Abstract
Antiretroviral Treatment (ART) has significantly decreased HIV-related morbidity and mortality among children despite the issue of drug resistance and subsequent treatment failure appearing as a challenge. Different studies have been conducted in Ethiopia regarding the prevalence of first-line ART failure among children but the magnitudes of these studies were inconsistent and had great variability. This review aimed to estimate the pooled prevalence of first line ART failure among children and its association with drug substitution and sex of children among first-line ART users in Ethiopia. The review was conducted using both published and unpublished studies until September 2020 in Ethiopia. MEDLINE, PubMed, Hinari, Web of Science, Google Scholar, Africa journal online (AJOL), Open gray literature, and online repository articles were searched. The quality of individual studies was assessed by Joanna Briggs Institute's (JBI) critical appraisal checklist. The statistical analysis was done by STATA-14 software and a random effect model was used. Heterogeneity was assessed using forest plot Cochrane Q–test and I-squared statistic. Publication bias was checked by using a funnel plot and Egger’s and Begg’s statistical tests. The interpretation was made by an odds ratio and with their respective 95% confidence intervals. The heterogeneity rate was 90% and Begg’s and Egger’s for publication bias were insignificant with p-values of 0.89 and 0.11 respectively. The pooled prevalence of pediatric first line ART failure in Ethiopia was 14.98% (95% CI 11.74, 18.21). Subgroup analysis showed that the highest failure rate was virological (9.13%). Female children had 1.4 times more risk of first-line ART failure (OR = 1.42; 95% CI 1.08, 1.85). First-line ART failure among children in Ethiopia is considerably high. Being female increases the likelihood of facing first line ART failure. More attention should be given to female children.
Subject terms: Immunology, Diseases, Health care
Introduction
Acquired Immunodeficiency Syndrome (AIDS), a disease of no cure, can take many years to be developed following Human Immunodeficiency Virus (HIV) infection. Globally 38 million people are affected by HIV. In 2019 nearly 53% of children infected with HIV globally were receiving antiretroviral treatment (ART)1. More than 2 million children worldwide are infected with HIV and 90% of them live in sub-Saharan Africa2. In Ethiopia, there is a significant pediatric HIV-1 burden with approximately 65,100 infected children, with an estimated 3200 AIDS-related child deaths occurring annually3 and nearly 60% of children under the age of 15 years living with HIV were on treatment in 20184.
Highly Active Antiretroviral Treatment (HAART) significantly decreased HIV-related morbidity and mortality3 intending to reduce the viral load to an undetectable level for further reduction of the risks of HIV transmission in addition to its role to live longer with healthier lives5. Many studies have reported the success of highly active antiretroviral therapy (HAART) in improving clinical and immunologic outcomes of children. However, the issue of drug resistance and subsequent treatment failure appears as a challenge6,7.
According to the World Health Organization (WHO) definition treatment failure (TF) could be clinical, immunological, and virological failure (VF)8. Virological failure is widely considered the criterion standard to detect treatment failure. Treatment failure rates of 10–34% were observed among children after 2–3 years of ART7. Viral load testing is a more sensitive and early indicator of TF9.
In Ethiopian clinical failure may be diagnosed if there is a new or recurrent clinical event indicating WHO clinical stage IV condition OR WHO clinical stage III conditions with pulmonary Tuberculosis (TB) and severe bacterial infections whereas Immunological failure is recorded if CD4 count at or below 250 cells/mm3 following clinical failure Or Persistent CD4 levels below 100 cells/mm3 and VF is defined as having Viral load above 1000 copies/mL based on two consecutive viral load measurements after 6 months of treatment start9.
Different factors contributed to the existence of TF like patients who didn’t change ART regimens, poor medication adherence, not taking Isoniazid (INH) prophylactic therapy, being on Zidovudine (AZT) based regimen, having lower baseline CD4 count, being bedridden during ART initiation, older age, Presence of WHO disease stage III/IV, history of injection drug use, previous protease inhibitor use, being on a second-line ART regimen, TB co-infection10–15. The main reasons for treatment modification in Ethiopia were toxicity, comorbidity, pregnancy, and treatment failure16.
Different studies have been conducted in Ethiopia to determine the prevalence of first-line ART failure among children but the magnitudes of these studies were inconsistent and characterized by great variability3,6,17–20. This review is conducted to fill the gaps regarding the problem of pediatric treatment failure and reduces the variability of results which was reported by individual studies. This study aimed to provide better estimates and greater power by assessing relationships that exist between first-line anti-retroviral treatment failure and its association with drug substitution and the sex of children.
Methods
Study setting and period
This review evaluated the relevant studies conducted in Ethiopia by using articles published until September 2020. Ethiopia is located in the Horn of Africa and has ten regional states and two administrative cities.
