual time for the second PD in both groups (Appendix Fig A1C). Survival and extended follow-up safety data were re-evaluated at the data cutoff of May 22, 2020.
In this updated analysis, the survival differences in the mean expected time to death were calculated as the restricted mean survival time (RMST) up to 5 and 7 years for a complementary to the log-rank test for OS.2,3 The RMST between the GCP and gefitinib groups represents the average gain in survival time within a time window from 0 to a specific threshold time point; the time was based on the areas under the survival curves for each group.
RESULTS
Patients and Treatment
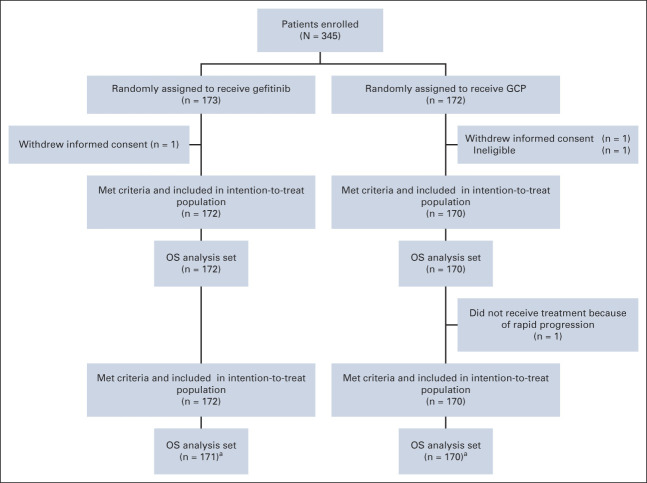
NEJ009 included 345 randomly assigned patients (gefitinib, n = 173; GCP, n = 172) from 47 institutions in Japan (Appendix Fig A2, online only). The median follow-up duration, which was defined as a median duration from patients' study enrollment dates to the cutoff date, was 84 months. Patient demographics and baseline clinical characteristics were generally well balanced between the groups (Table 1).
TABLE 1.
Patient Demographic and Disease Characteristics at Baseline
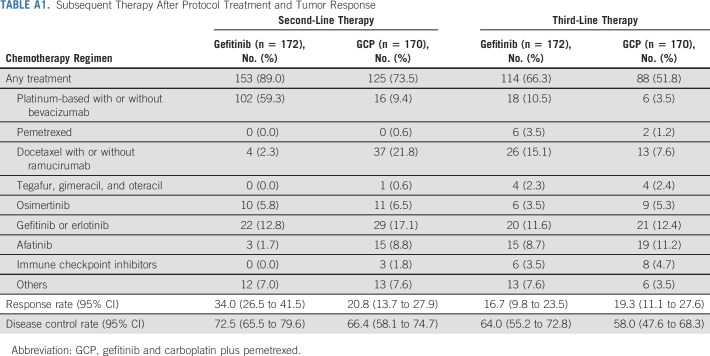
Patients in both groups received one or more subsequent chemotherapies after the protocol therapy. The regimens administered after the protocol treatment are summarized in Appendix Table A1 (online only). As osimertinib has been approved for treating patients with metastatic EGFR T790M mutation–positive NSCLC, 40 patients (23.3%) in the gefitinib group and 37 patients (21.8%) in the GCP group received osimertinib in any treatment line. In the gefitinib group, 46 patients (26.7%) did not receive platinum-based chemotherapy in any treatment line.
