Abstract
The lncRNA GAS5 acts as a tumor suppressor and is downregulated in gastric cancer (GC). In contrast, E2F1, an important transcription factor and tumor promoter, directly inhibits miR-34c expression in GC cell lines. Furthermore, in the corresponding GC cell lines, lncRNA GAS5 directly targets E2F1. However, lncRNA GAS5 and miR-34c remain to be studied in conjunction with GC. Here, we present a dynamic Boolean network to classify gene regulation between these two non-coding RNAs (ncRNAs) in GC. This is the first study to show that lncRNA GAS5 can positively regulate miR-34c in GC through a previously unknown molecular pathway coupling lncRNA/miRNA. We compared our network to several in-vivo/in-vitro experiments and obtained an excellent agreement. We revealed that lncRNA GAS5 regulates miR-34c by targeting E2F1. Additionally, we found that lncRNA GAS5, independently of p53, inhibits GC proliferation through the ATM/p38 MAPK signaling pathway. Accordingly, our results support that E2F1 is an engaging target of drug development in tumor growth and aggressive proliferation of GC, and favorable results can be achieved through tumor suppressor lncRNA GAS5/miR-34c axis in GC. Thus, our findings unlock a new avenue for GC treatment in response to DNA damage by these ncRNAs.
Subject terms: Bioinformatics, Biological models, Computer modelling, Dynamic networks, Regulatory networks, Computational biology and bioinformatics, Systems biology
Introduction
Gastric cancer (GC) is responsible for the second-highest number of cancer-related deaths worldwide, making it a major medical issue and an attractive target for drug development1. Dysregulation of dominant signaling pathways through alterations in key oncogenes and tumor suppressors is one of the hallmarks of GC, which initiates aberrant cell proliferation and survival2. In this context, Zheng et al.3 provide evidence that E2F transcription factor 1 (E2F1) remains highly expressed in GC and is accountable for GC tumorigenesis in BGC-823, MGC-803, and SGC-7901 cell lines. Furthermore, Zheng et al.3 found that microRNA-34c-5p (hereinafter referred to as miR-34c) is downregulated in the same GC cell lines. Interestingly, Zheng et al.3 determined that E2F1 directly inhibits miR-34c expression at the transcriptional level. Furthermore, Zheng et al.3 confirmed that knockdown (KO) of E2F1 triggers miR-34c expression, whereas overexpression (E1) of E2F1 shows the opposite result. In conclusion, Zheng et al.3 showed that upregulated miR-34c could suppress GC proliferation through induction of cell cycle arrest and apoptosis at the G1/S checkpoint following E2F1 knockdown.
The miR-34 family (miR-34a, miR-34b, and miR-34c) is a master regulator of tumor suppression and plays an essential role in the DNA damage response4. MiR-34c is downregulated in several types of cancer, including GC3. The transactivation of miR-34c by p53 is well known, although new evidence has shown that miR-34c activation occurs through the ATM4 or ATM/p38 MAPK pathway5, which is independent of de-novo p53-mediated transcription4,5. Furthermore, the study of Suzuki et al.6 provides evidence that miR-34b and miR-34c are found to be epigenetically silenced in GC cell lines. Moreover, Suzuki et al.6 further reported that there is no correlation found between miR-34b/c and p53 functionality in GC. Interestingly, it is well-recognized that cyclin-dependent kinase inhibitor 1 (p21) is transactivated through p53. Nevertheless, in GC cell lines, p21 was found to be governed by ATM/p38 pathway. In more detail, Liu et al.7 found that activation of p21 was strongly detected in GC cell lines with Wildtype (WT) p53 (MKN-45) and cells lacking WT p53 (MKN-28). Moreover, Liu et al.7 further explained that p21 was found to act as a switch between cell cycle arrest and apoptosis due to its ability to inhibit caspase 3 activity in GC cell lines. Furthermore, upregulation of p21 was determined through the p38 MAPK signaling pathway rather than p537. On the other hand, Subhash et al.8 provide evidence that p53 is not essential for cell cycle arrest and apoptosis in the G1/S checkpoint in GC. In more detail, Subhash et al.8 observed that ATM is required to induce cell fate decisions such as cell cycle arrest and apoptosis in GC cell lines (IM95, IST1, NUGC4, TMK1, YCC3, MKN45, and MKN1) at the G1/S checkpoint. Moreover, Subhash et al.8 found that overexpression of ATM in response to DNA damage induces robust induction of p21 and PUMA in CG cell lines, independently of p53. Interestingly, miR-34c regulates p21 expression independent of p53 in various cancer cell lines9.
It is well known that protein-coding genes account for above than 1% of the total genome, whereas large portions of the human genome can be transcribed into non-coding RNAs (ncRNAs)10,11. Indeed, miRNAs may be sufficient to inhibit proliferation in GC cells in response to DNA damage12. miRNAs are a class of small ncRNAs often associated with several biological processes such as cancer progression12. Recent studies identified a novel class of small ncRNAs, i.e., long noncoding RNAs (lncRNAs), which may regulate cell proliferation in GC through downstream DNA damage mechanisms13. lncRNAs have recently emerged as important players in cancer biology and can be used for cancer diagnosis, prognosis, and potential therapeutic targets13. In this context, a tumor suppressor lncRNA-GAS5 (growth arrest-specific transcript) was found to be dysregulated in various cancers, including GC14. Furthermore, in response to DNA damage, overexpression of the lncRNA-GAS5 can suppress cancer progression by induction of cell-cycle arrest or cellular apoptosis in many types of cancer, including GC14. Interestingly, recently, Sun et al.15 exposed the role of lncRNA-GAS5 in (SGC7901, BGC823, MGC803, MKN45, MKN28) GC cell lines. In detail, Sun et al.15 found that lncRNA-GAS5 expression was markedly downregulated in GC tissues and correlated with larger tumor size. Moreover, these authors15 further demonstrated through the Vivo/Vitro experiments that gain-of-function (GoF) of lncRNA-GAS5 inhibits gastric cancer cell proliferation by inducing cell fates at the G1/S checkpoint, while downregulation of lncRNA-GAS5 could promote cell proliferation. Furthermore, Sun et al.15 confirmed that E2F1 and G1/S-specific cyclin-D1 (Cyclin D1) were functional targets of lncRNA-GAS5 in GC cells. However, the advanced aspect of the lncRNA-GAS5 in the coordination of miR-34c expression in response to DNA damage at the G1/S checkpoint remains unexplained in GC cells.