Searching strategies
Both published and unpublished research articles conducted in different parts of Ethiopia focusing on HIV/AIDS treatment failure among children below the age of 18 years were searched using different searching techniques. PubMed/MEDLINE, Scopus, HINARI, Google scholar, AJOL, and Google were used as the main database for published articles. Institution repositories/libraries and research gate were used for searching unpublished studies. The advanced search for PMC was (First Line) OR Anti-Retroviral [MeSH Terms]) OR ART) OR Highly Active Antiretroviral) OR HAART) AND Treatment [MeSH Terms]) AND Failure) AND Children [MeSH Terms]) OR Pediatrics population) AND Ethiopia. Additionally, articles were searched by using keywords and phrases like “HIV/AIDS treatment Failure among Children in Ethiopia”, “Clinical Failure in Ethiopia”, “Immunological failure among Children in Ethiopia”, “Virological failure in Ethiopia”, Highly Active Antiretroviral treatment failure in Ethiopia” by using “AND, OR” bulletin. Searching for additional sources by using the reference lists of accessed articles was performed.
Inclusion and exclusion criteria
All observational studies (Cross-sectional, case–control, and Cohort) measuring ART failure among children in Ethiopia by using clinical, immunological, or virological criteria were included. All searches were limited to the English language and studies published from 2003 when Ethiopia started ART to September 2020. Studies determining ART failure by involving both children and adults at a time were excluded.
Measurement of the outcome variables
first line ART failure (outcome of interest) was estimated by using the national clinical, immunological, and virological criteria. Clinical failure is defined as a new or recurrent clinical event indicating WHO clinical stage IV condition OR WHO clinical stage III conditions with pulmonary Tuberculosis (TB) and severe bacterial infections whereas Immunological failure is recorded if CD4 count at or below 250 cells/mm3 following clinical failure Or Persistent CD4 levels below 100 cells/mm3 and VF is defined as having Viral load above 1000 copies/mL based on two consecutive viral load measurements after 6 months of treatment start9.
Study selection and data collection
All studies identified through searching different databases were managed by using Endnote version X8 software. Duplicated studies were removed and the full text of the articles was searched by Endnote software and manually. Authors evaluated the articles by using their titles and abstracts in first phase and full text in the final phase for inclusion, and data were extracted by two independent authors.
Quality assessment of individual studies
Two reviewers (SA & AA) independently assessed the quality of individual studies by using the JBI critical appraisal checklist for cross-sectional, case–control, and cohort studies. The tool is freely available at https://jbi.global/critical-appraisal-tools. The authors used different quality appraisal checklists for each study. On the critical appraisal process, 5 or more scores in the JBI criteria were considered to have good quality. Discrepancies in the quality assessment were resolved through a third author (GD).
Data extraction and management
Two authors extracted the data by using the First author's name, year of publication, study design; sample size, studied population, outcomes of interest, study area, and response rate.
Statistical analysis
The extracted data were exported to STATA/SE version 14.0 software for analysis. A descriptive summary of the included studies was presented. The pooled level of ART failure among children and its association with child sex and drug substitution was determined by using the random-effects model21. Since the studies retrieved were heterogeneous by study area, sample size, design, population, and study period, we declared to use a random effect model. The statistical heterogeneity was checked subjectively by using forest plot, and objectively by Cochrane Q-test and I2 statistics22 Subgroup analysis was carried out by region of studies. The presence of publication bias was checked by using a funnel plot and Egger’s and Begg’s statistical tests23. An odds ratio with a 95% confidence interval was used.
Results
Description of the studies
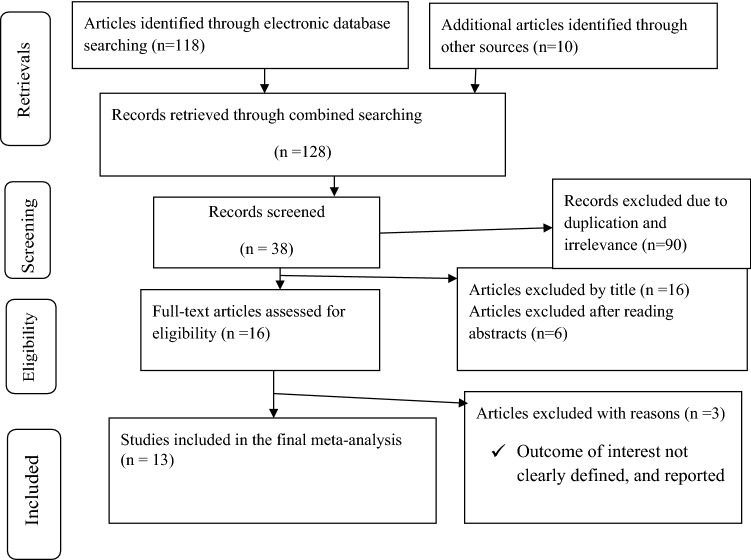
The authors retrieved a total of 128 retrievals by using different search engines. From a total of 16 full-text articles assessed, we rejected 3 of them because the outcome of interest was not clearly defined and reported; and because the estimates were different from the outcome of interest. Finally, 13 studies were supposed to be eligible for this review (Fig. 1).
