Abstract
Three major approaches of cancer therapy can be enunciated as delivery of biotherapeutics, tumor image analysis, and immunotherapy. Liposomes, artificial fat bubbles, are long known for their capacity to encapsulate a diverse range of bioactive molecules and release the payload in a sustained, stimuli-responsive manner. They have already been widely explored as a delivery vehicle for therapeutic drugs as well as imaging agents. They are also extensively being used in cancer immunotherapy. On the other hand, exosomes are naturally occurring nanosized extracellular vesicles that serve an important role in cell–cell communication. Importantly, the exosomes also have proven their capability to carry an array of active pharmaceuticals and diagnostic molecules to the tumor cells. Exosomes, being enriched with tumor antigens, have numerous immunomodulatory effects. Much to our intrigue, in recent times, efforts have been directed toward developing smart, bioengineered, exosome-liposome hybrid nanovesicles, which are augmented by the benefits of both vesicular systems. This review attempts to summarize the contemporary developments in the use of exosome and liposome toward cancer diagnosis, therapy, as a vehicle for drug delivery, diagnostic carrier for tumor imaging, and cancer immunotherapy. We shall also briefly reflect upon the recent advancements of the exosome-liposome hybrids in cancer therapy. Finally, we put forward future directions for the use of exosome/liposome and/or hybrid nanocarriers for accurate diagnosis and personalized therapies for cancers.
Keywords: extracellular vesicles (EVs), liposome, exosome-liposome hybrid, drug delivery, liquid biopsy, immunotherapy
Introduction
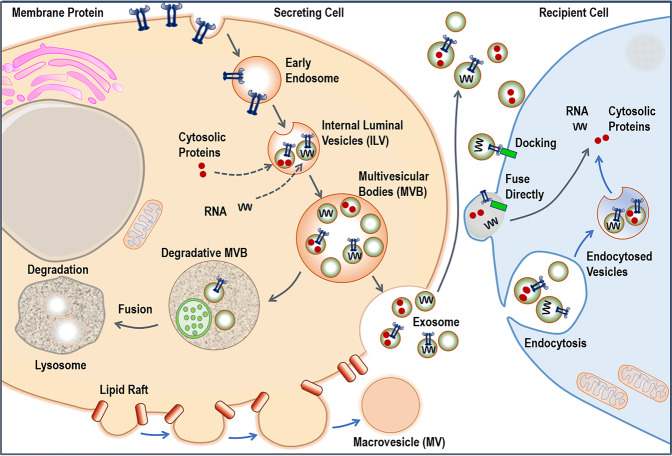
A rising number of research studies are being conducted to look at the function of cell-secreted membrane-bound vesicles to better understand the pathophysiology of cancer and develop therapeutic targets for patient care and cure. “Extracellular vesicles” (EVs) are defined as extracellular mobile, membrane-limited cell-derived vesicles released in the extracellular space. Considering their biogenesis, size, and membrane composition, they can be sub-categorized into three major groups: micro-vesicles (MVs), apoptotic bodies (ABs), and exosomes [1, 2]. Though secreted membrane vesicles’ functional existence was shrouded by skepticism, of late, their existence and role in regulating diverse biological functions have been well documented. Exosomes are evocative of their respective parent cells and contain the parent cell’s physiological state-specific proteins (e.g., transcription factors, surface receptors, heat-shock proteins, lipids, and nucleic acids (including DNA, mRNA, miRNA, and noncoding RNAs) [3]. Current research interest in the field primarily focuses on getting a deeper insight into the tumor by studying tumor cell-derived exosomes and EVs released into the blood and other body fluids. An exosome is a “nanosphere” and serves as a potential source of cancer biomarkers [1]. “Exosome” refers to EVs (30–150 nm in diameter), and their biogenesis occurs by the fusion of multivesicular bodies (MVBs) to the cell’s plasma membrane (Fig. 1). Exosomes are produced and secreted from all eukaryotic cell types so far tested and found in most body fluids, including serum/plasma, saliva, cerebrospinal fluid, breast milk, and urine and play a significant role in intercellular communication [1, 2]. The bilayer structure of exosomes comprises of different lipids like cholesterol, phosphatidylcholine, phosphatidylserine, ceramide, saturated fatty acids, etc. Exosomes differ from MVs and ABs in that they originate from the endocytotic compartment and are nanometric (<150 nm) in scale. Exosomes and EVs potentially serve as a noninvasive source of cancer-related information and are thereby widely explored as diagnostic and therapeutic agents [4].
Fig. 1. Exosomes are formed from late endosomes and are formed by the inward budding of the multivesicular body (MVB) membrane.
The ESCRT machinery is critical for the formation of exosomes. During exosome biogenesis, ESCRT-independent functions are played by nSMase2 and members of the RAB GTPase family. Micro-vesicles are formed by the plasma membrane budding, which is controlled by cytoskeletal and regulatory proteins. Phosphatidylserine (PS) and phosphatidylethanolamine (PEA) are homogeneously expressed throughout their membrane (PE). The development of apoptotic bodies occurs during the event of apoptosis. These vesicles are irregular in size and form, and they comprise nuclear fractions and cytoplasmic organelles, as well as phosphatidylserine in large quantities on their membrane. Exosomes are internalized by cells directly through a variety of pathways, including by phagocytosis, plasma membrane fusion, macropinocytosis, and endocytosis. Exosomes may have a significant impact on cellular processes by participating in genetic/protein transition, transcriptional control, and post-transcriptional regulation. Alternatively, exosomes can fuse with the lysosomes for degradation.
On the other hand, liposomes are artificial fat bubbles that constitute of a bilayer structure spontaneously achieved when natural or synthetic amphipathic lipids are dispersed into water. Since their inception, they have primarily been exploited as drug delivery vehicles because of biocompatibility and a favorable safety profile. Polyethylene glycol (PEG) may be coated on their surface to alter their half-life in the bloodstream [5]. Two major liposomal Doxorubicin (Dox), Doxil and Myocet, got Food and Drug Administration (FDA) clearance in 1995 and 1999, respectively, followed by several more in the category [6]. Several liposomal therapeutics have been clinically authorized for use in the market. According to recent studies, commercialized liposomal medicines improved overall survival (OS) compared to the parent drug [7, 8].
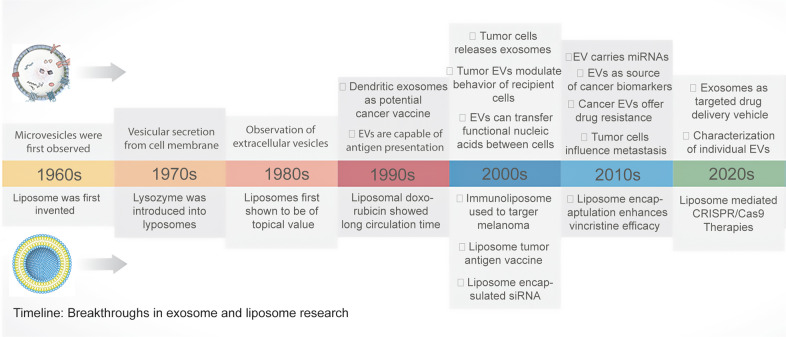
In recent years, numerous studies have focused on exploring the drug delivery potential of exosomes and liposomes, ranging from small molecules (paclitaxel (PTX), Dox, curcumin, etc.) to a variety of large molecules (siRNA, miRNA, proteins, etc.) [9]. The history of exosomes and liposomes has been an intellectually stimulating adventure and is summarized in Fig. 2. To harness unique ascendancies of liposomes and exosomes and overcome limitations related to restricted circulation time, structural breakdown, and cargo leakage, a new generation of a delivery system termed exosomes-liposome hybrids, has evolved that attracted significant attention. This review attempts to summarize recent accomplishments in using liposome, exosome, and bioengineered hybrid nanovesicles in cancer therapy, the pitfalls, and future possibilities.
Fig. 2. Timeline: Breakthroughs in exosome and liposome research.
The timeline and significant milestones in the progress of extracellular vesicle (EV) and liposome research in cancer drug delivery, detection and therapy. siRNA small interfering RNA, CRISPR clustered regularly interspaced short palindromic repeats.
Formation and composition
Defining the formation, composition, and functions of exosomes and liposomes can help understand how they can be better used in cancer therapy. Understanding the similarity of the nanosized, naturally occurring exosomes and their artificial mimic liposomes can help establish advanced platforms like engineered exosomes, exosome-mimetics, and exosome-liposome hybrids. The formation and composition of both the exosomes and liposomes are hereby discussed.
Exosomes
In 1983, Harding et al. and Pan et al. published two separate papers within a week and described transferrin receptor-linked small (~50 nM) vesicles being exuded from the reticulocyte cells to the extracellular space and therefore named “exosomes”. The term “exosome” was used by Canadian scientist Rose Johnstone but Trams et al., in 1981 and Mitchell et al., in 1997 also used the term for describing other membrane fragments [10]. Exosome formation is generally divided into three stages: (1) The development of ILVs and exosome biosynthesis inside MVBs, (2) MVB trafficking, and (3) fusion with the mother cell’s plasma membrane, resulting in the release of ILVs and exosomes through exocytosis [2] (Fig. 1). First, endocytic vesicles are generated from the plasma membrane, resulting in an early endosome, which matures into late endosomes during the second stage of the process late endosome’s limiting membranes bulge inward, resulting in the formation of vesicles inside the lumen. MVBs are the accumulation of these ILVs within the late endosomes. The creation of MVBs may be explained by two distinct routes, one is endosomal sorting complexes required for transport (ESCRT)-dependent, and the other one is ESCRT-independent. MVBs may either fuse with lysosomes for destruction or with the cell’s plasma membrane, releasing ILVs as exosomes into the extracellular area [11].
Approximately 98,769 proteins and 1116 lipids have been discovered to be linked with exosomes, according to ExoCarta (http://www.exocarta.org/), an exosome database [12]. Exosomes include proteins such as heat shock proteins (Hsp70 and Hsp90), membrane transport and fusion proteins (GTPases, Annexins, and flotillin), and many tetraspanins (CD9, CD63, CD81, and CD82) since they originate from the intracellular endosomal component [11]. Exosomes cargo includes a number of heat shock proteins, annexins, and Rab family proteins, which are involved in their intracellular formation and trafficking. Exosomes frequently contain tetraspanins, a transmembrane protein family. Tetraspanins have a role in cell fusion, motility, cell–cell adhesion, and intercellular communication. However, their function in exosomes is poorly understood [2]. Integrins, which are adhesion molecules that allow cell attachment to the extracellular matrix, are another common protein present in exosomes. Exosomal integrins are important in the adhesion of exosomes to their target cells [13]. These proteins are considered as molecular markers to detect exosomes. Elevated exosome secretion may be associated with malignancy and genotoxic stress [14]. Exosome production may be favorably modulated in tumor cells with aberrant signal transduction pathways, particularly those connected to p53 response elements like Steap3 [15].
Liposome
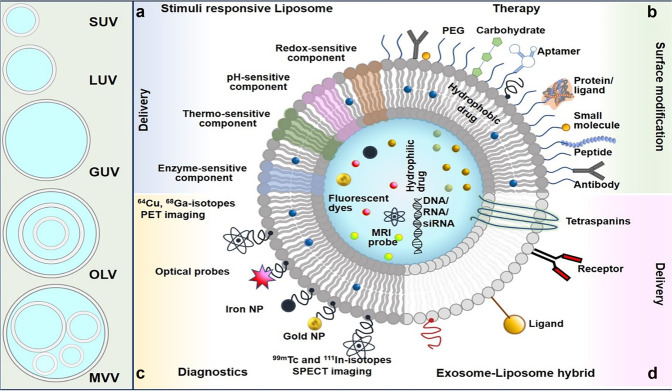
Liposomes are artificial nanosized vesicular systems with an aqueous center and encapsulating phospholipid bilayers. Liposomes have undergone substantial research as a drug delivery mechanism (DDS) for enhancing the delivery, safety, and efficacy of therapeutic molecules such as drugs, proteins, vaccines, enzymes, oligonucleotides, genetic material, and other biomolecules [9]. The size and lamellarity of the liposome can have a big impact on the half-life of liposomal formulations and the amount of encapsulated active pharmaceutical ingredients (API). Depending on the size and lamellarity of liposomes, they are categorized as unilamellar or multilamellar. Unilamellar vesicles can be small (20–100 nm), large (100–250 nm), and giant (>1000 nm). The other types includes, the Oligolamellar vesicles (2–5 concentric phospholipid layers; 100–1000 nm), multilamellar (> 5 concentric phospholipid layers; 1–5 µm), and multivesicular liposomes (500 nm–5 µm; with discontinuous non-concentric compartments filled with water) (Fig. 3) [16, 17]. Liposomes may also be categorized depending on their in vivo applications, such as conventional, sterically stabilized, or ligand-targeted, stimuli-responsive, stealth liposomes, catatonic, antibody-targeted liposomes, and so on.
