Abstract
Nasal carriage of Staphylococcus aureus has been identified as a risk factor for community-acquired and nosocomial infections. We screened 230 donors of diverse ethnic and socioeconomic backgrounds and identified 62 (27%) whose nasal secretions were colonized by S. aureus. In 18 donors in whom the various regions of the nasal luminal surface were separately sampled, the predominant region of S. aureus colonization was the moist squamous epithelium on the septum adjacent to the nasal ostium. Nasal fluid from carriers was defective in killing endogenous S. aureus and nasal carrier isolates of S. aureus but not a laboratory S. aureus strain. Transmission electron microscopy revealed that S. aureus isolates incubated in nasal fluid from carriers for 2 h at 37°C were less damaged than those incubated in noncarrier fluid and were coated with an electron-dense layer. Compared with that from healthy donors and patients with acute rhinitis, nasal fluid from carriers contained elevated concentrations of the neutrophil-derived defensins human neutrophil peptides 1 to 3 (47- and 4-fold increases, respectively), indicative of a neutrophil-mediated inflammatory host response to S. aureus colonization. The concentration of the inducible epithelial antimicrobial peptide human β-defensin 2 was also highly elevated compared to that in healthy donors, in whom the level was below the detection limit, or patients with acute rhinitis (sixfold increase). Thus, nasal carriage of S. aureus takes hold in nasal fluid that is permissive for colonization and induces a local inflammatory response that fails to clear the colonizing bacteria.
Nasal Staphylococcus aureus carriage, affecting about 20% of the population, has been identified as a risk factor for the pathogenesis of community-acquired and nosocomial infections (5, 12). The factors that determine carrier or noncarrier status are largely unknown. Various epithelial and mucous host factors, such as surface glycoproteins and proteoglycans, have been shown to mediate the binding of S. aureus, but the precise adhesive molecules on the host and bacteria have not been identified. S. aureus appears to attach to cell-associated and cell-free secretions (10) and to interact with receptor sites of secretory immunoglobulin A (1), glycolipids (6), and surfactant protein A (8). Various bacteria, including Staphylococcus epidermidis, are capable of reducing nasal ciliary activity in vitro (4). Enhanced adhesion and diminished mucociliary clearance could explain the retention of S. aureus within the nasal passageways but not its ability to grow to a high density in this normally nonpermissive environment.
Recent studies have highlighted the innate antimicrobial properties of nasal fluid (2) and airway fluid in general (11). The current study explored the factors that contributed to S. aureus colonization in a cohort of donors with nasal S. aureus carriage.
MATERIALS AND METHODS
Identifying carriers of S. aureus.
Samples of nasal flora and nasal fluid were collected from healthy volunteer donors according to a protocol approved by the UCLA Institutional Review Board. Donors were recruited by fliers and posters placed throughout the entire UCLA campus (currently >60,000 students and faculty and staff members of diverse ages and ethnic backgrounds).
A total of 230 people were screened by swabbing the anterior 1.5 cm of the nasal vestibule of both the right and the left nares with a sterile rayon swab. Rayon swab specimens from 100 donors were either placed in Ames transport medium or plated directly on tryptic soy agar (TSA) plates supplemented with 5% sheep blood (Microdiagnostics, Lombard, Ill.) to be typed for S. aureus at a remote laboratory (MRL Pharmaceutical Services, Cypress, Calif.). The remainder of the swab specimens (130 donors) were plated directly on TSA-blood plates and typed at the University of California at Los Angeles. After 18 h of incubation at 37°C, the UCLA samples were screened for S. aureus using a BBL Staphyloslide latex rapid agglutination test (Becton Dickinson, Cockeysville, Md.), which identifies coagulase-positive and protein A-positive bacteria. Samples exhibiting numerous strongly positive colonies were submitted for confirmatory microbiological testing (UCLA Clinical Microbiology Laboratory). The agglutination test correctly identified 100% of the first 15 isolates. Thus, subsequent screenings for S. aureus carriers were done with only the Staphyloslide agglutination test.
Collection and processing of nasal fluids.
Nasal secretions were collected by vacuum-aided suction without chemical stimulation. Gentle manipulation of a narrow rubber-tipped vacuum device inside the nasal passageways stimulated nasal secretions. Secretions were stored at 4°C for prompt usage or at −20°C for longer-term storage. As shown previously (2), secretions could be stored for several months at −20°C without alteration of antimicrobial properties or protein profile. When necessary, nasal secretions were fluidized by brief (10-s) microtip sonication or with 1% N-acetylcysteine, neither of which alters the antimicrobial properties of nasal fluid (3).
