Abstract
Graft-versus-host disease (GVHD) significantly contributes to patient morbidity and mortality after allogeneic hematopoietic cell transplantation (allo-HSCT). Sphingosine-1-phosphate (S1P) signaling is involved in the biogenetic processes of different immune cells. In the current study, we demonstrated that recipient sphingosine kinase 1 (Sphk1), but not Sphk2, was required for optimal S1PR1-dependent donor T-cell allogeneic responses by secreting S1P. Using genetic and pharmacologic approaches, we demonstrated that inhibition of Sphk1 or S1PR1 substantially attenuated acute GVHD (aGVHD) while retaining the graft-versus-leukemia (GVL) effect. At the cellular level, the Sphk1/S1P/S1PR1 pathway differentially modulated the alloreactivity of CD4+ and CD8+ T cells; it facilitated T-cell differentiation into Th1/Th17 cells but not Tregs and promoted CD4+ T-cell infiltration into GVHD target organs but was dispensable for the CTL activity of allogeneic CD8+ T cells. At the molecular level, the Sphk1/S1P/S1PR1 pathway augmented mitochondrial fission and increased mitochondrial mass in allogeneic CD4+ but not CD8+ T cells by activating the AMPK/AKT/mTOR/Drp1 pathway, providing a mechanistic basis for GVL maintenance when S1P signaling was inhibited. For translational purposes, we detected the regulatory efficacy of pharmacologic inhibitors of Sphk1 and S1PR1 in GVHD induced by human T cells in a xenograft model. Our study provides novel mechanistic insight into how the Sphk1/S1P/S1PR1 pathway modulates T-cell alloreactivity and validates Sphk1 or S1PR1 as a therapeutic target for the prevention of GVHD and leukemia relapse. This novel strategy may be readily translated into the clinic to benefit patients with hematologic malignancies and disorders.
Keywords: Sphk1, S1P, S1PR, GVHD, GVL, mitochondrial fission
Subject terms: Allotransplantation, T cells
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative option for the treatment of hematological malignancies and is primarily mediated by donor immune cells [1, 2]. More than 1 million HCTs have been performed, of which 40% were allogeneic [2, 3]. Acute graft-versus-host disease (aGVHD), mainly induced by transplanted donor T cells, is a major and life-threatening complication that drives a severe “cytokine storm” and damages multiple organs, contributing to high morbidity and mortality and thus limiting the success of allo-HSCT [4].
S1P, a bioactive lysophospholipid, is synthesized from sphingosine through Sphk1 or Sphk2, and its degradation by S1P lyase regulates cellular S1P abundance. Sphk1 and Sphk2 are highly homologous and execute distinct and overlapping functions [5, 6]. S1P produced by Sphk2 localizes to the nucleus and inhibits histone deacetylase (HDAC1/2) activity, leading to increased gene transcriptional activity [7]. In contrast, S1P generated by Sphk1 is mainly localized intracellularly or is secreted into the extracellular matrix [8, 9]. Extracellular spatial gradients of S1P, produced predominantly by hematopoietic and epithelial cells, tightly regulate and control fundamental processes such as hematopoietic cell trafficking, immune cell fate, and vascular integrity [10, 11]. We know that vertebrates process five G protein-coupled receptor S1PRs (S1PR1-5) that respond to extracellular S1P [12] and that S1P-S1PR-mediated signaling pathways contribute to the infiltration, activation, and survival of various immune cells. S1P gradients are essential for lymphocyte egress from lymphoid organs and homeostasis [10]. T cells with conditionally knocked out S1PR1 exhibit sustained deficiency of both CD4+ and CD8+ T cells in peripheral blood [13, 14]. S1PR1/4 on T cells and S1PR2 on lymphatic endothelial cells (LECs) are required for T-cell migration across LECs and into lymphatic vessels [15]. Activation of S1PR1 and subsequent activation of mTORC1 signaling directly antagonize Treg differentiation and promote Th1 differentiation [16]. S1PR1 phosphorylation on S351 is required to mediate IL-17-producing Th17 cells in adaptive immunity [17]. S1P-S1PR2 signaling controls B-cell maturation and clonal selection [18]. The positioning of NK cells in the medulla of lymph nodes is regulated by S1P-S1PR5 [19]. Herein, understanding the underlying mechanisms and more specific targeting of S1P/S1PR signaling molecules related to GVHD treatment are required for clinical translation.
LECs support the survival of T cells by secreting S1P via the transporter SPNS2, which depends on S1PR1 to maintain the mitochondrial contents of naive T cells and provide cells with the energy to continue their constant migration [20]. Mitochondrial ATP production is necessary for uropod maintenance and inhibits the mitochondria positioning at the trailing cell edge by blocking mitochondrial fission, which impairs lymphocyte migration [21–23]. These studies revealed that mitochondrial dynamics are among the driving forces for multiple T-cell biological functions positively associated with S1P/S1PR1 signaling. Mitochondrial proteins orchestrate the morphology of mitochondria in eukaryotic cells. The main protein implicated in mitochondrial fragmentation is GTPase dynamin-related protein 1 (Drp1) [24]. Optimal T-cell activation requires Drp1 and Drp1 phosphorylation (with activation site Ser616 and inhibitory site Ser637)-dependent accumulation of mitochondria at the immunological synapse (IS). The phosphorylation of the Ser616 site facilitates mitochondrial fission, while the phosphorylation of the Ser637 site plays the opposite role [24, 25]. Drp1 controls effective T-cell immune surveillance by regulating T-cell migration and proliferation through mTOR activation [26]. Similar to the upstream signaling of mTORC1, full activation of AKT requires phosphorylation at two key residues, Ser437 (S437) and Thr308 (T308) [27]. AKT and mTOR are serine/threonine kinases that play important roles in cell growth, proliferation, survival, and differentiation [28]. mTOR controls mitochondrial dynamics and Drp1 recruitment, and mTORC1 induces mitochondrial fragmentation [29]. The catalytic subunit of AMPK is encoded by two genes, PRKAA1 and PRKAA2, which encode AMPKα1 and AMPKα2, respectively. Only the AMPKα1 catalytic subunit is expressed in murine T cells, and AMPKα2 expression is not detected in resting or activated lymphocytes [30, 31]. In many types of cells, genetic deletion of PRKAA1 can abolish AKT-Ser473 activation [32], increase the number of mitochondrial fragments and promote mitochondrial fission [33]. In T cells, global deletion of PRKAA1 increases glycolysis and enhances the production of IFN-γ and IL-17 [34], and AMPK negatively regulates mTOR signaling and Drp1 phosphorylation in T cells [26, 35].
In the current study, we show that abrogation of Sphk1-dependent S1P/S1PR1 signaling, either by genetic excision or pharmacological blockade, reduced the T-cell alloreactivity and ameliorated pathogenicity in aGVHD by diminishing the mitochondrial fission induced by PRKAA1/AKT/mTOR/Drp1 activation. Targeting Sphk1 in recipient T cells or S1PR1 on donor T cells-impaired donor T-cell migration into target organs and attenuated pathogenic Th1, Th17, and Tc1 cell differentiation but exacerbated the suppression of iTreg generation. Interestingly, allogeneic CD4+ T cells were much more sensitive than CD8+ T cells to the Sphk1/S1P/S1PR1-mediated PRKAA1/AKT/mTOR/Drp1 activation axis, which may have contributed to the prevention of GVHD without impairing the GVL effect upon inhibition of the Sphk1/S1P/S1PR1 pathway. Taken together, our study reveals novel cellular and molecular mechanisms that explain how Sphk1/S1P/S1PR pathway regulates T-cell responses and provides a rationale and means to target this pathway for immunotherapy.
Materials and methods
Mice
C57BL/6 (H2Kb, Ly5.2+), BALB/c (H2Kd), FVB (H2Kq, Thy1.1+) and B6D2F1 (H2Kb/d) mice were purchased from the National Cancer Institute (Frederick, MD). Germline Sphk1−/− C57BL/6, Sphk2−/− C57BL/6, C57BL/6 (H2Kb, Ly5.1+), FVB (H2Kq, Thy1.2+), Rag1−/− C57BL/6, β-actin-luciferase transgenic C57BL/6 and FVB mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in the mouse-breeding facility at the Medical University of South Carolina (Charleston, SC). T-cell conditional S1PR1−/− mice (S1PR1flox/floxCD4-Cre, H2Kb, Ly5.2+) and PRKAA1−/− mice (PRKAA1flox/floxCD4-Cre, H2Kb, Ly5.2+) on a C57BL/6 background were used, and in these mice, both CD4+ and CD8+ T cells showed S1PR1 or PRKAA1 deficiency, as CD4Cre was expressed in the late double-negative to double-positive cell stage, leading to deletion of floxed genes in both CD4+ and CD8+ T cells. The breeder mice, S1PR1flox/floxCD4-Cre, PRKAA1flox/floxCD4-Cre, and NSG HLA-A2-Tg, were purchased from the Jackson Laboratory and bred in the mouse-breeding facility at the Medical University of South Carolina (Charleston, SC). β-Actin-luciferase transgenic S1PR1flox/floxCD4-Cre mice were generated by crossing β-actin-luciferase transgenic C57BL/6 mice with S1PR1flox/floxCD4-Cre mice, and luciferase transgenic with T-cell conditional S1PR1−/− mice were selected for in vivo experiments. Animals were maintained in pathogen-free facilities in the American Association for Laboratory Animal Care–accredited Animal Resource Center at the Medical University of South Carolina. All mouse procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina (IACUC #2018-00446).
Bone marrow transplantation, chimeric and xenograft models
Lethal-dose radiated was administered: 700 cGy for BALB/c mice and 1100 cGy (split) for C57BL/6 or B6D2F1 mice (X-RAD 320). T cells were purified from spleen and lymph node cells by negative selection using magnetic beads. MHC-mismatched BMT models were established as follows: 5 ×106 T-cell depleted bone marrow cells (TCD-BMCs) and 1 × 106 total T cells from FVB mice were transplanted into C57BL/6 mice, and 5 × 106 TCD-BMCs and 0.75 × 106 total T cells from C57BL/6 mice were transplanted into BALB/c mice. The haploidentical BMT model was established as follows: 5 × 106 TCD-BMCs plus 3 × 106 CD25-depleted T cells from C57BL/6 mice were transplanted into B6D2F1 mice. The MHC-matched BMT model was established as follows: 5 × 106 TCD-BMCs plus 1.25 × 106 CD44-depleted T cells from C3SWH mice were transplanted into C57BL/6 mice. As described in our previous study [36], recipient mice were monitored for weight loss and other clinical signs of GVHD twice per week. Clinical scores were assessed based on weight loss, posture, motility, fur texture, and skin integrity. Individual signs were scored from 0 to 2 for each criterion and from 0 to 10 overall. Recipients at the premorbid stage or with a body weight loss of more than 30% were euthanized and included in the count of mice killed by the treatment.
The GVL model was established with a haploidentical BMT model (C57BL/6 to D6D2F1) by additional P815 mastocytoma injection (5000 cells/mouse). Tumor growth was measured with bioluminescent imaging (BLI) using a Xenogen IVIS 200 preclinical in vivo imaging system (PerkinElmer) and analyzed by Living Image software (PerkinElmer). Tumor and GVHD mortality were distinguished by BLI signal intensity and clinical manifestation of GVHD.
A bone marrow chimeric model was established through syngeneic BMT.C57BL/6 mice (CD45.1) were lethally irradiated at 1100 cGy (split) and transplanted with 5 × 106 T cells from the depleted bone marrow of WT or Sphk1−/− donors on a C57BL/6 background (CD45.2). After 2 to 3 months, engraftment of donor hematopoietic cells was confirmed (>90%), and these BM chimeric mice were used as the recipients of a second bone marrow transplant.
As in our previous reports [37], a xenograft model was established with NSG HLA-A2+ mice, which were irradiated with 250 cGy and transfused with HLA-A2− human PBMCs (10 × 106 cells/mouse). Recipients were monitored for survival and body weight loss.