Updated PFS2
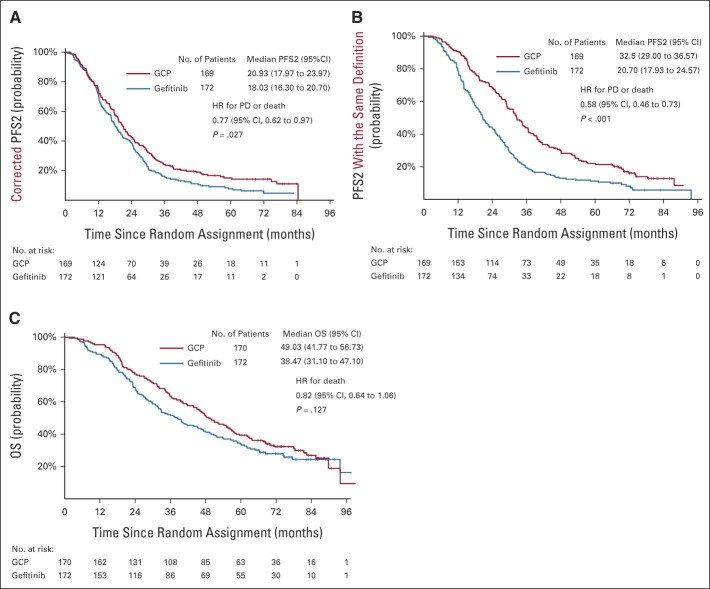
In the 341 evaluated patients, the updated median PFS2 (corrected PFS2) was 18.0 months (95% CI, 16.3 to 20.7) and 20.9 months (95% CI, 18.0 to 24.0) in the gefitinib and GCP groups, respectively (hazard ratio [HR], 0.77; 95% CI, 0.62 to 0.97; P = .027; Fig 1A). In addition, we performed an analysis to compare PFS in the two groups with the same definition (events are true for the second PD in both groups) as that used for reference. The updated median PFS2 with the same definition was 20.7 months (95% CI, 17.9 to 24.6) in the gefitinib group and 32.5 months (95% CI, 29.0 to 36.6) in the GCP group (HR, 0.58; 95% CI, 0.46 to 0.73; P < .001; Fig 1B).
FIG 1.
Updated PFS2 and OS. Kaplan-Meier curves for (A) corrected PFS2, (B) PFS2 with the same definition (events are true for the second PD in both groups), and (C) OS in patients treated with GCP and those treated with gefitinib-alone are shown. Plus symbols indicate censored patients at the data cutoff point. GCP, gefitinib and carboplatin plus pemetrexed; HR, hazard ratio; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
Updated OS
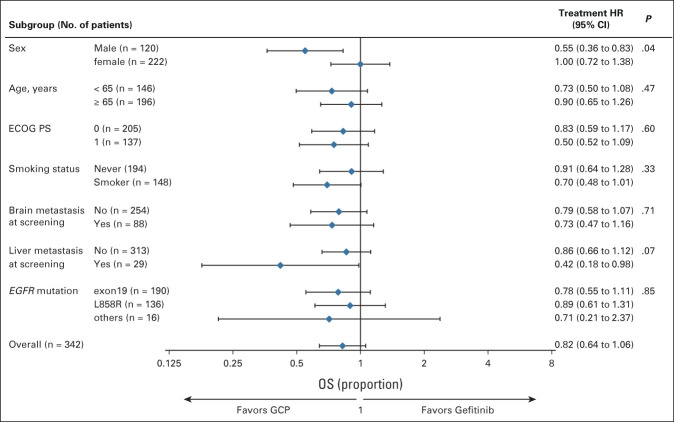
At data cutoff, 243 death events were recorded. The death event rate increased from 57% (195 events) in the previous report to 71% (243 events) in the current study. However, there was no significant difference in OS between the groups. The mean survival time, 2-year survival rate, and 5-year survival rate were 38.5 months, 69%, and 34% for the gefitinib group and 49.0 months, 77.1%, and 39% for the GCP group, respectively (HR, 0.822; 95% CI, 0.639 to 1.058; P = .127; Fig 1C). In the subgroup analysis, the OS benefit for GCP and gefitinib was comparable in the overall patient population, including the type of EGFR activation mutation and metastatic sites (Fig 2). However, larger numerical between-group differences in the HRs for OS were observed between male and female patients.
FIG 2.
Subgroup analysis of OS. Forest plots for OS are shown. A HR < 1 implies a lower risk of death with the GCP regimen than with gefitinib-alone. The Cox proportional hazards regression model includes randomly assigned treatments, subgroup covariates of interest, and treatment-by-subgroup interaction. ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; GCP, gefitinib and carboplatin plus pemetrexed; HR, hazard ratio; OS, overall survival.