Nasal topography of S. aureus colonization.
Donors with initial S. aureus carriage were reswabbed to confirm carriage and to define the topography of S. aureus colonization. Using a 1.5-mm-diameter fine rayon-tipped sterile swab (Becton Dickinson Microbiology Systems, Sparks, Md.), five distinct regions were sampled under direct vision (see Results). The tip of each swab was carefully streaked over the entire area of a TSA–5% sheep blood plate and incubated for 18 h at 37°C. S. aureus colonies were identified using the BBL Staphyloslide latex rapid agglutination test. Both the total number of bacteria and the number of S. aureus colonies were determined.
S. aureus isolation and culture conditions.
One or two isolates were obtained from the nose of each carrier. To prepare frozen master stocks of each S. aureus isolate, individual colonies originating from primary cultures were inoculated into 50 ml of tryptic soy broth (TSB) and incubated overnight at 37°C. A 300-μl portion of the overnight culture was combined with 1 ml of TSB–20% enzyme-grade glycerol (Fisher Scientific, Pittsburgh, Pa.), vortexed thoroughly, and stored at −80°C. A laboratory strain of S. aureus (7) was also used in this study. In preparation for antimicrobial assays, approximately 10 μl was scraped from the frozen stock, streaked onto TSA–blood plates, and incubated overnight at 37°C. Single colonies from these plates were inoculated into 50 ml of TSB for overnight culturing at 37°C. Each strain (500 μl of overnight culture) was subcultured 2.5 h prior to use in 50 ml of TSB to obtain midlogarithmic growth. Subcultures were then centrifuged at 1,400 × g for 10 min and washed once in Hanks balanced salt solution (HBSS; GibcoBRL). The bacteria were collected by centrifugation at 2,000 × g for 10 min and resuspended in 1 ml of HBSS. An optical density at 625 nm of 0.2 approximated 3 × 107 CFU/ml.
Antimicrobial CFU microassays.
Bacteria at an optical density at 625 nm of 0.2 were diluted 300-fold in HBSS for use in CFU microassays as described previously (3). Test samples consisted of 6 μl of bacterial dilution plus 24 μl of nasal fluid for each condition, which allowed triplicates for four time points (0, 1, 3, and 24 h of incubation). Separate tubes with 6 μl of bacteria and 24 μl of HBSS–0.013× TSB were used as controls for microbial growth. Each well of a 72-well Terasaki microtiter plate (Nalge Nunc, International, Roskilde, Denmark) was loaded with 1.5 μl of liquid wax (MJ Research, Watertown, Mass.) to prevent evaporation. Two microliters of test sample was loaded into each of 12 wells by pipetting directly underneath the liquid wax. The entire plate was incubated at 37°C with 5% CO2. At various times, wells were washed thoroughly with 46.5 μl of HBSS, and samples were diluted in HBSS if necessary and placed on ice in a microcentrifuge tube. The fluid was then hand spread on TSA plates. Plates were incubated overnight at 37°C, and colonies were counted.
Protein extraction and purification from acidified nasal fluids.
Nasal fluids were solubilized by 1:3 dilution in 10% acetic acid overnight at 4°C with gentle shaking. Fluid was twice cleared by centrifugation at 21,000 × g for 15 min, and the resulting supernatant was stored at −20°C. SepPak Vac C-18 columns (100 mg; Waters, Millford, Mass.) were wet with 10 ml of 100% methanol and washed twice with 10 ml of distilled H2O (dH2O)–0.1% trifluoroacetic acid (TFA). Clarified samples were diluted with 5 volumes of dH2O–0.1% TFA and slowly introduced into the primed column. The column was then washed twice with 10 ml of dH2O–0.1% TFA. Samples were eluted with 1 ml of 60% acetonitrile–0.1% TFA, vacuum dried to remove organic solvent, and resuspended in dH2O to the original nasal fluid volume for analysis by a sandwich enzyme-linked immunosorbent assay (ELISA).
Sandwich ELISA.