Fig. 3. Graphical presentation of the liposome as a theranostic nano-platform.
Left-hand panel: liposomes are classified depending on their size and lamellarity. Right-hand panel: the schematic shows the many permutations that may be utilized to create multifunctional liposomes, which can subsequently be employed for theranostic applications such as cargo transport, tumor targeting, and diagnostics. a Liposomes with stimuli-responsive structures. b Surface changes, including PEGylated liposomes, to create targeted liposomes. c Tumor screening diagnostics applications. d Hybrids of exosomes and liposomes for tumor targeting. Reproduced from Madamsetty et al. [144]. DOI: 10.1016/C2019-0-02790-2 Copyright © 2021 Elsevier Inc. In Press.
Though liposomes are a secure and efficient way to deliver therapeutic agents, they often suffer from opsonization. The mononuclear phagocyte system (MPS) or the reticuloendothelial system clears conventional liposomes from the bloodstream by binding with Opsonins (serum proteins) [18]. Conventional liposomes, also known as first-generation liposomes, are made up of cationic, anionic, or acidic phospholipids in conjunction with cholesterol and have been shown to improve the therapeutic index of APIs like Dox [19, 20]. Stealth liposomes or PEGylated liposomes are made by using a hydrophilic polymer, such as PEG, is applied to the liposomal surface to shield the liposomes from serum proteins [21]. Tumors overexpress particular receptors or ligands and, therefore, can be attacked with specific binding partners such as antibodies, polypeptides, proteins, and other molecules. Liposomal surface engineering with different ligands, such as protein, peptide, aptamer, and small molecule, could achieve targeted drug delivery (as shown in Fig. 3). Immune-liposomes characterized by the liposomal-surface coupling of a monoclonal antibody, specific for a particular tumor antigen are among the most specific approaches in targeting tumors. Since immune liposomes have reduced in vivo effectiveness, newer generations of liposomes are engineered utilizing a hybrid design strategy to improve tissue targeting and precise drug delivery [22].
Though Liposome and Micelle are excellent vehicles for therapeutic delivery but there exist some structural differences between liposomes and micelles. Liposomes are made up of bilayers of amphiphilic lipids which cocoon an aqueous interior from the external bulk aqueous phase. The non-polar hydrophobic lipid tails reside close to each other by a stabilizing van der waals force while hydrophilic head groups divulge outwards in the aqueous phase and are stabilized by polar interactions. Thin lipid-film hydration, ethanol injection are a few well-known methods of nanometric liposome synthesis. Clearly, in this structural orientation, hydrophilic drugs can be entrapped within the aqueous interior while hydrophobic drug molecules can be encapsulated in the lipid bilayer. Drug encapsulation is followed by a protracted circulation time and sustained systemic release of the therapeutic entities [23]. On the other hand, micelles are composed of closed lipid monolayers with a non-polar lipid tail core and the polar surface of the head group as a shell. There also exist some micelles where the polar core is observed with lipid tail on the surface, they are known as an inverted micelle. It has been observed that block-copolymers can aggregate in a micellar structure when dispersed in an aqueous phase. In nanotechnology, various methods such as nanoprecipitation or double emulsion have been described to synthesize polymeric NPs. These micellar self-assemblies of biodegradable amphiphilic are well adequate for systemic administration. The benefit derived from this core-shell structural orientation is the encapsulation of hydrophobic molecules with the sustained-release property having prolonged circulation time [24].
Interestingly, surface modification with PEG for both the delivery systems has drawn significant attention of the formulators worldwide. This actually improved the pharmacokinetic profiles of the active ingredients. Many FDA-approved and successfully commercialized liposomes contain DSPE-PEG as a co-polymer in the delivery system which was shown to render protection from the Kupffer cells. A polymeric NP, PLGA-mPEG based PTX delivery system (Genexol-PM), has also been approved by EMA for metastatic breast cancer therapy which is being used in Korea and the European Union [25]. It is worth mentioning here that the addition of DSPE-PEG (2000) in liposomes should be limited up to a particular concentration. The excess amount of the presence of the co-polymer DSPE-PEG (2000) breaks down the bilayer into an intermediate discoidal structure and further excess can lead to micelle formation [26].
Cancer drug delivery
Traditional chemotherapy exhibited some effectiveness, but its significant disadvantages include low bioavailability, high dosage requirement, adverse side effects, low therapeutic indices, multiple drug resistance generation, and non-specific targeting. Hence, an efficient drug delivery vehicle is needed to overcome these delivery-related challenges and move medications to the target sites for effective clinical intervention. To date, a significant number of nanotechnology-based products have demonstrated potential, including liposomes, polymeric nanoparticles (NP), albumin NPs, and inorganic NPs, and few among them have already gained access to clinical use [22]. Exosomes, packed with biological and chemical cargos, act as natural drug delivery carriers and currently being used to target tumor cells and are considered a potential alternative to artificial NPs. The use of exosomes as drug delivery vectors for cancer is described here, emphasizing on small molecule chemotherapeutics, proteins, and nucleic acid.
Exosome as a drug delivery vehicle
Owing to their endogenous origin, low immunogenicity, the innate ability to cross the blood-brain barrier (BBB), high target specificity, and biocompatibility, the therapeutic potential of exosome-mediated drug delivery has also been investigated by numerous research teams across the world [2, 3]. The recent advances are summarized in the following section.
Exosomal delivery of small molecule
The uptake of exosomes by specific recipient tumor cells is mediated by exosomal surface proteins and this can control successful drug delivery and distribution. Shrivastava et al. created an exo-Gold NP-based therapeutic delivery method for Dox, named “nanosomes” for lung cancer treatment. The data demonstrated the therapeutic effectiveness of nanosomes and effectual Dox distribution in H1299 and A549 non-small cell lung cancer cells [27]. In the last two decades, global efforts were witnessed toward exhibiting ramifications of curcumin treatment, including anti-inflammatory, antineoplastic, antioxidant, and chemo-preventive activity, both in vitro and in vivo [28]. Sun et al. isolated EL-4 cell-derived exosomes using sucrose gradient centrifugation and introduced curcumin into the exosomes, which enhanced solubility, curcumin stability in vitro, and its bioavailability, in vivo. In an LPS-induced murine septic shock model, curcumin, loaded and delivered in exosomes, showed an enhanced anti-inflammatory effect, protecting the mice, compared to its curcumin-free empty counterpart. They further showed that exosomal curcumin diminished the number of CD11b+Gr-1+ cells, which is increased upon LPS-shock [29]. The BBB is formed by a compact network of endothelial cells, astrocytes, and pericytes. The BBB inhibits many chemotherapeutic drugs from entering the brain. To breach the BBB, Yang et al. isolated exosomes from glioblastoma and brain endothelial cells, incorporated PTX and Dox into them, and demonstrated their successful transport across BBB in a zebrafish model [30]. In another study, Tian et al. isolated exosomes from mouse immature dendritic cells (DCs) pulsed with a plasmid construct to express Lamp2b (exosomal membrane protein) fused with αv integrin targeting iRGD peptide. This was followed by Dox loading in engineered exosomes through electroporation. Intravenous administration of Dox-loaded exosome in a MDA-MB-231 transplanted nude Balb/c murine model showed effective tumor growth inhibition [31]. Recently McAndrews et al. developed engineered exosomes loaded with “small molecule STING agonist cyclic GMP-AMP” (iExoSTINGa). Exosome-mediated transmission of STING agonist suppresses B16F10 subcutaneous tumor development and boosts antitumor immunity [32].
Exosomal delivery of protein
Engineered exosomes are ideal for delivering small molecules, genes, or proteins intracellularly to reprogram targeted cells and can be effectively used for tumor targeting. Surface engineering of exosomes by genetic modification and chemical alteration is done with the apparent aim of enhancing targeting precision. Many tumors overexpress a “don’t eat me” signal (CD47) that interacts with phagocytic cell’s signal regulatory protein (SIRP). CD47 prevents macrophage’s capacity to engulf tumor cells by activating SIRP. Koh et al. engineered exosomes harboring SIRPα variants (SIRPα-exosomes). SIRPα-exosomes could disrupt CD47-SIRP interaction, resulting in tumor cell death through macrophage-mediated phagocytosis [33]. Many other groups have demonstrated the broad potential of exosomal membrane-associated protein therapeutics, and the strategy can be employed to treat cancer.
Exosomal delivery of siRNA/miRNA
RNA-interference (RNAi) is a sequence-specific, unique spatiotemporal, post-transcriptional gene silencing process that has been preserved throughout evolution. Since the discovery of RNAi in cultured mammalian cells, studies aimed at demonstrating the therapeutic potential of small interfering RNAs (siRNAs) have been reported in mouse models and in non-human primates and more recently in humans [34]. Global attempts have been devoted to create vectors for therapeutic siRNA delivery in vivo [35]. FDA has approved Alnylam Pharmaceuticals Inc.‘s lipid-based RNAi therapy (ONPATTROTM), the first of its kind in 2018, which treats Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults [36]. Nevertheless, a few cellular and preclinical studies have already been performed for exosome-mediated RNAi delivery [37]. Here we summarize a few of them.
In 2011, Wood et al. made a successful attempt to load siRNA onto DC-derived exosomes (DEXs) by electroporation and delivered to the mouse brain via intravenous injection. Electroporation at 400 V and 125 μF yielded the highest retention of siRNA. Specificity to target the brain was introduced by triggering DCs to express CNS-specific rabies viral glycoprotein peptide (YTIWMPENPRPGTPCDIFTNSRGKRASNG) that specifically binds to the acetylcholine receptor fused with the N-terminus of murine Lamp2b, an exosomal membrane protein. To assess the therapeutic activity, they delivered siRNA against BACE1, a target for Alzheimer’s disease, and achieved substantial dose-dependent knockdown of mRNA and protein levels [38]. In another study performed by Banizs et al. in 2014, exosomes were obtained from mouse aortic endothelial cells expressing CD9 and CD63 and were examined to deliver luciferase siRNA to luciferase-expressing endothelial cells, where more than 40% gene knockdown was observed. In this study, electroporation was employed to load siRNA onto exosomes, specifically, at 400 mV/200 μF using 100 μl volume and the BTX ECM 600 electroporation system [39]. Similarly, Wahlgren et al. isolated exosomes from peripheral blood, loaded MAPK1 siRNA by electroporation at 0.150 kV/100 mF, and delivered into monocytes and lymphocytes, resulting in specific gene silencing [40]. In 2013, Filatov et al. also incorporated siRNAs against RAD51 and RAD52 into HeLa and HT1080 cells using chemical transfection and electroporation. HeLa cells, when treated with these exosomes, underwent massive cell death [41]. Notably in a study performed in 2013, Ohno et al. loaded let7a miRNA onto HEK293 cell-derived exosomes by lipofection and delivered to EGFR overexpressing murine RAG2–/– breast cancer xenografts upon intravenous administration. Tumor targeting was attained by modifying exosomes with GE11 peptide (YHWYGYTPQNVI), less mitogenic than EGF [42]. Considering the wide applications of exosome in cancer, Table 1 summarizes the recent clinical trials that involved exosomes.
Table 1.
List of exosomal formulations undergoing clinical trials aimed at diagnosis and treatment of cancer.
| Clinical Trial ID | Description | Duration | Cancer type | Sponsor/Agency |
|---|---|---|---|---|
| NCT01779583 | Circulating exosomes as prognostic and predictive biomarker in advanced gastric cancer patient | 2013–2016 | Gastric | Hospital Miguel Servet |
| NCT02393703 | Interrogation of Exosome-mediated Intercellular Signaling in Patients with Pancreatic Cancer | 2015–2019 | Pancreatic | Memorial Sloan Kettering Cancer Center |
| NCT01668849 | Edible plant-derived exosome ability to prevent oral Mucositis associated with chemoradiation treatment of head and neck cancer | 2012–2018 | Head and neck | James Graham Brown Cancer Centre |
| NCT01294072 | Study investigating the ability of plant exosome to deliver curcumin to normal and colon cancer tissue | 2011–2020 | Colon | James Graham Brown Cancer Centre |
| NCT02071719 | Predicting response to kinase inhibitors based on protein phosphorylation profiles in tumor tissue from advanced renal cell cancer patients | 2012–2017 | Renal cell | VU University Medical Center |
| NCT01550523 |
Pilot Immunotherapy trial for recurrent malignant gliomas IGF-1R/AS ODN |
2012–2013 | Malignant glioma of brain | Thomas Jefferson University |
| NCT02454930 | Evaluation of microRNA expression in blood and cytology for detecting Barrett’s Esophagus and associated neoplasia | 2015–2018 | Esophageal adenocarcinoma | Midwest Biomedical Research Foundation |
| NCT02310451 | Study of molecular mechanism implicated in the pathogenesis of melanoma | 2014–2016 | Metastatic melanoma | Center HospitalierUniversitaire de Nice |
| NCT02147418 | Exosome testing as a screening modality for human Papilloma-positive Oropharyngeal Squamous Cell Carcinoma | 2015–2019 | Oropharyngeal | New Mexico Cancer Care Alliance |
| NCT03542253 | Combined Diagnosis of CT and Exosome in Early Lung Cancer | 2018–2019 | Lung | Second Affiliated Hospital of Soochow University |
| NCT03317080 | Dynamic Monitoring Circulating Tumor DNA in Surgical Patients with Lung Cancer | 2017–2023 | Lung | West China Hospital |
Details obtained from https://clinicaltrials.gov/.
