Abstract
Enzyme-linked immunosorbent assay (ELISA) with crude extracts of adult Clonorchis sinensis has been reported to have a high degree of sensitivity with a moderate degree of specificity for the serodiagnosis of clonorchiasis. The cystatin capture ELISA was investigated for its usefulness for the serodiagnosis of human clonorchiasis. Cystatin bound specifically to cysteine proteinases in crude extracts of adult C. sinensis worms, and its binding capacity was not hindered competitively by the other proteinase inhibitors tested. The cystatin capture ELISA for clonorchiasis showed a higher degree of specificity than the conventional ELISA, which produced some cross-reactivities to sera from patients with cysticercosis, sparganosis, and opisthorchiasis. Immunoglobulin G antibodies to C. sinensis cysteine proteinases were produced in experimental rabbits at week 3, and their levels increased rapidly and remained at a plateau after 8 weeks of infection. Of the proteins from the C. sinensis crude extract captured with cystatin, seven proteins were reactive with the serum from patients with clonorchiasis. The cystatin capture ELISA is indicated to be a sensitive and highly specific immunodiagnostic assay for serodiagnosis of human clonorchiasis.
Clonorchis sinensis, the Chinese liver fluke, lives in biliary passages and provokes hepatobiliary disorders. Clonorchiasis, which is human infection with C. sinensis, is endemic in East Asian countries, and about 7 million persons are estimated to be infected with the flukes (16). Human infections have been diagnosed by microscopic fecal examinations and by finding the characteristic eggs. In an effort to develop a more cooperative serodiagnostic test and to circumvent difficulties associated with the collection of stool samples from individuals and for mass surveys, enzyme-linked immunosorbent assays (ELISAs) with various kinds of antigenic preparations have been evaluated for their usefulness for the serodiagnosis of human clonorchiasis. Several antigenic molecules have been characterized and proposed as potential serodiagnostic reagents (18, 33, 35).
The availability of a pure antigenic protein is a prerequisite for a diagnostic ELISA to have high degrees of sensitivity and specificity. An antigenic protein could be laboriously purified from a crude extract of the worm by multiple chromatographies or could be produced as a recombinant protein by molecular biological techniques. Among the antigenic proteins of parasites, cysteine proteinases have been recognized as molecules that may be useful for the serodiagnosis of parasitic infections. Cysteine proteinases are involved in the host-parasite relationship by facilitating invasion and nutrition uptake (26, 27) and, in immune evasion, by cleaving immunoglobulins (13). The proteinases render high degrees of specificity and sensitivity to ELISAs for trematode infections such as schistosomiasis, fascioliasis (28, 34), and paragonimiasis (21).
Among the proteinase inhibitors, members of the cystatin superfamily showed reversible and tight binding capacities specific to the papain-like cysteine proteinases such as cathepsins B, H, and L (1, 3). With this specific binding characteristic, cystatin has been used as an antigen capture agent in capture ELISAs, which have been reported to be excellent methods for the serodiagnosis of leishmaniasis (31) and for the serodiagnosis of fascioliasis and paragonimiasis (21).
This study was performed to evaluate the usefulness of the cystatin capture ELISA for the serodiagnosis of clonorchiasis and to identify which proteins are antigenic in this case.
MATERIALS AND METHODS
The protocol for this study was approved by the Institutional Review Board of Chung-Ang University College of Medicine.
Crude extract of C. sinensis.
Adult C. sinensis flukes were recovered from experimental rabbits infected with the metacercariae collected from topmouth gudgeons, Pseudorasbora parva, caught in the southern part of Korea. The flukes were homogenized in 10 mM Tris buffer containing 1× Complete Mini (Roche, Manheim, Germany), a proteinase inhibitor cocktail, and were kept at 4°C overnight. The homogenate was centrifuged at 20,000 × g for 20 min at 4°C, and the supernatant was stored at −20°C and used as a crude antigen.
Patient sera.