The protocol for the sandwich ELISA was modified from an established ELISA protocol (Pierce, Rockford, Ill). In brief, monoclonal antibody to human β-defensin 1 (anti-HBD-1), HBD-2 (anti-HBD-2), or human neutrophil peptides 1 to 3 (anti-HNP-1, anti-HNP-2, and anti-HNP-3) was diluted 1:5,000 in coating buffer (0.2 M sodium carbonate-bicarbonate, pH 9.4), added to 96-well polystyrene microtiter plates, and incubated overnight at 4°C. Wells were washed three times with dH2O and incubated in 1% bovine serum albumin–phosphate-buffered saline blocking bluffer for 1 h at room temperature (RT). Standards (HBD-1, HBD-2, or HNP-1, HNP-2, and HNP-3) and samples were subsequently diluted in blocking buffer and incubated at RT for 1 h. Wells were washed as before, 1:2,000-diluted secondary rabbit polyclonal antibody (anti-HBD-1, anti-HBD-2, or anti-HNP-1, anti-HNP-1, and anti-HNP-3) was added, and plates were incubated at RT for 2 h. After the wells were washed, goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (diluted 1:2,000 in blocking buffer) was added and incubated for 1.5 h at RT. O-Phenylenediamine–H2O2 developing solution was added, and plates were incubated for 10 min at RT. The reaction was stopped by the addition of 0.5 volume of 2.5 M H2SO4. Plates were immediately quantified at 492 nm with a SpectraMax 250 spectrophotometer (Molecular Devices, Sunnyvale, Calif.).
Transmission electron microscopy.
S. aureus clinical isolates were cultured and subcultured as described above. Bacteria (109 to 1010 CFU/ml) were incubated in unmanipulated nasal fluid (carrier or noncarrier) or HBSS–0.067× TSB (control) for 2 h at 1,000 rpm in an Eppendorf Thermomixer R (Brinkmann Instruments, Inc., Westbury, N.Y.). Bacteria were recovered by 10 min of centrifugation at 21,000 × g and immediately placed in 2% glutaraldehyde in a buffer solution of 0.08 M sodium cacodylate and 0.2% CaCl2 (pH 7.3). The samples were then washed in the buffer solution for 30 min, dehydrated in a graded ethanol series (50, 75, 95, and 100%) for 15 min each, and embedded in LR-White (Ted Pella, Redding, Calif.). Thin sections were viewed and photographed at 80 keV on a JEOL model 100XC electron microscope.
Statistical analyses.
Antimicrobial assays were performed at least in triplicate for each independent experiment. Analyses of the frequency of carriage in subgroups were done with the z test to compare the carriage frequency for a subgroup to that for the total population (SigmaStat; SPSS Inc., Chicago, Ill.). Sets of independent CFU and ELISA experiments were compared with a Student t test (SigmaStat). Error bars represent the standard error of the mean (SEM).
RESULTS
Population analysis of nasal S. aureus carriers.
Screening of 230 volunteers identified 62 donors (27%) whose nasal vestibules were colonized with S. aureus (Table 1). Analysis of carriage by race and gender indicated that only the white male and total white subgroups had significantly higher percentages of carriers than the total sampled population (P < 0.001).
TABLE 1.
Prevalence of S. aureus nasal carriers
| Race | No. of donors (% of staphylococcus carriers) in the following group:
|
||
|---|---|---|---|
| Female | Male | Total | |
| American Indian or Alaskan Native | 0 (0) | 0 (0) | 0 (0) |
| Asian or Pacific Islander | 31 (19) | 46 (15) | 77 (17) |
| Black, not of Hispanic origin | 6 (17) | 3 (67) | 9 (33) |
| Hispanic | 8 (25) | 12 (50) | 20 (40) |
| White, not of Hispanic origin | 23 (39) | 27 (67) | 50 (54) |
| Other or unknown | 33 (18) | 41 (12) | 74 (15) |
| Total | 101 (24) | 129 (29) | 230 (27) |
S. aureus predominantly colonizes the moist squamous epithelium of the anterior nasal vestibule.
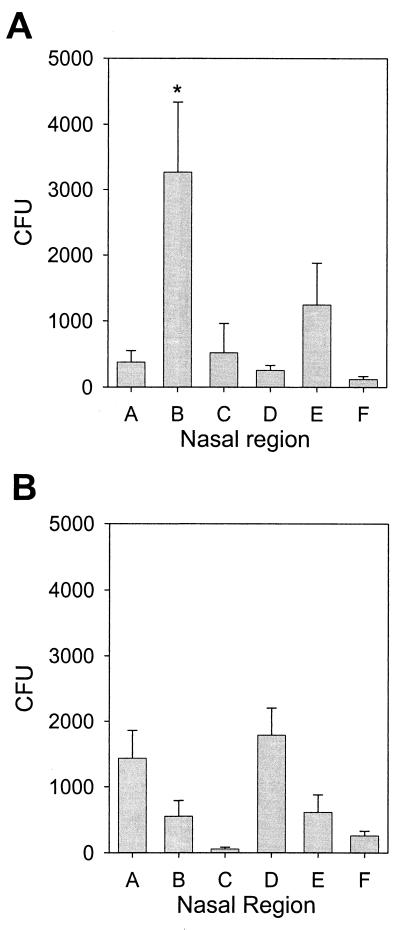
To determine if S. aureus preferentially colonized a specific area(s) of the nasal vestibule, bacteria were obtained with swabs, typed (S. aureus or coagulase-negative staphylococci), and quantified from five distinct regions in the nostrils of 18 donors: region A, the skin of the nasal septum; region B, moist squamous epithelium on the septum adjacent to the nasal ostium; region C, mucus-covered nonciliated epithelium deeper on the septum; region D, anterior, hair-covered epidermal portion of the lateral wall; and region E, deeper, hairless area of the lateral wall. In addition, bacteria were quantified from three clipped nose hairs (region F). As expected, coagulase-negative bacteria were found colonizing regions A and D, septal and lateral epidermal regions, respectively (Fig. 1B). However, S. aureus was localized primarily to one area, with >60% of bacteria colonizing region B (Fig. 1A). It is possible that in carriers, the epithelial cells in this area are altered and thus selectively permit the binding and colonization of S. aureus. S. aureus colonization could also result from nasal fluid being deficient in antimicrobial activity, since region B is moist but devoid of cilia that would sweep the bacteria toward the back of the nasal cavity.
FIG. 1.
S. aureus predominantly colonizes the moist squamous epithelium adjacent to the nasal ostium. The right nasal vestibule of each donor was swabbed in five distinct regions, A to E; in addition, bacteria were quantified from three clipped nose hairs (region F). The swab specimens were plated on TSA–5% sheep blood, and S. aureus CFU (A) and coagulase-negative staphylococcus CFU (B) were quantified for each region (18 donors). The majority (>60%) of S. aureus bacteria were isolated from region B (asterisk). Error bars represent the SEM.
Carrier nasal fluid supports the growth of carrier isolates of S. aureus.
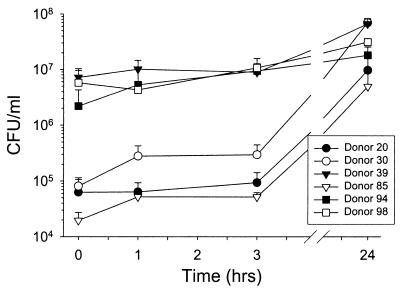
The ability of nasal secretions to support the growth of bacteria ex vivo was assayed with indigenous S. aureus. Minimally manipulated carrier nasal secretions were incubated at 37°C, and microbial counts were determined using CFU microassays (Fig. 2). By 24 h, all samples showed S. aureus CFU higher than initial counts (P < 0.01). Strains other than S. aureus were rarely observed at 24 h.
FIG. 2.
Nasal fluid from carriers supports the growth of endogenous S. aureus. Nasal fluid from Staphylococcus carriers was tested in a CFU microassay to measure the growth of endogenous bacteria. Error bars represent the SEM.
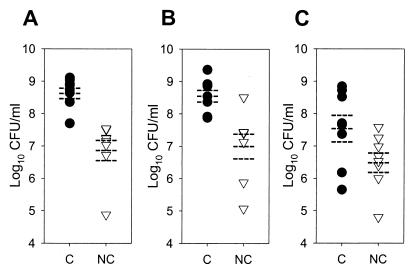
We next explored whether a similar effect would be observed with exogenously added laboratory strains and clinical isolates of S. aureus. Carrier (n = 8) and noncarrier (n = 8) nasal secretions were gamma irradiated (18 krads) to destroy the native microbiota. An inoculum of 105 S. aureus CFU/ml was used to model a host defense challenge in heavily colonized carriers (Fig. 2). In individual experiments, microbe-free nasal fluids were tested in CFU microassays with (i) an isolate of S. aureus from the carrier fluid donor; (ii) an isolate of S. aureus from another, randomly selected carrier; and (iii) a laboratory strain of S. aureus. At 24 h, S. aureus originally isolated from the carrier's own nose grew to significantly higher concentrations in carrier fluid than in noncarrier fluid (Fig. 3A) (P = 0.00016). Similar results were obtained with the S. aureus isolate from the random carrier (Fig. 3B) (P = 0.002). However, the laboratory strain of S. aureus grew less well in nasal fluid from carriers, so that the difference in CFU at 24 h between carrier fluid and noncarrier fluid did not reach significance (Fig. 3C) (P = 0.059). Thus, in addition to the increased ability of carrier fluid versus noncarrier fluid to support the growth of S. aureus, there may also be some adaptation or selection of the nasal strains for the carrier environment.