Liposome as a drug delivery vehicle
Several clinical studies are currently undergoing for nanotechnology-based products, including as liposomes, NP polymers, albumin NP, and inorganic particles etc., and few among them have already gained access to clinical use. Among these nanocarriers, one of the best characterized is “liposomes”. Ever since its discovery in 1965, liposomes have drawn enormous attention in nanomedicine research due to their unique properties, including targeting particular cells or tissues, protecting the payload, high biocompatibility, and low toxicity rendering enhanced bioavailability to the cargo, etc. Liposomes are artificially manufactured biomembrane-mimetic spherical vesicles containing a hydrophilic aqueous interior and lipidic bilayer exterior [24]. Owing to their unique structure, they can carry hydrophilic drugs in the cocooned aqueous core and hydrophobic biotherapeutics inside the lipid bilayer. An array of bioactive molecules, including anticancer agents, antiangiogenic agents, antimicrobial agents, chelating agents, peptides, hormones, enzymes, proteins, vaccines, and genetic materials, can be delivered via liposomes [43, 44]. It turns out that almost 17 different liposomal formulations are already out in the market for use against many pathological indications, and a plethora of them are undergoing various phases of clinical trials. The clinical translation of the liposomal vehicles can be attributed to their small nanometric particle size, high drug loading capacity, high bilayer permeability, colloidal stability (as indicated by surface charge), and surface modification PEGylation for obtaining better pharmacological profile and targetability [18, 45]. Liposomal formulations improved the pharmacokinetic profile of the drug and diminished off-target toxicity associated with the cytotoxic molecules [46, 47]. As mentioned above, liposomes have been successfully explored to deliver nucleic acids as well. For example, siRNA-based drug Onpattro (Alnylam) was recently approved by the FDA and EMA [48]. Here, in Table 2, we provide a brief account of liposomes undergoing clinical trials. Table 3 summarizes already marketed liposomes respectively for use in cancer therapy. Two very well-known liposomes, Doxil and Myocet, were approved by FDA in 1995 and 1999, with the subsequent approval of many of them [6]. As of now, 16 liposomal drugs received clinical approval and thereby marketed, including AmBisome, DaunoXome, DepoCyt, DepoDur, Visudyne, etc. Until 2017 no marketed liposomal drugs showed OS enhancement than the parent drug [7]. Recent phase III outcome of liposomal cytarabine-daunorubicin (Vyxeos; CPX-351) as contrasted with its individual counterparts cytarabine and daunorubicin (“7 + 3”) in 60–75 years old patients with high-risk acute myeloid leukemia, revealed enhanced OS of 9.56 months versus 5.95 months [8]. Liposomes are thus a promising vehicle for delivering anticancer drugs to specific locations and can be engineered for personalized cancer treatment.
Table 2.
List of liposomal formulations undergoing clinical trials aimed at diagnosis and treatment of cancer.
| Clinical Trial ID | Description | Duration | Cancer type | Sponsor/Agency |
|---|---|---|---|---|
| NCT02562378 | To determine MTD and PK for combination of Trastuzumab and non-pegylated liposomal doxorubicin (Phase 1) | 2015–2019 | Metastatic Breast Cancer | MedSIR |
| NCT02596373 | To study safety and efficacy of Mitoxantrone Hydrochloride Liposome Injection to shrink or slow the growth of advanced recurrent or metastatic breast cancer (Phase 2) | 2015–2018 | Advanced Recurrent/Metastatic Breast Cancer | CSPC ZhongQi Pharmaceutical Technology Co., Ltd. |
| NCT03088813 | Irinotecan Liposome Injection (ONIVYDE®) versus Topotecan in Patients with Small Cell Lung Cancer after Platinum-based First-Line Therapy (Phase 3) | 2018–2022 | Small Cell Lung Cancer | Ipsen |
| NCT02631733 | To evaluate safety and tolerability of veliparib when given together with liposomal irinotecan in treating patients with solid tumors (Phase 1) | 2016–2019 | Malignant Solid Neoplasm | National Cancer Institute (NCI) |
| NCT03164993 | To determine safety and efficacy of Atezolizumab when combined with immunogenic chemotherapy in subjects with metastatic triple-negative breast cancer (Phase 2) | 2017–2024 | Triple-Negative Breast Cancer | Oslo University Hospital |
| NCT03682224 | To assess pain management efficiency of liposomal Bupivacaine after elective thoracoscopic lobectomy (Phase 3) | 2018–2021 | Non-Small Cell Lung Cancer | Southern Illinois University |
| NCT01591356 | To learn about safety, MTD and efficacy of liposomal siRNA delivery against EphA2 gene (Phase 1) | 2015–2020 | Advanced, recurrent Cancers | M.D. Anderson Cancer Center |
| NCT01455389 | To find the highest dose of DOTAP: Chol-fus1 that can be safely given in combination with Tarceva (erlotinib hydrochloride) to patients with NSCLC (Phase 2) | 2014–2018 | Lung Cancer | Genprex, Inc |
| NCT02865811 | To study the combinatorial effect of Pegylated Liposomal Doxorubicin (PLD) and Pembrolizumab as a possible treatment for Recurrent Ovarian, Fallopian Tube or Peritoneal Cancer that is resistant to platinum therapy (Phase 2) | 2016–2024 | Recurrent Platinum Resistant Ovarian, Fallopian Tube Or Peritoneal Cancer | Dana-Farber Cancer Institute |
| NCT02580058 | To compare efficacy in prolonging Overall Survival: Avelumab alone to Avelumab plus Pegylated Liposomal Doxorubicin (PLD) and to PLD alone (Phase 3) | 2015–2018 | Platinum Resistant/Refractory Ovarian Cancer | Pfizer |
| NCT03207672 | To evaluate MTD, safety, PK and efficacy of E7389 Liposomal Formulation in breast cancer and adenoid cystic carcinoma (ACC) (Phase 1) | 2017–2020 | Solid Tumor | Eisai Co., Ltd. |
| NCT03409198 | Evaluating Immunogenic Chemotherapy PLD/Cyclophosphamide Combined with Ipilimumab and Nivolumab in Patients with Metastatic Luminal B Breast Cancer (Phase 2B) | 2018–2025 | Breast Cancer | Oslo University Hospital |
Details obtained from https://clinicaltrials.gov/.
Table 3.
Marketed anticancer formulations of liposomes.
| Trade name | Drug | Lipid component | Company | Approval year | Cancer |
|---|---|---|---|---|---|
| Doxil | Doxorubicin | HSPC:Cholesterol:PEG 2000-DSPE, stealth liposome |
Johnson and Johnson, Baxter Healthcare Dr. Reddy’s Lab Ltd |
FDA 1995 (USA) FDA 1999 (USA) EU 2000 |
Kaposi’ sarcoma Refractory ovarian CA Refractory Breast CA |
| LipoDox | Doxorubicin | DSPC:Cholesterol:DSPE-PEG stealth liposome | Sun Pharma Global FZE | FDA 2013 | Kaposi’ sarcoma |
| Myocet | Doxorubicin | EPC: Cholesterol |
Ban Corporation, Sepherion Therapeutics |
EU 2000, Canadian approval 2001, one | Metastatic breast cancer |
| DaunoXome | Daunorubicin | DSPC: Cholesterol:PEG stealth liposome | NeXstar Pharmaceuticals, Inc., CO, Gilead Science, UK | On the market since 1996 (USA and Europe) | Refractory Kaposi’ sarcoma |
| Marqibo | Vincristine | Egg Sphingomyelin: cholesterol | Talon Therapeutics | FDA 2012 | Acute Lymphoblastic leukemia |
| Onco TCS | Vincristine | Sphingomyelin: cholesterol | Inex pharmaceuticals | Phase II/IIIclinical trials | Refractory non-Hodgkin’s lymphoma |
| DepoCyt | Cytarabine |
DOPC:DPPG: Cholesterol:Triolein Multivesicular liposome |
Depo Tech, Enzon Pharmaceuticals, Pacira Pharmaceuticals | FDA 1999, 2007 | Malignant lymphomatous meningitis |
| Onivyde | Irinotecan | DSPC:Cholesterol:DSPE-PEG | Ipsen Biopharmaceuticals | FDA 1996 | Pancreatic adenocarcinoma |
| Caelyx | PC-PEG-methoxy polyethylene glycol | Johnson and Johnson, | EU 1996 | Metastatic breast cancer | |
| Mepact | Mifamurtide | Muramyl tripeptide-PE | Takeda France SAS | EU 2009 | Non-metastatic Osteosarcoma |
| CPX-351 (Vyxeos) | Daunorubicin; Cytarabine | DSPC: DSPG: Cholesterol | Jazz Pharmaceuticals | FDA 2017 | Acute myeloid leukemia |
| Lipusu | Paclitaxel | PC:Cholesterol | Luye Pharmaceutical Co. Ltd. |
China 2003 FDA 2005 |
Gastric, ovarian and lung cancer |
Reproduced from Madamsetty et al. [144]. 10.1016/C2019-0-02790-2 Copyright © 2021 Elsevier Inc. In Press.
DSPE distearoylphosphatidyl ethanolamine, DSPC distearoylphosphatidylcholine, DOPC dioleoyl-sn-glycero-3-phosphocholine, DPPG dipalmitoyl-sn-glycero-3-phosphoglycerol, PC phosphatidylcholine, HSPC hydrogenated soy phosphatidylcholine, EPC egg phosphatidylcholine, PEG polyethylene glycol.
Diagnostic carrier in cancer detection
Diagnostic testing entails scans and techniques that are used to confirm the existence of cancer and to determine the type, location, severity, and stage of the tumor. The most sought-after aim is to detect cancers early using sophisticated diagnostic technology to identify and analyze tumors and develop a therapeutic plan. Exosome and liposome-based molecular diagnostics are discussed below.
Exosome as diagnostic carrier
Exosomes were initially thought of as circulatory vehicles collecting and discarding scrap materials from cells; however, later a paradigm shift occurred in understanding their biological functions. It is quite clear now that exosomes’ content and physiological functions solely depend on the parent cell. Besides having tremendous possibilities as therapeutic delivery systems, they can also be viewed as potential prognostics and diagnostics agents with clinical significance [3]. It turns out that tumor cells discharge a higher number of exosomes compared to their normal counterpart and exosomes originated from tumor carry signature protein and nucleic acids of that particular tumor, which in turn, is capable of influencing normal healthy cells to instigate drug resistance, malignancy, immune-modulation, etc. Exosomes, thus, can be conceived as a diagnostic and prognostic tool for cancer detection, discerning the differences between histotypes [3]. For instance, in 2015, Melo et al. identified a cell surface proteoglycan, glypican-1 (GPC1) abundant in cancer cell-derived exosomes, found in pancreatic cancer patient’s serum with high specificity and sensitivity. This unequivocally enunciated that exosomes with plentiful of glypican-1 have the potential for prognosis and differentiate between healthy subjects, patients with benign tumor and early/late stage of pancreatic cancer. For pancreatic cancer diagnosis, the authors used flow cytometry to isolate exosomes from the serum of pancreatic cancer patients, and proposed an explanatory early detection method [49].
Imaging of exosomes
Spatiotemporal investigation of the metabolic activities of exosomes, EVs both in therapeutic and diagnostic fields of study requires the robust accompaniment of a variety of imaging techniques. They may be classified based on how they are detected [50].
Fluorescence
Optical imaging by fluorescence microscopy is a widely accepted technique to trace molecular and sub-cellular events. Exosomes have been traced using organic dyes, genetically encoded fluorescent proteins, fluorescent nanomaterials, and immunofluorescent reporters.