Figure 1.
PRISMA flow diagram showing search results for the inclusion of studies focusing on first line ART failure among Children in Ethiopia, 2020.
Characteristics of original studies
A total of 13 studies having a quality scale of 5 or more by the JBI critical appraisal criteria were included for this study. Eleven of the included studies were cohort and retrospective in nature, while the rest were cross-sectional. The included studies had sample sizes that ranged from 98 to 1186. The studies were conducted from 2009 to 2020. Individual studies showed that first line ART failure among children in Ethiopia Ranges from 5.45 to 23.76%. In this meta-analysis, a total of 4931 children under the age of 18 years who were on first-line ART were included. However, there were no included studies from Somali, Harari, Afar, Gambella, and Dire Dawa regions which fulfill the inclusion criteria (Table 1).
Table 1.
Characteristics of original studies on first line ART failure among children in Ethiopia, 2020.
| ID | First author name/publication year | Region | Study design | Studied population | TF prevalence | JBI score |
|---|---|---|---|---|---|---|
| 1 | Bacha et al./201217 | Addis Ababa | Retrospective follow-up | 1186 | 14.08 | 8 |
| 2 | Brhane et al./202024 | Amhara | Cross sectional | 238 | – | 6 |
| 3 | Sisay et al./201819 | Amhara | Retrospective follow-up | 824 | 7.65 | 10 |
| 4 | Getaneh Y et al./201925 | Multi Center | Prospective and Retrospective follow-up | 536 | 17.35 | 9 |
| 5 | Haile et al./201926 | Addis Ababa | Retrospective follow-up | 318 | 22.64 | 7 |
| 6 | Yassin et al./201727 | Oromia | Retrospective follow-up | 269 | 18.96 | 8 |
| 7 | Sibhat et al./202018 | Tigray | Retrospective follow-up | 404 | 23.76 | 8 |
| 8 | Sorsa et al./201820 | Oromia | Retrospective follow-up | 183 | 17.49 | 6 |
| 9 | Tadesse et al./201928 | Multi Center | Prospective follow up | 110 | 5.45 | 10 |
| 10 | Osman et al./202029 | Oromia | Cross sectional | 140 | 11.43 | 6 |
| 11 | Workneh et al./200930 | Oromia | Retrospective follow-up | 96 | 11.46 | 7 |
| 12 | Yihun et al./20192 | Amhara | Retrospective follow-up | 402 | 12.19 | 9 |
| 13 | Zeleke/20166 | Amhara | Retrospective follow-up | 225 | 18.22 | 9 |
TF- Treatment Failure.