Boolean models of regulatory networks were meant to simplify the dynamics of complex biological systems16. This method provides a qualitative description that captures advanced features of network dynamics17. For example, enclosed pathways connecting two or more nodes in a network (similar to feedback loops in the continuous model) may serve as regulatory circuits controlling the dynamics of the network18–21. Classification of molecular regulatory networks in a rational framework by a computational-experimental combinatorial process is recognized as a valuable approach for cell fate choice and the study of various biological processes19,20,22–28. For more details about Boolean modeling, see the Methods section.
Inspired by the facts mentioned above3,15, in this work, we proposed a mathematical model to uncover the unique function of the lncRNA-GAS5 in the gene regulation of GC at the G1/S checkpoint (see Fig. 1). Indeed, to our knowledge, this is the first fundamental study that uncovers a positive concrete nexus between lncRNA versus miRNAs, i.e., lncRNA-GAS5 controls miR-34c expression in GC.
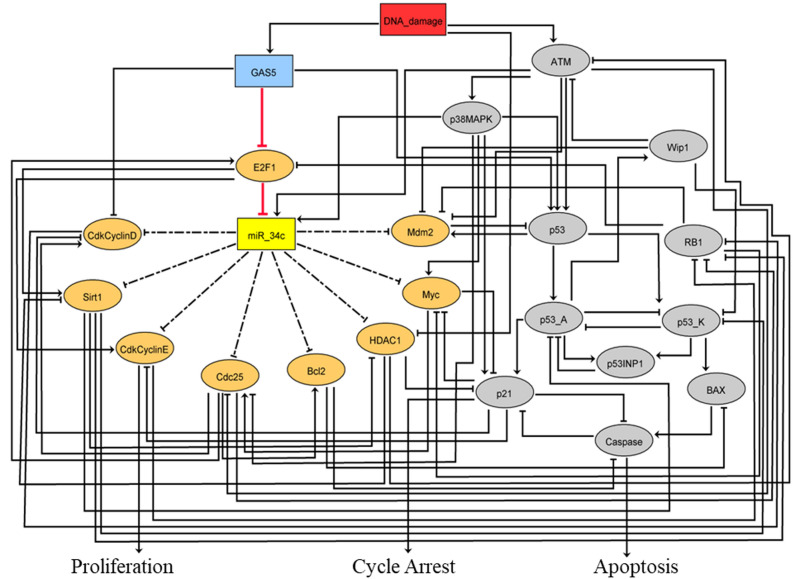
Figure 1.
Gene regulatory network of lncRNA-GAS5 for the GC. Arrows indicate activations, and hammer-head arcs indicate inhibitions, respectively. The blue rectangular node represents lncRNA-GAS5, the yellow rectangular node represents miR-34c, and the red one represents DNA damage which is the input to the Boolean model. Dashed hammer-head arcs represent the targets of miR-34c highlighted by orange-colored oval nodes while other proteins are in grey-colored nodes. Model outputs are proliferation (in the lack of DNA damage) or cell cycle arrest, or apoptosis in the occupation of DNA damage.
Results
Boolean model and its fixed points
There are 26 nodes in our Boolean network. The blue rectangular node represents lncRNA-GAS5, the yellow node signifies miR-34c, and the red one defines DNA damage which is the input of the Boolean network. Dashed hammer-head arches are highlighted by orange-colored oval nodes delineating the targets of miR-34c, while other proteins are in grey-colored nodes. The three outputs (in white nodes) of the network are proliferation, cell cycle arrest, and apoptosis, respectively. There are a total of 73 direct interactions within these nodes. See Fig. 1.
Our network simulations show 3 fixed points for the wild-type case dynamics (WT) that are associated with multiple phenotypes, Fig. 2. The first fixed point indicates a proliferative state (corresponding to input: DNA damage = OFF), i.e., no cycle arrest is identified, only cell cycle enhancers are active: cdk4/6-CycD, cdk2/CycE, Cdc25A, HDAC1, Myc and E2F1. Additional two fixed points (produced by the same input: DNA damage = ON), i.e., cell cycle arrest and apoptosis, respectively. Interestingly, all these three fixed points are consistent with in-vivo/in-vitro experiments3,15 in GC.
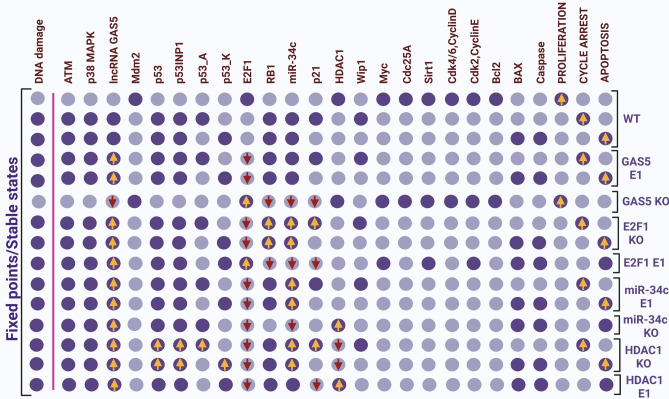
Figure 2.
Model fixed points for WT case and perturbations. The fixed points identified for distinct scenarios: WT, GAS5 E1, GAS5 KO, E2F1 KO, E2F1 E1, miR-34c E1, miR-34c KO, HDAC1 KO, HDAC1 E1, and p53 KO. Ectopic expression (E1) represents gain-of-function (GoF) while knockdown (KO) represents loss-of-function (LoF) of the respective network element. The leftmost column shows the level of DNA damage with other molecules separated by a pink line. At the same time, the rightmost columns show the model outputs: proliferation, cell cycle arrest, and apoptosis. Each line represents a fixed point or steady state corresponding to the input. Light purple cells indicate a zero value (inactivation), while dark purple cells indicate activation (value 1). The first three fixed points belong to the wild-type case. The remaining fixed points explain how GAS5 regulates the miR-34c expression, and at the end, three fixed points linked to the HDAC1/p21 pathway represent activation of p21 via knockdown of HDAC1 by miR-34c. The GoF of GAS5 accelerates miR-34c expression that triggers cell cycle arrest and apoptosis, whereas knockdown (KO) of GAS5 inhibits miR-34c expression but induces E2F1 that, triggers proliferation. Knockdown (KO) of E2F1 increases miR-34c expression that induces cycle arrest and apoptosis, whereas its gain of function (GoF) inhibits miR-34c expression. Overexpression of miR-34c induces cell cycle arrest and apoptosis, whereas knockdown (KO) triggers apoptosis. Next, the knockdown (KO) of HDAC1 enhanced p21 expression, which means induction of cell cycle arrest, and apoptosis. In comparison, its gain-of-function (GoF) inhibits p21 expression and induces apoptosis. Yellow arrows represent upregulation, while the red arrows facing down represent downregulation, respectively.