Bioluminescence
Luciferase, without an excitation source for emission of light, emits bioluminescence via reaction of its substrate with either ATP and Mg2+ or oxygen. In 2014, Lai et al. developed mbGluc-BAP, a membrane-bound (mb) version of Gluc (gaussia princeps luciferase) linked to a biotin acceptor peptide (BAP) for labeling and tracking EVs. Consequently, EVs isolated from HEK293T cells already overexpressing mbGluc-BAP were injected into nude mice. In vivo bioluminescence images revealed that the spleen and liver exhibited a prominent signal compared to control (only PBS) after 30 min of injection [51].
MRI
Magnetic resonance imaging is a robust contrivance in anatomy and physiology to produce 3D images of healthy and diseased organs with high sensitivity. The working principle is based on finding contrast in images that takes its origin from the differences in relaxation time of H nuclei in different tissue environments under study. Chemical contrast agents, such as superparamagnetic iron oxide nanoparticles (SPIONs) have been used in MRI to enhance sensitivity [52]. Recently, Hu et al. constructed melanoma exosomes for MRI tracking by loading SPIONs onto them using electroporation. Following standard in vivo MRI procedure, it was revealed that accumulation of SPIONs in the ipsilateral lymph node of C57BL/6 mice at 48 h was remarkably higher when delivered by exosomes compared to free SPIONs [53]. Interestingly, Marzola et al. made a tweak in the exosomes labeling strategy, i.e., they incubated ultra-small superparamagnetic iron oxide NPs with adipose stem cells (ASC), which can be viewed as an indirect labeling. The ASCs produced, labeled exosomes for MRI visualization [54]. These can eventually open a broad door for medical applications of noninvasive techniques in near future.
Computed tomography
Computed tomography (CT) scans, another vastly used noninvasive medical imaging technology with high spatiotemporal specificity, relies on cross-sectional images primarily arising from combinations of multiple X-ray absorptions by an object under investigation. A few studies have been performed to elucidate exosomes-tracing by CT as well. For instance, Lee et al. isolated exosome-mimetic nanovesicles (ENV) from RAW 264.7 cells, radio-labeled them with 99mTc-hexamethylpropyleneamineoxime (HMPAO) by 1 h incubation and tracked their distribution in mice. Single-photon emission computed tomography (SPECT)/CT images revealed a significant uptake of 99mTc-HMPAO-ENVs in mouse liver after 30 min and in salivary glands after 3 h but no accumulation in the brain even 5 h after injection. On the other hand, mice administered with only 99mTc-HMPAO show higher brain uptake [55]. In another study conducted by Bezter in 2017, MSC-secreted exosomes were labeled by glucose-coated Au-NP and administered via the intranasal route murine model of focal brain ischemia. Neuroimaging by CT revealed substantial brain accumulation of the exosomes [56].
Exosome and liquid biopsies
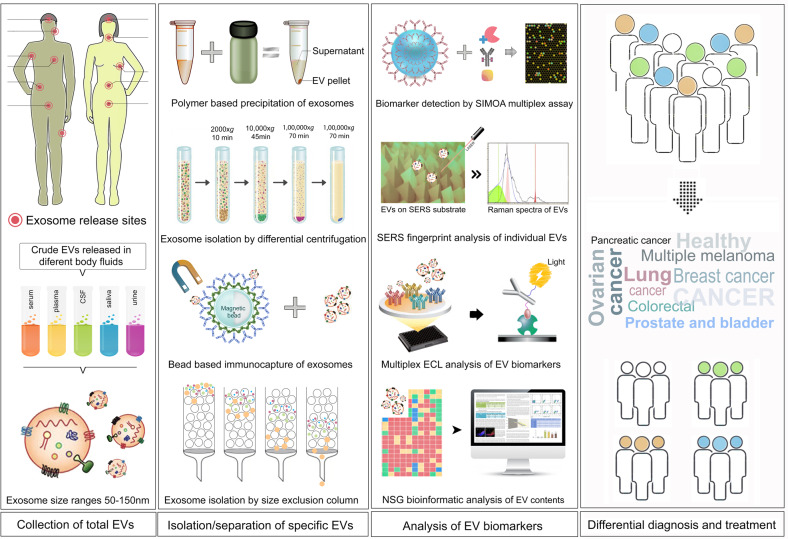
A liquid biopsy, popularly known as fluid phase biopsy or fluid biopsy, is the characterization and analysis of liquid-state biological tissue, primarily blood or other liquid specimens like saliva, urine etc. Liquid biopsy has been mainly used for the diagnostic and prognostic detection and monitoring tool for diseases like cancer. This technique aids in detection and characterization of molecules (e.g., tumor DNA), as well as whole cells (e.g., circulating tumor cells (CTC)) in a largely noninvasive mode instead of biopsies of tumor tissues [57]. Having the advantage of being barely invasive the detection is faster and can be tested more frequently for better monitoring tumors and mutations over time, monitor a patient’s reaction to therapy as a “surveillance” strategy for those who have concluded treatment yet are at high relapse of their cancer. Blood-based liquid biopsies offer easy access to altered tumor genetic material thus relieving the dependency on more invasive tissue biopsy [58]. These technological advances, of detecting rare mutations in a background of wild-type targets, have generated a boom in the field of liquid biopsies. Liquid biopsy source materials are of mainly three types: (1) tumor-derived exosomes (TEX) and EVs, (2) CTCs, and (3) circulating tumor DNA (ctDNA) [57, 58]. Analysis of the contents of specific exosomes, derived from a particular type of cancer, by using advanced state-of-the-art technologies like single molecular array, Surface-enhanced Raman spectroscopy, Luminex, AlphaLISA, Electroluminescence ELISA have triggered a lot of enthusiasm and hope in identifying disease biomarkers at early stage.
Exosomes and EVs are actively released into biofluids by most living cells including tumor cells (Figs. 1 and 4). Because most tumor cells have an overactive MAPK pathway, they actively secrete exosomes and EVs. Over the course of 48 h, a cancer cell is capable of releasing more than 20,000 of these vesicles. These vesicles carry cell- and cell-state-specific nucleic acids (RNA, DNA, and miRNAs) and signature proteins from the tumor, including relevant genetic material and proteins from the carcinogenic process. Exosomes have been shown to play a significant role in promoting tumor development, suppressing the immune response, inducing angiogenesis, and promoting metastasis, making them especially fascinating as cancer biomarkers.
Fig. 4. Liquid biopsy workflow for cancer diagnosis and treatment.
First panel showing exosome and EV release sites. Tumor-derived exosomes and EVs can be isolated from different types of bodily fluids. Second panel showing different methods for exosome isolation from bodily fluid and cell culture media. Third panel shows different methods used for the analysis of exosome biophysical properties and the molecular profiling of cargo. The available information can be used for diagnosis and treatment.
Since RNA is intrinsically fragile, it cannot exist in biofluids such as serum or plasma in its free form. Before it was discovered that exosomes comprise diagnostically important RNA, the liquid biopsy field was limited to CTCs and cfDNA [59]. When it was revealed that tumor-derived RNA could be stably extracted from exosomes from the serum or plasma of cancer patients, the arsenal for cancer diagnostics became much larger. Ratajczak et al. found Oct4 and other pluripotency factor mRNAs in vesicles produced from murine stem cells in 2006, which was the first discovery of RNA in vesicles from cell lines. Studies after this one established the existence of exosomal RNA, proved intercellular transport of exosomal RNA and offered a new medium for intercellular communication between cells [37].
Biomarker discovery in exosomes has traditionally focused on miRNA, although the exosomal long RNA (mRNA, lncRNA, etc.) has more usefulness in detecting somatic mutations and changes in gene transcription. Long RNAs provide new avenues for investigating disease states or progression. The study of KRAS and BRAF mutations in serum exosomal mRNA and tissue DNA from colorectal cancer patients revealed a high degree of agreement between these nucleic acids. For the first time, exosome long RNAs enabled for the detection of oncogenic fusion transcripts, as well as alternative splice variants, and the RNA transcriptome profile of a liquid biopsy from serum or plasma [60]. The existence of miRNAs has been associated to cancers of the lung, prostate, and pancreas. These various exosomal nucleic acids (exoNA) reflect benefits specific to exosomes that may not be detectable using cfDNA, short RNA, or protein tests, together with previously described RNA editing and circular RNAs.
It is worth noting that, in addition to RNA, exosomes contain other potential cargos that could be used as biomarkers, such as proteins including membrane proteins, lipids, and metabolites. Immuno-pulldowns can be designed in biofluids to enrich or deplete tissue-specific exosomes based on surface protein markers for subsequent biomarker analyses, including protein analytes (Fig. 4). Using plasma, this approach has been shown to significantly improve the clinical correlation of biomarkers with disease states, which is otherwise either poorly represented or undetectable when crude plasma was analyzed [57, 60].
After rigorous clinical validations, the first commercial prostate cancer exosome testing has lately been introduced. For the intended use population: males, this noninvasive urine test employs an unique expression profile of three RNA transcripts (two mRNAs and one lncRNA). PSA readings of 2 to 10 ng/ml, 50 years of age or older, no previous biopsy. In one extensive clinical validation involving over 1000 patients, the gene signature within exosomes analyzed from voided urine was indicated as a rule-out test for high-grade (>GS7) prostate cancer with an NPV of 91%, avoiding about 27% of biopsies [61]. The urine gene expression signature test is based on genes linked to prostate cancer development and progression, demonstrating the therapeutic value of exosome-derived RNA biomarkers. A workflow of the use of exosomes for the liquid biopsy of cancer is shown in Fig. 4.
Exosome enhances detection limit of liquid biopsy
Recent publications on cfDNA analysis reported detection of allelic frequency as little as 0.001%, i.e., ten mutant copies in a background of 1,000,000 wild-type alleles. While this approaches even the highest fidelity of DNA polymerase, strategies such as unique molecular indexing and bioinformatical background correction have helped improve the performance of next-generation sequencing (NGS) assays for mutant alleles at low frequencies in more targets. However, the biological limitation of low mutant copy number present in circulation, especially in patients with early-stage cancers, cannot be addressed by the improved methods for allelic frequency discrimination.
Exosome RNA has the potential to enhance the overall number of mutant copies accessible for sampling. A recent publication comparing ctDNA mutation detection vs. combined exoNA + ctDNA showed that the combined exosome assay had ~10-fold more mutated copies of activating EGFR mutations in NSCLC patients (median of 24 mutant copies/ml plasma on ctDNA vs. 234 copies/ml plasma with the combined approach) [62]. In addition, the allelic frequency of the mutations was ~3-fold higher when the mutation targets were analyzed by the combined exosome approach vs. ctDNA-only mutation detection by BEAMing. Furthermore, a recent longitudinal research examining the levels of BRAF, KRAS, and EGFR mutations in exosomes and cfDNA over time found that combining exoNA and cfDNA analysis increased the association of biomarkers with treatment success much more than cfDNA alone [63].
In all three kinds of liquid biopsy targets, the wild-type target is present in very high concentrations as compared to the tumor-derived components. Finding a single CTC in a backdrop of 106–107 leukocytes would be difficult assuming the cell in question were to be identified at all. Exosomes and cfDNA both face analogous limitations. A range of variables, including activity and disease status, have been shown to impact the absolute quantity of cfDNA, however there is no indication that only wild-type material, and not diseased material, is vulnerable to these perturbations. It is possible to get false negative responses because of the large background of wild-type nucleic acid and the modest, often undetectable, levels of mutations present in the sample. Positive identification of mutations, particularly when they occur in low allelic frequency, should, on the other hand, be read with care, since healthy persons, too, may display low allelic frequency of mutations as a result of clonal hematopoiesis, which is a rare occurrence [64].
Liposome in cancer diagnostics
In biomedical research, molecular imaging has vastly found its application for the diagnosis of pathological conditions as well as for monitoring of treatment progression [65]. To our intrigue, in addition to transporting a wide range of tiny and big molecules, liposomes have also been explored to deliver a myriad of diagnostic agents, namely, 64Cu [66], 14C isotopes [67], quantum dots [68], gadolinium (Gd)-based contrast agents [69] SPIONs [70], and fluorescent probes [71–73]. Moreover, liposomes loaded with these probes can be targeted passively or actively to the diseased tissue. Here, we shall briefly reflect upon few important studies. Mostly, long circulating PEGylated liposomes are used to carry radionuclides with short half-life, which is necessary to better signal-to-noise ratio. The radionuclides are loaded into various parts of the liposomes such as (1) aqueous core, (2) lipid bilayer, (3) outer surface, and (4) remote loading via a transmembrane gradient [18]. Generally, surface chelation or remote loading are preferred for higher and faster encapsulation, while chelators are routinely used in the aqueous interior of the liposomes for entrapping the radionuclides toward improving efficacy. The widely applied technologies for detecting and imaging of various radio-labels (99mTc, 111In, 123I, 18F, 68Ga, etc.) are—positron emission tomography and SPECT [45]. For instance, remote loading via a transmembrane gradient of 99mTc-N,N-bis(2-mercaptoethyl)-N′,N′-diethyl-ethylenediamine onto liposomes with variable composition was examined by Li et al. in a report [74].