FIG. 3.
Nasal fluid from carriers supports the growth of S. aureus isolates. Nasal fluids from S. aureus carriers (C; solid circles) and healthy noncarriers (NC; open triangles) were tested in CFU microassays with an isolate of S. aureus from the donor's own nose (A); an isolate of S. aureus from another, randomly selected carrier (B); and a laboratory strain of S. aureus (C) (eight donors for each condition, with four or five replicates per condition). Each point represents the log10 CFU per milliliter at 24 h. Broken lines indicate the mean and SEM. Compared to noncarrier fluid, carrier fluid permitted more rapid growth of isolates derived from either the same (P = 0.00016) or a different (P = 0.002) carrier. In contrast, the difference in the growth of the laboratory strain of S. aureus in carrier versus noncarrier fluids did not reach significance (P = 0.059).
Carrier nasal fluid causes less ultrastructural damage to S. aureus than noncarrier nasal fluid.
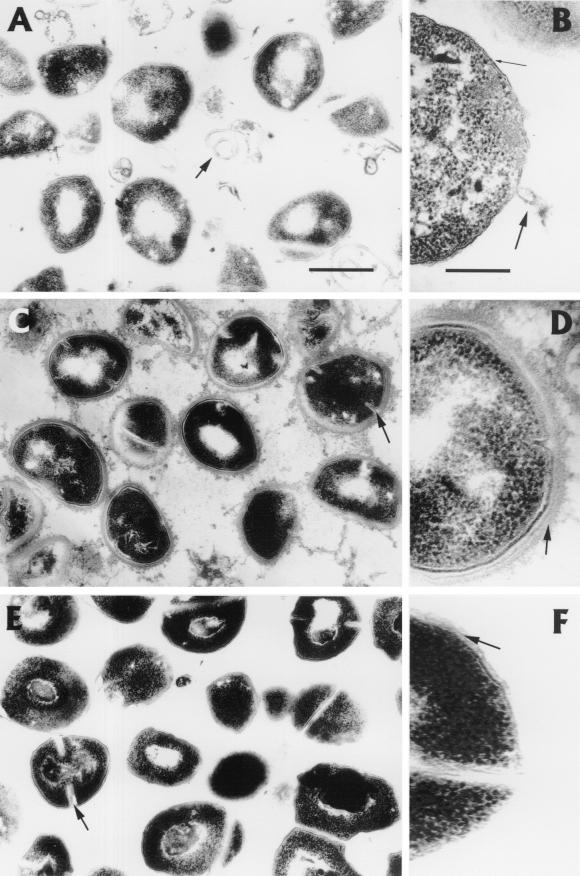
Next we asked whether differences in S. aureus growth in carrier fluid versus noncarrier fluid correlate with ultrastructural changes in the bacteria. Transmission electron microscopy was performed on S. aureus nasal isolates incubated with gamma-irradiated (18 krads) carrier and noncarrier nasal fluids for 2 h at 37°C. S. aureus (isolated from donor 20) incubated with noncarrier fluid (donor 530) exhibited signs of damage: peptidoglycan denudation, membrane rupture, and the formation of membrane ghosts (Fig. 4A and B). S. aureus incubated with carrier fluid (donor 30) was intact or only minimally damaged (Fig. 4C and D). Comparable bacterial morphologies were also seen with another set of nasal fluids (carrier, donor 20; noncarrier, donor 9; isolate from donor 20) (data not shown). In contrast, bacteria incubated for 2 h in HBSS-TSB remained intact (Fig. 4E and F), and S. aureus added to noncarrier fluid, carrier fluid, or buffer and immediately fixed in gluteraldehyde was undamaged (data not shown). Also notable was an additional electron-dense layer covering the peptidoglycan of S. aureus incubated with carrier fluid (Fig. 4D). Although the composition of this layer was similar in density and form to intercellular debris, it was not clear whether this coating was derived from the host fluid or was a bacterial product.