lncRNA-GAS5 modulates miR-34c expression through the targeting of E2F1
To uncover the advanced aspect of lncRNA-GAS5 in the regulation of miR-34c expression through the knockdown of E2F1, which is the main focus here. We interrogated lncRNA-GAS5/E2F1 and miR-34c, i.e., we performed GoF/LoF perturbation. The results are presented in Fig. 2. We found that overexpression (E1) of lncRNA GAS5 diminished E2F1 expression and increased the expressions of miR-34c, RB1, and p21. In comparison, knockdown (KO) of lncRNA GAS5 gives the opposite effect. On the other hand, overexpression of E2F1 declined miR-34c, RB1, and p21 expressions. At the same time, the knockdown of E2F1 presents opposite outcomes. In the last, overexpression (E1) of miR-34c inhibits proliferation through the induction of cell cycle arrest and apoptosis. Whereas knockdown (KO) of miR-34c enhanced apoptosis. As can be seen in Fig. 2, overexpression (E1) or knockdown (KO) of miR-34c did not affect the lncRNA-GAS5 expression.
Interestingly, all these results are in excellent agreement with in-vivo/in-vitro experiments3,15 in GC. Our results suggest that lncRNA-GAS5 is an important regulator of miR-34c activity in GC. Additionally, these results further pinpointed that lncRNA-GAS5 can also regulate RB1 and p21 expressions in GC.
Induction of p21 through the knockdown of HDAC1 by miR-34c
HDAC1 is a well-known tumor promoter in GC cells. Growing evidence suggests that targeting HDAC1 can upregulate p21 by miR-34c9,29,30. Therefore, we conducted the node perturbations to examine the similarity between phenotypes (model attractors) and experimental observations. Results are presented in Fig. 2. We found that the direct suppression of HDAC1 due to overexpression of miR-34c induces p21 expression, while the gain-of-function (GoF) of HDAC1 downregulates p21 (see Fig. 2). Additionally, Liu et al.7 provide evidence that p21 acts as a switch between cell cycle arrest and apoptosis, and the functionality of this switch does not depend on p53 status, implying that p21 can regulate cell cycle arrest and apoptosis in p53-deficient GC cell lines. To test it, we carried out another perturbation of loss-of-function (LoF) of p53. Results are presented in Fig. 2. These results show that miR-34c indirectly regulates p21 in GC. Thus, miR-34c functions to regulate cycle arrest and apoptosis through the HDAC1/p21 pathway that does not depend on the p53 functionality. Our results are in the finest agreement with these in-vivo/in-vitro experiments7,9,29,30.
The ATM/p38 MAPK pathway is required for the lncRNA-GAS5-mediate cell fate decision in GC
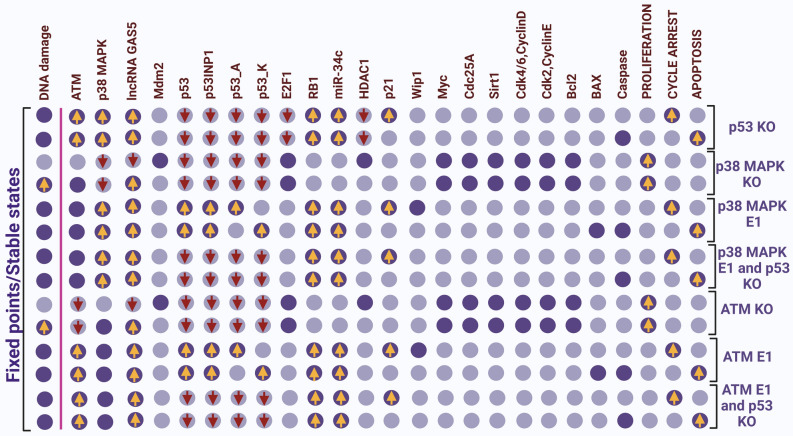
Indeed, Liu et al.7 confirmed that G1/S arrest and apoptosis are independent of p53 but dependent on the p38 MAPK pathway in GC cell lines. Furthermore, Subhash et al.8 provide further evidence that ATM mediates p53-independent regulation of apoptosis and cell-cycle arrest at the G1/S checkpoint in GC cells. Interestingly, various studies have recently shown that lncRNA-GAS5 is associated with the ATM/p38 MAPK pathway31–34. To address whether this ATM/p38 MAPK signaling pathway, independent of p53 and can regulate cell cycle arrest and apoptosis at the G1/S checkpoint. We performed node perturbation to examine the agreement between the phenotypes (fixed points of the model) and in-vivo/in-vitro experiments. The results are presented in Fig. 3. As expected, we found that loss-of-function (LoF) of p38 MAPK or ATM, i.e., knockdown of p38 MAPK or ATM, induces GC proliferation. Whereas gain-of-function (GoF) of p38 MAPK and ATM triggers cell cycle arrest and apoptosis in GC cell lines. We conducted two separate perturbations of each node. In more detail, first, we performed the single node perturbation, i.e., we overexpressed (E1) of p38 MAPK, and second, double node perturbation, which means we performed loss-of-function (LoF) of p53 together with gain-of-function (GoF) of p38 MAPK. We repeated the same technique with ATMs. First, we overexpressed (E1) ATM alone, and next, we overexpressed (E1) ATM along with knockdown (KO) of p53. In this way, we can determine that ATM/p38 MAPK may regulate cell fate decisions such as cell cycle arrest and apoptotic cell death beyond the functionality of p53 in GC cell lines. Interestingly (see Fig. 3). We observed that p38 MAPK and ATM knockdown (KO) cells, lncRNA-GAS5, were stimulated in the presence of DNA damage; however, lncRNA-GAS5 failed to regulate cycle arrest and/or apoptosis. These results provide two important conclusions. First, the ATM/p38 signaling pathway, rather than p53, is required for modulating tumor growth and proliferation in GCs. Second, the lncRNA-GAS5 regulates cell fates in an ATM/p38 signaling pathway-dependent manner. Our results are in excellent agreement with the in-vivo/in-vitro experiments7,8,31,33,34.
Figure 3.