Similarly, different imaging agents are required for different imaging instruments, such as, paramagnetic metals for MRI, contrast agents for CT imaging, or microbubbles for US sonography. For example, Erdogan et al. subsumed an amphiphilic polymeric chelator inside the lipid bilayer to heavily load Gd and modify the surface of the liposome with 2C5 mAb for specific targeting the cancer cell and effectively MR imaged the tumors in murine model after 4 h of injection [75]. In 2016, Ghaghada et al. demonstrated the efficacy of liposomal iodine (275 mg/kg) toward CT visualization of sub-cm sized primary as well as metastatic liver carcinoma in companion dogs [76]. Furthermore, facilitating the application of contrast-enhanced targeted ultra-sound imaging, an Annexin V-conjugated lipidic nanobubble was prepared and tested to observe the cisplatin induced apoptotic response in mice harboring MDA-MB 231 tumor. It demonstrated its efficacy by detecting apoptosis in tumor cells within 70 s post-injection [77].
Interestingly, toward achieving a multimodal diagnostic, Wu et al. loaded liposomes with IR820, Iohexol and Gd-chelates for fluorescent, CT and MRI imaging of C6 tumor in nude mice [78]. In recent times, cancer theranostics (therapy plus diagnostics) have attracted significant attention of the researchers, where a therapeutic drug and an imaging agent are co-delivered to the site of action [17, 79]. Individuals with advanced pleural mesothelioma exhibited higher (99mTc)-liposomal DOX uptake with ameliorated survival compared to the control cohort. Higher uptake as predicted by the image analysis of the theranostic liposome was directly proportional to the efficacy of the chemotherapeutic drug [80]. Importantly, Martinez et al. used biomimetic liposomes such as leukosomes (with leukocyte membrane protein) to load DOPE-Rh and imaged activated endothelial cells corroborating enhanced affinity (~14 times) of the leukosomes for the endothelium [81]. In addition, target activatable probes have also found their application in liposomal delivery [82, 83]. Taken these facts into consideration, scientists are nurturing high hopes for clinical translation of liposomes as a diagnostic nanocarrier for use in cancer in near future.
Cancer immunotherapy
In recent years, cancer immunotherapy has made significant strides. By optimizing cell type-specific distribution and enhancing medication effectiveness, nanocarriers such as exosomes and liposomes have the ability to further enhance cancer immunotherapy and help generate an even stronger immune response. Both exosomes and liposomes may help overcome some of the challenges that cancer immunotherapies pose and is described below.
Exosomes and cancer immune modulation
Several reports showed that cancer cells produce and release exosomes that are abundantly found in body fluids. Based on the cancer type the respective exosomes show specific differences in the expression of tumor-associated antigens, especially those found in association with the cell membrane. A comparison of whole-tumor-cell lysates with the TEX exhibits interesting enrichment of tumor antigens within the exosomes. Several reports suggest the expression of tumor antigen in respective exosomes, e.g., such as HER2/neu, melan-A [84], carcinoembryonic antigen [85], mesothelin [86], and others. There are several examples of cancer exosomes exerting a negative influence on the immune system and may be very highly relevant in the clinical setting. Regardless of the fact that cancer exosomes can activate immune response, but current theories rely on the fact that cancer exosome would encourage immune suppression as this will suffice the cancer cell’s interest. The fact that the cancer pursues a progressive course thereby contradicting the role of cancer exosomes in immune activation.
Dendritic cell-derived exosomes
DC secrete a high number of exosomes to cause successful anticancer effects as the most efficient antigen-presenting cell. In recent years, owing to its immunotherapeutic effects and immunoregulatory capacity in management of cancer, DEXs have attracted substantial attentions of the scientists across the globe. DEX presents antigens directly as well as indirectly to T cells, and in turn, they not only induce T cells dependent antitumor responses but also circumvent some technical limitations of DC based immunotherapy [87]. DEX’s therapeutic potential is obviously controlled by its composition. DEX contains a copious amount of MHC Class I and II molecules, as well as costimulatory molecules (CD80 and CD86), Ig family member ICAM-1, tetraspanins (CD63, CD81, CD9), milk fat globule EGF factor 8 (MFG-E8), and the heat shock protein hsc73 [88–90]. DEX has the potential to trigger a tumor-specific cytotoxic T lymphocyte (CTL)-response by presenting MHC-peptide complexes to T cells, which is aided by costimulatory molecule and tetraspanin. ICAM-1, MFG-E8, and hsc73 may also boost immune response by triggering a T-cell-dependent antitumor response [90, 91]. IL-15R, TNF, NKp30 ligand BAT3 and NKG2D expressed on the surface of DEX can trigger an NK cell-dependent antitumor immune response [92–94]. With this background, researchers have conducted studies to develop DEX vaccine for different tumor cells [87, 95]. Much to our intrigue, phase I clinical trials in melanoma and advanced NSCLC showed promising results [95, 96].
Tumor-derived exosomes
TEX are responsible for cancer growth, progression, induce malignancy, and suppress antitumor functions of T cells. It has been reported earlier that TEX engulfed by DCs could induce antigen-specific CTL response and is covered in details in other reviews [97, 98]. The different aspects of TEX-mediated pro-tumorigenic role are shown in Fig. 5. DCs loaded with ascites exosomes from melanoma increased the amount of peripheral blood CD8+ T cells and stimulated lymphocytes to destroy tumor cells or produce IFN, according to Andre et al. [99].
Fig. 5. Schematic showing potential pro-tumorigenic role of tumor-derived exomes (TEX) in cancer.
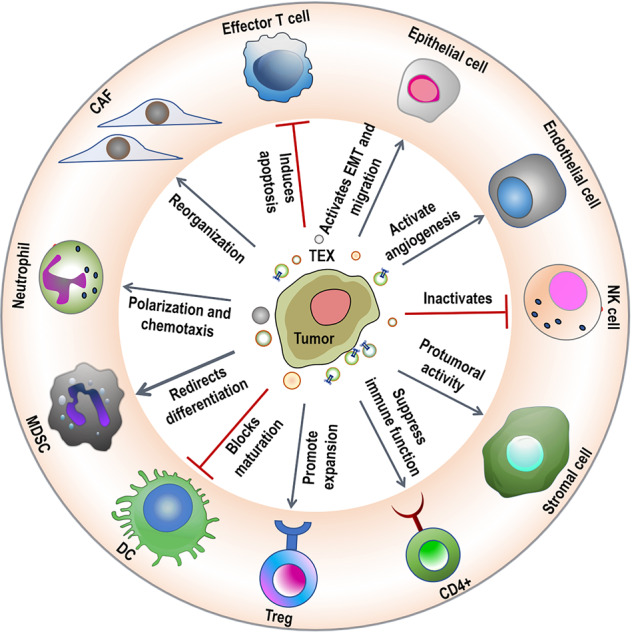
This figure is inspired by Zhang et al. [93].
Bu et al. discovered that TEX-stimulated DCs could activate T lymphocytes to become glioma-specific CTLs, and that CD8 antibodies could block CTL-mediated cytotoxicity to tumor cells, but not CD4 antibodies [100]. Marton et al. found that the number of CD8+ T lymphocytes was remarkably enhanced in serum and tumor tissues of mice treated with TEX-loaded DCs in immunotherapy of hepatocellular carcinoma [101]. Yao et al. also demonstrated that in vivo, EXOEG7-targeted DCs stimulated CD8+ T-cell differentiation into CTL effectors [102].
Liposome in cancer immunotherapy
It is needless to mention that liposomes have found tremendous application in cancer immunotherapy. We will briefly reflect upon in the following sections.
As antigen carriers
Antigen-presenting cells (APCs) take up exogenous antigens and subsequently process them in endosome/lysosomes into antigenic peptides. These peptides, after being tethered to MHC class II molecules, are presented to CD4+ T cells, which triggers helper T cells-based humoral immune responses. On the other hand, endogenous antigenic peptide present in APCs’ cytoplasm are degraded in proteasome and are then tied to MHC class I molecules. Matured APCs migrate to lymph nodes to present these complexes to CD8+ T cells which produce CTL-based cellular immune responses. A portion of the exogenous antigen, being transferred from endosome to cytosol, gets bound MHC class I molecules. This presentation of exogenous antigens on MHC class I molecules, coined as cross-presentation, is crucial for the commencement of CD8+ T-cell activation and ensuing responses [103]. Hence, the effective antigen delivery to APCs in the body, intracellular distribution of antigen in APCs for induction of antigen-specific CTLs is critically important to achieve cancer immunotherapy. To date, among various kinds of antigen carriers, liposomes are widely used for immune induction by cross-presentation, APC activation and counterbalancing immunosuppression in various cancers [104]. Here, we provide a brief account of these attempts.
Cross-presentation
In general, cross-presentation can follow two paths: cytosolic pathway and vacuolar pathway. In cytosolic pathway, as obvious from its name, cytoplasmic delivery of antigen is of primary importance. To achieve this, pH-sensitive liposomes have extensively been used as they release the payloads in pH-dependent manner and can destabilize the endosomal membrane. For example, in 2013, Yuba et al. developed a liposome with Egg-PC and polymer-lipids consisting of pH-sensitive fusogenic polymer moieties such as 3-methylglutarylated poly(glycidol) and 2-carboxycyclohexane-1-carboxylated poly(glycidol), grafted to a PE head group. These liposomes, when administered subcutaneously to mice, delivered antigenic protein ovalbumin (OVA) in the cytosol of DC followed by induction of antigen-specific cellular immunity with subsequent rejection of OVA-expressing E.G7-OVA cells and significant regression of E.G7-OVA tumors [105]. In another study performed in 2017, IMP-3-LPs (Insulin-like growth factor II mRNA-binding protein 3 derived long peptides) entrapped in these pH-sensitive liposomes were successfully cross-presented in vitro, and this LP efficaciously potentiated CTLs in HLA-A2 transgenic mice in vivo. Importantly, one of the IMP-3-LPs primed IMP-3-specific Th cells from PBMCs of head-and-neck malignant tumor patients [106].
Activation of antigen-presenting cells (APC)
Consolidation of an adjuvant to liposomal antigen delivery system is a beneficial way to activate APCs and reinforce immune responses. In a study carried out by Yoshizaki in 2017, an adjuvant named CpG-DNA, which happens to be a ligand to Toll-like receptor 9 expressed in DC-endosomes, was introduced to cationic liposomes modified with 3-methylglutarylated hyperbranched poly(glycidol). They reported liposomally stimulated production of cytokine from DC and costimulatory molecules in vitro and induced antigen-specific immune responses in vivo [107]. Yuba et al., in 2017, fabricated liposomes with Curdlan and mannan based pH-sensitive polymers to activate DCs (by interacting with Dectin-1 and Dectin-2 respectively) which is indicated by Th1 cytokine release from DC. These liposomes showed better in vivo antigen-specific immune responses and stronger antitumor effects than dextran derivative modified liposomes [108].
Negating immunosuppression
Owing to secretion of immunosuppressive cytokines like IL-10 or TGF-β from Myeloid-derived suppressor cells or regulatory T-cell (Treg), tumor microenvironment remains immunosuppressive and thwarts conventional immunotherapy. In order to circumvent this immunoinhibitory behavior of tumor microenvironment, Park et al., in 2012, developed liposomal polymeric gel for simultaneous delivery of IL-2 and TGF-β type I receptor inhibitor. These liposomes, releasing their content in the tumor microenvironment, demonstrated a marked tumor growth inhibition, enhanced CD8+ T-cell infiltration and improved survival rate [109]. In 2014, Xu et al. synthesized two NPs, namely, a mannose-modified lipid-calcium-phosphate NP and liposome-protamine-hyaluronic acid NP. The former, although effectively delivered Trp 2 peptide vaccine and adjuvant CpG oligonucleotide to DC, remained less effective against later stage B16F10 tumor in a subcutaneous syngeneic model. The latter delivered TGF-β siRNA to augment the vaccine efficacy and inhibited tumor growth by 52% compared with vaccine treatment alone [110].
As RNA carriers
RNAs encoding for tumor-specific antigens or tumor-associated antigens have emerged as a potent immunogenic trigger. In a phase Ib/IIa clinical trial, the safety and efficacy of cationic liposomes encapsulating siRNA (Atu027) directed against protein kinase N3 in the vascular endothelium in combination with conventional gemcitabine therapy for patients with advanced or metastatic pancreatic adenocarcinoma were assessed (NCT01808638) [111]. In another ongoing investigational phase I clinical trial, knockdown of EphA2 is being achieved by siRNA entrapped in neutral DOPC liposomes in patients with advanced recurrent solid tumors (NCT01591356) [112].