FIG. 4.
Ultrastructural effects of carrier and noncarrier fluids on S. aureus. S. aureus was incubated with unmanipulated noncarrier fluid (A, overview; B, detail), carrier fluid (C and D), or HBSS-TSB control (E and F) for 2 h at 37°C. Note the effects of noncarrier fluid, including membrane ghosts of S. aureus (A, arrow), the denudation of peptidoglycan (B, thin arrow), and membrane rupture (B, thick arrow). The appearance of intact peptidoglycan is shown by the arrow in panel F. Arrows in panels C (carrier fluid) and E (control) indicate clefts of actively dividing cells, which are less prominent or absent in noncarrier fluid (A). S. aureus exposed to carrier fluid was not damaged (C), and an additional, possibly protective layer surrounds peptidoglycan (D, arrow). Overview bar, ∼500 nm; detail bar, ∼100 nm.
Nasal secretions from carriers contain markers of inflammation.
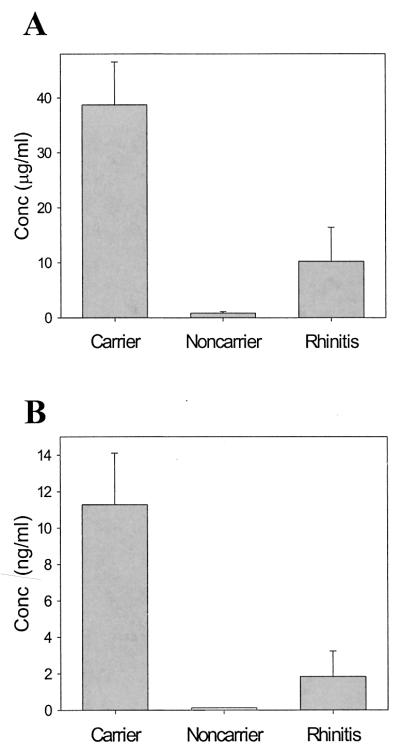
To examine the innate host defense response to S. aureus colonization, the concentrations of antimicrobial peptides were quantified for fluids from three types of donors: normal donors (healthy, noncarrier), donors with acute rhinitis, and persistently colonized carriers. Persistently colonized carriers tested positive for nasal S. aureus in 100% of cultures (n = 3 to 9 cultures per donor; Staphyloslide test) during the 6-month period prior to collection of nasal fluid for peptide analyses (data not shown). Three types of human antimicrobial peptides were analyzed by a quantitative sandwich ELISA: the α-defensins HNP-1 to HNP-3 as markers of neutrophil accumulation, the β-defensin HBD-2 as an inducible marker of epithelial host defense, and the β-defensin HBD-1 as a constitutively expressed antimicrobial peptide. The concentrations of HNP-1 to HNP-3 were nearly 4-fold higher in fluid from Staphylococcus carriers than in patients with acute rhinitis (P = 0.029) (Fig. 5A) and 40-fold higher than in normal donors (P = 0.009) (Fig. 5A). The levels of HBD-2 were nearly sixfold higher in Staphylococcus carriers than in donors with rhinitis (P = 0.044) (Fig. 5B). HBD-2 levels in normal donors were below the limit of detection (<0.125 ng/ml). The noninducible HBD-1 was not detected in the three groups (<0.125 ng/ml) (data not shown). The increased concentrations of neutrophil and inducible epithelial antimicrobial peptides are evidence of an inflammatory response to colonization.
FIG. 5.
Concentrations of neutrophil-derived and epithelial defensins are elevated in carrier nasal fluid. Concentrations (Conc) of the antimicrobial peptides HNP-1 to HNP-3 (A) and HBD-2 (B) are shown for carriers, noncarriers (normal, healthy donors), and patients with acute rhinitis. Levels of HNP-1 to HNP-3 were significantly higher in carriers than in acute rhinitis donors (P = 0.029) and noncarriers (P = 0.009). Similarly, the level of HBD-2 was higher in carriers than in rhinitis donors (P = 0.044). Noncarriers had an HBD-2 concentration below the limit of detection (<0.125 ng/ml). Error bars represent the SEM.