Interrogation of the ATM/p38 MAPK and p53 signaling pathway in GC. Ectopic expression (E1) represents gain-of-function (GoF) while knockdown (KO) represents loss-of-function (LoF) of the respective network element. The leftmost column shows the level of DNA damage with other molecules separated by a pink line. In contrast, the rightmost columns show the model outputs: proliferation, cell cycle arrest, and apoptosis. Each line represents a fixed point or steady state corresponding to the input. Light purple cells indicate a zero value (inactivation), while dark purple cells indicate activation (value 1). The first two fixed points are related to loss-of-function (LoF) of p53, that is, all node knockdown (KO) of p53. As you can see, only the ATM/p38 MAPK pathway is activated and triggers cell cycle arrest and apoptosis in response to DNA damage. The next two fixed points are described as loss-of-function (LoF) of p38 MAPK, that is, induction of proliferation. Apparently, the first fixed point knockdown (KO) of p38 MAPK represents a proliferation state due to the inactivation of DNA damage. Even in the presence of DNA damage, knockdown (KO) of p38 MAPK accelerated proliferation due to activation of cell cycle promoters such as E2F1, Myc, and CDK-cyclin complex. As you can see, loss-of-function (LoF) of p38 MAPK in response to DNA damage, lncRNA-GAS5 expression is accelerated but fails to trigger cell cycle arrest and/or apoptosis except in the proliferation state. After that, the other two fixed points represent the gain-of-function (GoF) of p38 MAPK, which means cell cycle arrest and apoptosis as well as activation of the p53 pathway. The ensuing fixed points are associated with gain-of-function (GoF) of p38 MAPK as well as loss-of-function of p53. Clearly, activation of the expression of the lncRNA-GAS5, miR-34c, p21, and RB1 triggers arrest and apoptosis (independently of p53). The other two following fixed points indicate the knockdown (KO) of ATM. Equally, this was observed in the loss-of-function (LoF) of p38MAPK. This means that at a certain point in the absence of DNA damage and another in response to DNA damage triggered proliferation; similarly, the lncRNA GAS was activated but refused to induce cell cycle arrest and apoptosis-like phenotypes. The other two fixed points of ATM represent the gain-of-function (GOF) of ATM, and then, more two fixed points (at last) represent the overexpression (E1) of ATM with p53 KO (knockdown), i.e., expression of the lncRNA-GAS5, miR-34c, p21, and RB1, induces cell cycle arrest and apoptosis (independently of p53). Yellow arrows represent upregulation, while the red arrows facing down represent downregulation, respectively.
Clinical trials proposed by the Boolean network
The concept of experimental design is driven by Guo et al.35. Who found that lncRNA-GAS5 blocks the G1/S checkpoint by modulating CDK6. Indeed, forced expression of lncRNA-GAS5 enhanced p21 activation, which directly inhibits CDK4/6-CyclinD and CDK2-CyclinE at the G1/S checkpoint. However, so far, there is no study/evidence yet that can uncover the functional concrete relationship between lncRNA-GAS5 and miR-34c. Therefore, we propose a new clinical framework defined by the network that a lncRNA-GAS5 functioning as an inhibitor of E2F1 may inhibit cancer progression through the establishment of miR-34c and can induce cell cycle arrest and apoptosis at the G1/S checkpoint.
A conceivable outcome of this experiment would be the suppression of tumor growth and proliferation in GC. To test this development, we insist on these potential perturbations scenarios predicted by the network.
lncRNA-GAS5 (E1) → E2F1 (KO) → miR-34c ↑
lncRNA-GAS5 (E1) → E2F1 (KO) → RB1 ↑
lncRNA-GAS5 (E1) → E2F1 (KO) → p21 ↑
Discussion
It is widely known that E2F1 is involved in both cancer cell growth and apoptosis36,37. Petrocca et al.38 went into further depth, showing that transfection of E2F1 overexpression and suppressing expression in GC cells might promote cell proliferation and prevent apoptosis. Similar to this, Guo et al.39 recently discovered that miR-537/E2F1 has a positive (double negative) feedback loop which inhibits GC development and proliferation by activating cell cycle arrest and apoptosis. Furthermore, Zhang and colleagues40 discovered that overexpression of E2F1 and p53 cause cancer cells to undergo apoptotic cell death in response to DNA damage. We have also revealed the dual functionality of E2F1 in both proliferation and apoptosis in glioblastoma multiform (GBM)41. High expression of E2F1 has been linked to poor outcomes in GC15. As no genetic aberrations have been found so far that could describe the upregulation of E2F1 in GC42. The lncRNA-GAS5 is a key regulator of E2F1 expression and is downregulated in GC15. Similarly, miR-34c is downregulated in GC3. Interestingly, miR-34c was inhibited by E2F1 in GC3. It was unknown whether the lncRNA-GAS5/E2F1 axis plays an important role in the regulation of miR-34c in GC. Here, we constructed a Boolean network to identify lncRNA-GAS5 interactions in GC (see Fig. 1). The model suggested the lncRNA-GAS5 as a key regulator of miR-34c through its ability to repress E2F1 expression. Consistent with this concept, high expression of the lncRNA-GAS5/miR-34c in GC was correlated with suppression of GC progression and proliferation through induction of cell cycle arrest and/or apoptotic in GC3,15.
Our results suggest the mechanism by which increased expression of lncRNA-GAS5 may enhance miR-34c expression in GC. lncRNA-GAS5 targets E2F1 expression, which is the main inhibitor of miR-34c activity in GC. Knockdown of E2F1 was followed by increased expression of the tumor suppressor miR-34c, i.e., inhibition of tumor growth and proliferation at the G1/S checkpoint. The following assumption is confirmed by the observation of lncRNA-GAS5 expression at three fixed points (attractors). The results are shown in Fig. 2. In the first fixed point, lncRNA-GAS5/miR-34c was downregulated, whereas E2F1, CDK4/6-CyclinD, CDK2-CyclinE, Cdc25A were highly expressed. In response to DNA damage, two fixed points were found to be associated with lncRNA-GAS5, conferring constitutive activation of miR-34c in both fixed points. On the other hand, E2F1 was downregulated in both fixed points, which was expected.
Indeed, our Boolean network captured a specific molecular mechanism related to the lncRNA-GAS5 that had never been reported before. Nevertheless, all these marked results are established on the discrete core of the model elements. Therefore, the prediction of time-dependent features as the evolution of precise expression levels over time is one of the limitations of our model.