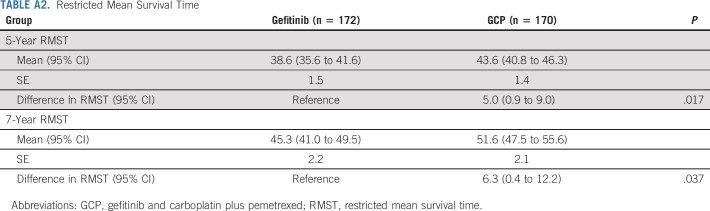
The RMST was calculated to further compare survival between the groups (Appendix Table A2, online only). The 5-year RMST for the GCP group was longer than those for the gefitinib group (43.6 v 38.6 months, P = .017). Over a 5-year period, RMST analysis demonstrated that GCP was indeed associated with a 5-month OS benefit. In addition, this tendency was still detectable when the 7-year period was selected. The 7-year RMST for the GCP group was longer than those for the gefitinib group (51.6 v 45.3 months, P = .037). RMST analysis indicated a statistically significant 6.3-month survival advantage associated with GCP over a 7-year follow-up period.
Safety
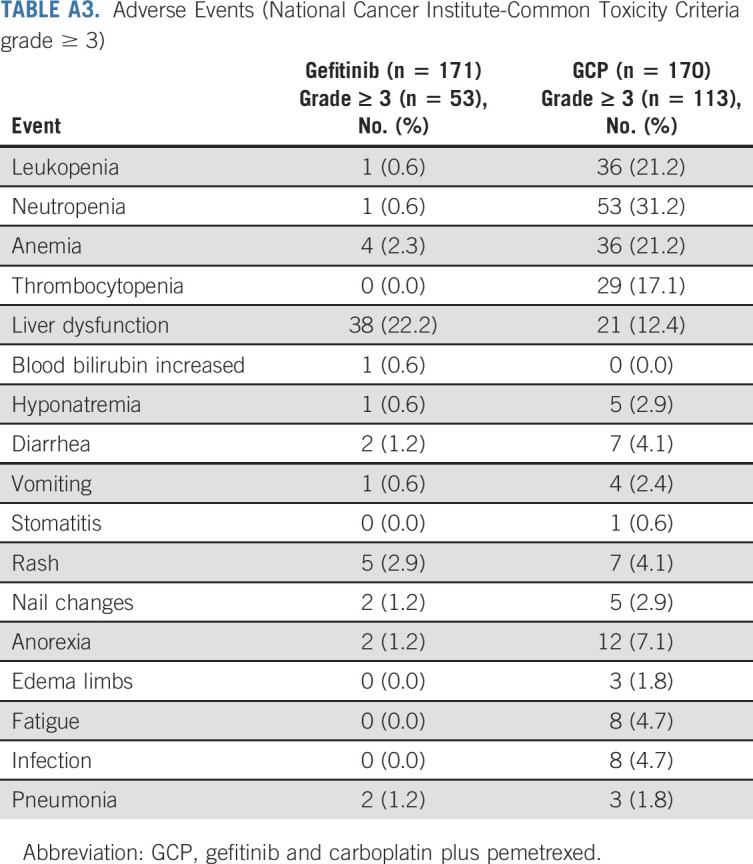
In the extended follow-up analysis, fewer patients reported grade ≥ 3 treatment-related adverse events in the gefitinib group than those in the GCP group (31.0% v 66.5%; odds ratio, 0.23; 95% CI, 0.15 to 0.36; P < .001; Appendix Table A3, online only). One grade 5 treatment-related adverse event (infection) was observed in the GCP group. No new severe interstitial lung disease occurred during the extended follow-up since the first report.
DISCUSSION
In the first report of NEJ009, PFS benefit in the GCP group was consistent with the results from other phase III trials with EGFR tyrosine kinase inhibitors (TKIs), such as the FLAURA study.1,4-20 Of note, this updated analysis demonstrated a significantly prolonged corrected PFS2 in the GCP group. These persistent survival benefits confirmed that combination therapy with EGFR-TKI and chemotherapy boosted the tumor response and duration of response than EGFR-TKI monotherapy in patients with EGFR-mutated NSCLC.