Sera were collected from patients with clonorchiasis, paragonimiasis westermani, and opisthorchiasis viverinii. The patients were infected with the respective flukes, and their infections were confirmed parasitologically by microscopic examination. Sera were obtained from patients with cysticercosis, which was diagnosed by computed tomography-magnetic resonance imaging findings. Sera were collected from patients with sparganosis, which was proved by surgically removal of the worm(s). Sera from humans not infected with helminths were included as a control group. All sera were stored at −20°C until they were used.
ELISA.
The wells of a micro-ELISA plate (Costar Co., Cambridge, Mass.) were coated with 0.5 μg of the C. sinensis crude extract overnight at 4°C. After the plate was washed with phosphate-buffered saline (PBS)–Tween 20, the sera from helminth-infected humans, diluted 1:300, were added to the wells, and the plates were incubated for 2 h at room temperature. After the plates were washed with PBS-Tween 20, the secondary antibody, peroxidase-conjugated anti-human immunoglobulin G (IgG; Cappel Co., St. Louis, Mo.), was diluted 1:1,000 and was applied to the wells. The color was allowed to develop for 30 min by using a substrate, o-phenylenediamine (Sigma Chemical Co., St. Louis, Mo.), and the absorbance was measured at a wavelength of 490 nm.
Cystatin capture ELISA.
The cystatin capture ELISA was performed as described previously (21), with partial modification. The optimal conditions for the cystatin capture ELISA for clonorchiasis were determined through the use of combinations in a matrix of various amounts of chicken egg cystatin (Sigma Chemical Co.), C. sinensis crude extract, and serial dilutions of sera from patients with clonorchiasis (data not shown). Each well of the micro-ELISA plate (Costar Co.) was sensitized with 1 μg of cystatin in 0.1 ml of 0.1 M carbonate buffer (pH 9.6) at 4°C overnight. After masking of the well with 2% bovine serum albumin, C. sinensis crude extract containing 1.5 μg of protein was added to the well and the plate was incubated for 4 h at 4°C. Patient sera diluted 1:300 were incubated with the captured antigen for 2 h at room temperature, and then the secondary antibody, peroxidase-conjugated anti-human IgG (Cappel Co.) diluted 1:1,000, was applied. Color development and measurement of the absorbance were done as described above.
Competitive capture assay with cystatin.
It was evaluated whether the capacity of cystatin to capture cysteine proteinases in the C. sinensis crude extract was hindered by proteinase inhibitors (see Table 1). The crude extract was preincubated with a proteinase inhibitor for 4 h at 4°C and was transferred to the cystatin-sensitized well. A serum sample from a patient with clonorchiasis with a high antibody titer was used, and the procedure described above was followed.
TABLE 1.
Effects of proteinase inhibitors on capture capacity of cystatin for cysteine proteinases of C. sinensis
| Inhibitorb | Concn (mM) | A490a |
|---|---|---|
| Control | —c | 0.278 |
| Cystatin | 0.1 | 0.029 |
| E-64 | 0.01 | 0.147 |
| Leupeptin | 0.1 | 0.127 |
| Pepstatin | 0.1 | 0.307 |
| IAA | 0.1 | 0.302 |
| Phosphotatin | 0.1 | 0.326 |
| PMSF | 0.1 | 0.285 |
| EDTA | 0.1 | 0.276 |
The values are means of triplicate measurements.
Abbreviations: E-64, l-trans-epoxysuccinyl-leucylamide-(4-guanidino)- butane; IAA, iodoacetic acid; PMSF, phenylmethylsulfonyl fluoride; EDTA, ethylenediaminetetraacetic acid.
—, the C. sinensis crude extract preincubated with the respective inhibitors was added to the cystatin-coated well and was then used for the capture ELISA.
Rabbit sera.
Rabbit sera were collected at 1- to 6-week intervals for 1 year from four New Zealand White rabbits infected with 500 C. sinensis metacercariae. The sera were diluted by 1:300 and used for the cystatin capture ELISA by the procedure described above. Peroxidase-conjugated anti-rabbit IgG (Cappel Co.) was used as the secondary antibody.