Plenty of studies are available that highlight the essential role of lncRNAs sponging miRNAs in controlling mRNAs. For example, numerous studies found that the lncRNA-GAS5 sponged miR-2143, miR-22231, and miR-106a-5p44 in several cancers, including GC. On the other hand, the lncRNA-GAS5 is also known to directly (positively or negatively) regulate mRNAs expressions such as E2F115, p5345, and mTOR46. However, there is no evidence yet that lncRNAs can positively regulate miRNAs expression by sponging/decoys of other molecules (e.g., other lncRNAs, miRNAs, mRNA). Our results highlighted a very convincing relationship between the lncRNA-GAS5 and miR-34c via E2F1. Unraveling this novel molecular mechanism associating lncRNA-GAS5/miR-34c for the first time confirms that lncRNAs can positively modify miRNAs and that collectively they can effectively inhibit the tumor growth and proliferation in GC.
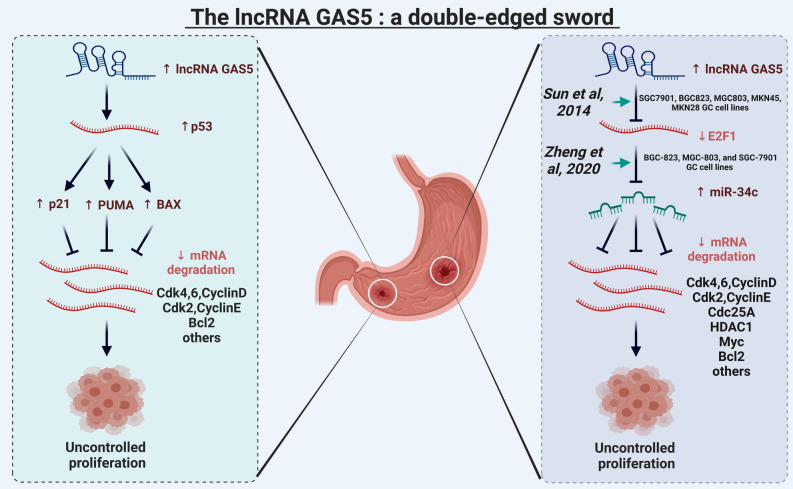
Additionally, our results revealed that the ATM/p38 MAPK pathways are essential for regulating cell cycle arrest and apoptosis at the G1/S checkpoint in GC cell lines7,8. It is well known that p53 is the main regulator of cell fate in response to DNA damage and plays an important role in controlling tumor growth47. However, in GC cell lines, p53 mutations have been found in up to 70%48, and there is much evidence confirming that the ATM/p38 MAPK pathway compensates for p53 deficiency in GC cells7,8,49. So, we tested it, and the results are shown in Fig. 3. We found that cell fate decisions such as cell cycle arrest and/or apoptosis are independent of p53 status in GC cells as suggested by these in-vivo/in-vitro studies7,8,49. Although WT p53 is defined in some GC cell lines, it will induce cell cycle arrest and/or apoptosis, as both ATM/p38 MAPK are upstream regulators of p5350,51. Whereas p53-deficient GC cell lines, ATM/ p38MAPK pathway eliminates the tumor growth and proliferation by the activation of cell cycle arrest and apoptosis in GC cell lines. Thus, lncRNA-GAS5 acts as a double-edged sword in GC progression through the activation of miR-34c expression as well as p21 and RB1. For more detail, see Fig. 4. Consequently, Our results provide a broad and relevant background in this new direction. miRNAs are already well-known players in cancer research52. The molecular mechanisms associated with lncRNAs are not yet fully understood, and there is still much to be revealed. The results presented in this work will provide a new direction to many researchers with diverse expertise in fighting cancer (development of new technologies and new experiments) based on accumulated knowledge.
Figure 4.
lncRNA-GAS5: a double-edged sword in tumor growth and proliferation in GC. On the left (highlighted in the green box), p53 was directly activated by lncRNA-GAS5 in GC45. Once p53 expression is activated, it may induce p2168, PUMA/BAX68. Although, the expression of p21 does not depend on the p53 functionality in GC7,8. Despite this fact, activated p21 and PUMA/BAX, which directly inhibit the expression of the CDK4/6-CyclinD and CDK2-CyclinE, Cdc25A, Bcl2, and others and induce cell cycle arrest and apoptosis at the G1/S checkpoint. On the right (highlighted in the purple box), the novel tumor suppressor mechanism of lncRNA-GAS5 is captured by the Boolean network. Overexpression of lncRNA-GAS5 directly inhibits E2F1 expression15, which triggers miR-34c expression3. Then, overexpression of miR-34c directly targets the CDK4/6-CyclinD and CDK2-CyclinE, Cdc25A, HDAC1, Myc, Sirt1, Mdm2, and Bcl2, see the review by Xiong et al.67. Targeting these molecules activates cycle arrest and apoptotic cell death at the G1/S checkpoint in GC. Thus, lncRNA-GAS5/miR-34c axis inhibits tumor growth and uncontrolled proliferation in GC. Consequently, p53-dependent or independently of p53, lncRNA-GAS5 can prevent tumor progression and cell proliferation in GC.
In summary, we developed a Boolean model of lncRNA-GAS5 at the G1/S checkpoint based on the literature and public databases in GC. Our Boolean model simulations uncover very complex scenarios between lncRNA-mRNA-miRNA, i.e., lncRNA-GAS5, E2F1, and miR-34c in response to DNA damage. Our results suggested that lncRNA-GAS5 is an important regulator of miR-34c activity in GC. Thus, our approach may contribute to proposing alternative therapeutic strategies for cancer through drugs targeting this lncRNA-mRNA-miRNA axis that might significantly reduce the tumor growth and proliferation in GC cancer cells.
Methods
Development of the gene regulatory network and collection of the public databases & tools
Building a gene regulatory network of lncRNA-GAS5 activity GC cells. We have only used PubMed studies53 and databases such as BioGrid54. The focus was to identify genes or proteins that were targeted by the lncRNA-GAS5 (Fig. 1), such as E2F1 and cyclin D1. Furthermore, we identify genes or proteins that are directly targeted through the miR-34c. For that, we have used public databases like; miRTargetLink55 and TargetScan56, respectively.
GINsim 3.0.0b was used for the construction and simulation of the Boolean network and visualization of the results57, which is a Java-based software and is freely available to researchers (http://www.ginsim.org/downloads). GINsim algorithms recognize all the attractors for the wild-type case as well as for various mutant situations57.
The model file is available in the “Code availability” section.