By contrast, improvements in PFS and PFS2 did not translate to an OS benefit in the present analysis. One possible reason for this was the availability of osimertinib as the subsequent therapy. The long-term survival postprogression diluted the OS differences between treatment groups; nevertheless, we observed consistently favorable survival benefit (HR, 0.82; OS) in the GCP group than in the gefitinib group. With contrast analyses using RMST for 5 years in both treatment groups, the GCP appeared to have a clinically meaningful survival benefit compared with gefitinib.2,3,21-24
Previous studies have demonstrated that exon 19 deletion and exon 21 L858R mutations can distinguish clinical characteristics among patients.25-30 In this study, GCP was found to confer more survival benefits than gefitinib-alone in a subgroup of patients regardless of their EGFR mutation type. A consistent result was reported in a similar phase III trial in India.5 In addition, male patients demonstrated a more evident OS benefit in GCP in the subgroup analysis; however, no clear explanation exists for the differences observed.
In conclusion, to our knowledge, NEJ009 is the first phase III study to evaluate the efficacy of a combination of EGFR-TKI and platinum-doublet chemotherapy in patients with untreated advanced NSCLC with EGFR mutations. The GCP regimen improved PFS and PFS2 with an acceptable safety profile than with gefitinib-alone. Clinical trials are ongoing to compare efficacy of osimertinib monotherapy with osimertinib combined with platinum plus pemetrexed as first-line treatment for patients with untreated NSCLC with EGFR mutations.31,32
ACKNOWLEDGMENT
We thank all the persons who participated in this study, including all patients, their families, physicians, and data managers. Members of the North-East Japan Study Group were as follows: advisors for study coordination: S. Kudoh, T. Nukiwa, and M. Kurihara; Independent Data and Safety Monitoring Committee: K. Nagao, Y. Nakai, and T. Yoshioka; and Study Personnel: T. Isobe, S. Oizumi, M. Kamimura, Y. Osaki, S. Koyama, H. Isobe, N. Morikawa, T. Ishida, Y. Ishii, H. Watanabe, Y. Mori, T. Tabata, S. Kuyama, K. Kiura, K. Hagiwara, K. Usui, K. Soejima, M. Nishitsuji, I. Kinoshita, K. Taima, N. Nishimura, K. Kishi, K. Ito, M. Akai, K. Takahashi, T. Kato, A Gemma, K. Hagiwara, and T. Nukiwa.
APPENDIX
FIG A1.
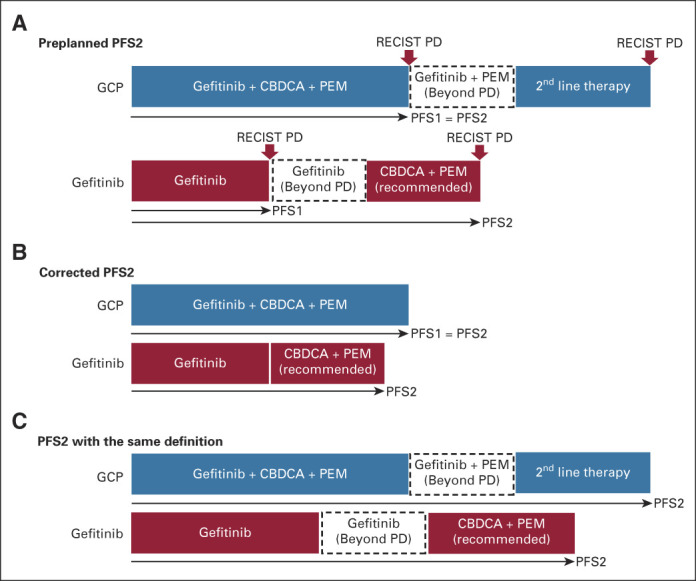
Definition of (A) preplanned PFS2, (B) corrected PFS2, and (C) PFS2 with the same definition. CBDCA, carboplatin; GCP, gefitinib combined with carboplatin plus pemetrexed; PD, progressive disease; PEM, pemetrexed; PFS, progression-free survival.