Exosome and liposome-based theranostics
Most important advancements in nanotechnology are directed toward the identification and treatment of cancerous development. The fundamental technology for theranostics is the creative combining diagnostic and therapeutic agents on a single nanocarrier such as exosomes and/or liposome. These small molecule-based theranostic (therapeutic and diagnostic) capacities can be used in different cancers. Exosome or liposome imaging may be a reliable way to establish effective diagnostic and prognostic modalities. The distribution of tumor-targeted exosomes or liposomes can be verified in real-time, and the exact position of tumors can be determined [17, 79]. A vast array of imaging strategies includes bioluminescence, photoacoustic imaging (FPA, SPECT, MRA), radioactive (PET), and MR imaging tumors. Srivastava et al. generated a structure called “fexosomes” by attaching Dox and 5–10 nm SPION to standard lung fibroblast cell (MRC9)-derived exosomes. Flexible oligonucleotide aptamer with high affinity and specificity for targets has been widely used as molecular probes in exosome research and theranostic applications [113]. Exosomes are natural carriers ideal for developing theranostics, and detailed descriptions are provided in other reviews [17, 114–116].
Similarly, the prospect of the use of liposomes in cancer theranostics is promising. Cheng et al. investigated a novel EGFR-binding peptide GE11 with DOX-loaded liposomes and found that the GE-11 modified liposomes had higher deposition and retention than the control unmodified liposomes using a near-infrared fluorescence imaging system [117]. Lin et al. engineered double ligand (anti-carbonic anhydrase IX antibody and CPP33)-modified triptolide-loaded liposomes (dl-TPL-lip) with enhanced tumor accumulation and cytotoxicity in NSCLC in vitro and in vivo models [118]. MSEE et al. generated theranostic dextran core-based stealth liposomal formulation using Iron oxide for MRI contrast, Dox for tumor targeting, PEGylated for immune system evasion, and BODIPY for optical fluorescence detection of Lewis lung carcinoma tumors in vivo [119]. Cittadino et al. designed liposomes with a cargo of glucocorticoid prednisolone phosphate (PLP) and a paramagnetic contrast agent [Gd-DOTAMA(C18)2] for MRI-guided in vivo imaging of PLP delivery, biodistribution, and therapeutic monitoring suing a B16 melanoma syngeneic mouse model [120]. Other liposomal theranostics are described in detail in other reviews [17, 121–123].
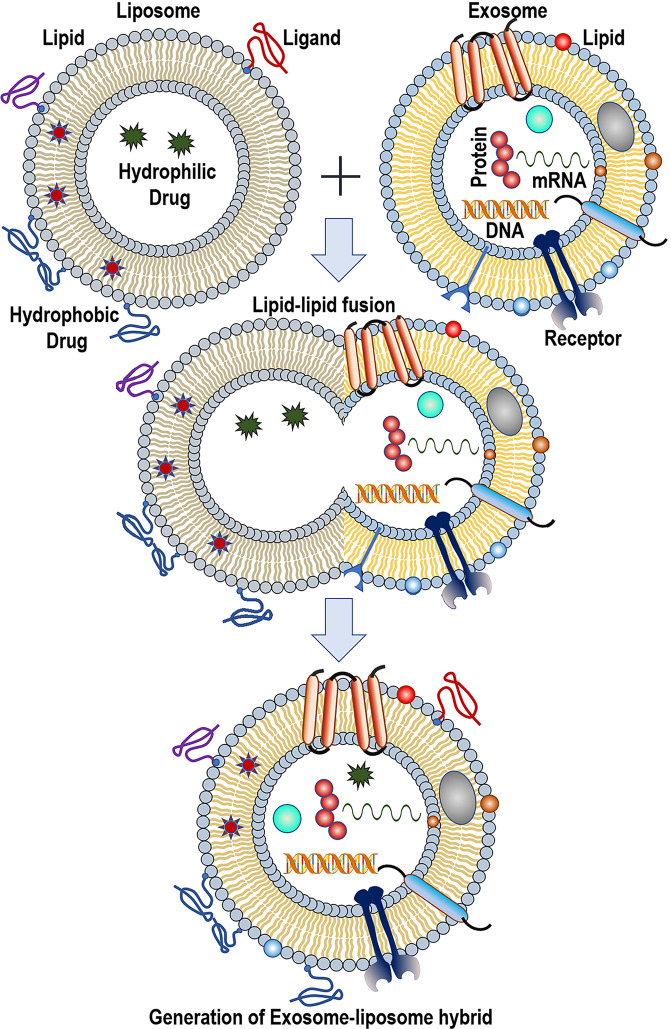
Novel hybrid bioengineered nanovesicle, exosome-liposome hybrid
Much to our intrigue, membrane fusion techniques are employed to generate exosomes-liposome hybrids, a next-generation drug delivery vehicle (Fig. 6) [124]. Three methods are generally adopted to accomplish this membrane fusion—incubation, sonication, and freeze-thaw cycles [22]. For instance, in 2016, Sato et al. isolated exosomes from Raw 264.7 cells and HER2-expressing CMS7 cells, fused them with liposomes made up of different lipids by freeze-thaw cycles, and evaluated the cellular uptake efficiency. The primary goal of the research was to alter the exosome surface toward diminishing the immunogenicity, enhancing the colloidal stability and circulation half-life [125]. Liposomes from anionic and neutral lipids showed better uptake compared to cationic lipids. It is now well known that for in vivo gene editing targeted delivery of the CRISPR/Cas9 system to the recepient cells is critical. In a study conducted by Lin et al. in 2018, exosomes extracted from sgRNA expressing HEK293FT cell line, were fused to liposomes, and loaded with dCas9 expressing vector by incubating 12 h at 37 °C. It has been demonstrated that the hybrids could successfully deliver CRISPR–Cas9 to mesenchymal stem cells [126]. In reality, membrane fusion is a crucial cellular process by which a mingled membrane architecture is formed by merging two separate lipid bilayers. While examining whether vascular stomatitis virus-G protein expression on the surface of exosome can enable the delivery of therapeutic membrane proteins to the targeted cells, Yang et al. developed a FRET-based imaging assay for measuring fusion efficacy of exosomes with liposomes that mimic lipidic plasma membranes [127]. Similar ideation was conceived by Gao et al., and they designed a virus mimicking fusogenic vesicle to detect exosomal miRNAs in a fast and fruitful manner [128].
Fig. 6. Schematic diagram showing the process to engineer the exosome-liposome hybrids.
Figure inspired from Sato et al. [118].
In 2018, Piffoux et al. demonstrated that PEG facilitated membrane fusion of EVs with synthetic cargo-carrying liposomes. These complexed membrane-bound hybrid nanovesicles showed enhanced drug delivery efficacy (3–4 times) compared to only liposomal drugs and free drugs [129]. Interestingly, in a recent report, Rayamajhi et al. prepared bioengineered vesicles by hybridizing exosomes from murine macrophage with liposomes using a thin-film hydration technique followed by sequential membrane extrusion. Furthermore, they loaded DOX into the hybrid vesicles and found higher toxicity toward cancer cells and enhanced pH-triggered drug releases in the acidic tumor microenvironment [129]. In all these exercises, the targeting property of endogenous exosomes is coupled with the drug delivery property of the synthetic liposomes [130]. The added benefits of the hybrid nanovesicles are enhanced circulation stability, better pharmacokinetic profiles, less immunogenicity, etc. [131]. We envisage that many possibilities will be opened up in the future for this field of study to move ahead into clinical translation. Further research into the exosome-liposome hybrid will include the development of theranostic applications and personalized medicine.
Effect of protein corona on NPs, liposomes and exosomes
During the last decade, the astounding progress in nanotechnology brought around a heterogeneous and wide range of NP-based platforms for the diagnosis and treatment of cancers including several other diseases. In most of the cases, the drug-loaded liposomes/NPs are administered intravenously. When inside the body, during the exposure with the biological materials, a dynamic interactions occur between NPs and the circulating proteins. During this encounter, the set of proteins that get adsorbed to the NP surface, forming a spontaneous coating, is referred to as the protein corona. The protein corona plays an important role in making the NPs easily recognizable by the immune system [132] which attributes to the immunogenicity of NPs. Additionally, the protein corona may cause agglomeration and destabilization of NPs. This is one of the major hurdles in achieving efficient NP-based drug targeting, therefore, manipulation of NP surface has proven to be an alternative way for stabilizing NPs in circulation and prolonging their half-lives. Therefore, NPs-protein interaction must be carefully studied to predict and, thereby, control the fate of drug-loaded NPs inside the body. The protein corona, also impedes the efficient translation of liposome-mediated targeted drug delivery technology. After introduction into the circulation, the liposomal lipid surface immediately gets modified by the adsorption of the protein corona causing hindrance to the surface functionality. However, a long-standing protein corona with receptor-binding sites could possibly associate with the target cell long enough to activate the internalization machinery of the cell, thereby, triggering the liposome endocytosis and functional cargo delivery [133]. Nonetheless, researchers have shown that internalization of liposome–protein corona complexes by cancer cells was greater than that of control liposomes [134].
Though the protein corona on NPs have been looked upon, to date, very limited information is available regarding the protein corona of EVs, especially of exosomes. Earlier, researchers have hypothesized that, being biological and having specific surface markers, EVs, in theory, should not have any corona other than specific receptors for their surface antigens and this idea was not explored until recently. The formation of protein corona around EVs in blood plasma has been tested very recently [135]. It has been observed that plasma protein corona‐coated EVs had a higher density compared to that of nascent EVs and carries several newly associated proteins. By using advanced methods researchers identified that a few EV-corona proteins are shared with viruses and synthetic NPs in blood plasma. Other studies have indicated that presence of the protein corona may have an influence on the vesicle diameter [136] as well as on mobility [137]. It has also been shown that pro‐metastatic EVs could possibly establish a distinct association with low-density lipoprotein, and that interaction affects the internalization of EVs by monocytes [138]. Although our understanding of the protein corona composition, relevance, and manipulation has significantly expanded in the last few years, additional detailed studies are required to fully understand the functional significance of the protein corona around EVs.
Design strategies of NPs for effective clinical use
Till a couple of decades earlier, targeted drug delivery was extremely challenging. In the recent times our knowledge about the molecular biology in general as well as disease biomarkers, drug development and delivery mechanisms has substantially been improved and as a result several novel therapeutics have been developed. It’s been a while now that liposomes are being used to circumvent concerns associated with the low efficiency of anticancer drugs. Recently, the idea started to unfold that the limited success of liposomal drugs in clinical practice is mostly due to our poor understanding of the NPs–biomolecules interactions, that too, in species specific manner. Literature study suggests, enrichment of protein corona could be used to predict the targeting ability of NP formulations and optimization of NP design, and surface modifications toward clinical translation are currently being carried out. In a recent study, researchers have shown that the predicted targeting capability of liposome–protein corona complexes significantly correlated with cellular uptake in cancer models [139].
In this aspect, the engineered NPs hold tremendous promise to better disease diagnosis and treatment strategies by improving the stability and solubility of encapsulated cargos which could help overcome the limitations of conventional drug delivery systems. Apart from the surface modification, other physical properties of the NPs should be taken in to serious consideration as this could define the success or failure of the application. It is to be noted that beside size, the shape and the overall charge of the NPs are equally important factors that influences lymph node clearance of NPs, as well as cellular internalization and activation of the immune response. Earlier NP preparations were mainly spherical, but the recent advances in engineered NPs have resulted in the generation of a wide variety of shapes [140]. It has been demonstrated that, the non-spherical particles, with higher aspect ratios, shown to have a higher blood circulation time, and higher penetration capacities within tumors [141]. Therefore, detailed investigations and understanding of the interactions between biomolecules and the immune system are essential for designing of nanomaterials appropriate for anticancer therapy. Nonetheless, with proper understanding, scientists should have the opportunity of developing new multifunctional NPs and adapting the material design to the precise requirements.