Proteins of C. sinensis captured with cystatin.
Ten microcentrifuge tubes were each sensitized with 100 μl of 20 μg of cystatin per ml at 4°C overnight. After the contents of the tubes were washed three times with PBS-Tween 20, 100 μl of C. sinensis crude extract (200 μg/ml) was added to the sensitized tubes, and the tubes were incubated at 4°C for 4 h. The proteins captured by cystatin were collected by adding 100 μl of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer to the microcentrifuge tubes and boiling at 85°C for 3 min. The proteins displayed in an SDS–12.5% polyacrylamide gel were stained with Coomassie brilliant blue or were electrotransferred onto a nitrocellulose membrane. After masking of the membrane with 2% skim milk, the membrane was incubated in the C. sinensis-infected human serum sample that showed the highest absorbance in the cystatin capture ELISA. Then, alkaline phosphatase-conjugated anti-human IgG (Cappel Co.) was used as the secondary antibody. The antigenic proteins captured by cystatin were recognized by color development with the substrates 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Sigma Chemical Co.).
Data analysis.
Statistical analysis was performed as appropriate. The diagnostic sensitivity, specificity, and predictive values were calculated as described previously (25). These values were calculated and expressed as follows: the number of true-negative samples was the number of control samples (samples from patients with other helminthiases and healthy controls) that were negative by the assay; the number of true-positive samples was the number of samples from patients with proven clonorchiasis that were positive by the assay; the number of false-negative samples was the number of samples from patients with proven clonorchiasis that were negative by the assay; the number of false-positive samples was equal to the number of control samples that were positive by the assay; sensitivity was equal to the [number of true-positives samples/(number of true-positives samples + number of false-negative samples)] × 100; specificity was equal to the [number of true-negative samples/(number of false-positive samples + number of true-negative samples)] × 100; the positive predictive value was equal to the [number of true-positive samples/(number of true-positive samples + number of false-positive samples)] × 100; the negative predictive value was equal to the [number of true-negative samples/(number of true-negative samples + number of false-negative samples)] × 100.
RESULTS
Noncompetitive capture of cysteine proteinases by cystatin.
The results of an evaluation of the effect of pretreatment of the C. sinensis crude extract with various proteinase inhibitors is shown in Table 1. Exposure of the crude extract to cystatin reduced significantly (90%) the high degree of ELISA reactivity. Compared to cystatin, pretreatment of the crude extract with the cysteine proteinase inhibitors such as l-trans-epoxysuccinyl-leucylamide(4-guanidino)butane and leupeptin reduced the ELISA reactivity by 47 and 54%, respectively. Exposure of the crude extract to other proteinase inhibitors of aspartic, serine, and metalloproteinases did not competitively reduce the ELISA reactivity compared to that obtained with the control.
Comparison of conventional and cystatin capture ELISAs.
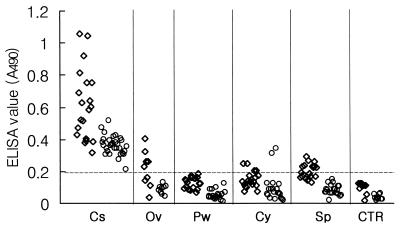
Sera from 20 patients with clonorchiasis (30 patients with clonorchiasis in the case of the cystatin capture ELISA), 20 patients with paragonimiasis westermani, 9 patients with opisthorchiasis viverinii, 20 patients with cysticercosis cellulosae, and 20 patients with sparganosis and sera from 10 uninfected controls were assayed by conventional and cystatin capture ELISAs; and the absorbances at 490 nm were plotted (Fig. 1). The cutoff value for clonorchiasis was put at an absorbance of 0.2, which was established in the Department of Parasitology, Chung-Ang University College of Medicine. Conventional ELISA with the crude extract showed high absorbance values above the cutoff value for sera from patients with clonorchiasis and, unfavorably, moderate values for sera from patients with opisthorchiasis, cysticercosis, and sparganosis (Table 2, Fig. 1). The cystatin capture ELISA had absorbance values lower than those measured by conventional ELISA. However, the absorbance values remained positive for sera from patients with clonorchiasis. Cross-reactions were found only for two serum samples from patients with cysticercosis (Table 2, Fig. 1). Although the mean absorbance values of the cystatin capture ELISA were lower than those of the conventional ELISA, the smaller standard deviations indicate its degree of higher reliability (Table 2).
