Abstract
Immunotherapy, in particular immune checkpoint blockade (ICB) therapy targeting the programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) axis, has remarkably revolutionized cancer treatment in the clinic. Anti-PD-1/PD-L1 therapy is designed to restore the antitumor response of cytotoxic T cells (CTLs) by blocking the interaction between PD-L1 on tumour cells and PD-1 on CTLs. Nevertheless, current anti-PD-1/PD-L1 therapy suffers from poor therapeutic outcomes in a large variety of solid tumours due to insufficient tumour specificity, severe cytotoxic effects, and the occurrence of immune resistance. In recent years, nanosized drug delivery systems (NDDSs), endowed with highly efficient tumour targeting and versatility for combination therapy, have paved a new avenue for cancer immunotherapy. In this review article, we summarized the recent advances in NDDSs for anti-PD-1/PD-L1 therapy. We then discussed the challenges and further provided perspectives to promote the clinical application of NDDS-based anti-PD-1/PD-L1 therapy.
Keywords: cancer immunotherapy, immune checkpoint blockade, PD-1/PD-L1 axis, nanomedicine, nanosized drug delivery systems.
Nanosized drug delivery systems (NDDS) targeting the programmed cell death protein-1 (PD-1) and programmed death ligand-1 (PD-L1) axis have remarkably potentiated cancer immunotherapy. In this review, the NDDS exploited for anti-PD-1/PD-L1 therapy, including dimerization of PD-L1 protein, downregulation of PD-L1 expression, and blockade of PD-1/PD-L1 interaction were summarized. The perspectives and challenges of NDDS-based anti-PD-1/PD-L1 therapy were discussed as well.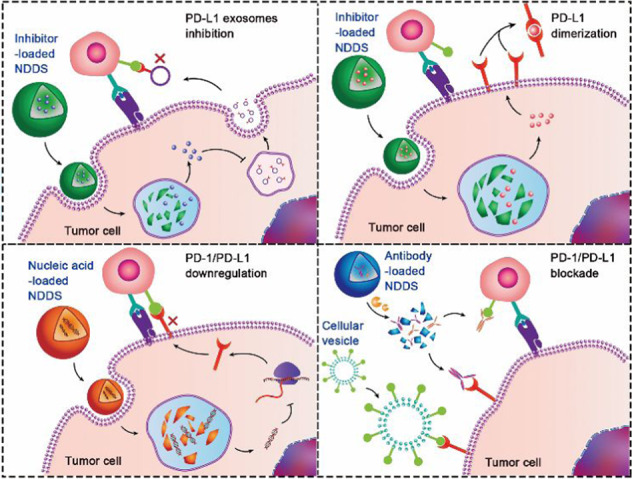
Introduction
Cancer immunotherapy has gained significant momentum in the clinic to boost the systemic antitumor immune response [1–5]. In particular, immune checkpoint blockade (ICB) therapy has presented impressive therapeutic efficacy against multiple types of cancers and shifted the paradigm of cancer management [6–8]. Immune checkpoints play a crucial role in maintaining immune homeostasis and preventing autoimmunity [9–11]. However, tumour cells may overexpress certain kinds of immune checkpoints to evade immunologic surveillance and thereby generate an immunosuppressive tumour microenvironment (ITM) [12]. Among the immune checkpoints investigated thus far, the programmed cell death protein-1 and programmed death ligand 1 (PD-1/PD-L1) axis has attracted the most attention. During the process of PD-1/PD-L1 axis-mediated immune evasion, tumour cells send a “don’t find me” signal to T cells via the interaction of PD-L1 on the surface of tumour cells with PD-1 present on the surface of T lymphocytes or antigen-presenting cells (APCs) to consequently suppress the antitumor immunity of cytotoxic T lymphocytes (CTLs) [13, 14]. Therefore, anti-PD-1/PD-L1 therapy has been extensively exploited to block immune checkpoints and revitalize exhausted cytotoxic T cells (CTLs) [15]. Compared to anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy, anti-PD-1/PD-L1 therapy has shown a favourable response rate, a low grade of immune-related adverse effects (irAE), and long-term clinical benefits in a subset of cancer patients [16–19]. Furthermore, anti-PD-1/PD-L1 therapy was combined with other therapy modalities, such as chemotherapy, radiotherapy, molecular-targeted therapy, and even the recently developed anti-T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) therapy [20–23].