In some experiments, W146 (1 mg/kg daily) or PF543 (0.5 mg/kg every other day) was administered to the recipients from Day 0 to 14 after BMT. Representative samples of GVHD target organs were collected from recipients on Day 14 or 21 after BMT and subjected to pathology scoring or flow cytometry.
Flow cytometry antibodies and assay
The following antibodies were used for cell-surface staining: anti-CD4 (clone RM4-5, BD Biosciences), anti-CD8 (clone 53-6.7, BD Biosciences), anti-CD25 (clone pc61.5, eBioscience), anti-HK2q (clone KH114, BioLegend), anti-HK2b (clone AF6-88.5, BD Biosciences), anti-CD45.1 (clone A20, BD Biosciences), anti-CD45.2 (clone 104, BD Biosciences), anti-CD90.1 (clone H1S51, eBioscience), anti-CD90.2 (clone 30-H12, BD Biosciences), anti-CD69 (clone H1.2F3, BD Biosciences), anti-CD45R (clone RA3-6B2, BD Biosciences), anti-CD11b (clone M1/70, eBioscience), anti-CD11c (clone HL3, BD Biosciences), anti-F480 (clone T45-2342, BD Biosciences), anti-CD40 (clone 1C10, eBioscience), anti-CD86 (clone GL1, eBioscience), anti-IAb (clone AF6-120.1, BioLegend), anti-CD107 (clone 1D4B, BD Biosciences), anti–CXCR3-biotin (clone CXCR3-173, eBioscience), anti-CCR6 (clone 29-2L17, BioLegend), anti-α4β7 (clone DAK32, BD Biosciences), anti-CD44 (clone IM7, BioLegend), anti-CD62L (clone MEL-14, eBioscience), and anti–S1PR1-PE (clone #713412, R&D systems) antibodies. Biotinylated antibodies were detected using APCcy7 (BD Biosciences, catalog no. 554063) or PEcy7 (BD Biosciences, catalog no. 557598) conjugated to streptavidin. To measure intracellular cytokine levels, cells were stimulated for 4–5 h at 37 °C with PMA (100 ng/mL, Sigma‒Aldrich) and ionomycin (100 ng/mL; Calbiochem, EMD) in the presence of GolgiStop (BD Biosciences). Fixation and permeabilization were performed using Cytofix/Cytoperm Plus (BD Biosciences), and the samples were subsequently stained with the appropriate antibodies, including anti-IFNγ (clone XMG1.2, eBioscience), anti–IL-17 (clone TC11-18H10.1, BioLegend), anti–IL-4 (clone 11B11, BD Biosciences), anti–IL-5 (clone TRFK5, eBioscience), anti-FOXP3, (clone FJK-16s, eBioscience), anti-Ki67 (16A8, BioLegend), anti–pS6-AF467 (Cell Signaling Technology, clone D57.2.2E), anti–AKT (pS473)-V450 (BD Biosciences, catalog no. 560858) and anti-–AKT (pT308)-PE (BD Biosciences, catalog no. 558275). A Live/Dead Yellow Cell Staining Kit (catalog no. L-34968) and CFSE (catalog no. C1157) were purchased from Invitrogen. The data were analyzed with FlowJo Software (Tree Star).
S1P measurement
Mouse serum was used to extract lipids, and analyses were performed by the Lipidomics Shared Resource, Analytical Unit (MUSC). Further preparation of samples and advanced analyses of endogenous bioactive sphingolipids were performed on a Thermo Fisher TSQ Quantum liquid chromatography/triple-stage quadrupole mass spectrometer system operating in multiple reaction monitoring and positive ionization mode as previously described [38, 39].
Bioactive S1P was obtained from Dr Ogretmen’s laboratory. A similar dose of S1P-specific antibody (LT10002, Lpath INC) was used in the in vitro MLR assay, as previously reported [15].
Gut permeability assay
Mouse gut permeability was detected as described in our previous study (75). Food and water were withheld from all mice for 4 h on Day 7 post-BMT. FITC-dextran (Millipore Sigma) was administered by a 20-gauge 1.5-inch flexible intragastric gavage needle (Braintree Scientific) at a concentration of 800 mg/kg (approximately 16 mg/mouse). Four hours later, serum was collected from peripheral blood, diluted 1:1 with PBS, and analyzed on a plate reader (BioTek Instruments, catalog FLx800) at excitation and emission wavelengths of 485 nm and 535 nm, respectively. The concentrations of FITC-dextran in experimental samples were determined based on serum from the BM-alone group or naive mice.
Mitochondrial dynamics and Drp1 measurement
Purified CD25− CD4+ or CD8+ T cells were cocultured with allogenic TCD-splenocytes, classified as APCs, for 3 days. Activated T cells were enriched from bulk culture on the basis of CD25-positive cell detection using microbeads (Miltenyi Biotec). To assess the degree of mitochondrial fission, cell lysates were prepared from CD25+ T cells, and the protein levels of Drp1 (BD Biosciences, 611112), Drp1-Ser637 (Cell Signaling Technology, 4867), Drp1-Ser616 (Cell Signaling Technology, 3455 S) and AMPKα1 (ThermoFisher, MA5-14922) were measured by western blotting.
GFP-EV, GFP-Drp1 Ser637A, and GFP-Drp1 Ser637D DNA plasmid vectors were obtained from Dr Ogretmen’s laboratory [40]. T cells were transfected with Cell Line Nucleofector TM Kit V (Lonza, VCA-1003). After 6 h of transfection, the T cells were collected and cocultured with allogenic TCD-splenocytes, serving as APCs, for an additional 3 days.
Other experimental procedures
T-cell purification, T-cell depleted bone marrow, APC purification, lymphocyte isolation from recipient liver, gut, and spleen, and histological and western blot analysis were performed as described in our previously published work [36, 37, 41–44].
Statistical analysis
For comparison of recipient survival and tumor mortality rates in all experiments, log-rank (Mantel-Cox) test (GraphPad Prism software, version 8) was performed to determine statistical significance. To compare clinical scores and percentage of body weight loss, a nonparametric Mann‒Whitney U test was performed. In the analyses of cell frequency, cytokine level, and immune cell marker expression, 2-tailed Student t-test was performed to identify a statistical differences between the 2 groups. For multiple pairwise comparisons between conditions, ordinary 1-way or 2-way analysis of variance (ANOVA) with Sisak multiple comparisons test was performed to adjust P values. P < 0.05 was considered to be statistically significant, and the data are shown as the mean±1 SD.
Results
Recipient secretory S1P promotes acute GVHD development
S1P produced by Sphk1 is mainly localized intracellularly or secreted into the extracellular matrix, whereas S1P produced by Sphk2 is located and exerts biofunctions in the nucleus [9]. We compared the immune phenotypes of Sphk1−/− and Sphk2−/− in mice under hemostatic conditions; specifically, we assessed the numbers of DC, B cell, macrophage, monocyte, and T cell population. We found that WT, Sphk1−/− and Sphk2−/− mice carried similar proportions of various immune cell populations in their spleens, namely DCs (CD11C+MHCII+); monocytes (CD11b+Ly6c+, CD11b+Ly6c+); B cells (B220+CD11c−); macrophages (CD11b+F480+); CD4, naïve CD8 (CD44−CD62L+), and effector memory (CD44+CD62L−) T cells; and nTregs (CD4+CD25+Foxp3)(Fig. S1). In one exception, Sphk1−/− and Sphk2−/− mice showed an increased frequency of a CD4 central memory (CD44+CD62L+) T cell subset. To investigate the role played by S1P in regulating T-cell alloreactivity and pathogenicity in the induction of aGVHD, we isolated T cells from Sphk1−/− or Sphk2−/− mice and tested the ability of these cells to induce aGVHD in a murine model of major histocompatibility complex (MHC)-mismatched BMT. We observed that T cells deficient in either Sphk1 or Sphk2 showed a similar ability to induce aGVHD, which differed from that induced by WT T cells (Fig. S2A–C). These mutant T cells were similarly activated in allogeneic recipients regardless of the donor genotype (Fig. S2D, E), suggesting that the autocrine production of S1P was not essential for T-cell allogeneic responses in vivo.
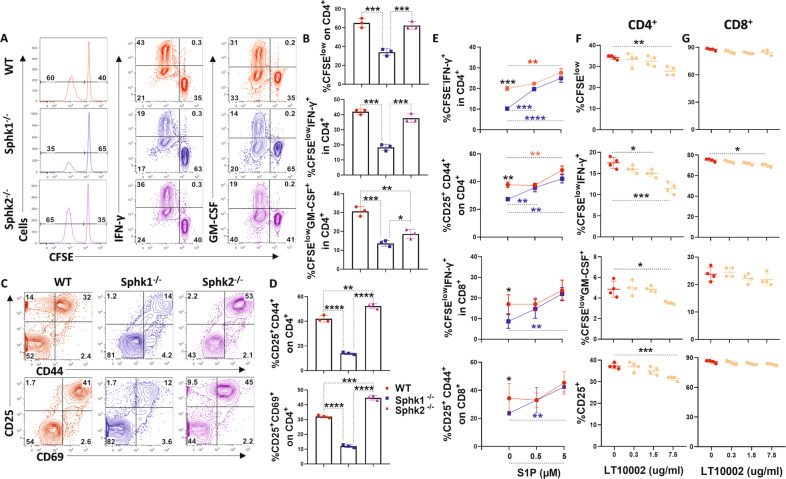
Indeed, S1P is generated predominantly by epithelial and hematopoietic cells [10], and the S1P gradient in plasma plays a major role in the egress of T lymphocytes from lymphoid organs and entry into the bloodstream and in the survival of T lymphocytes [45, 46]. Therefore, we hypothesized that recipient secretory S1P may play an essential role in enhancing donor T-cell pathogenicity in aGVHD. To test this hypothesis, we initially employed mixed lymphocyte reaction (MLR) assays and found that APCs deficient for Sphk1 but not Sphk2 showed a significantly reduced ability to activate allogeneic CD4+ T cells, as reflected by attenuated proliferation, activation, and cytokine production (Fig. 1A–D), which was reversed by exogenous S1P supplementation (Fig. 1E). Moreover, we observed similar results when S1P was blocked with a specific antibody in an in vitro MLR system (Fig. 1F, G).
Fig. 1.