FIG. 1.
ELISA (◊) and cystatin capture ELISA (○) of the helminth-infected human sera. A crude extract of an adult C. sinensis worm was used as the antigen for the ELISAs. Cs, clonorchiasis; Ov, opisthorchiasis viverinii; Pw, paragonimiasis westermani; Cy, cysticercosis cellulosae; Sp, sparganosis; CTR, uninfected control group. The dashed line indicates the cutoff value.
TABLE 2.
Comparison of absorbances between conventional and cystatin capture ELISAs
| Disease of patient used as serum source | Conventional ELISA
|
Cystatin capture ELISA
|
||||
|---|---|---|---|---|---|---|
| Range | Mean ± SD | No. positivea/total no. (%) | Range | Mean ± SD | No. positivea/total no. (%) | |
| Clonorchiasis | 0.320–1.056 | 0.615 ± 0.220 | 20/20 (100) | 0.214–0.518 | 0.361 ± 0.060 | 30/30 (100) |
| Opisthorchiasis | 0.040–0.406 | 0.215 ± 0.113 | 5/9 (55.6) | 0.045–0.130 | 0.086 ± 0.027 | 0/9 (0) |
| Paragonimiasis | 0.082–0.186 | 0.127 ± 0.035 | 0/20 (0) | 0.014–0.126 | 0.050 ± 0.029 | 0/20 (0) |
| Cysticercosis | 0.074–0.251 | 0.148 ± 0.049 | 4/20 (20) | 0.020–0.340 | 0.092 ± 0.086 | 2/20 (10) |
| Sparganosis | 0.133–0.237 | 0.200 ± 0.046 | 6/20 (30) | 0.021–0.152 | 0.083 ± 0.033 | 0/20 (0) |
| Control | 0.058–0.127 | 0.099 ± 0.035 | 0/10 (0) | 0.020–0.060 | 0.036 ± 0.015 | 0/10 (0) |
The cutoff value is an absorbance of 0.2 at 490 nm.
By the conventional ELISA, the cutoff absorbance value of 0.2 for clonorchiasis produced sensitivity, specificity, and positive and negative predictive values (serodiagnostic indices) of 100, 84, 57.1, and 100%, respectively. When a statistical cutoff absorbance value of 0.45 (which was the sum of the mean absorbance values and 10 standard deviations for the negative control group) was used, the sensitivity and the negative predictive value were lowered to 76.9 and 92.9%, respectively, while the specificity and the positive predictive value were increased to 100% each. By the cystatin capture ELISA, the cutoff value of 0.2 produced sensitivity, specificity, and positive and negative predictive values (serodiagnostic indices) of 100, 97.5, 93.8, and 100%, respectively. The statistical cutoff value was calculated to be 0.186, but it did not cause any differences in the values of the serodiagnostic indices (Table 2).
Antibody responses of experimental rabbits.
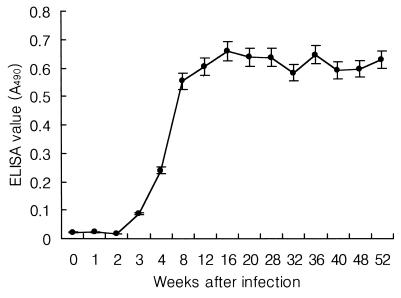
After 1 year of metacercarial infection, the rabbits were killed and adult C. sinensis flukes were recovered from the biliary passages. A total of 116 ± 85 flukes were recovered. By the cystatin capture ELISA, IgG antibodies were detected as early as week 3 after infection, and the levels increased rapidly between 4 and 8 weeks after infection. From 12 weeks after infection, the cystatin-captured protein-specific IgG level remained relatively stable for 1 year (Fig. 2).