The development of NP-based cancer detection, therapy, and theranostics approaches has exploded in recent years, and thus the importance of absorption, distribution, metabolism and excretion (ADME) investigations for future FDA approval. Nanocarriers tend to localize in the tumor through the leaky tumor vascularization by the enhanced permeability and retention (EPR) effect. The smaller (sub-200 nm) nanocarriers are excellent for manipulating the EPR effect at the tumor while protecting healthy cells. Moreover, ligands may be grafted onto the surface of nanotransporters to target cancer cells and enhance drug delivery systems (DDS) pharmacokinetics [142]. Bioinspired NPs have been extensively studied. Therapeutic carrier systems may enhance API effectiveness and reduce adverse effects [143]. Defective cellular absorption, targeting precision, conducive pharmacokinetics, and undesirable toxicity are common clinical challenges for all DDS. Liposomal formulations may not be able to distribute API to specified sites. The surface features, material composition, size, and production process can modulate the pharmacokinetic properties, circulation time, and biodistribution of liposome formulations. The lipid phase transition temperature (TC) governing the alteration of lipid phase from ordered gel phase to liquid crystalline phase is critical. Multiple criteria like bond unsaturation, hydrocarbon length, charge distribution, and lipid head group are important determinants regulating liposomal membrane permeability, drug release kinetics, and fluidity. The degree of unsaturation also governs the TC, where lipid bilayer with long and saturated hydrocarbons tends to be less permeable [144, 145]. The liposomal formulation method may also affect clearance and pharmacokinetics [143].
Exosomes and exosome-liposome hybrids are nanocarriers that may be bioengineered for achieving better biocamouflage, specific targeting, and enhanced API delivery [146]. When developing exosome-based formulations for preclinical and clinical use, pharmacokinetic aspects must be studied early to better predict tissue distribution, uptake, and elimination rate to avoid delays in clinical approval. Multiple studies are done to understand and maximize exosome uptake. Exosome surface glycoproteins augment milk-exosome endocytosis in vitro in Caco-2 cells and rat small intestine epithelial cells [147], and the role of clathrin-mediated endocytosis in exosome absorption in PC12 cells [148]. Exosome tissue biodistribution is critically dependent on membrane composition, surface features, and membrane transport. Exosome source plays a key role and is under investigation to avoid clearance by the MPS. As an example, macrophage-derived exosomes injected into mice’s circulation largely target MPS organs such as the liver, spleen, lungs, and kidneys and can be used to target the same [149, 150]. Bioengineering exosomes, including exosome-liposome hybrids, may include disguise methods for avoiding phagocytosis and long-term circulation of therapeutic exosomes [146]. The delivery method is also crucial since it may determine whether the exosomal-API is delivered to target tissue or not and if it is circulated throughout the body. Exosomes post-cellular uptake should operate in the cytosol without being trapped in endosomes or destroyed by acidic pH or hydrolases. Morisitha et al. modified tumor-derived exosomes by presenting a pH-sensitive fusogenic peptide and achieved cytosolic cargo delivery, and displayed class I tumor antigens better [151].
For nanocarriers, physiologically based pharmacokinetic (PBPK) modeling has been used to predict ADME, effectiveness, and toxicity of medicines and should include specified organs or tissues, along with physiological, physicochemical, and biochemical characteristics. The PBPK model and pharmacokinetics simulation have recently been used in liposomal and exosomal-based delivery systems and are discussed in detail in other reviews [152–154].
Future outlook
According to preliminary reports, the membrane-engineering technique will provide a novel way for creating rationally tailored exosomes as hybrid nanocarriers for use in sophisticated and targeted drug delivery systems. To assist the identification of low-copy targets in liquid biopsies, there has been a push to build and enhance downstream analytical tools. For example, in the field of NGS, broader panels with greater sequencing depth have been created. For NGS solutions, novel technologies such as molecular barcoding and background correction have evolved. Patients with low-copy targets will continue to be a problem because of the challenge of stochastic detection of single-digit copy numbers. There is also a need for a front end that is both fast and precise when it comes to sample acquisition and isolation. Therefore, validated sample collection and isolation kits for exoNA + cfDNA under cGMP manufacturing can enable robust next-generation liquid biopsy diagnostics. Liquid biopsy of CTC has been proven and authorized by the FDA to be a valuable prognostic tool for several forms of cancer, but its clinical application is still in its infancy in terms of general adoption. In conclusion, in the future, exosomes, detection agents, and therapeutic drugs can be used together to formulate diagnostic and therapeutic integrated NPs that could amalgamate their advantages for applications, both in noninvasive diagnostics and precision cancer treatments.
Acknowledgements
BB acknowledges Dr. Jay M. Lee for providing research support. MKP acknowledges S. Dubinett and B. Gomperts from UCLA for providing constant support and mentoring.
Competing interests
The authors declare no competing interests.
References
- 1.Sinha D, Roy S, Saha P, Chatterjee N, Bishayee A. Trends in research on exosomes in cancer progression and anticancer therapy. Cancers. 2021;13:326. doi: 10.3390/cancers13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modani S, Tomar D, Tangirala S, Sriram A, Mehra NK, Kumar R, et al. An updated review on exosomes: biosynthesis to clinical applications. J Drug Target. 2021;29:925–40. doi: 10.1080/1061186X.2021.1894436. [DOI] [PubMed] [Google Scholar]
- 5.De Leo V, Milano F, Agostiano A, Catucci L. Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles. Polymers. 2021;13:1027. doi: 10.3390/polym13071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Petersen GH, Alzghari SK, Chee W, Sankari SS, La-Beck NM. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Release. 2016;232:255–64. doi: 10.1016/j.jconrel.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Chen EC, Fathi AT, Brunner AM. Reformulating acute myeloid leukemia: liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML. Onco Targets Ther. 2018;11:3425–34. doi: 10.2147/OTT.S141212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olusanya T, Haj Ahmad R, Ibegbu D, Smith J, Elkordy A. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23:907. doi: 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–71. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Janowicz K, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020;9:436. doi: 10.3390/jcm9020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–4. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimaoka M, Kawamoto E, Gaowa A, Okamoto T, Park E. Connexins and integrins in exosomes. Cancers. 2019;11:106. doi: 10.3390/cancers11010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52. doi: 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 16.Khan YY, Suvarna V. Liposomes containing phytochemicals for cancer treatment—an Update. Int J Curr Pharm Res. 2016;9:20–4. doi: 10.22159/ijcpr.2017v9i1.16629. [DOI] [Google Scholar]
- 17.Mukherjee A, Paul M, Mukherjee S. Recent progress in the theranostics application of nanomedicine in lung cancer. Cancers. 2019;11:597. doi: 10.3390/cancers11050597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal V, Paul MK, Mukhopadhyay AK. 6-mercaptopurine and daunorubicin double drug liposomes—preparation, drug-drug interaction and characterization. J Liposome Res. 2008;15:141–55. doi: 10.1080/08982100500364081. [DOI] [PubMed] [Google Scholar]
- 20.Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- 21.Gu Z, Da Silva C, Van der Maaden K, Ossendorp F, Cruz L. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics. 2020;12:1054. doi: 10.3390/pharmaceutics12111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkhoury K, Koçak P, Kang A, Arab-Tehrany E, Ellis Ward J, Shin SR. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics. 2020;12:849. doi: 10.3390/pharmaceutics12090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharmacol Res. 2006;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomed. 2019;14:1937–52. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM, Kyung SY, et al. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2014;74:277–82. doi: 10.1007/s00280-014-2498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsson M, Edwards K. Liposomes, disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly(ethylene glycol)-phospholipids. Biophys J. 2003;85:3839–47. doi: 10.1016/S0006-3495(03)74798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep. 2016;6:38541. doi: 10.1038/srep38541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20:791. doi: 10.1186/s12885-020-07256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharmacol Res. 2015;32:2003–14. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 32.McAndrews KM, Che SPY, LeBleu VS, Kalluri R. Effective delivery of STING agonist using exosomes suppresses tumor growth and enhances antitumor immunity. J Biol Chem. 2021;296:100523. doi: 10.1016/j.jbc.2021.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh E, Lee EJ, Nam G-H, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–9. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristen AV, Ajroud-Driss S, Conceição I, Gorevic P, Kyriakides T. Obici L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener Dis Manag. 2019;9:5–23. doi: 10.2217/nmt-2018-0033. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 39.He J, Banizs A, Huang T, Dryden K, Berr S, Stone J, et al. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed. 2014;9:4223–30. [DOI] [PMC free article] [PubMed]
- 40.Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130–e. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shtam TA, Kovalev RA, Varfolomeeva E, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938–66. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee A, Madamsetty VS, Paul MK, Mukherjee S. Recent advancements of nanomedicine towards antiangiogenic therapy in cancer. Int J Mol Sci. 2020;21:455. doi: 10.3390/ijms21020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–309. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patra JK, Das G, Fraceto LF, Campos EVR. Rodriguez-Torres MdP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–7. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 49.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuo ST-Y, Chien JC-Y, Lai CP-K. Imaging extracellular vesicles: current and emerging methods. J Biomed Sci. 2018;25:91. [DOI] [PMC free article] [PubMed]
- 51.Lai CP, Tannous BA, Breakefield XO. Noninvasive in vivo monitoring of extracellular vesicles. Biolumin Imaging. 2014;19:249–58. doi: 10.1007/978-1-62703-718-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, Yan Y, Zou Q, Chen J, Li C. Superparamagnetic iron oxide nanoparticles for MR imaging of pancreatic cancer: potential for early diagnosis through targeted strategies. Asia Pac J Clin Oncol. 2016;12:13–21. doi: 10.1111/ajco.12437. [DOI] [PubMed] [Google Scholar]
- 53.Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. 2015;74:266–71. doi: 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marzola P, Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomed. 2016;11:2481–90. doi: 10.2147/IJN.S104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99mTc-HMPAO. Sci Rep. 2015;5:15636. [DOI] [PMC free article] [PubMed]
- 56.Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–93. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 57.Smania MA. Liquid biopsy for cancer screening, diagnosis, and treatment. J Am Assoc Nurse Pract. 2020;32:5–7. doi: 10.1097/JXX.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 58.Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297–312. [DOI] [PubMed]
- 59.Fici P. Cell-free DNA in the liquid biopsy context: role and differences between ctDNA and CTC marker in cancer management. Methods Mol Biol. 2019;4:47–73. doi: 10.1007/978-1-4939-8973-7_4. [DOI] [PubMed] [Google Scholar]
- 60.Vitale SR, Helmijr JA, Gerritsen M, Coban H, van Dessel LF, Beije N, et al. Detection of tumor-derived extracellular vesicles in plasma from patients with solid cancer. BMC Cancer. 2021;21:315. [DOI] [PMC free article] [PubMed]
- 61.Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144. [DOI] [PMC free article] [PubMed]
- 62.Jouida A, McCarthy C, Fabre A, Keane MP. Exosomes: a new perspective in EGFR-mutated lung cancer. Cancer Metastasis Rev. 2021;40:589–601. [DOI] [PMC free article] [PubMed]
- 63.Krug AK, Enderle D, Karlovich C, Priewasser T, Bentink S, Spiel A, et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann Oncol. 2018;29:700–6. doi: 10.1093/annonc/mdx765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif. 2019;17:100087. [DOI] [PMC free article] [PubMed]
- 65.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 66.Petersen AL, Hansen AE, Gabizon A, Andresen TL. Liposome imaging agents in personalized medicine. Adv Drug Deliv Rev. 2012;64:1417–35. doi: 10.1016/j.addr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Al-Jamal WT, Al-Jamal KT, Tian B, Cakebread A, Halket JM, Kostarelos K. Tumor targeting of functionalized quantum dot−liposome hybrids by intravenous administration. Mol Pharmacol. 2009;6:520–30. doi: 10.1021/mp800187d. [DOI] [PubMed] [Google Scholar]
- 68.Wang QCY. Multifunctional quantum dots and liposome complexes in drug delivery. J Biomed Res. 2018;32:91–106. doi: 10.7555/JBR.31.20160146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamichhane N, Udayakumar T, D’Souza W, Simone Ii C, Raghavan S, Polf J, et al. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:288. [DOI] [PMC free article] [PubMed]
- 70.Martínez-González R, Estelrich J, Busquets M. Liposomes loaded with hydrophobic iron oxide nanoparticles: suitable T2 contrast agents for MRI. Int J Mol Sci. 2016;17:1209. [DOI] [PMC free article] [PubMed]