Translation of PubMed literature into boolean rules and simulations of the boolean network
The Boolean method is based on the characterization of a regulatory graph, where an individual node defines a molecule, and each directed edge (or arc) signifies an activation or inhibition among two nodes. Nodes are Boolean variables that only consider 0/OFF and 1/ON values. Based on the description of the biochemical information, each node in the network is assigned a logical rule, which determines its activation level concerning the position of its regulators58.
Our Boolean network model of lncRNA-GAS5 was produced by translating the biological interactions of the DNA damage-induced G1/S checkpoint, which is an ATM/p53-dependent, described in the gene regulatory network (Fig. 1) into Boolean rules. For more details, see Supplementary Table S1 (PubMed links included). Classical Boolean operators were used to write these rules “AND,” “OR,” and “NOT”. Attractors are the main outcome of simulations using a Boolean network58. The dynamical performance of a Boolean model can be interpreted by a state transition graph (STG). In this graph, each node describes the state of the network variables, and the arcs signify transitions between these states58. The STG serves all possible trajectories that one initial state can drive to a final state. Terminal nodes that have no outgoing edges are called stable states (or fixed points), while a set of transitions trapped among a fixed group of states in the STG defines a cyclic state58. For the updates of states, synchronous updates were considered, which has the potential to describe deterministic behavior observed in molecular networks16,57. In addition, in silico Gain-of-Function (GoF) or Loss-of-function (LoF) perturbations, we force node values to be ON or OFF, respectively, to examine the effect of particular nodes on network dynamics and the resulting phenotype57,58.
Expected performance of the lncRNA-GAS5 Model
Simulations using the lncRNA-GAS5 Boolean network should describe the biology of a cancer cell (with or without DNA damage in GC). Indeed, cancer cells can be simplified as either remains a proliferative state due to downregulation of lncRNA-GAS5/miR-34c3,15 i.e., upregulation of E2F115 (in the absence of DNA damage). Whereas, in the case when DNA damage is present in the cell, upregulation of lncRNA-GAS5/miR-34c is required that can repress the proliferation by the induction of cell fate decisions such as cell cycle arrest and/or apoptosis at the G1/S checkpoint3,14,15. Consequently, at least three fixed points/stable states (attractors) are expected.
lncRNA-GAS5-mediate molecular mechanism of the G1/S checkpoint in GC
It is well-known that cell fate decisions such as cell cycle arrest or apoptosis are induced by DNA damage response at both G1/S and G2/M checkpoints22,59–61. Interestingly, lncRNA-GAS5 can be induced by DNA damage as suggested by Liu et al.14 and Zhou et al.62. In addition, in the event of the G1/S checkpoint, about 20 molecules are involved in its main regulatory network63. DNA double-strand breaks (DSBs) can be affected by the radiomimetic chemicals or reactive oxygen species (ROS) or radiation, which triggers autophosphorylation of ATM at serine 1981 by initiating its kinase activity63,64. Downstream phosphorylation at the ATM pathway leads to the activation of p53 in response to DNA damage64. In GC cells, lncRNA-GAS5 is required for the induction of the G1/S checkpoint, which is our focus here; in fact, GC cells knocked down for lncRNA-GAS5 cannot arrest at the G1/S phase15.
In DNA damage response, In this model, based on its different phosphorylation events, p53 is denoted by different nodes65. The p53 node is linked with its interaction with Mdm2, which is required to initiate p53-A and p53-K65. p53-A represents p53 phosphorylated at Ser-15 and Ser-20 that activated p21, while p53-K represents p53 further phosphorylation at Ser-46, which leads to the activation of the apoptotic pathway via BAX65. P53-A and p53-K are attached by a positive circuit65, and the conversion between p53-A and p53-K is ruled by the protein phosphatase 1D (Wip1) and the tumor protein p53 inducible nuclear protein 1 (p53-INP1)65. In the network, apoptosis is triggered by caspase, which is regulated by Bcl2, Bax, and p21. In more detail, activation of Bax and inactivation of Bcl2 and p21 are associated with caspase activation, which causes apoptotic cell death.
In addition, p53 is a well-known activator of the miR-34 family (miR-34a, miR-34b, and miR-34c) in the DNA damage response66. However, the expression of miR-34c modulates by ATM and p38 MAPK pathway, which is independent of de-novo p534,5. Therefore, in our network, miR-34c is directly activated by the ATM/p38 MAPK pathway4,5 or indirectly by the lncRNA GAS5/E2F1 pathway3,15.
miR-34c directly targets E3 ubiquitin-protein ligase Mdm2 (Mdm2), Myc proto-oncogene protein (Myc), Cyclin-dependent kinase 4/6 (cdk4/6-Cyclin-D), and the Cyclin-dependent kinase 2-G1/S-specific cyclin-E1 (cdk2/Cyclin-E) complex, Cell division cycle 25A (Cdc25A), NAD-dependent protein deacetylase sirtuin-1 (Sirt1), Histone deacetylase 1 (HDAC1), and Apoptosis regulator Bcl-2 (Bcl2), for more details about miR-34c targets see review by Xiong et al.67. Worth noting is that in the lncRNA-GAS5 Boolean network, we selected only experimentally verified targets of miR-34c. Forced expression of miR-34c induces cycle arrest by targeting cdk4/6-Cyclin-D, cdk2/Cyclin-E complex, HDAC1, Myc, and Cdc25A3,66, whereas it triggers apoptotic phenotype by knockdown Bcl23,66.
Supplementary Information
Acknowledgements
S. G. and R. F. H. are thankful to the São Paulo Research Foundation (FAPESP) for the financial support, Grant No. 2021/09070-8 and 2015/22308-2, respectively. P.K.P., W.L., and R.A. acknowledge the financial support from the Swedish Research Council (VR Grant No. 2016-06014).
Author contributions
S.G. designed and conceptualized the study, S.G, P.K.P, and R.F.H analyzed the results, S.G. and P.K.P. wrote the manuscript; S.G., P.K.P designed all the illustrations; R.F.H. verified and provided feedback to the illustrations; W.L. and R.A. provided critical feedback and edited the manuscript. All authors approved the final version of the manuscript.
Funding
Open access funding provided by Uppsala University.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Code availability
The model code is available in the Github repository: https://github.com/pritampanda15/lncRNA-GAS5.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shantanu Gupta, Pritam Kumar Panda and Ronaldo F. Hashimoto.
Contributor Information
Ronaldo F. Hashimoto, Email: ronaldo@ime.usp.br
Rajeev Ahuja, Email: rajeev.ahuja@physics.uu.se.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21492-x.