FIG. 2.
Cystatin capture ELISA values according to infection period in rabbits infected with C. sinensis. The bars represent one standard deviation of triplicate measurements.
Cystatin-captured proteins.
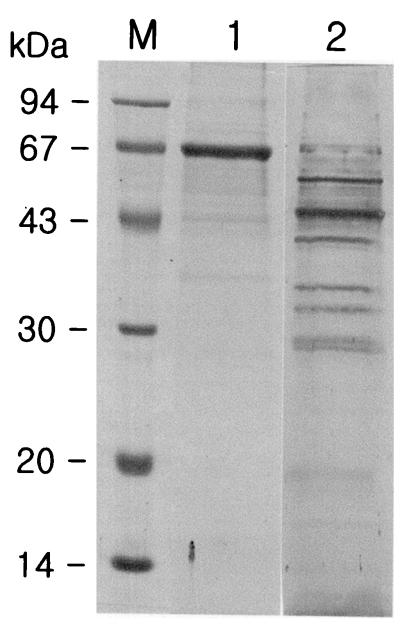
Among the cystatin-captured proteins, a 66-kDa protein was the thickest and most prominent, with minor 44- and 36-kDa proteins found in SDS-polyacrylamide gels stained with Coomassie blue. An immunoblot of the cystatin-captured proteins against a serum sample from a patient with clonorchiasis showed a profile of positive bands different from the profile obtained by Coomassie blue staining. The major 66-kDa protein appeared to be less antigenic, while the 45-kDa protein appeared to be potentially antigenic. There were also moderately antigenic proteins with molecular masses of 55, 40, 35, 32, 30, and 29 kDa and proteins with low levels of antigenicity with molecular masses of 26, 24, 19, 16, and 13 kDa (Fig. 3).
FIG. 3.
Analysis of cystatin-captured proteins of adult C. sinensis. Lane M, molecular mass marker; lane 1, SDS-PAGE and Coomassie blue staining of cystatin-captured C. sinensis proteins; lane 2, immunoblot of the cystatin-captured antigenic proteins with a serum sample from a patient with clonorchiasis.
DISCUSSION
Cystatin is a 13-kDa protein that specifically inhibits the enzymatic activity of papain-like proteinases (4). In the present study, cystatin showed a higher and more specific capacity to capture the antigenic proteinases in C. sinensis crude extracts than the other proteinase inhibitors tested (Table 1). The tertiary structure of cystatin consists of five α helices and five β sheets (6). Of the two loops of the N-terminal wedge-shaped edge, one is complementary to the cleft active site of papain-like cysteine proteinases and the other can bind to legumain, another family of cysteine proteinases, but not cathepsin B (2). These structural features may be attributes that allow cystatin to capture noncompetitively those antigenic cysteine proteinases of C. sinensis.
The cystatin capture ELISA showed a higher degree of specificity for clonorchiasis compared to that of the conventional ELISA, which used a crude extract of the worm as the antigen (Fig. 1, Table 2). In general, the conventional ELISA showed a superior reactivity to the infection than the ELISA with a purified antigenic protein. The crude extract of C. sinensis worms contains many kinds of antigenic proteins cross-reactive to the sera of patients with paragonimiasis westermani and metagonimiasis (19, 23). The cross-reactive antigens in the crude extract are responsible for the higher degree of reactivity of the conventional ELISA.