- 71.Portnoy E, Nizri E, Golenser J, Shmuel M, Magdassi S, Eyal S. Imaging the urinary pathways in mice by liposomal indocyanine green. Nanomed Nanotechnol Biol Med. 2015;11:1057–64. doi: 10.1016/j.nano.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Xing J, Liu D, Zhou G, Li Y, Wang P, Hu K, et al. Liposomally formulated phospholipid-conjugated novel near-infrared fluorescence probe for particle size effect on cellular uptake and biodistribution in vivo. Colloids Surf B Biointerfaces. 2018;161:588–96. doi: 10.1016/j.colsurfb.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 73.Shen J, Kim H-C, Wolfram J, Mu C, Zhang W, Liu H, et al. A liposome encapsulated ruthenium polypyridine complex as a theranostic platform for triple-negative breast cancer. Nano Lett. 2017;17:2913–20. doi: 10.1021/acs.nanolett.7b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Goins B, Phillips WT, Bao A. Remote-loading labeling of liposomes with99mTc-BMEDA and its stability evaluation: effects of lipid formulation and pH/chemical gradient. J Liposome Res. 2010;21:17–27. doi: 10.3109/08982101003699036. [DOI] [PubMed] [Google Scholar]
- 75.Erdogan S, Medarova ZO, Roby A, Moore A, Torchilin VP. Enhanced tumor MR imaging with gadolinium-loaded polychelating polymer-containing tumor-targeted liposomes. J Magn Reson Imaging. 2008;27:574–80. doi: 10.1002/jmri.21202. [DOI] [PubMed] [Google Scholar]
- 76.Xu B, Ghaghada KB, Sato AF, Starosolski ZA, Berg J, Vail DM. Computed tomography imaging of solid tumors using a liposomal-iodine contrast agent in companion dogs with naturally occurring cancer. PLoS ONE. 2016;11:e0152718. [DOI] [PMC free article] [PubMed]
- 77.Zhou T, Cai W, Yang H, Zhang H, Hao M, Yuan L, et al. Annexin V conjugated nanobubbles: A novel ultrasound contrast agent for in vivo assessment of the apoptotic response in cancer therapy. J Control Release. 2018;276:113–24. doi: 10.1016/j.jconrel.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Wu B, Wan B, Lu ST, Deng K, Li XQ, Wu BL, et al. Near-infrared light-triggered theranostics for tumor-specific enhanced multimodal imaging and photothermal therapy. Int J Nanomed. 2017;12:4467–78. doi: 10.2147/IJN.S137835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madamsetty VS, Paul MK, Mukherjee A, Mukherjee S. Functionalization of nanomaterials and their application in melanoma cancer theranostics. ACS Biomater Sci Eng. 2019;6:167–81. doi: 10.1021/acsbiomaterials.9b01426. [DOI] [PubMed] [Google Scholar]
- 80.Muthu MS, Feng SS. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin Drug Deliv. 2012;10:151–5. doi: 10.1517/17425247.2013.729576. [DOI] [PubMed] [Google Scholar]
- 81.Martinez JO, Molinaro R, Hartman KA, Boada C, Sukhovershin R, De Rosa E, et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics. 2018;8:1131–45. doi: 10.7150/thno.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Wang B, Xia Y, Zhao S, Tian Z, Ning P, et al. In vivo and in situ activated aggregation-induced emission probes for sensitive tumor imaging using tetraphenylethene-functionalized trimethincyanines-encapsulated liposomes. ACS Appl Mater Interfaces. 2018;10:25146–53. doi: 10.1021/acsami.8b07727. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q, Liang C, Sun X, Chen J, Yang Z, Zhao H, et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc Natl Acad Sci USA. 2017;114:5343–8. doi: 10.1073/pnas.1701976114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 85.Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, et al. More efficient induction of HLA-A*0201-Restricted and carcinoembryonic antigen (CEA)–specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11:7554–63. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 86.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–66. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 87.Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19:160. [DOI] [PMC free article] [PubMed]
- 88.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol Res. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 89.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 90.Choi JU, Park IK, Lee YK, Hwang SR. The biological function and therapeutic potential of exosomes in cancer: exosomes as efficient nanocommunicators for cancer therapy. Int J Mol Sci. 2020;21:7363. doi: 10.3390/ijms21197363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segura E, Nicco C, Lombard BRR, Véron P, Raposo GA, Batteux FDR, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 92.Zimmer J, Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS ONE. 2008;3:e3377. [DOI] [PMC free article] [PubMed]
- 93.Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2014;1:1074–83. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. [DOI] [PMC free article] [PubMed]
- 95.Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olejarz W, Dominiak A, Żołnierzak A, Kubiak-Tomaszewska G, Lorenc T. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res. 2020;2020:1–11. doi: 10.1155/2020/6272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. [DOI] [PMC free article] [PubMed]
- 99.Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 100.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 101.Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, et al. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett. 2012;148:34–8. doi: 10.1016/j.imlet.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Yao Y, Chen L, Wei W, Deng X, Ma L, Hao S. Tumor cell-derived exosome-targeted dendritic cells stimulate stronger CD8+ CTL responses and antitumor immunities. Biochem Biophys Res Commun. 2013;436:60–5. doi: 10.1016/j.bbrc.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 103.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 104.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2:159–82. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuba E, Kono Y, Harada A, Yokoyama S, Arai M, Kubo K, et al. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials. 2013;34:5711–21. doi: 10.1016/j.biomaterials.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 106.Hirayama M, Tomita Y, Yuno A, Tsukamoto H, Senju S, Imamura Y, et al. An oncofetal antigen, IMP-3-derived long peptides induce immune responses of both helper T cells and CTLs. Oncoimmunology. 2016;5:e1123368. [DOI] [PMC free article] [PubMed]
- 107.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials. 2017;141:272–83. doi: 10.1016/j.biomaterials.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Yuba E, Yamaguchi A, Yoshizaki Y, Harada A, Kono K. Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials. 2017;120:32–45. doi: 10.1016/j.biomaterials.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 109.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636–45. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32:4141–8. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 112.Wagner MJ, Mitra R, McArthur MJ, Baze W, Barnhart K, Wu SY, et al. Preclinical mammalian safety studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA) Mol Cancer Ther. 2017;16:1114–23. doi: 10.1158/1535-7163.MCT-16-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yi K, Rong Y, Huang L, Tang X, Zhang Q, Wang W, et al. Aptamer–exosomes for tumor theranostics. ACS Sensors. 2021;6:1418–29. doi: 10.1021/acssensors.0c02237. [DOI] [PubMed] [Google Scholar]
- 114.He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–55. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cordonnier M, Chanteloup G, Isambert N, Seigneuric R, Fumoleau P, Garrido C, et al. Exosomes in cancer theranostic: diamonds in the rough. Cell Adh Migr. 2017;11:151–63. doi: 10.1080/19336918.2016.1250999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen D-W, Cheng L, Huang F, Cheng L, Zhu Y, Hu Q, et al. GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomed. 2014;9:921–35. doi: 10.2147/IJN.S53310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin C, Zhang X, Chen H, Bian Z, Zhang G, Riaz MK, et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;25:256–66. doi: 10.1080/10717544.2018.1425777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erten A, Wrasidlo W, Scadeng M, Esener S, Hoffman RM, Bouvet M, et al. Magnetic resonance and fluorescence imaging of doxorubicin-loaded nanoparticles using a novel in vivo model. Nanomed Nanotechnol Biol Med. 2010;6:797–807. doi: 10.1016/j.nano.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cittadino E, Ferraretto M, Torres E, Maiocchi A, Crielaard BJ, Lammers T, et al. MRI evaluation of the antitumor activity of paramagnetic liposomes loaded with prednisolone phosphate. Eur J Pharm Sci. 2012;45:436–41. doi: 10.1016/j.ejps.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Saraf S, Jain A, Tiwari A, Verma A, Panda PK, Jain SK. Advances in liposomal drug delivery to cancer: an overview. J Drug Deliv Sci Technol. 2020;56:101549.
- 122.Seleci M, Ag Seleci D, Scheper T, Stahl F. Theranostic liposome–nanoparticle hybrids for drug delivery and bioimaging. Int J Mol Sci. 2017;18:1415. doi: 10.3390/ijms18071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Madamsetty VS, Mukherjee A, Mukherjee S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front Pharmacol. 2019;10:1264. doi: 10.3389/fphar.2019.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19:242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. [DOI] [PMC free article] [PubMed]
- 126.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5:1700611. [DOI] [PMC free article] [PubMed]
- 127.Yang Y, Hong Y, Nam G-H, Chung JH, Koh E, Kim I-S. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv Mater. 2017;29:1605604. [DOI] [PubMed]
- 128.Gao X, Li S, Ding F, Fan H, Shi L, Zhu L, et al. Rapid detection of exosomal microRNAs using virus‐mimicking fusogenic vesicles. Angew Chem Int Ed. 2019;58:8719–23. doi: 10.1002/anie.201901997. [DOI] [PubMed] [Google Scholar]
- 129.Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 130.Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. [DOI] [PubMed]
- 131.Xu M, Yang Q, Sun X, Wang Y. Recent advancements in the loading and modification of therapeutic exosomes. Front Bioeng Biotechnol. 2020;8:586130. [DOI] [PMC free article] [PubMed]
- 132.Rampado R, Crotti S, Caliceti P, Pucciarelli S, Agostini M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “Stealthy” nanomaterials. Front Bioeng Biotechnol. 2020;8:166. [DOI] [PMC free article] [PubMed]
- 133.Caracciolo G. Liposome–protein corona in a physiological environment: challenges and opportunities for targeted delivery of nanomedicines. Nanomed Nanotechnol Biol Med. 2015;11:543–57. doi: 10.1016/j.nano.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Tasciotti E, Molinaro R, Taraballi F, Toledano Furman N, Sherman M, Parodi A, et al. Effects of the protein corona on liposome-liposome and liposome-cell interactions. Int J Nanomed. 2016;11:3049–63. doi: 10.2147/IJN.S109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tóth EÁ, Turiák L, Visnovitz T, Cserép C, Mázló A, Sódar BW, et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles. 2021;10:e12140. [DOI] [PMC free article] [PubMed]
- 136.Varga Z, Fehér B, Kitka D, Wacha A, Bóta A, Berényi S, et al. Size measurement of extracellular vesicles and synthetic liposomes: the impact of the hydration shell and the protein corona. Colloids Surf B Biointerfaces. 2020;192:111053. [DOI] [PubMed]
- 137.Skliar M, Chernyshev VS, Belnap DM, Sergey GV, Al-Hakami SM, Bernard PS, et al. Membrane proteins significantly restrict exosome mobility. Biochem Biophys Res Commun. 2018;501:1055–9. doi: 10.1016/j.bbrc.2018.05.107. [DOI] [PubMed] [Google Scholar]
- 138.Busatto S, Yang Y, Walker SA, Davidovich I, Lin WH, Lewis-Tuffin L, et al. Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J Nanobiotechnol. 2020;18:162. doi: 10.1186/s12951-020-00722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Palchetti S, Caputo D, Digiacomo L, Capriotti A, Coppola R, Pozzi D, et al. Protein corona fingerprints of liposomes: new opportunities for targeted drug delivery and early detection in pancreatic cancer. Pharmaceutics. 2019;11:31. doi: 10.3390/pharmaceutics11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24. doi: 10.1186/s40824-018-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Velpurisiva P, Gad A, Piel B, Jadia R, Rai P. Nanoparticle design strategies for effective cancer immunotherapy. J Biomed Sci. 2017;2:64–77. doi: 10.7150/jbm.18877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parak K. Rational drug loading of liposomes revisited. J Control Release. 2017;252:125. [DOI] [PubMed]
- 143.Ait-Oudhia S, Mager D, Straubinger R. Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology. Pharmaceutics. 2014;6:137–74. doi: 10.3390/pharmaceutics6010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madamsetty VS, Mukherjee A, Paul MK. Bioinspired nanoparticles-based drug delivery systems for cancer theranostics. In: Patra CAI, Ayaz M, Khalil AT, Mukherjee S, Ovais M, editors. Biogenic nanoparticles for cancer theranostics, chap 9. Elsevier; 2021. p. 189–228.
- 145.Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10:11.
- 146.Kim DH, Kothandan VK, Kim HW, Kim KS, Kim JY, Cho HJ, et al. Noninvasive assessment of exosome pharmacokinetics in vivo: a review. Pharmaceutics. 2019;11:649. [DOI] [PMC free article] [PubMed]
- 147.Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J Nutr. 2015;145:2201–6. doi: 10.3945/jn.115.218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–67. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed Nanotechnol Biol Med. 2018;14:195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 150.Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morishita M, Takahashi Y, Nishikawa M, Ariizumi R, Takakura Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol Pharmacol. 2017;14:4079–86. doi: 10.1021/acs.molpharmaceut.7b00760. [DOI] [PubMed] [Google Scholar]
- 152.He H, Yuan D, Wu Y, Cao Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics. 2019;11:110. [DOI] [PMC free article] [PubMed]
- 153.Rowland M. Physiologically-based pharmacokinetic (PBPK) modeling and simulations principles, methods, and applications in the pharmaceutical industry. CPT Pharmacomet Syst Pharmacol. 2013;2:e55. doi: 10.1038/psp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yellepeddi V, Rower J, Liu X, Kumar S, Rashid J, Sherwin CMT. State-of-the-art review on physiologically based pharmacokinetic modeling in pediatric drug development. Clin Pharmacokinet. 2018;58:1–13. doi: 10.1007/s40262-018-0677-y. [DOI] [PubMed] [Google Scholar]