Despite remarkable advances, only a subset of cancer patients benefits from current ICB therapy [24]. Certain types of malignant tumours are intrinsically or adaptively resistant to anti-PD-1/PD-L1 antibody drugs due to multiple mechanisms, including immune heterogeneity and complex immune tolerance pathways [25–29]. First, certain types of solid tumours (e.g., immune-excluded tumours) lack tumour-infiltrating T cells to exert antitumor effects. It is essential to promote intratumoural infiltration of T lymphocytes to potentiate anti-PD-1/PD-L1 therapy. Second, tumour cells generally display low immunogenicity. CTLs cannot recognize tumour cells due to insufficient expression of tumour antigens. Therefore, it is crucial to elicit immunogenicity of the tumour cells to prompt ICB therapy. Third, the antitumor activity of CTLs is coordinately impaired by other kinds of immune checkpoints (e.g., CD47) or immune suppressive cells (e.g., regulatory T cells and M2-type macrophages). It is essential to concurrently block multiple immune checkpoints or regulate the ITM. Finally, immune checkpoints can be upregulated with IFN-ɤ secretion by tumour-infiltrating CTLs, which induces adaptive immune evasion. Therefore, promoting anti-PD-1/PD-L1 therapy remains a formidable challenge [30–32].
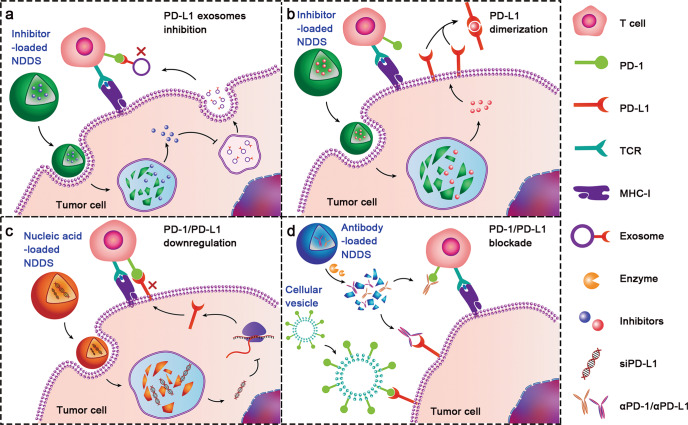
Encouragingly, nanosized drug delivery systems (NDDSs) have displayed valuable potential to address these concerns [33–36]. The NDDSs are endowed with tumour-targeting capability, biological barrier permeability, enhanced tissue accumulation, sufficient cellular uptake, and controllable payload release [37–40]. Specifically, nanoparticles modified with specific ligands can actively target tumour cells [41, 42]. Moreover, stimuli-responsive nanoparticles have been generated to systemically deliver immunotherapeutics, which can be triggered to dissociate and release drugs in the TME while remaining stable in blood circulation [43–47]. For example, an endogenous enzyme-liable prodrug of immune modulator prodrug could be activated to trigger immune responses by intracellular enzymes after tumour cell adsorption [48]. Last but not least, consisting of advanced biomaterials, including polymers, liposomes, silica, and metal-organic frameworks (MOFs), NDDSs with high loading capability and good biocompatibility can safely codeliver multiple drugs to the tumour site and tumour cells [49–53]. To fully exert the therapeutic effects, a variety of NDDSs have been designed to deliver different therapeutic cargoes, including antibodies, small molecule inhibitors, and nucleic acids (Table 1). Herein, we summarize the recent advances in NDDS-based anti-PD-1/PD-L1 therapy, with an emphasis on the delivery of therapeutic cargoes with 4 different PD-1/PD-L1 targeting strategies (Fig. 1): PD-L1 exosome inhibition, PD-L1 dimerization, PD-1/PD-L1 downregulation, and PD-1/PD-L1 blockade [54–58]. The recently rising NDDS of engineered cellular vesicles (CVs) for anti-PD-1/PD-L1 therapy are also discussed. Finally, the current challenges and perspectives of NDDS-based anti-PD-1/PD-L1 therapy are briefly overviewed.
Table 1.
A table summary of various anti-PD-1/PD-L1 therapeutics.
| Agents | Examples | Mechanisms | Advantages | Delivery barriers | Status | Refs. |
|---|---|---|---|---|---|---|
| Antibody | Nivolumab | PD-1/PD-L1 blockade | Well-studied, great efficacy | Low penetration, irAE, inadequate pharmacokinetics | Marketed | [56, 98, 101, 110] |
| Inhibitor | BMS-1 | PD-L1 dimerization | Good penetration, low cost, fewer irAE | Tumor-targeted delivery, rapid clearance | Preclinical/phase I | [54, 57, 111] |
| GW4869 | PD-L1 exosomes inhibition | |||||
| Nucleic acid | siPD-1/PD-L1 | PD-1/PD-L1 transcription inhibition | Gene regulation, fewer irAE | Cytosolic delivery, electronegativity, sensitive to nuclease (RNA), off-target effect | Preclinical | [55, 88, 109] |
| CRISPER-Cas9 system | PD-1/PD-L1 genome editing |
Fig. 1. Schematic illustration of NDDS-based anti-PD-1/PD-L1 therapy to circumvent immune evasion and elicit a T-cell antitumor immune response.