Cystatin captured seven major and five minor antigenic proteins from the C. sinensis crude extract (Fig. 3) and allowed the capture ELISA to have a higher degree of specificity. The cysteine proteinases that have been purified and characterized from C. sinensis are, so far, a 15-kDa cathepsin B-like isoenzyme of the adult stage (33), a 20-kDa isoenzyme of the juvenile and adult stages and a 30-kDa isoenzyme of the metacercarial stage (32), and a 24-kDa isoenzyme of the excretory-secretory product of adult worms (30). These cysteine proteinases that have been identified have, on the basis of cystatin capture immunoblotting, proved to be less antigenic and are thus minimal contributors to the cystatin capture ELISA for clonorchiasis.
The cysteine proteinases of schistosomes are secreted into the gastrointestinal lumen of the flukes and are involved in the degradation of host hemoglobins (7, 8). Several protozoan and helminthic cysteine proteinases have been found to cleave the host IgG (5, 13, 14). These offensive proteolytic activities of parasitic cysteine proteinases may account for their lower levels of antigenicity to the host immune system.
In this work, the antigenic proteins captured from C. sinensis with cystatin were found to have cysteine proteinase homologues, on the basis of their approximate molecular weights, in parasitic trematodes such as Paragonimus westermani (12, 14, 22, 24, 29), Fasciola hepatica (15, 34), Schistosoma mansoni (9, 17), and other parasites. Of the homologues, cysteine proteinases with molecular masses ranging from 24 to 35 kDa have been reported to be specifically reactive to IgG in the sera from patients infected with the respective trematode (10, 11, 15, 24). The two antigenic proteins with molecular masses of 40 and 45 kDa are found not to have homologues that contain a cystatin-binding motif, and thus, their biochemical and immunological properties remain to be elucidated. The cystatin-captured C. sinensis proteins with molecular masses of 40 and 45 kDa might be the naturally glycosylated proenzymes of cysteine proteinases whose mature enzymes have smaller molecular masses (7).
In C. sinensis-infected rabbits, IgG antibodies specific for cystatin-captured cysteine proteinases were detected at week 3, and their levels increased rapidly between 4 and 8 weeks after infection. This finding indicates that human clonorchiasis can be serodiagnosed by cystatin capture ELISA as early as 3 weeks after the infection. A similar pattern was seen in experimental mice infected with Paragonimus, Fasciola, or Schistosoma, in which IgG antibodies specific for cysteine proteinases were detected in the animals at week 2 and in which IgG antibody levels increased rapidly between 3 and 5 weeks after infection (20). One might speculate that the delay in the appearance of C. sinensis-specific antibodies compared to the times of appearance of antibodies specific for tissue-invading trematodes reflects an outcome derived from the luminal parasitism in the biliary tract without migration through tissue and, consequently, less effective antigen presentation through the mucosae of the biliary and intestinal tracts.
In conclusion, a cystatin capture ELISA with multiple cysteine proteinases was evaluated and was found to be sensitive and more specific than an ELISA with crude extracts for the serodiagnosis of human clonorchiasis. The capture of cysteine proteinases with cystatin was a simple and practical procedure for the purification of antigenic proteins from the fluke lysate.
ACKNOWLEDGMENTS
Ok-Kyung Lim is gratefully acknowledged for technical assistance.
This work was supported by a Chung-Ang University special research grant in 1999.