FIG A2.
CONSORT diagram. All patients except one ineligible patient and two who withdrew consent were randomly assigned to the gefitinib group or GCP group. One patient in the gefitinib group did not receive gefitinib-alone but was treated with the GCP regimen at the patient's request; thus, this patient's safety data were evaluated as if the patient was in the GCP group, whereas the PFS and OS data were evaluated as if the patient was in the gefitinib group on the basis of the intention to treat. One patient in the GCP group was excluded from the PFS analysis because the patient did not receive any protocol treatment; this patient was included in the OS analysis only. aOne patient received GCP instead of gefitinib monotherapy with a breach of allocation. GCP, gefitinib combined with carboplatin plus pemetrexed; OS, overall survival; PFS, progression-free survival.
TABLE A1.
Subsequent Therapy After Protocol Treatment and Tumor Response
TABLE A2.
Restricted Mean Survival Time
TABLE A3.
Adverse Events (National Cancer Institute-Common Toxicity Criteria grade ≥ 3)
Eisaku Miyauchi
Honoraria: AstraZeneca, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Lilly, Taiho Pharmaceutical, Kyowa Kirin, Novartis, Daiichi Sankyo, Bristol Myers Squibb, Merck
Consulting or Advisory Role: Chugai Pharma, Boehringer Ingelheim, Lilly, Kyowa Kirin
Research Funding: Boehringer Ingelheim, Lilly (Inst), Chugai Pharma (Inst)
Satoshi Morita
Honoraria: AstraZeneca Japan, Bristol Myers Squibb Company, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, MSD K.K, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer Japan Inc, Eisai, Novartis, Kyowa Kirin Co Ltd
Research Funding: Eisai (Inst)
Atsushi Nakamura
Honoraria: AstraZeneca, Chugai Pharma, Lilly Japan, Kyowa Kirin, Taiho Pharmaceutical, Novartis, Thermo Fisher Scientific
Yukio Hosomi
Speakers' Bureau: AstraZeneca, Taiho Pharmaceutical, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD, Kyowa Kirin International
Satoshi Ikeda
Honoraria: Chugai Pharma, Boehringer Ingelheim, Taiho Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Takeda
Research Funding: Chugai Pharma, AstraZeneca
Masahiro Seike
Honoraria: AstraZeneca, MSD K.K, Chugai Pharma, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, Nippon Boehringer Ingelheim, Novartis, Kyowa Hakko Kirin, Nippon Kayaku, Daiichi-Sankyo Company
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Lilly, MSD K.K, Nippon Boehringer Ingelheim, Nippon Kayaku
Yuka Fujita
Research Funding: Novartis (Inst), Asahi Kasei (Inst), Ono Pharmaceutical (Inst), Otsuka (Inst), Lilly Japan (Inst)
Koichi Minato
Honoraria: Bristol Myers Squibb Japan, Chugai Pharma
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst)
Ryo Ko
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly Japan, Boehringer Ingelheim, Pfizer, AstraZeneca, Ono Pharmaceutical, Daiichi Sankyo/UCB Japan, Takeda
Research Funding: MSD
Koichi Hagiwara
Honoraria: AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Novartis, Kyorin, Takeda, Lilly, Nippon Shinyaku, Kyowa Kirin, Nippon Kayaku, Bayer Yakuhin
Research Funding: Ono Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim (Inst), Eiken Chemical
Patents, Royalties, Other Intellectual Property: LSI Medience
Kunihiko Kobayashi
Speakers' Bureau: AstraZeneca, Takeda
Toshihiro Nukiwa
Consulting or Advisory Role: Nippon Boehringer Ingelheim, Grifols
Akira Inoue
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Bristol Myers Squibb Japan, Shionogi, Daiichi Sankyo, Kyowa Hakko Kirin, Nippon Kayaku, Mundipharma, Novartis, Pfizer
Consulting or Advisory Role: AstraZeneca, Lilly Japan, MSD
Research Funding: Boehringer Ingelheim (Inst), Lilly Japan (Inst), Kyowa Hakko Kirin (Inst), Chugai Pharma (Inst), Shionogi (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2021 ASCO annual meeting, virtual, June 4, 2021.