– denotes no estimation due to lack of information from the original studies.
Prevalence of first line ART failure among children
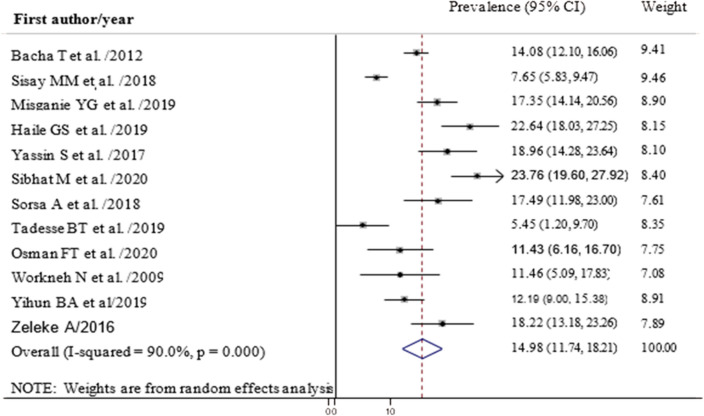
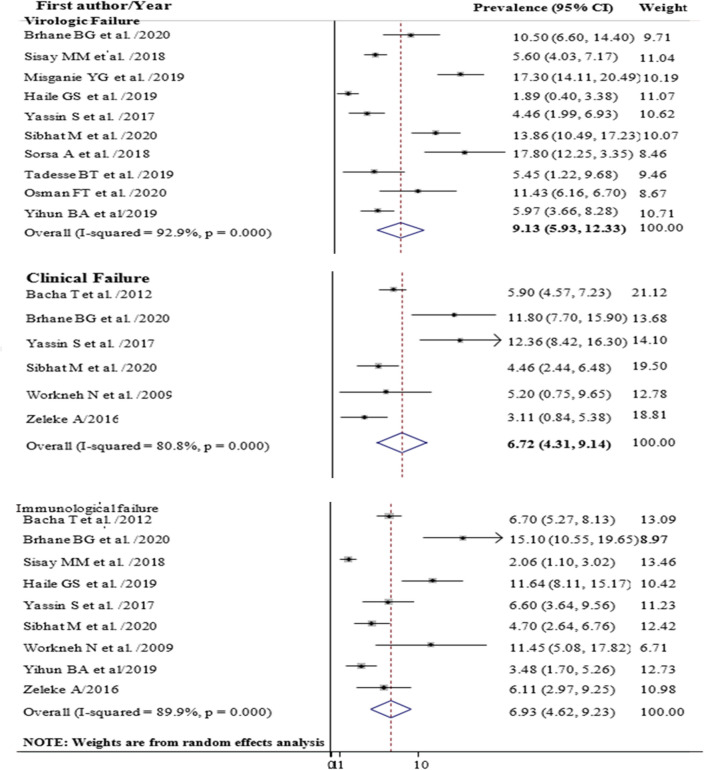
The pooled prevalence of first line ART failure among children in Ethiopia was 14.98% (95% CI 11.74, 18.21) with high heterogeneity between studies (I-squared = 90.0%) (Fig. 2). Subgroup analysis showed that the highest failure was Virological (9.13%) followed by Immunological (6.93%) and clinical failure (6.72%) (Fig. 3). Analysis by region indicated that the highest virological treatment failure rate was recorded in Tigray (13.86%) and Oromia (10.95%) (Table 2).
Figure 2.
Forest plot showing the prevalence of anti-retroviral treatment failure among children in Ethiopia, 2020.
Figure 3.
Forest plot showing the prevalence of virologic, clinical and immunological treatment failure among children in Ethiopia, 2020.
Table 2.
Subgroup analysis by region showing the prevalence of clinical, immunological and VF among children in Ethiopia, 2020.
| Subgroup analysis (by region) | Overall TF prevalence (95%CI) | CF prevalence (95%CI) | IF prevalence (95%CI) | VF prevalence (95%CI) |
|---|---|---|---|---|
| Addis Ababa | 18.10 (9.72, 26.47) | 5.90 (4.57, 7.23) | 8.90 (4.09, 13.71) | 1.89 (0.40, 3.38) |
| Amhara | 12.27 (6.78, 17.76) | 11.80 (7.70, 15.90) | 6.03 (2.42, 9.64) | 6.76 (4.49, 9.04) |
| Oromia | 15.07 (11.11, 19.03) | 8.86 (1.84, 15.87) | 8.17 (3.72, 12.62) | 10.95 (2.76, 19.13) |
| Tigray | 23.76 (19.61, 27.92) | 4.46 (2.44, 6.48) | 4.70 (2.64, 6.76) | 13.86 (10.49, 17.231 |
CF clinical failure, IF immunological failure, VF virologic failure, TF treatment failure.
Factors associated with first line ART failure
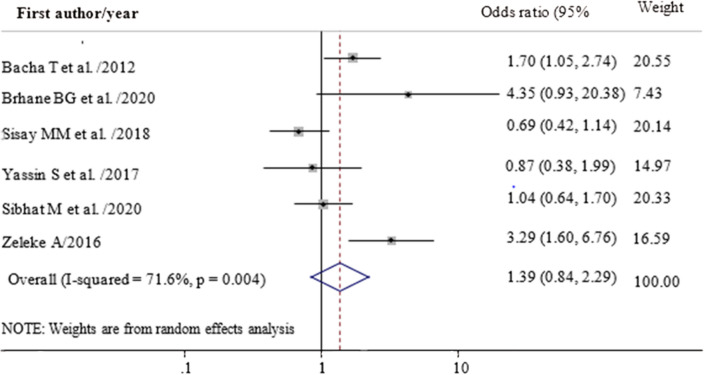
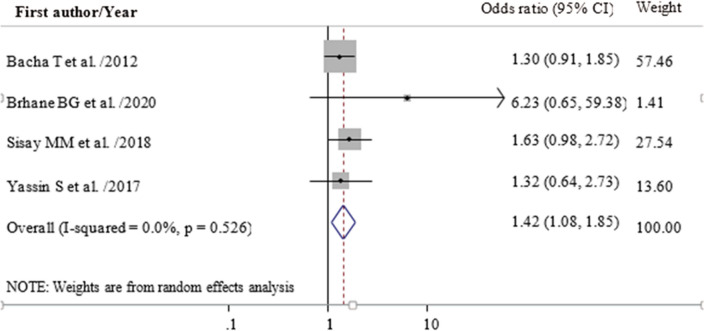
In this meta-analysis, we examined the association between drug substitution and the sex of children with first line ART failure. Accordingly, the odds of first line ART failure were 1.4 times higher among children who had a history of drug substitution (OR = 1.39; 95% CI 0.84, 2.29) (Fig. 4). Female children had an increased risk of first line ART failure (OR = 1.42; 95% CI 1.08, 1.85) as compared to their counterparts (Fig. 5).