REFERENCES
- 1.Abrahamson M, Ritonja A, Brown M A, Grubb A, Machleidt W, Barrett A J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987;262:9688–9694. [PubMed] [Google Scholar]
- 2.Alvarez-Fernandez M, Barrett A J, Gerhartz B, Dando P M, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- 3.Anastasi A, Brown M A, Kembhavi A A, Nicklin M J, Sayers C A, Sunter D C, Barret A J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983;211:129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett A J. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta. 1986;45:1363–1374. [PubMed] [Google Scholar]
- 5.Bastida-Corcuera F, Butler J E, Heyermann H, Thomford J W, Corbeil L B. Tritrichomonas foetus extracellular cysteine proteinase cleavage of bovine IgG2 allotypes. J Parasitol. 2000;86:328–332. doi: 10.1645/0022-3395(2000)086[0328:TFECPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Bode W, Engh R, Musil D, Thiele U, Huber R, Karshikov A, Brzin J, Kos J, Tujk V. The 2.0 Å X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988;7:2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady C P, Dowd A J, Brindley P J, Ryan T, Day S R, Dalton J P. Recombinant expression and localization of Schistosoma mansoni cathepsin L1 support its role in the degradation of host hemoglobin. Infect Immun. 1999;67:368–374. doi: 10.1128/iai.67.1.368-374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brindley P J, Kalinna B H, Dalton J P, Day S R, Wong J Y M, Smythe M L, McManus D P. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol. 1997;89:1–9. doi: 10.1016/s0166-6851(97)00098-4. [DOI] [PubMed] [Google Scholar]
- 9.Chappell C L, Dresden M H. Antibody response to a purified parasite proteinase (SMw32) in Schistosoma mansoni infected mice. Am J Trop Med Hyg. 1988;39:66–73. doi: 10.4269/ajtmh.1988.39.66. [DOI] [PubMed] [Google Scholar]
- 10.Chappell C L, Dresden M H, Gryseels B, Deelder A M. Antibody response to Schistosoma mansoni adult worm cysteine proteinases in infected individuals. Am J Trop Med Hyg. 1990;42:335–341. doi: 10.4269/ajtmh.1990.42.335. [DOI] [PubMed] [Google Scholar]
- 11.Chappell C L, Dresden M H. Purification of cysteine proteinases from adult Schistosoma mansoni. Arch Biochem Biophys. 1987;256:560–568. doi: 10.1016/0003-9861(87)90613-8. [DOI] [PubMed] [Google Scholar]
- 12.Choi M H, Choe S C, Lee S H. A 54 kDa cysteine protease purified from the crude extract of Neodiplostomum seoulense adult worms. Korean J Parasitol. 1999;37:39–46. doi: 10.3347/kjp.1999.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung Y B, Kong Y, Yang H J, Kang S Y, Cho S Y. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol. 1997;83:902–907. [PubMed] [Google Scholar]
- 14.Chung Y B, Yang H J, Kang S Y, Cho S Y. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J Parasitol. 1997;35:139–142. doi: 10.3347/kjp.1997.35.2.139. [DOI] [PubMed] [Google Scholar]
- 15.Cordova M, Herrera P, Nopo L, Bellatin J, Naquira C, Guerra H, Espinoza J R. Fasciola hepatica cysteine proteinases: immunodominant antigens in human fascioliasis. Am J Trop Med Hyg. 1997;57:660–666. doi: 10.4269/ajtmh.1997.57.660. [DOI] [PubMed] [Google Scholar]
- 16.Crompton D W. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397–403. [PubMed] [Google Scholar]
- 17.Davis A H, Nanduri J, Watson D C. Cloning and gene expression of Schistosoma mansoni protease. J Biol Chem. 1987;262:12851–12855. [PubMed] [Google Scholar]
- 18.Hong S J, Seong K Y, Sohn W M, Song K Y. Molecular cloning and immunological characterization of phosphoglycerate kinase from Clonorchis sinensis. Mol Biochem Parasitol. 2000;108:207–216. doi: 10.1016/s0166-6851(00)00220-6. [DOI] [PubMed] [Google Scholar]