The impact of Sphk1/S1P on T-cell alloreactivity. CFSE-labeled CD4+ T cells from FVB mice were cocultured with T-cell depleted (TCD)-splenocytes serving as APCs from WT, Sphk1−/− or Sphk2−/− mice for 4 days. A, B Representative flow figures and percentages of CFSE, CFSE-diluted, and IFN-γ+ or GM-CSF+ cells are shown on gated CD4+ T cells. C, D Representative dot plots and the average frequencies of CD25 and CD69 or CD44 double-positive expression on gated CD4+ T cells are shown. CFSE-labeled T cells from FVB mice were stimulated with WT or Sphk1−/− APCs in the absence or presence of S1P for 4 days. E Percentages of CFSE-diluted and IFN-γ+ cells and the average frequencies of CD25 and CD44 double-positive gated CD4+ or CD8+ T cells are shown. CFSE-labeled T cells from B6 mice were cocultured with BALB/c mouse APCs and treated with a specific anti-S1P antibody (0, 0.3, 1.5, 7.5 μg/ml) for 4 days. F, G Percentages of CFSE-diluted and IFN-γ+ or GM-CSF+ cells and the average frequencies of CD25 on gated CD4+ or CD8+ T cells are shown. The experiments were repeated 2 to 3 independent times. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001 and **** P < 0.0001
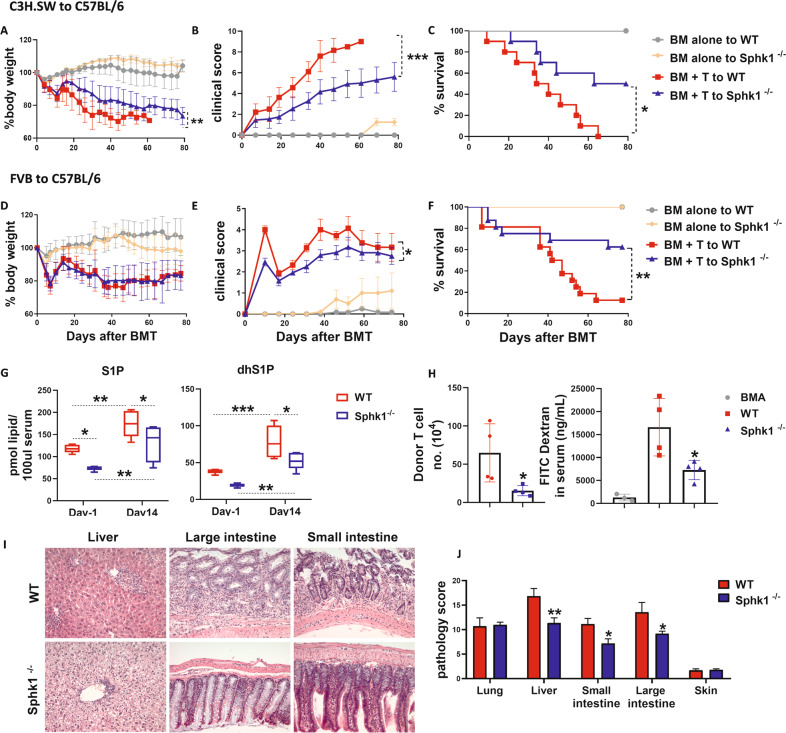
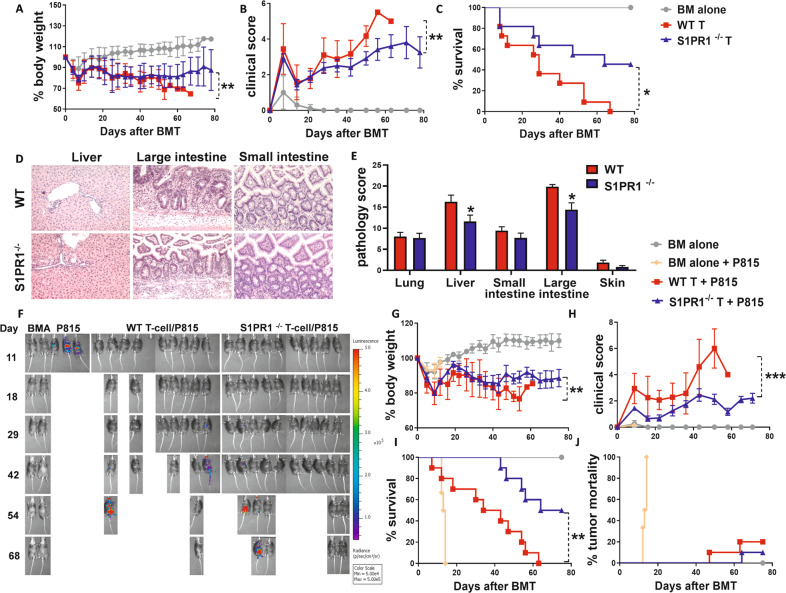
Considering these in vitro studies, we focused on the impact of host S1P on the development of aGVHD. Previous research revealed that mice that underwent Sphk1 germline KO showed a decrease in serum S1P recycling by as much as 50% [47]. Using a clinically relevant MHC-matched BMT model, we found that aGVHD was significantly less severe in Sphk1−/− recipients than in their WT counterparts, as reflected by survival, body weight loss and clinical scores (Fig. 2A–C). These results were reproduced in an MHC-mismatched BMT model (Fig. 2D–F). Compared with those in WT controls, the levels of S1P and dihydroS1P (dhS1P) were significantly reduced in Sphk1−/− recipients after allo-BMT. Notably, the levels of S1P and dhS1P were markedly increased in the recipients with aGVHD compared to those in the unmanipulated mice (Fig. 2G). Because the gastrointestinal tract is not only a major target organ of GVHD but also a critical amplifier of systemic GVHD [48], we further evaluated how Sphk1/S1P deficiency impacts recipient gut barrier integrity after allogeneic BMT. Compared to WT mice, Sphk1−/− mice showed improved gut barrier function after conditioning and donor T-cell transfer. Furthermore, fewer donor T cells infiltrated the gut of Sphk1−/− recipients than that of WT controls (Fig. 2H). aGVHD severity in the recipient liver and small and large intestines was verified by histopathologic analysis (Fig. 2I–J). Collectively, these results indicate that recipient S1P generated through Sphk1 expressed in the host promoted aGVHD development.
Fig. 2.
The role played by recipient Sphk1 in the development of aGVHD. Lethally irradiated C57BL/6 WT and Sphk1−/− mice (1100 cGy) were transplanted with 5 × 106 TCD-BM alone or with 1.25 × 106 CD44-depleted T cells isolated from a C3H cell population of SW donors. Recipients were monitored for A body weight loss, B clinical score, and C survival over time (n = 10 mice/group). Lethally irradiated C57BL/6 WT and Sphk1−/− mice (1100 cGy) were transplanted with 5 × 106 TCD-BM alone or with 1 × 106 total T cells isolated from FVB donors, and recipients were monitored for D body weight loss, E clinical score, and F survival over time (n = 10 mice/group). G Serum was collected from the recipient mice before and 14 days after allo-BMT and subjected to HPLC‒MS analysis for S1P and dhS1P measurement (n = 5 mice/group). Seven days after allo-BMT, WT and Sphk1−/− recipients were administered FITC-dextran (80 mg/mL, 200 μL/mouse) by gavage. H Gut permeability was evaluated on the basis of the concentration of secreted FITC-dextran in serum after an additional 4 h, and the absolute numbers of infiltrating donor T cells were calculated (n = 4 mice/group). Tissues from WT and Sphk1−/− FVB mice and transplanted into C57BL/6 mouse recipients were collected on 21 days after allo-BMT and analyzed for pathology (I, J). Representative photomicrographs showing hematoxylin and eosin staining of liver, small and large intestine at the original 200 x magnification, and pathology the scores are shown (n = 10 mice/group). “BMA” in the summary graph indicates BM alone. The experiments were repeated 2 independent times, and the combined data are presented. Log-rank (Mantel‒Cox) test (C and F) and nonparametric Mann‒Whitney U tests (A, B, D, and E) were performed to compare groups. A two-way ANOVA test (G) was used to compare the levels of S1P and dhS1P between groups. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001
Recipient Sphk1/S1P contributes to T-cell distribution and differentiation
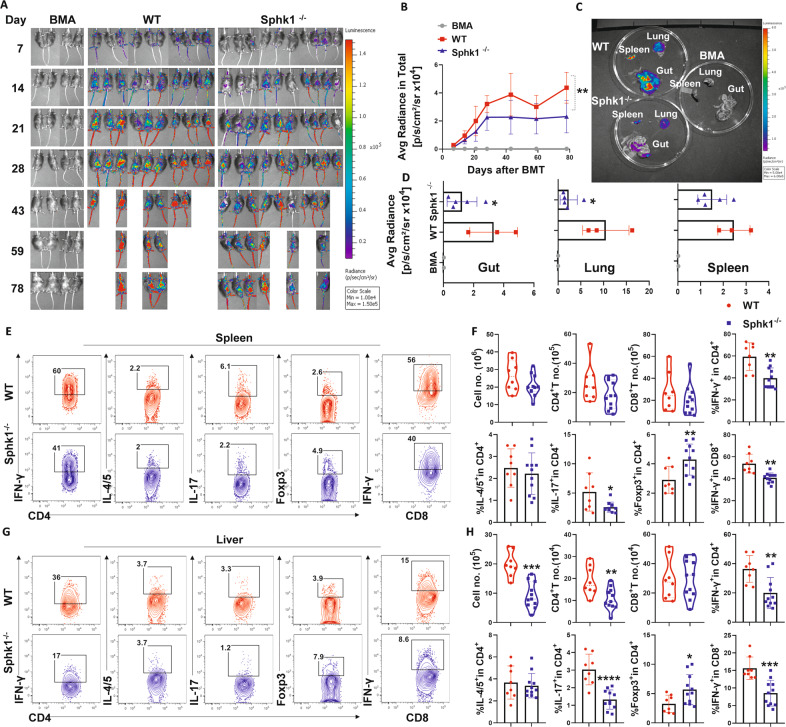
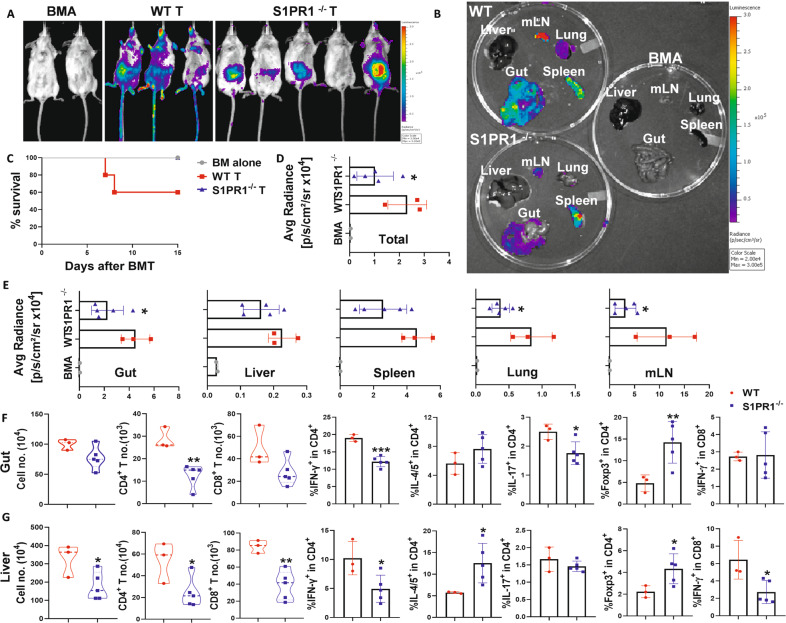
The development of aGVHD requires donor T-cell expansion, cytokine production, and migration into the target organs of recipients [49]. Secretory chemoattractant S1P allows T cells to maintain mitochondrial content, thereby maintaining ATP production, which in turn plays a crucial role in naive T-cell migration and survival [20]. We sought to determine how recipient-derived S1P affects the fate of donor T cells after allo-BMT. To evaluate the impacts of S1P on donor T-cell migration and expansion, we isolated donor T cells from β-actin-driven luciferase transgenic mice on a FVB background and transferred them into lethally irradiated C57BL/6 mouse recipients. The migration and expansion of donor T cells were monitored through bioluminescent imaging (BLI) over time. The overall abundance of donor T cells in Sphk1−/− recipients was significantly reduced, as reflected by decreased total-body BLI signal intensity (Fig. 3A, B). Furthermore, Sphk1−/− recipients showed significantly reduced BLI signal intensity emitted by donor T cells in GVHD target organs, including the gut and lungs, but not in lymphoid organs or the spleen (Fig. 3C, D). Th1, Th17 and Tc1 cell subsets have been implicated in mediating aGVHD pathogenesis, whereas iTregs play a protective role in disease development [41, 50, 51]. We further examined donor T-cell differentiation and found that donor CD4+ and CD8+ T cells produced lower levels of IFN-γ and IL-17, while CD4+ T cells exhibited increased Foxp3 expression in the spleen of Sphk1−/− recipients compared to that in WT recipients 14 days after BMT (Fig. 3E, F, Fig. S3A). Consistently, significantly fewer donor T cells were found in the livers of Sphk1−/− recipients than in WT controls (Fig. 3H). In the absence of recipient Sphk1, donor lymphocytes produced significantly less IFN-γ and IL-17 while expressing more Foxp3 in recipient livers (Fig. 3G, H, Fig. S3B). The expression of Sphk1 in recipients did not impact the levels of TNF-α, granzyme B, perforin or CD107a on donor CD8+ T cells (Fig. S4A–D). On the other hand, donor CD4+ and CD8+ T cells underwent comparable rates of cell death in WT or Sphk1−/− recipients after allo-BMT (Fig. S5A–D). Taken together, these results show that recipient Sphk1/S1P not only contributed to donor T-cell migration/expansion but also exerted impacts on T-cell differentiation, enhancing their pathogenicity in aGVHD.