References
- 1.Ajani JA, et al. Gastric adenocarcinoma. Nat. Rev. Dis. Primers. 2017;3:1–19. doi: 10.1038/nrdp.2017.36. [DOI] [PubMed] [Google Scholar]
- 2.Smith M-G, Hold G-L, Tahara E, El-Omar E-M. Cellular and molecular aspects of gastric cancer. World J. Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng H, Wang J-J, Yang X-R, Yu Y-L. Upregulation of miR-34c after silencing E2F transcription factor 1 inhibits paclitaxel combined with cisplatin resistance in gastric cancer cells. World J. Gastroenterol. 2020;26:499–513. doi: 10.3748/wjg.v26.i5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzman DW, et al. miR-34 activity is modulated through 5’-end phosphorylation in response to DNA damage. Nat. Commun. 2016;7:10954. doi: 10.1038/ncomms10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannell IG, et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. PNAS. 2010;107:5375–5380. doi: 10.1073/pnas.0910015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, et al. Methylation-associated silencing of microRNA-34b/c in gastric cancer and its involvement in an epigenetic field defect. Carcinogenesis. 2010;31:2066–2073. doi: 10.1093/carcin/bgq203. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z-M, et al. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503–513. doi: 10.1038/sj.onc.1207173. [DOI] [PubMed] [Google Scholar]
- 8.Subhash VV, et al. ATM expression predicts veliparib and irinotecan sensitivity in gastric cancer by mediating P53-independent regulation of cell cycle and apoptosis. Mol. Cancer Ther. 2016;15:3087–3096. doi: 10.1158/1535-7163.MCT-15-1002. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Lammers P, Torrance CJ, Bader AG. TP53-independent function of miR-34a via HDAC1 and p21(CIP1/WAF1.) Mol. Ther. 2013;21:1678–1686. doi: 10.1038/mt.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L, et al. Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer. 2013;119:1618–1626. doi: 10.1002/cncr.27903. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, et al. LncRNAs: Emerging biomarkers in gastric cancer. Future Oncol. 2015;11:2427–2441. doi: 10.2217/fon.15.175. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, et al. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci. Rep. 2015;5:10159. doi: 10.1038/srep10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun M, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwab JD, Kühlwein SD, Ikonomi N, Kühl M, Kestler HA. Concepts in Boolean network modeling: What do they all mean? Comput. Struct. Biotechnol. J. 2020;18:571–582. doi: 10.1016/j.csbj.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozum JC, Gómez Tejeda Zañudo J, Gan X, Deritei D, Albert R. Parity and time reversal elucidate both decision-making in empirical models and attractor scaling in critical Boolean networks. Sci. Adv. 2021;7:eabf8124. doi: 10.1126/sciadv.abf8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R, Thieffry D, Kaufman M. Dynamical behaviour of biological regulatory networks–I. Biological role of feedback loops and practical use of the concept of the loop-characteristic state. Bull. Math. Biol. 1995;57:247–276. doi: 10.1007/BF02460618. [DOI] [PubMed] [Google Scholar]
- 19.Deritei D, Rozum J, Ravasz Regan E, Albert R. A feedback loop of conditionally stable circuits drives the cell cycle from checkpoint to checkpoint. Sci. Rep. 2019;9:16430. doi: 10.1038/s41598-019-52725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silveira DA, Gupta S, Mombach JCM. Systems biology approach suggests new miRNAs as phenotypic stability factors in the epithelial-mesenchymal transition. J. R. Soc. Interface. 2020;17:20200693. doi: 10.1098/rsif.2020.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Hashimoto RF. Dynamical analysis of a boolean network model of the oncogene role of lncRNA ANRIL and lncRNA UFC1 in non-small cell lung cancer. Biomolecules. 2022;12:420. doi: 10.3390/biom12030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Silveira DA, Mombach JCM. Towards DNA-damage induced autophagy: A boolean model of p53-induced cell fate mechanisms. DNA Repair. 2020;96:102971. doi: 10.1016/j.dnarep.2020.102971. [DOI] [PubMed] [Google Scholar]
- 23.Guberman E, Sherief H, Regan ER. Boolean model of anchorage dependence and contact inhibition points to coordinated inhibition but semi-independent induction of proliferation and migration. Comput. Struct. Biotechnol. J. 2020;18:2145–2165. doi: 10.1016/j.csbj.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wooten DJ, et al. Mathematical modeling of the Candida albicans yeast to hyphal transition reveals novel control strategies. PLoS Comput. Biol. 2021;17:e1008690. doi: 10.1371/journal.pcbi.1008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Silveira DA, Hashimoto RF, Mombach JCM. A boolean model of the proliferative role of the lncRNA XIST in non-small cell lung cancer cells. Biology. 2022;11:480. doi: 10.3390/biology11040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, et al. Dynamical modeling of miR-34a, miR-449a, and miR-16 reveals numerous DDR signaling pathways regulating senescence, autophagy, and apoptosis in HeLa cells. Sci. Rep. 2022;12:4911. doi: 10.1038/s41598-022-08900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silveira DA, Gupta S, Sinigaglia M, Mombach JCM. The Wnt pathway can stabilize hybrid phenotypes in the epithelial-mesenchymal transition: A logical modeling approach. Comput. Biol. Chem. 2022;99:107714. doi: 10.1016/j.compbiolchem.2022.107714. [DOI] [PubMed] [Google Scholar]
- 28.Mazaya M, Kwon Y-K. In silico pleiotropy analysis in KEGG signaling networks using a boolean network model. Biomolecules. 2022;12:1139. doi: 10.3390/biom12081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, et al. By recruiting HDAC1, MORC2 suppresses p21Waf1/Cip1 in gastric cancer. Oncotarget. 2015;6:16461–16470. doi: 10.18632/oncotarget.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisnieski F, et al. Differential expression of histone deacetylase and acetyltransferase genes in gastric cancer and their modulation by trichostatin A. Tumour Biol. 2014;35:6373–6381. doi: 10.1007/s13277-014-1841-0. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, et al. Long non-coding RNA growth arrest specific transcript 5 acts as a tumour suppressor in colorectal cancer by inhibiting interleukin-10 and vascular endothelial growth factor expression. Oncotarget. 2017;8:13690–13702. doi: 10.18632/oncotarget.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S-D, et al. GAS5 promotes myocardial apoptosis in myocardial ischemia-reperfusion injury via upregulating LAS1 expression. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8447–8453. doi: 10.26355/eurrev_201812_16544. [DOI] [PubMed] [Google Scholar]