- 19.Hong S T, Lee M, Sung N J, Cho S R, Chai J Y, Lee S H. Usefulness of IgG4 subclass antibodies for diagnosis of human clonorchiasis. Korean J Parasitol. 1999;37:243–248. doi: 10.3347/kjp.1999.37.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda T. Antibody responses to fluke cysteine proteinases in Paragonimus- and Fasciola-infected rats. J Helminthol. 1998;72:187–191. doi: 10.1017/s0022149x00016424. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda T. Cystatin capture enzyme-linked immunosorbent assay for immunodiagnosis of human paragonimiasis and fascioliasis. Am J Trop Med Hyg. 1998;59:286–290. doi: 10.4269/ajtmh.1998.59.286. [DOI] [PubMed] [Google Scholar]
- 22.Kang S Y, Cho M S, Chung Y B, Kong Y, Cho S Y. A cysteine protease of Paragonimus westermani eggs. Korean J Parasitol. 1995;33:323–330. doi: 10.3347/kjp.1995.33.4.323. [DOI] [PubMed] [Google Scholar]
- 23.Kim S I. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol. 1998;36:37–45. doi: 10.3347/kjp.1998.36.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Kim T S, Na B K, Park P H, Song K J, Hontzeas N, Song C Y. Cloning and expression of a cysteine proteinase gene from Paragonimus westermani adult worms. J Parasitol. 2000;86:333–339. doi: 10.1645/0022-3395(2000)086[0333:CAEOAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Maleewong W, Wongkham C, Intapan P M, Pipitgool V. Fasciola gigantica-specific antigens: purification by a continuous-elution method and its evaluation for the diagnosis of human fascioliasis. Am J Trop Med Hyg. 1999;61:648–651. doi: 10.4269/ajtmh.1999.61.648. [DOI] [PubMed] [Google Scholar]
- 26.McKerrow J H. Parasite proteinases. Exp Parasitol. 1989;68:111–118. doi: 10.1016/0014-4894(89)90016-7. [DOI] [PubMed] [Google Scholar]
- 27.North M J. Comparative biochemistry of the proteinases of eukaryotic microorganisms. Microbiol Rev. 1982;46:308–340. doi: 10.1128/mr.46.3.308-340.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neill S M, Parkinson M, Strauss W, Angles R, Dalton J P. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am J Trop Med Hyg. 1998;58:417–423. doi: 10.4269/ajtmh.1998.58.417. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Hong K M, Ryu J S, Shin C H, Lee J B, Soh C T, Paik M K, Min D Y. Cloning of a cysteine proteinase cDNA of adult Paragonimus westermani by polymerase chain reaction. Mol Cell. 1997;7:335–339. [PubMed] [Google Scholar]
- 30.Park H, Ko M Y, Paik M K, Soh J T, Seo J H, Im K. Cytotoxicity of cysteine proteinase of adult Clonorchis sinensis. Korean J Parasitol. 1995;33:211–218. doi: 10.3347/kjp.1995.33.3.211. [DOI] [PubMed] [Google Scholar]
- 31.Scharfstein J, Abrahamson M, de Souza C B P, Barral A, Silva I V. Antigenicity of cystatin-binding proteins from parasitic protozoan detection by a proteinase inhibitor based capture immunoassay (PINC-ELISA) J Immunol Methods. 1995;182:63–72. doi: 10.1016/0022-1759(95)00023-4. [DOI] [PubMed] [Google Scholar]
- 32.Song C Y, Dresden M H, Rege A A. Clonorchis sinensis: purification and characterization of a cysteine proteinase from adult worms. Comp Biochem Physiol. 1990;97B:825–829. doi: 10.1016/0305-0491(90)90129-h. [DOI] [PubMed] [Google Scholar]
- 33.Song C Y, Rege A A. Cysteine proteinase activity in various developmental stages of Clonorchis sinensis: a comparative analysis. Comp Biochem Physiol. 1991;99B:137–140. doi: 10.1016/0305-0491(91)90018-9. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki H, Aoki T, Oya H. A cysteine proteinase from the liver fluke Fasciola spp.: purification, characterization, localization and application to immunodiagnosis. Jpn J Parasitol. 1989;38:373–384. [Google Scholar]
- 35.Yong T S, Yang H J, Park S J, Kim Y K, Lee D H, Lee S M. Immunodiagnosis of clonorchiasis using a recombinant antigen. Korean J Parasitol. 1998;36:183–190. doi: 10.3347/kjp.1998.36.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]