Fig. 3.
The effect of host secretory S1P on donor T-cell migration and differentiation after allo-BMT. Lethally irradiated WT and Sphk1−/− mice were transplanted with 5 × 106 TCD-BM isolated from FVB donors plus 0.5 × 106 total T cells isolated from β-actin-luciferase transgenic FVB mice (n = 10 mice/group). T-cell migration was monitored using bioluminescent imaging (BLI). Macrophotos show BLI for A the total body over time and C individual organs in a region of interest (ROI) summary (B, D). Lethally irradiated WT and Sphk1−/− mice (H2b) were transplanted with 5 × 106 TCD-BM (Thy1.2+) plus 0.75 × 106 total T cells (Thy1.1+) isolated from FVB mice (H2q). Two weeks after allo-BMT, the spleens and livers of the recipients were collected and analyzed by flow cytometry (n = 8–11 mice/group). The numbers of infiltrated donor cells, representative dot plots, and the average levels of IFN-γ, IL4/5, IL-17, and Foxp3 on gated donor CD4+ or CD8+ T cells from E, F spleens and G, H livers are shown. “BMA” in the summary graph indicates BM alone. The experiments were repeated 2 times independently, and the combined data are presented. Nonparametric Mann‒Whitney U test (C) was performed to compare between groups. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Sphk1/S1P increases host APC activation, contributing to GVHD development
APCs are critical for the initiation and maintenance of GVHD [52, 53], and they are rapidly activated after conditioning [54]. We showed that Sphk1−/− APCs showed a reduced capacity to activate allogeneic T cells, which was reversed by exogenous S1P supplementation (Fig. 1). Furthermore, upon LPS stimulation, Sphk1 but not Sphk2 was required for the optimal activation of APCs, including B cell, dendritic cell, monocyte, and macrophage activation, as reflected by the expression of MHC II, CD40, and CD86 (Fig. S6A, B). Consistent with the in vitro experiments, Sphk1−/− APCs (B cells, DCs, and macrophages) displayed lower levels of MHC II, CD40 and CD86 expression than WT controls soon after allo-BMT (Fig. S7).
Given that S1P can be produced by various hematopoietic cells, including macrophages and dendritic cells [55], to further evaluate the impact of S1P produced by host hematopoietic APCs in the induction of aGVHD, we established chimeric mice in which Sphk1 was deficient in hematopoietic cells by transferring BM from WT or Sphk1−/− donors (Ly5.2+) into lethally irradiated WT C57BL/6 Ly5.1+ mice (Fig. S8A). When using these mice as recipients for allo-BMT, the Sphk1−/− chimeric mice showed attenuated GVHD severity, as reflected by attenuated body weight loss and lower clinical scores than WT chimeric recipients (Fig. S8B, C). However, in contrast to recipients with germline Sphk1 deficiency, Sphk1−/− BM-chimeric recipients died from GVHD after only slightly prolonged survival compared to WT controls (Fig. S8D). Mechanistically, genetic deletion of Sphk1 in hematopoietic cells significantly reduced the percentage of DCs and macrophages in chimeric recipients and the expression of MHCII and CD86 in host DCs and B cells (Fig. S8E), which subsequently attenuated IFN-γ production by donor CD4+ T cells early posttransplantation (Fig. S8F). Collectively, these results indicate that Sphk1/S1P increased host APC activation soon after allo-BMT, which partially contributed to the pathogenesis of aGVHD. S1P produced by both hematopoietic and nonhematopoietic compartments contributed to the development of aGVHD.
Sphk1-derived S1P promotes alloreactivity of CD4+ but not CD8+ T cells via S1PR1 activation
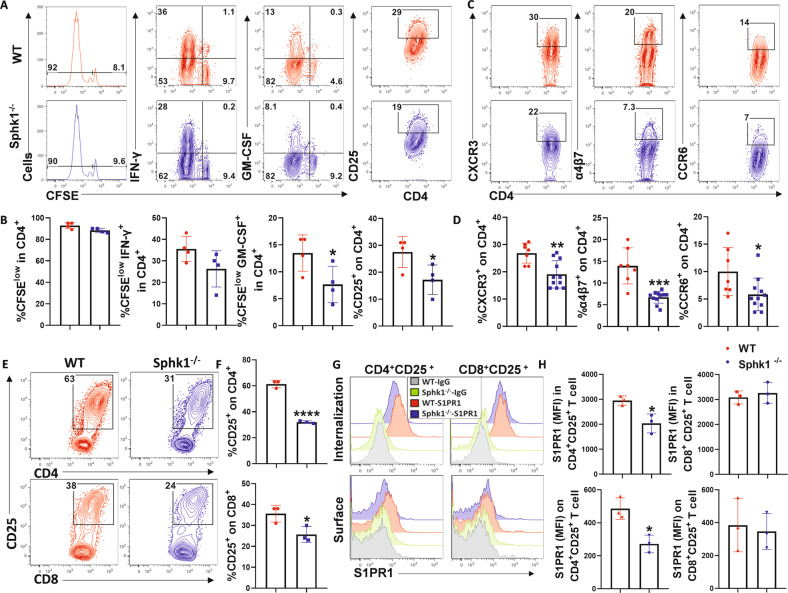
Our data illuminated that T-cell differentiation and distribution were modulated by Sphk1-generated S1P, which played a dominant role in deteriorating aGVHD (Fig. 3). To further define whether CD4+ and CD8+ T cells respond consistently to host-S1P, we studied allogeneic T-cell activation soon after transfer into WT or Sphk1−/− mice and found that the proliferation (CSFE dilution) and IFN-γ production of donor CD4+ and CD8+ T cells were similar in the spleen of WT and Sphk1−/− recipients. However, activation (CD25 expression) and GM-CSF production in donor CD4+ but not CD8+ T cells were significantly reduced in Sphk1−/− recipients compared to WT controls (Fig. 4A, B, Fig. S9A, B). Similar results were observed in recipient lymph nodes (Fig. S10A, B). These results demonstrated that host S1P plays a dominant role in promoting the activation and cytokine production of donor CD4+ T cells but not CD8+ T cells, at least in the early stage of allogeneic response. Α4β7 integrin [56, 57] and chemokine receptors such as CXCR3 [58] and CCR6 [59] have been shown to promote donor T-cell migration to GVHD target tissues. Interestingly, the frequencies of α4β7-, CXCR3- or CCR6-expressing cells among donor CD4+ but not CD8+ T cells were significantly reduced in Sphk1−/− recipients compared to those in WT controls 14 days after allo-BMT (Fig. 4C, D, Fig. S9C, D). Moreover, after being stimulated with allogeneic WT or Sphk1−/− APCs, CD8+ T cells showed comparable CTL activity (Fig. S9E, F). Taken together, these results demonstrate that Sphk1-derived S1P differentially regulated the allogeneic responses of CD4+ and CD8+ T cells.
Fig. 4.
S1P/Sphk1 upregulates S1PR1 expression in alloantigen-activated CD4+ T cells but not CD8+ T cells. Purified T cells from FVB mice (H2Kq) were labeled with CFSE and transferred into lethally irradiated WT or Sphk1−/− (H2Kb) mice at 2 × 106 cells per mouse. Four days after cell transfer, recipient spleens were harvested and analyzed by flow cytometry. A, B Representative flow figures and percentages of CFSE, CFSE-diluted, and IFN-γ+ or GM-CSF+ and CD25+ cells are shown on gated live H2Kq+ CD4+ cells. (n = 4 mice/group). C, D The same experiments are shown in Fig. 2E–H. Representative dot plots and the average frequencies of CXCR3, α4β7, and CCR6 expression on gated donor CD4+ T cells from the recipient spleen are shown. Purified FVB T cells were cocultured with APCs from WT or Sphk1−/− mice for three days. E Representative dot plots and F the average levels of CD25 on gated CD4+ or CD8+ T cells are shown. G Representative histograms and H graphical summary for MFI of S1PR1 on (surface staining) or in (permeable staining) gated CD4+ CD25+ T cells and CD8+ CD25+ T cells are shown. PE-conjugated anti-rat IgG was used as the negative control antibody. The experiments were repeated 2 to 3 times. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Cells sense environmental S1P through S1PRs on their surface. Among these receptors, S1PR1 directs T-lymphocyte trafficking [13, 15] and activates various cytokine-related signaling pathways [16, 17]. Upon interacting with Sphk1−/− APCs, although both CD4 and CD8 T cells showed reduced frequency of activated cells (Fig. 4E, F), activated CD4+ but not CD8+ T cells showed decreased abundance of total or membrane S1PR1 (Fig. 4G, H). Additionally, STAT1 [60] and STAT3 [61] signals have been reported to mediate the activation and expression of S1PR1. Stimulation with Sphk1−/− APCs decreased the phosphorylation of STAT3 in alloreactive CD4+ T cells but not in alloreactive CD8+ T cells (Fig. S11A, B). Collectively, these results indicate that Sphk1-derived S1P promoted CD4+ T-cell alloreactivity and modulated S1PR1 expression in the activated subsets but not in CD8+ T cells.
S1PR1 is required for the optimal pathogenicity of donor T cells in aGVHD but is dispensable for the GVL response
Given that S1PR1 expression was differentially regulated in the activated subsets of allogeneic CD4+ and CD8+ T cells via Sphk1-generated S1P in APCs (Fig. 4G, H), we asked whether S1PR1 directly influences T-cell alloreactivity in acute GVHD and GVL activity. After demonstrating that WT and S1PR1flox/floxCD4-Cre mice presented with similar frequencies of CD4, CD8, and associated memory T-cell subsets, including naive (CD44−CD62L+), central memory (CD44+CD62L+), and effector memory (CD44+CD62L−) T cells and nTregs (CD4+CD25+Foxp3) in the thymus and spleen (Fig. S12A–D), we evaluated the role played by S1PR1 in regulating T-cell pathogenicity during GVHD induction using an MHC-mismatched allo-BMT mouse model (BM T cells from C57BL/6 mice transplanted in to BALB/c mice). As expected, the recipients transplanted with WT T cells developed severe and lethal aGVHD, whereas the recipients receiving S1PR1−/− T cells survived longer with less body weight loss and lower GVHD severity scores (Fig. 5A–C). Clinical manifestations were confirmed via a pathological analysis of multiple GVHD target organs (Fig. 5D, E). Furthermore, S1PR1−/− CD4+ T cells expressed significantly lower levels of α4β7, CXCR3 and CCR6 in the recipient spleen (Fig. S13A), and fewer of these cells differentiated into toward Th1, Th17, and Tc1 cells but more of these cells differentiated into iTregs in the recipient spleen and liver on 21 days after allo-BMT (Fig. S13B, C).
Fig. 5.