SUPPORT
Supported by a grant-in-aid from the Japan Society for Promotion of Science and the Japanese Foundation for the Multidisciplinary Treatment of Cancer. This study was also supported by the North-East Japan Study Group.
AUTHOR CONTRIBUTIONS
Conception and design: Eisaku Miyauchi, Satoshi Morita, Toshiyuki Harada, Koichi Hagiwara, Toshihiro Nukiwa, Akira Inoue
Financial support: Kunihiko Kobayashi, Toshihiro Nukiwa
Administrative support: Eisaku Miyauchi, Toshiyuki Harada, Kunihiko Kobayashi, Toshihiro Nukiwa
Provision of study materials or patients: Atsushi Nakamura, Kana Watanabe, Koichi Minato, Ryo Ko, Toshiyuki Harada, Koichi Hagiwara, Kunihiko Kobayashi
Collection and assembly of data: Eisaku Miyauchi, Atsushi Nakamura, Yukio Hosomi, Kana Watanabe, Satoshi Ikeda, Yuka Fujita, Koichi Minato, Ryo Ko, Toshiyuki Harada, Koichi Hagiwara, Kunihiko Kobayashi
Data analysis and interpretation: Eisaku Miyauchi, Satoshi Morita, Masahiro Seike, Toshiyuki Harada, Kunihiko Kobayashi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Updated Analysis of NEJ009: Gefitinib-Alone Versus Gefitinib Plus Chemotherapy for Non–Small-Cell Lung Cancer With Mutated EGFR
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eisaku Miyauchi
Honoraria: AstraZeneca, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Lilly, Taiho Pharmaceutical, Kyowa Kirin, Novartis, Daiichi Sankyo, Bristol Myers Squibb, Merck
Consulting or Advisory Role: Chugai Pharma, Boehringer Ingelheim, Lilly, Kyowa Kirin
Research Funding: Boehringer Ingelheim, Lilly (Inst), Chugai Pharma (Inst)
Satoshi Morita
Honoraria: AstraZeneca Japan, Bristol Myers Squibb Company, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, MSD K.K, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer Japan Inc, Eisai, Novartis, Kyowa Kirin Co Ltd
Research Funding: Eisai (Inst)
Atsushi Nakamura
Honoraria: AstraZeneca, Chugai Pharma, Lilly Japan, Kyowa Kirin, Taiho Pharmaceutical, Novartis, Thermo Fisher Scientific
Yukio Hosomi
Speakers' Bureau: AstraZeneca, Taiho Pharmaceutical, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD, Kyowa Kirin International
Satoshi Ikeda
Honoraria: Chugai Pharma, Boehringer Ingelheim, Taiho Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Lilly, Pfizer, Takeda
Research Funding: Chugai Pharma, AstraZeneca
Masahiro Seike
Honoraria: AstraZeneca, MSD K.K, Chugai Pharma, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, Nippon Boehringer Ingelheim, Novartis, Kyowa Hakko Kirin, Nippon Kayaku, Daiichi-Sankyo Company
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Lilly, MSD K.K, Nippon Boehringer Ingelheim, Nippon Kayaku
Yuka Fujita
Research Funding: Novartis (Inst), Asahi Kasei (Inst), Ono Pharmaceutical (Inst), Otsuka (Inst), Lilly Japan (Inst)
Koichi Minato
Honoraria: Bristol Myers Squibb Japan, Chugai Pharma
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst)
Ryo Ko
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly Japan, Boehringer Ingelheim, Pfizer, AstraZeneca, Ono Pharmaceutical, Daiichi Sankyo/UCB Japan, Takeda
Research Funding: MSD
Koichi Hagiwara
Honoraria: AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Novartis, Kyorin, Takeda, Lilly, Nippon Shinyaku, Kyowa Kirin, Nippon Kayaku, Bayer Yakuhin
Research Funding: Ono Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim (Inst), Eiken Chemical
Patents, Royalties, Other Intellectual Property: LSI Medience
Kunihiko Kobayashi
Speakers' Bureau: AstraZeneca, Takeda
Toshihiro Nukiwa
Consulting or Advisory Role: Nippon Boehringer Ingelheim, Grifols
Akira Inoue
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Ono Pharmaceutical, Bristol Myers Squibb Japan, Shionogi, Daiichi Sankyo, Kyowa Hakko Kirin, Nippon Kayaku, Mundipharma, Novartis, Pfizer
Consulting or Advisory Role: AstraZeneca, Lilly Japan, MSD
Research Funding: Boehringer Ingelheim (Inst), Lilly Japan (Inst), Kyowa Hakko Kirin (Inst), Chugai Pharma (Inst), Shionogi (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hosomi Y, Morita S, Sugawara S, et al. : Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol 38:115-123, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Royston P, Parmar MK: Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 13:152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uno H, Claggett B, Tian L, et al. : Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol 32:2380-2385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawara S, Oizumi S, Minato K, et al. : Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol 26:888-894, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Noronha V, Patil VM, Joshi A, et al. : Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 38:124-136, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Oizumi S, Sugawara S, Minato K, et al. : Updated survival outcomes of NEJ005/TCOG0902: A randomised phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations. ESMO Open 3:e000313, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Wu YL, Cheng Y, Zhou X, et al. : Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer(ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18:1454-1466, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Cheng Y, Zhou X, et al. : Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 36:2244-2250, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Saito H, Fukuhara T, Furuya N, et al. : Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 20:625-635, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Garon EB, Seto T, et al. : Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:1655-1669, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, et al. : Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380-2388, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Morita S, Yatabe Y, et al. : Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11:121-128, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Wu YL, Chen G, et al. : Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 26:1877-1883, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Zhou C, Liam CK, et al. : First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 26:1883-1889, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Rosell R, Carcereny E, Gervais R, et al. : Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239-246, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Sequist LV, Yang JC, Yamamoto N, et al. : Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327-3334, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Inoue A, Kobayashi K, Maemondo M, et al. : Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24:54-59, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi E, Inoue A, Kobayashi K, et al. : Efficacy of chemotherapy after first-line gefitinib therapy in EGFR mutation-positive advanced non-small cell lung cancer-data from a randomized Phase III study comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Jpn J Clin Oncol 45:670-676, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339-2349, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghaei H, Gettinger S, Vokes EE, et al. : Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 39:723-733, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Garon EB, Kim DW, et al. : Five year survival update from KEYNOTE-010: Pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol 16:1718-1732, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Pak K, Uno H, Kim DH, et al. : Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol 3:1692-1696, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riely GJ, Pao W, Pham D, et al. : Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12:839-844, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Jackman DM, Yeap BY, Sequist LV, et al. : Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 12:3908-3914, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Won YW, Han JY, Lee GK, et al. : Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol 64:947-952, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Castellanos E, Feld E, Horn L: Driven by mutations: The predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol 12:612-623, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Choi YW, Jeon SY, Jeong GS, et al. : EGFR Exon 19 deletion is associated with favorable overall survival after first-line gefitinib therapy in advanced non-small cell lung cancer patients. Am J Clin Oncol 41:385-390, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Li WQ, Cui JW: Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: Distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol 146:2329-2338, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asahina H, Tanaka K, Morita S, et al. : A phase II study of osimertinib combined with platinum plus pemetrexed in patients with EGFR-mutated advanced non-small-cell lung cancer: The OPAL study (NEJ032C/LOGIK1801). Clin Lung Cancer 22:147-151, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Planchard D, Feng PH, Karaseva N, et al. : Osimertinib plus platinum-pemetrexed in newly diagnosed epidermal growth factor receptor mutation-positive advanced/metastatic non-small-cell lung cancer: Safety run-in results from the FLAURA2 study. ESMO Open 6:100271, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]