Figure 4.
Forest plot of adjusted odds ratio showing the association between having drug substitution and treatment failure among children in Ethiopia, 2020.
Figure 5.
Forest plot of adjusted odds ratio showing the association between being female children and ART failure among children in Ethiopia, 2020.
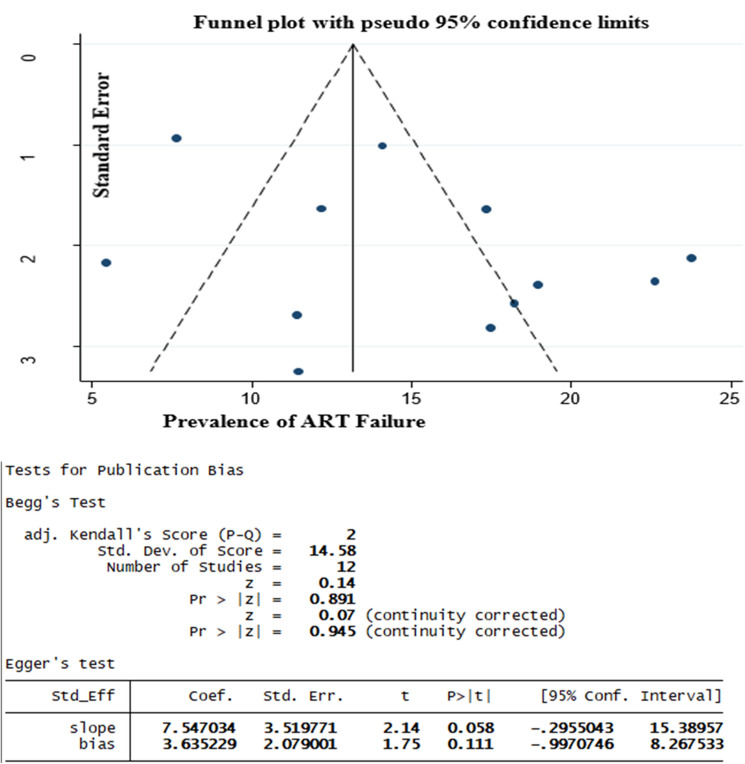
Publication bias
Begg’s and Egger’s test showed that there was no publication bias (Fig. 6).
Figure 6.
Test of publication bias for prevalence studies by using Begg’s and Egger’s test, 2020.
Discussion
Based on the results of this review the pooled prevalence of first-line ART failure among children in Ethiopia was 14.98%. The result was closer to previous studies conducted in Ethiopia at the general population level which were 15.9%31 and 15.3%32. This review showed that the magnitude of VF among children was 9.13% which was lower than studies conducted in the USA (40%)33, India (29%)34, Iran (29.2%)35 South Africa (16.9%)36. Tanzania (34%)37, Zambia (40%), and Senegal (64%)38. The possible reasons for the lower magnitude in this review may be associated with the difference in defining VF which was more than 1000 copies/ml in the current review, while more than 400 copies/ml were considered as VF in the above studies36, the differences in the populations to be studied whereby adults and children were included and the use of virologically failed children to estimate VF after some point in time33; and the difference in the types of HIV infections whereby dual HIV infections (Infection by both HIV-1 and 2) become a problem in west African countries38. The presence of a high level of ART adherence in Ethiopia which was more than 93%32–41 than studies conducted in Tanzania (65.3–84%)42,43, Uganda (79%)44 and Nigeria (65.6–91%)45,46 may contribute to the low level of TF in this study.
According to the results of this review being female was 1.4 times more likely to have first line ART failure even though a study conducted in Gondar showed that being male had higher odds of ART failure (AOR = 3.15)47. The result was consistent with the findings of previous studies in the country11 though, it was slightly higher than a study conducted in Addis Ababa17. The possible reason for this variation could be attributed to our review result was the pooled estimate from many studies. The highest risk of delaying ART initiation and experiencing more side effects among females, and the biological differences existed in metabolizing ART drugs between females and males48,49 might contribute to the high rate of first line ART failure among female children. Studies also indicated that female children had a higher risk of severe anemia50 and being low weight51 which may further affect their level of immunity and could contribute to the rapid progression of disease stages.