The role played by S1PR1 on donor T cells in GVH and GVL responses. Lethally irradiated BALB/c mice (700 cGy) underwent BMT with 5 × 106 TCD-BMCs alone or with 0.75 × 106 total T cells isolated from WT or S1PR1flox/floxCD4-Cre mice, and recipients were monitored for A body weight loss, B clinical score and C survival over time (n = 10 mice/group). Tissues from BALB/c recipients were collected on 21 days after allo-BMT and analyzed for pathology. D Representative photomicrographs showing hematoxylin and eosin staining of livers, small and large intestines at the original magnification 200x, and E pathology scores are shown (n = 6–10 mice/group). Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD-BMCs alone or with 3 × 106 total CD25-depleted T cells isolated from WT or S1PR1flox/floxCD4-Cre mice and with or without 5000 β-actin-luciferase-transduced P815 mastocytoma cells. Mice were monitored for tumor burden. F BLI images taken throughout the experiment were used to determine tumor growth. G Body weight loss, H clinical score, I survival, and J tumor mortality of recipients were monitored over time (n = 10 mice/group). The experiments were repeated 2 to 3 independent times, and the combined data are presented. Log-rank (Mantel‒Cox) test (C, I, and J) and nonparametric Mann‒Whitney U test (A, B, G and H) were performed to compare between groups. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001
To further test the effect of S1PR1 on GVL activity, we employed a haploidentical BMT model (BM T cells from C57BL/6 mice transplanted in to B6D2F1 mice) with P815 mastocytoma infiltration. All recipients of P815 without donor T cells died from leukemia relapse within 15–20 days of BMT, whereas recipients of P815 with WT T cells died from GVHD within 60 days of BMT. In contrast, the recipients of P815 with S1PR1−/− T cells showed significantly less severe GVHD, as reflected by survival, body weight loss, and clinical scores (Fig. 5G–I). Donor T cells, regardless of their levels of S1PR1 expression, showed abrogated or substantially delayed progression to leukemia relapse after HCT (Fig. 5F, J). Taken together, these results demonstrate that S1PR1 was required for the optimal pathogenicity of donor T cells but was dispensable for the GVL response.
S1PR1 regulates the migration and differentiation of donor T cells in aGVHD target organs
To further determine whether damage to GVHD target organs is associated with S1PR1-mediated donor T-cell migration and/or expansion, we transferred purified β-actin-luciferase-transduced T cells from WT or S1PR1flox/floxCD4-Cre mice into irradiated allogeneic BALB/c recipients. Fourteen days after allo-BMT, two of five recipients of the WT T cells died from aGVHD (Fig. 6C), and the remaining recipients exhibited a greater abundance of donor T cells, as reflected by total-body BLI signal intensity, compared to those who received S1PR1−/− T cells (Fig. 6A, D). Furthermore, S1PR1 deficiency substantially decreased donor T-cell infiltration into target organs, including the gut, lungs, and mLNs, but not the spleen (Fig. 6B, E) and resulted in lower production of IFN-γ and IL-17 by T cells while promoting Foxp3 expression in recipient intestine and liver cells (Fig. 6F, G). These results demonstrate that S1PR1 regulates T-cell migration and differentiation and thus impacts T-cell pathogenicity in aGVHD.
Fig. 6.
S1PR1 regulates T-cell differentiation and migration. Lethally irradiated BALB/c mice underwent BMT with 5 × 106 TCD-BMCs alone (Ly5.1+) or plus 0.5 × 106 β-actin-luciferase transgenic total T cells isolated from WT or S1PR1flox/floxCD4-Cre mice (Ly5.2+). Two weeks after allo-BMT, T-cell expansion and migration were monitored using BLI. Macrophotos show the BLI of A the total body and B individual organs with D, E a region of interest (ROI) summary. C Mouse survival is shown (n = 3-5 mice/group). Recipient livers and intestines were collected and analyzed with flow cytometry. The numbers of infiltrated donor cells and the average levels of IFN-γ, IL4/5, IL-17, and Foxp3 on gated donor CD4+ or CD8+ T cells from F livers and G intestines are shown. “BMA” in the summary graph indicates BM alone. The experiments were repeated 2 independent times. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001
Pharmacological inhibition of the Sphk1/S1P/S1PR1 pathway alleviates aGVHD while maintaining the GVL response
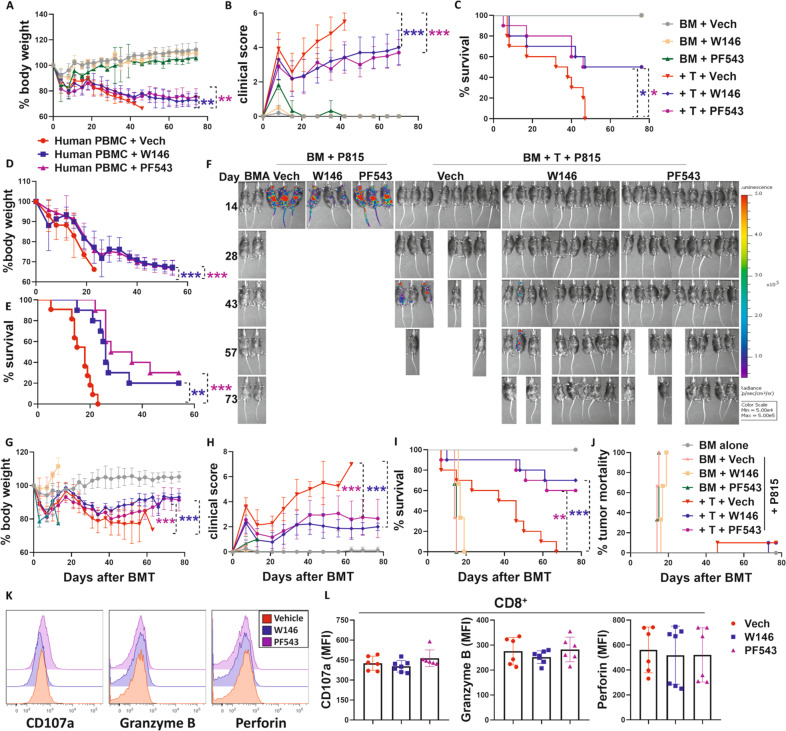
To investigate potential clinical translation, we next evaluated the efficacy of Sphk1/S1P/S1PR pathway blockade in GVHD prevention via inhibition of Sphk1 activation using the small-molecule PF543 [62] or S1PR1 using the specific antagonist W146 [63, 64]. Compared with vehicle-treated controls, MHC-mismatched BMT model mice, treated with PF543 or W146 showed less body weight loss, lower clinical GVHD severity scores, and prolonged survival (Fig. 7A–C). Human PBMCs from an HLA-A2– health donor were transferred into sublethally irradiated NSG mice, and these transgenic mice expressed human HLA-A2 but were deficient for murine MHC-I. Compared to vehicle-treated controls, the NSG recipients treated with PF543 or W146 showed significantly ameliorated GVHD, as reflected by less body weight loss and prolonged survival (Fig. 7D, E). To determine whether pharmacological targeting of Sphk1 or S1PR1 would impact the GVL response, we injected a haploidentical BMT model with P815 mastocytoma cells. All recipients of P815 cells without donor T-cell infusion died from leukemia relapse within 15–20 days after BMT, regardless of treatment, whereas most of the recipients with donor T cells died from GVHD after BMT. Compared to that of the vehicle control, PF543 or W146 treatment significantly reduced GVHD severity, as reflected by reduced body weight loss, lower clinical severity scores, and prolonged survival (Fig. 7G–I). Importantly, the recipients treated with W146 or PF543 maintained sufficient GVL capacity, as the vast majority of these recipients did not undergo leukemia relapse (Fig. 7F, J).
Fig. 7.
Pharmacological inhibition of S1P/S1PR1 signaling impacts GVH and GVL responses. Lethally irradiated BALB/c mice (700 cGy) underwent transplantation with 5 × 106 TCD-BMCs alone and with or without 0.75 × 106 total T cells isolated from C57BL/6 donors. Recipient mice were injected i.p. with Sphk1 inhibitor (PF543) at 0.5 mg/kg or S1PR1 inhibitor (W146) at 1 mg/kg every other day from Day 0 to Day 14. Recipients were monitored for A body weight loss, B clinical score, and C survival over time (n = 10 mice/group). NSG-HLA-A2+ mice were irradiated (250 cGy) and transfused with HLA-A2− human PBMCs (10 × 106/mouse). Recipient (D) body weight loss and E survival were monitored for 60 days (n = 10 mice/group). Recipients were treated with PF543 or W146 as shown in A–C. Lethally irradiated B6D2F1 recipients (1100 cGy) were transplanted with 5 × 106 TCD-BMCs alone or with 3 × 106 total CD25-depleted T cells isolated from C57BL/6 donors with or without 5000 β-actin luciferase-transduced P815 mastocytoma. Recipients were treated with PF543 or W146 as in A–C. G Body weight loss, H clinical score, I survival, and J tumor mortality of recipients were monitored over time. F BLI images taken throughout the experiment were used to reflect tumor growth. In a separate experiment, spleens were collected from recipients on14 days after allo-BMT. K, L Representative histograms and summary graphs of CD107a, granzyme B, and perforin level on gated donor CD8+ T cells are shown (n = 6-7 mice/group). “BMA” in the summary graph indicates BM alone. The experiments were repeated 2 independent times, and combined data are presented. Log-rank (Mantel‒Cox) test (C, E, I, and J) and nonparametric Mann‒Whitney U test (A, B, D, G, and H) were performed to compare groups. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001
Mechanistically, upon treatment with PF543 or W146 in vitro, fewer T cells differentiated into Th1 and Th17 cells subsets but more differentiated into iTregs under the respective polarization conditions (Fig. S14A, B). Then, we measured the alloreactivity and CTL activity of donor T cells 14 days after GVH/GVL induction in vivo. Treatment of recipients with W146 or PF543 significantly reduced the activation of both donor CD4+ and CD8+ T cells (Fig. S15A), but the expression of α4β7 and CXCR3 was reduced only on CD4+, not CD8+ T cells (Fig. S15B). Moreover, pharmacological inhibition of Sphk1 or S1PR1 also reduced IFN-γ, IL-17, and TNF-α production in CD4+ T cells while enhancing iTreg generation (Fig. S15C, D). In CD8+ T cells, W146 or PF543 treatment reduced IFN-γ production but did not affect the levels of CD107a, perforin, granzyme B, or TNF-α in recipient spleens and livers (Fig. 7K, L, Fig. S15E).
To further determine the specificity of W146 and PF543, we transferred allogeneic T cells into WT and Sphk1−/− mice and treated the recipients with vehicle control, W146 or PF543. Fourteen days after allo-BMT, the activation, cytokine production, and chemokine receptor expression of donor T cells were measured in the recipient spleens and livers. We consistently observed that treatment with W146 or PF543 reduced the activation and cytokine production of donor T cells in WT recipients. In contrast, treatment with W146 or PF543 exerted no impact on the donor T-cell response in Sphk1−/− mice (Fig. S16 A–D). These results indicate that the effect of PF543 and W146 on the T-cell response was indeed Sphk1/S1P/S1PR1 pathway-dependent in vivo.
Hence, we conclude that specific pharmacological inhibition of the Sphk1/S1P/S1PR1 pathway profoundly ameliorated aGVHD by modulating donor T-cell activation, migration, and differentiation while maintaining the GVL response via preservation of CD8 T cell CTL activity.