- 33.Long X, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J. Exp. Clin. Cancer Res. 2019;38:345. doi: 10.1186/s13046-019-1329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke K, Sun Z, Wang Z. Downregulation of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion in esophageal squamous cell carcinoma. Oncol. Lett. 2018;16:1801–1808. doi: 10.3892/ol.2018.8797. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Guo X, et al. GAS5 inhibits gastric cancer cell proliferation partly by modulating CDK6. Oncol. Res. Treat. 2015;38:362–366. doi: 10.1159/000433499. [DOI] [PubMed] [Google Scholar]
- 36.Polager S, Ginsberg D. E2F - at the crossroads of life and death. Trends Cell Biol. 2008;18:528–535. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Poppy Roworth A, Ghari F, La Thangue NB. To live or let die - complexity within the E2F1 pathway. Mol. Cell Oncol. 2015;2:e970480. doi: 10.4161/23723548.2014.970480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrocca F, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Gao S, et al. The miR-532-E2F1 feedback loop contributes to gastric cancer progression. Cell Death Dis. 2022;13:1–10. doi: 10.1038/s41419-022-04832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X-P, Liu F, Wang W. Coordination between cell cycle progression and cell fate decision by the p53 and E2F1 pathways in response to DNA damage. J. Biol. Chem. 2010;285:31571–31580. doi: 10.1074/jbc.M110.134650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silveira DA, Gupta S, Mombach JCM. p53/E2F1/miR-25 axis regulates apoptosis induction in glioblastoma cells: A qualitative model. J. Phys. Complex. 2020;1:035001. doi: 10.1088/2632-072X/aba3bb. [DOI] [Google Scholar]
- 42.Liu X, Hu C. Novel potential therapeutic target for E2F1 and prognostic factors of E2F1/2/3/5/7/8 in human gastric cancer. Mol. Ther. Methods Clin. Dev. 2020;18:824–838. doi: 10.1016/j.omtm.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, et al. The long noncoding RNA, growth arrest-specific 5, suppresses gastric cancer by downregulating miR-21 expression. Pharmacology. 2020;105:434–444. doi: 10.1159/000504674. [DOI] [PubMed] [Google Scholar]
- 44.Dong S, Zhang X, Liu D. Overexpression of long noncoding RNA GAS5 suppresses tumorigenesis and development of gastric cancer by sponging miR-106a-5p through the Akt/mTOR pathway. Biol. Open. 2019;8:bio041343. doi: 10.1242/bio.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Yin L, Chen C, Zhang X, Wang S. Long non-coding RNA GAS5 inhibits migration and invasion in gastric cancer via interacting with p53 protein. Dig. Liver Dis. 2020;52:331–338. doi: 10.1016/j.dld.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Frank F, et al. The lncRNA growth arrest specific 5 regulates cell survival via distinct structural modules with independent functions. Cell Rep. 2020;32:107933. doi: 10.1016/j.celrep.2020.107933. [DOI] [PubMed] [Google Scholar]
- 47.Purvis JE, et al. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanchet A, Bourgmayer A, Kurtz J-E, Mellitzer G, Gaiddon C. Isoforms of the p53 family and gastric cancer: A ménage à trois for an unfinished affair. Cancers (Basel) 2021;13:916. doi: 10.3390/cancers13040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Q, et al. Irinotecan induces autophagy-dependent apoptosis and positively regulates ROS-related JNK- and P38-MAPK pathways in gastric cancer cells. OncoTargets Ther. 2020;13:2807–2817. doi: 10.2147/OTT.S240803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi T, van Soest DMK, Polderman PE, Burgering BMT, Dansen TB. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic. Biol. Med. 2021;172:298–311. doi: 10.1016/j.freeradbiomed.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Q, Chen J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle. 2010;9:472–478. doi: 10.4161/cc.9.3.10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu H, Gatti RA. MicroRNAs: New players in the DNA damage response. J. Mol. Cell Biol. 2011;3:151–158. doi: 10.1093/jmcb/mjq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NCBI Resource Coordinators Database resources of the national center for biotechnology information. Nucl. Acids Res. 2016;44:D7–19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oughtred R, et al. The BioGRID interaction database: 2019 update. Nucl. Acids Res. 2019;47:D529–D541. doi: 10.1093/nar/gky1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamberg M, et al. MiRTargetLink–miRNAs, genes and interaction networks. Int. J. Mol. Sci. 2016;17:564. doi: 10.3390/ijms17040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naldi A, et al. Logical modeling and analysis of cellular regulatory networks with GINsim 3.0. Front. Physiol. 2018;9:646. doi: 10.3389/fphys.2018.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abou-Jaoudé W, et al. Logical modeling and dynamical analysis of cellular networks. Front. Genet. 2016;7:94. doi: 10.3389/fgene.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Silveira DA, Mombach JCM. Modeling the role of microRNA-449a in the regulation of the G2/M cell cycle checkpoint in prostate LNCaP cells under ionizing radiation. PLoS ONE. 2018;13:e0200768. doi: 10.1371/journal.pone.0200768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S, Silveira DA, Mombach JCM. ATM/miR-34a-5p axis regulates a p21-dependent senescence-apoptosis switch in non-small cell lung cancer: A Boolean model of G1/S checkpoint regulation. FEBS Lett. 2020;594:227–239. doi: 10.1002/1873-3468.13615. [DOI] [PubMed] [Google Scholar]
- 61.Gupta S, Silveira DA, Barbé-Tuana FM, Mombach JCM. Integrative data modeling from lung and lymphatic cancer predicts functional roles for miR-34a and miR-16 in cell fate regulation. Sci. Rep. 2020;10:2511. doi: 10.1038/s41598-020-59339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Chen B. GAS5-mediated regulation of cell signaling (Review) Mol. Med. Rep. 2020;22:3049–3056. doi: 10.3892/mmr.2020.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 64.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X-P, Liu F, Wang W. Two-phase dynamics of p53 in the DNA damage response. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8990–8995. doi: 10.1073/pnas.1100600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 67.Xiong S, Hu M, Li C, Zhou X, Chen H. Role of miR-34 in gastric cancer: From bench to bedside (Review) Oncol. Rep. 2019;42:1635–1646. doi: 10.3892/or.2019.7280. [DOI] [PubMed] [Google Scholar]
- 68.Bi E, et al. Oridonin induces growth inhibition and apoptosis in human gastric carcinoma cells by enhancement of p53 expression and function. Br. J. Med. Biol. Res. 2018;51:e7599. doi: 10.1590/1414-431x20187599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
The model code is available in the Github repository: https://github.com/pritampanda15/lncRNA-GAS5.