Regarding drug substitution, even though it was not statistically significant, first-line ART drug substitution increased the risk of ART failure (OR = 1.39; 95% CI 0.84, 2.29). This finding was supported by studies conducted in Ethiopia and Myanmar6,32,47,52. The possible reason may be due to the availability of limited facilities for viral load testing in resource-limited countries like Ethiopia, forced to use clinical and immunologic criteria, which have low specificity and positive predictive values, to diagnose TF and then to substitute or change drugs may result in unnecessary switches/substitutions of ART drugs53. Drug substitution/change in Ethiopia may be a result of problems in the supply system or client-related factors without consideration of risks of drug resistance before changing a particular regimen54 which may fail. This highlights the need for a drug resistance test before switching/substituting ART drugs.
Strength and limitations
The use of internationally accepted critical appraisal tools and searching for the inclusion of unpublished data was the strength. The retrieved studies represent only four of the twelve regions within the country so the finding may not represent the nation. High heterogeneity within the included studies was detected in this analysis. The criteria’s used to determine first-line ART failure across the included studies were different, which may under or overestimate the prevalence.
Conclusion
First-line ART failure among children in Ethiopia is considerably high. Being female increases the likelihood of facing ART failure. More attention should be given to female children.
Acknowledgements
Our everlasting appreciation is to all authors of the included studies without them having such alike manuscript may be very challenging.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- ART
Antiretroviral treatment
- HAART
Highly active anti-retroviral treatment
- HIV
Human immunodeficiency virus
- TB
Tuberculosis
- TF
Treatment failure
- VF
Virological failure
- WHO
World Health Organization
Author contributions
S.A.M., A.A.K., G.D.A., A.A.D., and T.M.D. were involved in the design, selection of articles, data extraction, statistical analysis, and manuscript writing of this review. All authors read and approved the final manuscript.
Data availability
The data set used and analyzed for the review is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. HIV/AIDS fact Sheet. https://www.who.int/news-room/fact-sheets/detail/hiv-aids (Accessed 6 July 2020).
- 2.Yihun BA, Kibret GD, Leshargie CT. Correction: Incidence and predictors of treatment failure among children on first-line antiretroviral therapy in Amhara Region Referral Hospitals, northwest Ethiopia 2018: A retrospective study. PLoS ONE. 2019;14(6):e0217901. doi: 10.1371/journal.pone.0217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haile GS, Berha AB. Predictors of treatment failure, time to switch and reasons for switching to second line antiretroviral therapy in HIV infected children receiving first line anti-retroviral therapy at a Tertiary Care Hospital in Ethiopia. BMC Pediatr. 2019;19(1):37. doi: 10.1186/s12887-019-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS, HIV/AIDS in Ethiopia (2018) https://www.unaids.org/en/regionscountries/countries/ethiopia (Accessed 17 Sept 2020).
- 5.U.S Department of Health and Human Service. HIV Treatment: The Basics (2020) https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/51/hiv-treatment--the-basics (Accessed 27 Aug 2020).
- 6.Zeleke A. Prevalence of antiretroviral treatment failure and associated factors in HIV infected children on antiretroviral therapy at Gondar University Hospital, retrospective cohort study. Int. J. Med. Med. Sci. 2016;8(11):125–132. doi: 10.5897/IJMMS2015.1164. [DOI] [Google Scholar]
- 7.Mulisa D, et al. Determinants of first line antiretroviral therapy treatment failure among adult patients on ART at central Ethiopia: Un-matched case control study. BMC Infect. Dis. 2019;19(1):1024. doi: 10.1186/s12879-019-4651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization; 2013. [PubMed] [Google Scholar]
- 9.Ethiopian Minstry of Health, National Guidelines For Comprehensive HIV Prevention, Care And Treatment. (2017).