The Sphk1/S1P/S1PR1 pathway promotes CD4+ T-cell alloreactivity via mitochondrial fission
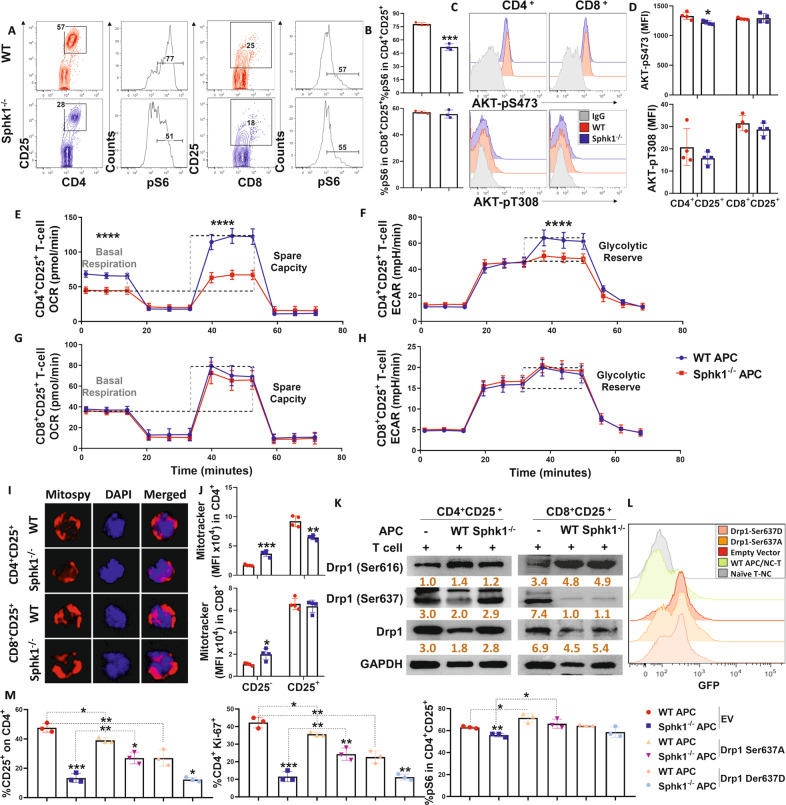
Recent studies have demonstrated that lymphatic endothelial [20] and monocyte-derived [65] S1P is essential for T-cell immune responses. S1P is required not only for the circulation of naive T cells but also for their survival because it modifies mitochondrial contents and dynamics [20]. Therefore, we asked how S1P/S1PR1 signaling affects T-cell mitochondria after allogeneic stimulation. mTORC1, which is activated downstream of activated AKT [66], regulates mitochondrial dynamics and induces mitochondrial fragments [29]. We found that activated (CD25+) CD4+ but not CD8+ T cells expressed less phosphorylated S6 and activated AKT (Ser473) when stimulated by Sphk1−/− APCs than when stimulated with the WT counterparts (Fig. 8A–D). Increased pS6 can enhance mitochondrial respiration and glycolysis in human and murine T cells, which impacts immune responses after allo-BMT [67–69]. We measured the glycolysis-to-OXPHOS shift in alloreactive T cells by evaluating OCR and ECAR. The alloreactive CD4+ T cells showed significantly decreased mitochondrial respiration and glycolytic activity after stimulation with allogeneic Sphk1−/− than with WT APCs, while the metabolic profiles were comparable in activated CD8+ T cells regardless of Sphk1 abundance on APCs (Fig. 8E–H). Similarly, activated CD4+ but not CD8+ T cells showed reduced mitochondrial content after stimulation with Sphk1−/− APCs, as reflected by mitochondrial staining (Fig. 8I, J). We also observed similar results in T cells deficient in S1PR1 (Fig. S17A–G). These results reveal a potential positive relationship between the Sphk1/S1P/S1PR1 pathway and mitochondrial dynamics in alloantigen-activated T cells.
Fig. 8.
S1P regulates CD4+ T-cell pathogenicity through Akt/pS6-mediated mitochondrial fission. Purified CD25-depleted T cells from FVB mice were cocultured with APCs from either WT or Sphk1−/− mice. A, B pS6, C, D AKT-pS473 and AKT-pT308 expression in gated CD4+ CD25+ or CD8+ CD25+ T cells was measured after 3 days. Representative dot plots or histograms and average frequencies are shown. E–H Purified CD4+ or CD8+ T cells from FVB mice were cocultured with APCs from either WT or Sphk1−/− mice for 3 days, and activated CD4+ or CD8+ T cells were obtained from bulk culture with CD25 using microbeads and further analyzed with a Seahorse XF96e machine. Glycolysis and OXPHOS shifts in alloreactive T cells were determined by evaluating OCR and ECAR. I Fluorescence figures showing costaining of DAPI and MitoSpy in CD4+ and CD8+ T cells after CD25 selection are shown. J Expression of MitoTracker in CD25+ or CD25− on gated CD4+ or CD8+ T cells was detected by flow cytometry, and summary graphs are shown. K Purified CD4+ or CD8+ T cells from FVB mice were cocultured with APCs from either WT or Sphk1−/− mice for 3 days, and activated CD4+ or CD8+ T cells were obtained from bulk culture with CD25 using microbeads. Drp1, Drp1-Ser616, Drp1-Ser637 and GAPDH expression in CD4+ CD25+ or CD8+ CD25+ T cells was detected by western blotting. Purified naive CD4+ T cells from FVB mice were transfected with GFP-EV, GFP-Drp1 Ser637A, and GFP-Drp1 Ser637D DNA plasmid vectors. After 6 h, transfected T cells were collected and cocultured with TCD-splenocytes serving as APCs from WT or Sphk1−/− mice for three days, and then, CD25, pS6, and Ki-67 expression was analyzed. L GFP signal and M the average levels of CD25, pS6, and Ki-67 on gated CD4+ T cells are shown. The experiments were repeated 2 to 3 independent times. The gray intensity of each band in the western blot was measured by ImageJ. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
It has been reported that inhibiting the positioning of mitochondria at the trailing cell edge by blocking mitochondrial fission impairs lymphocyte migration [21–23, 70]. The main protein implicated in mitochondrial fragmentation and fission is Drp1 [24], and the levels of pS6 have been found to be markedly reduced in Drp1-deficient T cells [26]. Phosphorylation of Drp1 controls lymphocyte migration in vitro [21] and Th1 and Th17 cell abundance after Treg polarization in the CNS [71]. Thus, we hypothesized that the Sphk1/S1P/S1PR1 pathway might influence aGVHD development through Drp1-mediated mitochondrial fission in donor allogeneic T cells. We found a lower level of phosphorylated Drp1 at the Ser616 site but a higher level at Ser637 in activated CD4+ but not CD8+ T cells after stimulation with allogeneic Sphk1−/− APCs compared to the effects of WT controls (Fig. 8K). Similar results were observed when comparing WT and S1PR1−/− T cells (Fig. S17H). To evaluate a potential causative relationship between S1P/S1PR1 signaling and Drp1 activation in T-cell alloreactivity, we asked whether augmentation of Drp1 activation rescues defective T-cell alloreactivity when S1P/S1PR1 signaling is impaired. Compared with the effect of GFP-empty vector (EV)-transfected CD4+ T cells (Fig. 8L), reconstitution of phospho-null S637A-Drp1 (Drp1 Ser637A) partially reversed the impairment to CD25, pS6 and Ki-67 expression caused by Sphk1−/− APC stimulation, whereas reconstitution of the inactive phospho-mimic mutant (Drp1 Ser637D) decreased CD25 and Ki-67 expression but not pS6 expression in CD4+ T cells after stimulation with WT APCs (Fig. 8M). These results indicate that Drp1 indeed plays a crucial role in Sphk1/S1P/S1PR1-mediated allogeneic T-cell activation and proliferation.
Augmenting mitochondrial fission by blocking PRKAA1 reversed the alloreactivity of pathogenic T cells with an impaired Sphk1/S1P/S1PR1 pathway
PRKAA1, one of the AMPK catalytic subunits, lies upstream of AKT/pS6/Drp1. Inhibition of AMPK can abolish AKT-Ser473 activation [32] and thus suppress pS6 [35] and Drp1 [72] activity. Moreover, genetic deletion of PRKAA1 promotes mitochondrial fission and increases mitochondrial fragments in multiple types of cells [33]. We asked whether PRKAA1 deficiency can reverse the alloreactivity of T cells with impaired Drp1-mediated mitochondrial fission due to the absence of S1P/S1PR1 signaling. To validate our hypothesis, we exploited PRKAA1flox/flox CD4-Cre+ and Cre− mice to implicate PRKAA1 deficiency in T-cell alloreactivity and GVHD pathogenesis regulated by the Sphk1S1P/S1PR1 pathway. While WT T cells exhibited impaired activation, proliferation, and cytokine production, the PRKAA1−/− T-cell response was not impacted when Sphk1 expression was inhibited or absent in APCs (Fig. 9A, B). At the molecular level, compared with WT CD4+ T cells, PRKAA1−/− CD4+ T cells showed comparable level of pS6 and a higher level of phosphorylated Drp1 (ser616) when activated with WT APCs. Moreover, in contrast to their WT counterparts, PRKAA1-deficient CD4+ T cells maintained pS6 and Drp1 activation levels in upon allogeneic stimulation when Sphk1 was genetically deleted in APCs or pharmacologically inhibited (Fig. 9A, C). However, the proliferation and the levels of pS6 and phosphorylated Drp1 (Ser616/Ser637) in CD8+ T cells were not disturbed regardless of Sphk1 or PRKAA1 levels (Fig. 9A–C). Alloreactive CD4+ T cells expressed higher levels of AMPK1α after stimulation with WT but not Sphk1−/− APCs. However, the expression of AMPK1α in CD8+ T cells was not altered by allogeneic stimulation regardless of Sphk1 expression (Fig. 9D). Therefore, we conclude that the tolerance of CD8+ T cells to profoundly reduced S1P was independent of PRKAA1 level. To extend these findings to in vivo studies, we purified T cells from PRKAA1flox/flox CD4-Cre+ and Cre− mice and transferred them into lethally irradiated BALB/c mouse recipients that were treated with vehicle or PF543. While Sphk1 inhibition effectively prevented GVHD induced by WT T cells, it had no impact on GVHD induced by PRKAA1-/- T cells (Fig. 9E–G). Taken together, these data demonstrate that S1P/S1PR1 signaling regulates AKT/pS6/Drp1-mediated mitochondrial fission in pathogenic T cells via PRKAA1 action during GVHD development.
Fig. 9.
S1P regulates mitochondrial fission of pathogenic T cells via PRKAA1. A Upon soluble anti-CD3 mAb stimulation (1 μg/mL), purified CD25-depleted T cells from WT or PRKAA1flox/floxCD4-Cre mice were cocultured with APCs from WT or Sphk1−/− mice for 3 days. CD25 abundance on gated CD4+ or CD8+ T cells and pS6 abundance on gated CD4+CD25+ or CD8+CD25+ T cells were measured. Summary graphs of the average levels are shown. B Purified CD25-depleted T cells from either WT or PRKAA1flox/floxCD4-Cre mice were cocultured with APCs from BALB/c mice in the presence or absence of PF543 (1 μM). The percentages of CFSE-diluted and IFN-γ+ cells on gated CD4+ or CD8+ T cells were detected after 4 days. Summary graphs of the average frequencies are shown. C Purified CD4+ or CD8+ T cells from either WT or PRKAA1flox/floxCD4-Cre mice cocultured with APCs from BALB/c mice in the presence or absence of PF543 (1 μM). Activated T cells were enriched from bulk culture through CD25−positive selection after 3 days. Drp1, Drp1-Ser616, Drp1-Ser637, and GAPDH expression in CD4+CD25+ and CD8+CD25+ T cells was detected by western blotting. D Purified CD4+ or CD8+ T cells from FVB mice were cocultured with APCs from either WT or Sphk1−/− mice for 3 days, and activated CD4+ or CD8+ T cells were obtained from bulk culture with CD25 using microbeads. AMPKα1 and GAPDH expression in CD4+CD25+ and CD8+CD25+ T cells was detected by western blotting. Lethally irradiated BALB/c mice underwent BMT with 5 × 106 TCD-BMCs alone and with or without 0.75 × 106 total T cells isolated from WT or PRKAA1flox/floxCD4-Cre donors. Recipients were injected i.p. with PF543 at 0.5 mg/kg every other day from Day 0 to Day 14. E Body weight loss, F clinical score, and G survival were monitored over time (n = 10 mice/group). The experiments were repeated 2 to 3 independent times. Log-rank (Mantel‒Cox) test (G) and nonparametric Mann‒Whitney U test (E, F) were performed to compare groups. The gray intensity of each band in the western blot was measured by ImageJ. Statistical data are presented as the mean ± 1 SD, and significance was determined by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001
Discussion
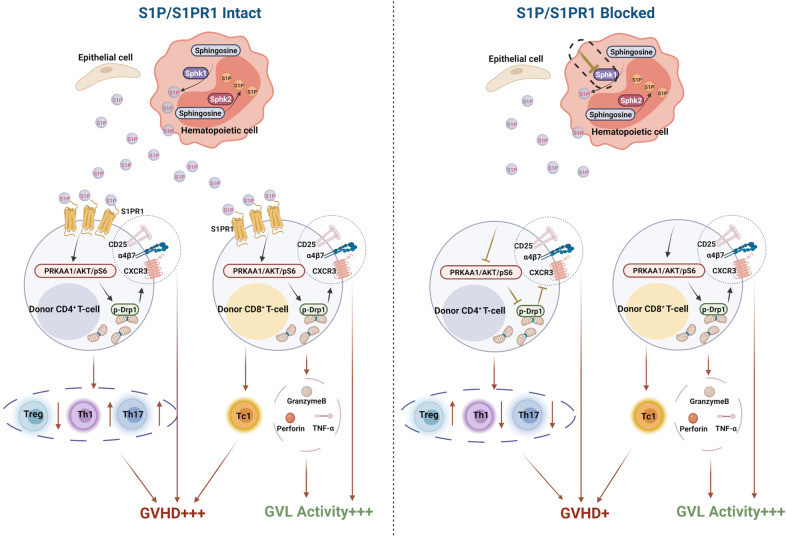
Sphk1 can be activated by numerous stimuli and promotes S1P production. In turn, S1P can be exported by tissue-specific transporters to activate its receptors via autocrine and paracrine signaling. This process, known as “inside-out signaling”, is important for various immune responses, including autoimmunity [5, 11, 73]. In the current study, we first examined the impact of Sphk1-deprived S1P on the allogeneic response of T cells and found that host-derived S1P predominantly promoted the alloreactivity of donor CD4+ T cells but not that of CD8+ T cells. At the cellular level, the lack of host Sphk1 or donor S1PR1 expression restrained pathogenic CD4+ T-cell infiltration into the GVHD target organs and inhibited Th1/Th17 cell differentiation while promoting iTreg generation. At the molecular level, S1P/S1PR1 signaling is essential for mitochondrial fission because it enhances AMPK/AKT/pS6/Drp1 activation in alloantigen-activated CD4+ T cells but dispensable in CD8+ T cells (Fig. 10). This evidence provides novel mechanistic insight into how the inhibition of the Sphk1/S1P/S1PR1 pathway can prevent GVHD while preserving GVL activity.