DISCUSSION
The nasal vestibule has a complex epithelial surface that can be histologically classified into five distinct regions (9). These regions range from an anterior hairy epidermis continuous with the skin to a moist pseudostratified columnar layer characterized by many ciliated cells which transport the mucus and entrapped particles to the pharynx, where they are swallowed. Consequently, in a normal individual, the fluid layer turns over every 10 to 20 min and must be replenished by secretions of submucosal glands. We have shown that in nasal carriers, the highest concentrations of S. aureus are found immediately distal to the anterior hairy epidermis in a moist squamous epithelium devoid of hair, cilia, and microvilli (Fig. 1, region B). This localization may be due to the lack of ciliary clearance of nasal fluid from this area, so that its resistance to colonization depends largely on the intrinsic antimicrobial properties of the nasal fluid. Evidence was presented that in carriers, the intrinsic antimicrobial activity of nasal fluid for S. aureus is impaired. We conclude that the combination of defective nasal fluid and the lack of alternative mechanical clearance mechanisms results in the colonization of region B in carriers of S. aureus.
The host defense response to S. aureus colonization had not been previously studied. Surprisingly, we found that colonization by S. aureus is accompanied by the release of epithelial and neutrophil-derived host defense peptides into nasal secretions. The high concentrations of HNP-1 to HNP-3 present in carrier nasal secretions indicate that neutrophils are recruited in response to S. aureus colonization. Light microscopic analysis of Wright-stained nasal fluid confirmed that neutrophils were present in carrier and acute rhinitis donor fluids but not in fluid from normal donors (data not shown). Apparently, neither recruited neutrophils nor epithelial products can eliminate S. aureus in the mucus of carriers.
The current study suggests that bacterial factors may also promote colonization by S. aureus. An electron-dense material that coats and possibly protects the bacterial surface was observed on S. aureus incubated with carrier fluid. More importantly, the origin of S. aureus that was used in CFU microassays influenced bacterial proliferation in carrier nasal fluid. All S. aureus nasal isolates, presumably selected for survival in carriers or adapted to the nasal carrier environment, grew better in carrier nasal fluid than did a standard laboratory strain originally isolated from a urinary catheter. Bacterial resistance mechanisms, together with host-associated determinants, may contribute to nasal colonization by S. aureus.
In order to collect useful amounts of nasal fluid, mild mechanical stimulation was used. It is possible that the concentrated resting fluid of both carriers and noncarriers is more potently antimicrobial and thus less supportive of bacterial growth than the fluid collected by mechanical stimulation. Nevertheless, this study identified an underlying difference between carriers and noncarriers. The molecular basis of the defects that make S. aureus carriers susceptible to nasal colonization remains unknown. Based on our data, biochemical analysis of nasal fluid may hold the key to identifying the primary cause of this condition and to developing more specific interventions to control this important source of nosocomial infections.
ACKNOWLEDGMENTS
We thank IntraBiotics, Inc. (Sunnyvale, Calif.) for funding diagnostic tests through MRL Pharmaceutical Services. This work was supported by grants P50 HL67665, HL46809, and AI48167 from the National Institutes of Health (to T.G.), by grants from the Cystic Fibrosis Foundation and Cystic Fibrosis Research, Inc. (to T.G.), and by a postdoctoral fellowship from the American Lung Association (to A.M.C.).
REFERENCES
- 1.Biesbrock A R, Reddy M S, Levine M J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole A M, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole A M, Wu M, Kim Y-H, Ganz T. High resolution techniques for analyzing antimicrobial properties of scant human fluids. J Microbiol Methods. 2000;41:135–143. doi: 10.1016/s0167-7012(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson J L, McCaffrey T V, Kern E B, Martin W J. The effects of sinus bacteria on human ciliated nasal epithelium in vitro. Otolaryngol Head Neck Surg. 1988;98:299–304. doi: 10.1177/019459988809800405. [DOI] [PubMed] [Google Scholar]
- 5.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1–4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I H, Cho Y, Lehrer R I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect Immun. 1997;65:2898–2903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeely T B, Coonrod J D. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Mygind N, Pedersen M, Nielsen M H. Morphology of the upper airway epithelium. In: Proctor D F, Andersen I, editors. The nose: upper airway physiology and the atmospheric environment. New York, N.Y: Elsevier Biomedical Press; 1982. pp. 71–97. [Google Scholar]
- 10.Shuter J, Hatcher V B, Lowy F D. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis S M, Conway B A, Zabner J, Smith J J, Anderson N N, Singh P K, Greenberg E P, Welsh M J. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 12.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]