- 10.Alene M, et al. Incidence and predictors of second-line antiretroviral treatment failure among adults living with HIV in Amhara region: A multi-centered retrospective follow-up study. BMC Infect. Dis. 2019;19(1):599. doi: 10.1186/s12879-019-4243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asgedom SW, et al. Immunologic and clinical failure of antiretroviral therapy in people living with human immunodeficiency virus within two years of treatment. Biomed. Res. Int. 2020;2020:5474103–5474103. doi: 10.1155/2020/5474103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaborative Initiative for Paediatric, H.I.V.E. C. Research Global Cohort Incidence of switching to second-line antiretroviral therapy and associated factors in children with HIV: An international cohort collaboration. Lancet. HIV. 2019;6(2):e105–e115. doi: 10.1016/S2352-3018(18)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inzaule S, et al. Incidence and predictors of first line antiretroviral regimen modification in western Kenya. PLoS ONE. 2014;9(4):e93106–e93106. doi: 10.1371/journal.pone.0093106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thao VP, et al. Second-line HIV therapy outcomes and determinants of mortality at the largest HIV referral center in Southern Vietnam. Medicine. 2015;94(43):e1715–e1715. doi: 10.1097/MD.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aung ZZ, et al. Survival rate and mortality risk factors among TB–HIV co-infected patients at an HIV-specialist hospital in Myanmar: A 12-year retrospective follow-up study. Int. J. Infect. Dis. 2019;80:10–15. doi: 10.1016/j.ijid.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Woldemedhin B, Wabe NT. The reason for regimen change among HIV/AIDS patients initiated on first line highly active antiretroviral therapy in southern Ethiopia. N. Am. J. Med. Sci. 2012;4(1):19–23. doi: 10.4103/1947-2714.92898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infect. Dis. 2012;12(1):197. doi: 10.1186/1471-2334-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibhat M, Kassa M, Gebrehiwot H. Incidence and predictors of treatment failure among children receiving first-line antiretroviral treatment in general hospitals of two zones, Tigray, Ethiopia, 2019. Pediatr. Health Med. Ther. 2020;11:85. doi: 10.2147/PHMT.S243656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sisay MM, et al. Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: A retrospective follow-up study. BMJ Open. 2018;8(4):e019181. doi: 10.1136/bmjopen-2017-019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorsa A. Clinical, immunological and virological responses of zidovudine-lamivudine-nevirapine versus zidovudine-lamivudine-efavirenz antiretroviral treatment (ART) among HIV-1 infected children: Asella Teaching and Referral Hospital, South-East Ethiopia. Open Med. Inform. J. 2018;12:11. doi: 10.2174/1874431101812010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. (2008).
- 23.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brhane, B. G., NIbret, E., Abay, G. K., Nega, D. & Tsegay, Y. G. Virologic Failure and its Determinant Factors among Children in First Line on Highly Active Anti Retroviral Therapy at Felegehiwot Referral Hospital, Bahir Dar, Northwest, Ethiopia: cross-sectional study. (2020).
- 25.Getaneh Y, Yizengaw A, Likie A, Getahun M, Feleke A, Kidane E, Yilma A, Mulugeta A, Moshago T, Assefa Y. Rate and predictors of Treatment Failure among pediatric population taking Highly Active Antiretroviral Therapy in Ethiopia. J AIDS HIV Treat. 2019;1(2):58–68. [Google Scholar]
- 26.Haile GS, Berha AB. Predictors of treatment failure, time to switch and reasons for switching to second line antiretroviral therapy in HIV infected children receiving first line anti-retroviral therapy at a Tertiary Care Hospital in Ethiopia. BMC pediatrics. 2019;19(1):1–9. doi: 10.1186/s12887-019-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yassin S, Gebretekle GB. Magnitude and predictors of antiretroviral treatment failure among HIV‐infected children in Fiche and Kuyu hospitals, Oromia region, Ethiopia: a retrospective cohort study. Pharmacol. res. perspect. 2017;5(1):e00296. doi: 10.1002/prp2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadesse BT, Chala A, Mukonzo J, Chaka TE, Tadesse S, Makonnen E, Brumme ZL, Brumme CJ, Aklillu E. Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study. Pathogens. 2019;8(4):161. doi: 10.3390/pathogens8040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman, F.T. & Yizengaw, M.A. Virological failure and associated risk factors among HIV/AIDS pediatric patients at the ART clinic of Jimma University Medical Center, Southwest Ethiopia. Open AIDS J.14(1) (2020).
- 30.Workneh, N., Girma, T. & Woldie, M. Immunologic and clinical outcomes of children on HAART: a Retrospective cohort analysis at Jimma University specialized hospital. Ethiop. J. Health Sci.19(2) (2009).