Fig. 10.
Graphic summary
Our data showed that genetic deletion of Sphk1 or Sphk2 in T cells did not affect T-cell alloreactivity or GVHD development (Fig. S2). The S1P spatial gradient is important for “inside-out signaling” and diverse biological activities, and sufficient secretion of S1P in circulation allows immune cell development and anchoring in target organs [11]. Sphk1−/− mice have been shown to display a 50% decrease in serum S1P concentrations [74], whereas Sphk2−/− mice have shown increased S1P concentrations in whole blood and plasma [75]. Sphk2- null mice and WT mice treated with Sphk2 inhibitor developed more severe inflammation in a dextran-induced colitis model and were more prone to colitis-associated cancer [61, 76]. Consistent with these previous studies, our results showed that despite similar proliferation and cytokine production rates, CD4+ T cells cocultured with allogeneic Sphk2−/− APCs showed higher activation than CD4+ T cells cocultured with WT controls. However, APCs deficient for Sphk1 showed a reduced ability to stimulate allogeneic T cells in vitro (Fig. 1A–D). Furthermore, Sphk1−/− recipients showed significantly less severe GVHD than WT control recipients (Fig. 2). Our findings suggest that Sphk1, but not Sphk2, is a potential therapeutic target for controlling GVHD.
In parallel to targeting Sphk1/S1P, we evaluated how S1PR1 regulates T-cell allogeneic responses because S1PR1 plays a dominant role among S1PRs [13, 14, 16, 17]. As observed with a deficiency in Sphk1 in recipient mice, S1PR1−/− donor T cells induced significantly less severe GVHD than their WT counterparts (Fig. 5). Given that S1PR4 was shown to cooperate with S1PR1 in T-cell migration [15], it is possible that S1PR4 on T cells may contribute to their pathogenicity in the induction of GVHD. Signaling via multiple S1PRs can be induced by S1P, and a reduction in S1P by inhibiting Sphk1 expression may be more effective against GVHD than targeting S1PR1 or individual S1PRs. Nonetheless, we found similar GVHD prevention efficiency when inhibiting either Sphk1 activity (with PF543) or S1PR1 activity (with W146) in a murine allo-BMT and xenograft model (Fig. 7), suggesting that S1PR1 serves as a dominant receptor, although inhibition of multiple S1PRs, such S1PR1, and S1PR4, is expected to result in higher efficacy in the control of GVHD.
The Sphk1/S1P/S1PR1 pathway exerted little impact on the proliferation or death of activated donor T cells in allogeneic recipients (Fig. 4 and Figs. S5, 9). In contrast, we observed that blocking the Sphk1/S1P/S1PR1 pathway decreased donor T-cell infiltration into target organs and activation of IFN-γ and IL-17 production in pathogenic T cells and promoted iTreg generation after allo-BMT (Figs. 3, 6 and Fig. S13, 15). Interestingly, the absolute cell numbers in the Th17 cell subset were comparable in the spleens from WT and Sphk1−/− recipients but significantly reduced in the livers of Sphk1−/− mice posttransplantation (Fig. S3). These data suggest that Sphk1 deficiency in recipients impairs the migration of donor Th17 cells but not other T-cell subsets. Moreover, allogeneic CD4+ T cells were more sensitive than CD8+ T cells to the S1P signal (Fig. 1F, G). Genetic or pharmacological inhibition of the Sphk1/S1P/S1PR1 pathway did not impact donor CD8+ T cells in terms of migration (Figs. 3H, 6F and Fig. S9C, D, S15B) or CD107a, perforin and granzyme B expression (Fig. 7K, L and Fig. S4, S15C–E). At the molecular level, CD4+ T cells with Sphk1/S1P/S1PR1 pathway activation inhibited showed reduced PRKAA1/AKT/pS6-induced mitochondrial respiration, glycolysis, and mitochondrial fission (Figs. 8, 9 and Fig. S17), which resulted in diminished T-cell infiltration and differentiation (Figs. 3 and 6) [25, 71]. However, this impairment was reversed by genetic abrogation of PRKAA1 (Fig. 9), which drives incessant activation of mitochondrial fission [33]. Notably, allogeneic CD8+ T cells exhibited nonalternative S1PR1 expression and comparable CTL activity in the absence of S1P and were much less sensitive to the PRKAA1/AKT/pS6/Drp1 axis or mitochondrial dynamics (Figs. 4E–H, 8 and Figs. S4, 9, 17). Under these circumstances, Drp1 and mTOR signaling in CD8+ T cells was rapidly activated, rendering the Sphk1/S1P/S1PR1 pathway dispensable (Fig. 7A, B, K and Fig. S17A, B, H). The difference in sensitivity to the Sphk1/S1P/S1PR1 pathway between CD4+ and CD8+ T cells provides a rational explanation for why S1P/S1PR1 signaling can modulate GVHD pathogenesis and GVL activity separately.
Fingolimod (FTY720), a well-known immune modulator used for the treatment of multiple sclerosis [77], is phosphorylated by nuclear Sphk2. FTY720 is a high-affinity agonist for 4 of the 5 known S1PRs, S1PR1, S1PR3, S1PR4, and S1PR5 [78]. Recent studies suggested FTY720 is an anti-GVHD agent that prevents allogeneic T-cell migration [78, 79]. In mammals, S1PR1, S1PR2, and S1PR3 are found in all tissues, whereas S1PR4 is highly expressed in lymphoid tissues and lungs, and S1PR5 is found in NK cells and the central nervous system [80]. Due to its extensive involvement in S1P/S1PR signaling and potential associated biological functions, the benefits and risks in clinical applications cannot be determined. In addition, several previous studies [77, 78, 81] and our preliminary study [82] showed that FTY720 inhibits CTL activity and GVL responses. FTY720 can impair CD8+ T-cell function independently of the S1P pathway [78, 81]. By applying specific inhibitors against Sphk1 (PF543) [62] and S1PR1 (W146) [63, 64], we consistently observed that treatment with W146 or PF543 preserved the GVL effects while potently attenuating GVHD (Fig. 7A–J). Previous research illuminated that FTY720 did not prevent effector T cells migration into the liver, the major GVHD parenchymal target organ [77]. However, we showed that either genetic deletion or pharmacologic inhibition of Sphk1 and S1PR1 significantly reduced effector T-cell numbers and cytokine production by pathogenic T cells in the liver after allo-HSCT (Figs. 3G, H, 6G, and Fig. S13C, 15D). The data indicate that selectively inhibiting Sphk1 or S1PR1 shows advantages over broad inhibitors such as FYT720.
In summary, we clearly demonstrated that S1P production by recipient or S1PR1 in donor T cells was required for an optimal allogeneic T-cell response and a full-blown development of GVHD. We tested specific inhibitors of Sphk1 and S1PR1 and found that these inhibitors were effective in the prevention of GVHD and maintained the GVL effect, which is an advantage over the broad S1PR modulator FTY720. We validated the efficacy of Sphk1- or S1PR1-specific inhibitors in the prevention of GVHD induced by human T cells in a xenograft transplant model. Furthermore, we discovered a novel mechanism in which the Sphk1/S1P/S1PR1 pathway differentially modulated the alloreactivity of CD4+ and CD8+ T cells. By activating the AMPK/AKT/mTOR/Drp1 pathway, S1P/S1PR1 signaling augmented mitochondrial fission and mass primarily in allogeneic CD4+ but not CD8+ T cells, which may contribute to GVL maintenance when S1P/S1PR1 signaling is inhibited. Thus, specifically targeting Sphk1 or S1PR1 is a novel therapeutic strategy that may benefit patients with hematologic malignancies.
Supplementary information
Acknowledgements
The authors are grateful for the technical support provided by the Department of Laboratory Animal Research, Flow Cytometry Core and Imaging Core as part of the Hollings Cancer Center at the MUSC, which is funded by Cancer Center Support Grant P30 CA138313 from the National Institutes of Health, National Cancer Institute (NCI).
Author contributions
LT participated in the research design and execution of the experiments, analyzed and interpreted the data, and wrote the manuscript; YW performed long-term and short-term allo-BMT experiments (WT, Sphk1−/− and Sphk2−/− transplantation from C57BL/6 mice to BALB/c mice); HC, XS, XL and HS participated in some of the experiments; MK performed the T-cell transfection assay; BO, SK, and XC participated in the research design, provided essential reagents, and/or edited and revised the manuscript; and X-ZY designed the research, interpreted the data, and edited and revised the manuscript.
Funding
This work is supported in part by SmartState Cancer Stem Cell Biology & Therapy Program and by R01 grants from the National Institutes of Health, including AI118305, HL140953 and CA258440 (X.-Z.Y.).
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00921-x.