- 31.Endalamaw A, et al. HIV/AIDS treatment failure and associated factors in Ethiopia: Meta-analysis. BMC Public Health. 2020;20(1):82. doi: 10.1186/s12889-020-8160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assemie MA, et al. Treatment failure and associated factors among first line patients on highly active antiretroviral therapy in Ethiopia: A systematic review and meta-analysis. Glob. Health Res. Policy. 2019;4(1):1–10. doi: 10.1186/s41256-019-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairlie L, et al. CD4+ and viral load outcomes of antiretroviral therapy switch strategies after virologic failure of combination antiretroviral therapy in perinatally HIV-infected youth in the United States. AIDS. 2015;29(16):2109–2119. doi: 10.1097/QAD.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandrasekaran P, et al. Long-term virological outcome in children receiving first-line antiretroviral therapy. AIDS Res. Ther. 2018;15(1):23. doi: 10.1186/s12981-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasoolinejad M, et al. Virologic Failure in different antiretroviral regimens among pediatric patients with HIV referring to a Voluntary Counseling and Testing (VCT) Center in Tehran, Iran (2004–2017) Arch. Pediatr. Infect. Dis. 2019;7(4):e80318. doi: 10.5812/pedinfect.80318. [DOI] [Google Scholar]
- 36.Fox MP, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J. Acquir. Immune Defic. Syndr. 2012;60(4):428–437. doi: 10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitwale NZ, et al. Prevalence and factors associated with virological treatment failure among children and adolescents on antiretroviral therapy attending HIV/AIDS care and treatment clinics in Dodoma Municipality, Central Tanzania. J. Pediatr. Infect. Dis. Soc. 2021;10:131–140. doi: 10.1093/jpids/piaa030. [DOI] [PubMed] [Google Scholar]
- 38.Cissé A-M, et al. High level of treatment failure and drug resistance to first-line antiretroviral therapies among HIV-infected children receiving decentralized care in Senegal. BMC Pediatr. 2019;19(1):47. doi: 10.1186/s12887-019-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biressaw S, et al. Adherence to Antiretroviral Therapy and associated factors among HIV infected children in Ethiopia: Unannounced home-based pill count versus caregivers’ report. BMC Pediatr. 2013;13(1):132. doi: 10.1186/1471-2431-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zegeye S, Sendo E. Adherence to antiretroviral therapy among hiv-infected children attending Hiwot Fana and Dil-Chora art clinic at referral hospitals in Eastern Ethiopia. J. HIV Clin. Sci. Res. 2015;2(1):008-14. [Google Scholar]
- 41.Feyera B, Letta S, Kumie A. Level of adherence and factors associated with antiretroviral therapy among HIV infected children in selected public hospitals, Addis Ababa, Ethiopia. East Afr. J. Health Biomed. Sci. 2016;1(1):23–30. [Google Scholar]
- 42.Nyogea D, et al. Determinants of antiretroviral adherence among HIV positive children and teenagers in rural Tanzania: A mixed methods study. BMC Infect. Dis. 2015;15(1):28. doi: 10.1186/s12879-015-0753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martelli G, et al. Adherence to antiretroviral treatment among children and adolescents in Tanzania: Comparison between pill count and viral load outcomes in a rural context of Mwanza region. PLoS ONE. 2019;14(3):e0214014. doi: 10.1371/journal.pone.0214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadunde I, et al. Factors associated with adherence to antiretroviral therapy among HIV infected children in Kabale district, Uganda: A cross sectional study. BMC. Res. Notes. 2018;11(1):466–466. doi: 10.1186/s13104-018-3575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akahara C, et al. Assessment of antiretroviral treatment adherence among children attending care at a tertiary hospital in southeastern Nigeria. J. Trop. Med. 2017;2017:3605850. doi: 10.1155/2017/3605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zubayr B, et al. Adherence to highly active antiretroviral therapy among HIV-infected children in Kano, Nigeria. J. Hum. Virol. Retrovirol. 2015;2(2):1–6. [Google Scholar]
- 47.Getawa S, et al. Antiretroviral treatment failure and associated factors among HIV-infected children on antiretroviral therapy: A retrospective study. HIV/AIDS. 2021;13:229. doi: 10.2147/HIV.S294046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempf MC, et al. Gender differences in discontinuation of antiretroviral treatment regimens. J. Acquir. Immune Defic. Syndr. 2009;52(3):336–341. doi: 10.1097/QAI.0b013e3181b628be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monforte AD, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS. 2000;14(5):499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 50.Alemayehu M, et al. Prevalence and correlates of anemia among children aged 6–23 months in Wolaita Zone, Southern Ethiopia. PLoS ONE. 2019;14(3):e0206268. doi: 10.1371/journal.pone.0206268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chernet, A. G., Nega, T. & Biru, M. D. Prevalence and Associated Factors of Anaemia severity among Children in Ethiopia (2019).
- 52.Kyaw NT, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005–2015. PLoS ONE. 2017;12(2):e0171780. doi: 10.1371/journal.pone.0171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babo YD, Alemie GA, Fentaye FW. Predictors of first-line antiretroviral therapy failure amongst HIV-infected adult clients at Woldia Hospital, Northeast Ethiopia. PLoS ONE. 2017;12(11):e0187694. doi: 10.1371/journal.pone.0187694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sisay M, Edessa D, Ayele Y, Getachew M. Pattern of and reasons for antiretroviral therapy regimen change among adult HIV/AIDS patients at regional hospital in Eastern Ethiopia: A 10-year retrospective study. SAGE Open Med. 2019;7:2050312119827092. doi: 10.1177/2050312119827092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used and analyzed for the review is available from the corresponding author upon reasonable request.