References
- 1.Voermans C, Hazenberg MD. Cellular therapies for graft-versus-host disease: a tale of tissue repair and tolerance. Blood. 2020;136:410–7. doi: 10.1182/blood.2019000951. [DOI] [PubMed] [Google Scholar]
- 2.Zeiser R, Blazar BR. Acute graft-versus-host disease–biologic process, prevention, and therapy. N. Engl J Med. 2017;377:2167–79. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2:e91–100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 4.Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E. Pathophysiology of GvHD and other HSCT-related major complications. Front Immunol. 2017;8:79. doi: 10.3389/fimmu.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel S, Milstien S. The outs and the ins of Sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–9. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 7.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by Sphingosine-1-Phosphate. Science. 2009;325:1254–7. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong EK, Li X, Hylemon PB, Zhou H. Sphingosine Kinases/Sphingosine 1-Phosphate signaling in hepatic lipid metabolism. Curr Pharm Rep. 2017;3:176–83. doi: 10.1007/s40495-017-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi H. Sphingosine-1-Phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32:16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartier A, Hla T. Sphingosine 1-Phosphate: Lipid signaling in pathology and therapy. Science. 2019;366:eaar5551. doi: 10.1126/science.aar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proia RL, Hla T. Emerging biology of Sphingosine-1-Phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–87. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte Egress from Thymus and peripheral lymphoid organs is dependent on S1p Receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 14.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the Sphingosine 1-Phosphate receptor, S1p1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, et al. Cd4 T Cell Sphingosine 1-Phosphate Receptor (S1pr)1 and S1pr4 and Endothelial S1pr2 regulate afferent lymphatic migration. Sci Immunol. 2019;4:eaav1263. doi: 10.1126/sciimmunol.aav1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1p(1)-Mtor Axis directs the reciprocal differentiation of T(H)1 and T(Reg) cells. Nat Immunol. 2010;11:1047–56. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y, et al. Defective Sphingosine 1-Phosphate Receptor 1 (S1p1) Phosphorylation Exacerbates Th17-mediated autoimmune neuroinflammation. Nat Immunol. 2013;14:1166–72. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green JA, Cyster JG. S1pr2 links germinal center confinement and growth regulation. Immunol Rev. 2012;247:36–51. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-Bet-dependent S1p5 Expression in Nk cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–81. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, Muller J, et al. Lymphatic Endothelial S1p promotes mitochondrial function and survival in naive T cells. Nature. 2017;546:158–61. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–86. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678–84. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, et al. Miro-1 links mitochondria and microtubule dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–26. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833:1256–68. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Baixauli F, Martín-Cófreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, et al. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30:1238–50. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simula L, Pacella I, Colamatteo A, Procaccini C, Cancila V, Bordi M, et al. Drp1 controls effective T cell immune-surveillance by regulating T cell migration, proliferation, and Cmyc-dependent metabolic reprogramming. Cell Rep. 2018;25:3059–73.e3010. doi: 10.1016/j.celrep.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 28.Chadha R, Meador-Woodruff JH. Downregulated Akt-Mtor signaling pathway proteins in dorsolateral prefrontal cortex in Schizophrenia. Neuropsychopharmacology. 2020;45:1059–67. doi: 10.1038/s41386-020-0614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, et al. mTOR controls mitochondrial dynamics and cell survival via Mtfp1. Mol Cell. 2017;67:922–35.e925. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Ma EH, Poffenberger MC, Wong AH, Jones RG. The role of Ampk in T cell metabolism and function. Curr Opin Immunol. 2017;46:45–52. doi: 10.1016/j.coi.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, et al. Regulation of the energy sensor Amp-activated protein kinase by antigen receptor and Ca2+ in T Lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing SS, Yang XY, Zheng T, Li WJ, Wu D, Chi JY, et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related Ampk/Pi3k/Akt/Enos pathway. Vasc Pharmacol. 2015;72:141–52. doi: 10.1016/j.vph.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Wu S, Zhu H, Ding Y, Dai X, Ouyang C, et al. Deletion of Prkaa triggers mitochondrial fission by inhibiting the autophagy-dependent degradation of Dnm1l. Autophagy. 2017;13:404–22. doi: 10.1080/15548627.2016.1263776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, et al. The Liver Kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–98. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, et al. The energy sensor Ampk regulates Tcell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen H, Alawieh A, Bastian D, Kuril S, Dai M, Daenthanasanmak A, et al. Targeting the complement alternative pathway permits graft versus leukemia activity while preventing graft versus host disease. Clin Cancer Res. 2020;26:3481–90. doi: 10.1158/1078-0432.CCR-19-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinrichs J, Li J, Nguyen H, Wu Y, Bastian D, Daethanasanmak A, et al. Cd8(+) Tregs promote Gvhd prevention and overcome the impaired Gvl effect mediated by Cd4(+) Tregs in mice. Oncoimmunology. 2016;5:e1146842. doi: 10.1080/2162402X.2016.1146842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sofi MH, et al. Ceramide synthesis regulates T cell activity and Gvhd development. JCI Insight. 2017;2:e91701. doi: 10.1172/jci.insight.91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Sphingolipid analysis by High Performance Liquid Chromatography-Tandem Mass Spectrometry (Hplc-Ms/Ms) Adv Exp Med Biol. 2010;688:46–59. doi: 10.1007/978-1-4419-6741-1_3. [DOI] [PubMed] [Google Scholar]
- 40.Dany M, Gencer S, Nganga R, Thomas RJ, Oleinik N, Baron KD, et al. Targeting Flt3-Itd signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in Aml. Blood. 2016;128:1944–58. doi: 10.1182/blood-2016-04-708750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daenthanasanmak A, Iamsawat S, Chakraborty P, Nguyen HD, Bastian D, Liu C, et al. Targeting Sirt-1 controls GVHD by inhibiting T-Cell Allo-response and promoting treg stability in mice. Blood. 2019;133:266–79. doi: 10.1182/blood-2018-07-863233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betts BC, Bastian D, Iamsawat S, Nguyen H, Heinrichs JL, Wu Y, et al. Targeting Jak2 reduces GVHD and Xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci USA. 2018;115:1582–7. doi: 10.1073/pnas.1712452115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Schutt S, Paz K, Zhang M, Flynn RP, Bastian D, et al. Microrna-17-92 is required for T-Cell and B-cell pathogenicity in chronic graft-versus-host disease in mice. Blood. 2018;131:1974–86. doi: 10.1182/blood-2017-06-789321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sofi MH. A single strain of bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight. 2021;6:e136841. doi: 10.1172/jci.insight.136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 46.Baeyens A, Fang V, Chen C, Schwab SR. Exit strategies: S1p signaling and T cell migration. Trends Immunol. 2015;36:778–87. doi: 10.1016/j.it.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakraborty P, Vaena SG, Thyagarajan K, Chatterjee S, Al-Khami A, Selvam SP, et al. Pro-survival lipid Sphingosine-1-Phosphate metabolically programs T cells to limit anti-tumor activity. Cell Rep. 2019;28:1879–93.e1877. doi: 10.1016/j.celrep.2019.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9. doi: 10.1182/blood.V95.9.2754.009k25_2754_2759. [DOI] [PubMed] [Google Scholar]
- 49.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–36. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–12. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of Th1 and Th17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–13. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of. nce. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 53.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, et al. Donor APCs are required for maximal GVHD but not for Gvl. Nat Med. 2004;10:987–92. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 54.MacDonald KPA, Kuns RD, Rowe V, Morris ES, Banovic T, Bofinger H, et al. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood. 2007;109:5049–57. doi: 10.1182/blood-2007-01-067249. [DOI] [PubMed] [Google Scholar]
- 55.Smith P, O’Sullivan C, Gergely P. Sphingosine 1-phosphate signaling and its pharmacological modulation in allogeneic hematopoietic stem cell transplantation. Int. J. Mol. Sci. 2017;18:2027. doi: 10.3390/ijms18102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutt S, Ermann J, Tseng D, Liu YP, George TI, Fathman CG, et al. L-Selectin and Beta7 Integrin on Donor Cd4 T cells are required for the early migration to host mesenteric lymph nodes and acute colitis of graft-versus-host disease. Blood. 2005;106:4009–15. doi: 10.1182/blood-2005-06-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, et al. Absence of Beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107:1703–11. doi: 10.1182/blood-2005-08-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, et al. Role of Cxcr3-induced donor T-cell migration in Acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/S0301-472X(03)00198-X. [DOI] [PubMed] [Google Scholar]
- 59.Varona R, Cadenas V, Gómez L, Martínez AC, Márquez G. Ccr6 regulates Cd4+ T-cell-mediated acute graft-versus-host disease Responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- 60.Xin Q, Cheng G, Kong F, Ji Q, Li H, Jiang W, et al. Stat1 transcriptionally regulates the expression of S1pr1 by binding its promoter region. Gene. 2020;736:144417. doi: 10.1016/j.gene.2020.144417. [DOI] [PubMed] [Google Scholar]
- 61.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, et al. Sphingosine-1-Phosphate links persistent Stat3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–20. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, et al. Modulation of cellular S1p levels with a novel, potent and specific inhibitor of Sphingosine Kinase-1. Biochem J. 2012;444:79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- 63.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1p1 antagonist in vivo. Nat Chem Biol. 2006;2:434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 64.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-Phosphate (S1p) Receptor subtypes S1p1 and S1p3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839––13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 65.Baeyens A, Bracero S, Chaluvadi VS, Khodadadi-Jamayran A, Cammer M, Schwab SR. Monocyte-derived S1p in the lymph node regulates immune responses. Nature. 2021;592:290–5. doi: 10.1038/s41586-021-03227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dan HC, Ebbs A, Pasparakis M, Van Dyke T, Basseres DS, Baldwin AS. Akt-dependent activation of Mtorc1 complex involves phosphorylation of mTOR (Mammalian Target of Rapamycin) by Iκb Kinase Α (Ikkα) J Biol Chem. 2014;289:25227–40. doi: 10.1074/jbc.M114.554881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones N, Cronin JG, Dolton G, Panetti S, Schauenburg AJ, Galloway S, et al. Metabolic adaptation of human Cd4(+) and Cd8(+) T-cells to T-cell receptor-mediated stimulation. Front Immunol. 2017;8:1516. doi: 10.3389/fimmu.2017.01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen HD, Chatterjee S, Haarberg KMK, Wu Y, Bastian D, Heinrichs J, et al. Metabolic reprogramming of alloantigen-activated T Cells after hematopoietic cell transplantation. J Clin Invest. 2016;126:1337–52. doi: 10.1172/JCI82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–70. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desdín-Micó G, Soto-Heredero G, Mittelbrunn M. Mitochondrial activity in T cells. Mitochondrion. 2018;41:51–7. doi: 10.1016/j.mito.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 71.Li YH, Xu F, Thome R, Guo MF, Sun ML, Song GB, et al. Mdivi-1, a mitochondrial fission inhibitor, modulates T helper cells and suppresses the development of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2019;16:149. doi: 10.1186/s12974-019-1542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr, Losón OC, Hellberg K, et al. Metabolism. Amp-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–81. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 74.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in Sphingosine Kinase 1 are rendered lymphopenic by Fty720. J Biol Chem. 2004;279:52487–92. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 75.Kharel Y, Raje M, Gao M, Gellett AM, Tomsig JL, Lynch KR, et al. Sphingosine Kinase Type 2 inhibition elevates circulating sphingosine 1-Phosphate. Biochem J. 2012;447:149–57. doi: 10.1042/BJ20120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai HC, Han MH. Sphingosine-1-Phosphate (S1p) and S1p signaling pathway: therapeutic targets in autoimmunity and inflammation. Drugs. 2016;76:1067–79. doi: 10.1007/s40265-016-0603-2. [DOI] [PubMed] [Google Scholar]
- 77.Taylor PA, Ehrhardt MJ, Lees CJ, Tolar J, Weigel BJ, Panoskaltsis-Mortari A, et al. Insights into the mechanism of Fty720 and compatibility with regulatory T cells for the inhibition of Graft-Versus-Host Disease (GVHD) Blood. 2007;110:3480–8. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ntranos A, Hall O, Robinson DP, Grishkan IV, Schott JT, Tosi DM, et al. Fty720 impairs Cd8 T-cell function independently of the Sphingosine-1-Phosphate pathway. J Neuroimmunol. 2014;270:13–21. doi: 10.1016/j.jneuroim.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Ryu J, Jhun J, Park MJ, Baek JA, Kim SY, Cho KH, et al. Fty720 ameliorates GVHD by blocking T lymphocyte migration to target organs and by skin fibrosis inhibition. J Transl Med. 2020;18:225. doi: 10.1186/s12967-020-02386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of the S1p/S1pr axis in health and disease. J Dent Res. 2011;90:841–54. doi: 10.1177/0022034510389178. [DOI] [PubMed] [Google Scholar]
- 81.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance Liquid Chromatography-Tandem Mass Spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Sofi MH, Tian L, Schutt S, Khan I, Choi HJ, Wu Y, et al. Ceramide Synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-Ras/Erk Pathway. Leukemia. 2022;36:1907–15. doi: 10.1038/s41375-022-01581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.