Inhibitors, nucleic acids and antibodies are therapeutic cargoes loaded in NDDSs. Among different therapeutic strategies targeting the PD-1/PD-L1 axis, 4 unique strategies are highlighted in this review, including a restraining the extracellular secretion of PD-L1-expressing exosomes by delivering small molecular inhibitors (e.g., GW4869) [57]. b Dimerizing PD-L1 on the surface of the tumour cells by delivering small molecular inhibitors (e.g., BMS-1) [54]. c Downregulating PD-1/PD-L1 expression by delivering gene-editing tools or inhibitors (PD-1 downregulation is omitted in the scheme) [55, 74, 88]. d Blocking the PD-1/PD-L1 interaction by antibodies or engineered cellular vesicles [56, 58].
NDDSs for the delivery of small molecule PD-L1 inhibitors
Small molecule inhibitors have been recently explored for ICB therapy [59, 60]. It has been reported that there are approximately 15 small-molecule PD-1/PD-L1 inhibitors in preclinical or phase I clinical trials [61]. However, due to high toxicity and nonspecific biodistribution, a tumour-targeted delivery system is of paramount importance. Additionally, as ICB monotherapy may lead to drug resistance in tumours, it would be a wise choice to codeliver therapeutic cargoes with different antitumor mechanisms for synergistic antitumor therapy [62, 63]. For example, recent studies have discovered that exosomes derived from tumour cells are rich in PD-L1, which can travel to the draining lymph node and downregulate T-cell activity [64]. Moreover, ferroptosis refers to the antioxidant capacity impairment of cells caused by intracellular iron accumulation, which has recently been exploited to elicit antitumor immunogenicity [65–68].
To suppress extracellular secretion of PD-L1-expressing exosomes and elicit ferroptosis, Wang et al. engineered a nanosystem by coassembling the exosome inhibitor GW4869 with 5-β-cholanic acid (CA)-grafted and Fe3+-chelated hyaluronic acid (HA) [57]. In combination with αPD-L1-based anti-PD-L1 therapy, this nanosystem significantly reduced exosomal PD-L1 expression and thereby augmented the proportion of immune-active CD8+ T cells in the tumour-draining lymph nodes, which led to constant tumour inhibition.
BMS-1 is another small molecule that inhibits the PD-1/PD-L1 axis by promoting PD-L1 dimerization [60]. Yu et al. developed an immunotherapy nanoparticle codelivering BMS-1 and CCL19 (an immunostimulatory chemokine)-encoding plasmid (pCCL19) to synergistically suppress cancer immune evasion and reprogram the ITM [54]. The nanoparticles contained the tumour-targeting peptide cyclo(Arg-Gly-Asp-D-Phe-Lys), which ensured enhanced accumulation at tumour sites. After administration of the nanoparticles, the levels of CCL19 and IFN-γ were significantly improved, which indicated that the TME was reprogrammed to an immunoactive state.
The intrinsic immune resistance caused by low tumour immunogenicity is another reason for the limited response rate of anti-PD-1/PD-L1 therapy [69]. Tumour cells conceal tumour-specific antigens and reduce their immunogenicity by autophagy-mediated degradation of major histocompatibility complex class I (MHC-I) (an indispensable cellular component for antigen presentation) [70–73].
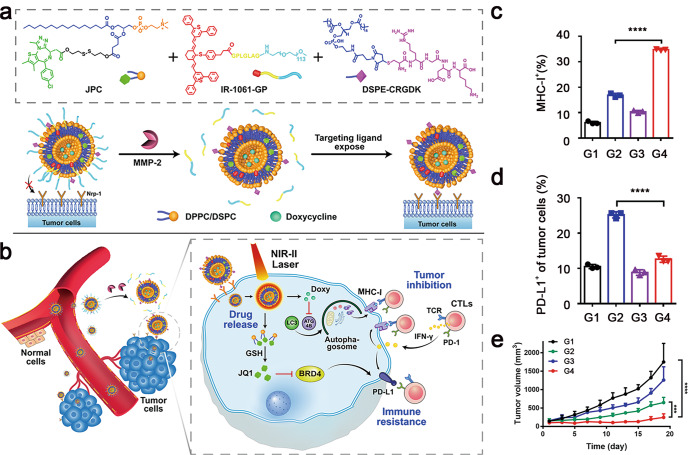
To address this challenge, Zhou et al. developed liposome-based versatile nanovesicles for tumour-targeted inhibition of autophagy and restoration of MHC-I expression on the surface of tumour cells [74]. The nanovesicles were rationally designed for codelivery of the autophagy inhibitor doxycycline (Doxy) and the PD-L1 inhibitor JQ1. The nanovesicles were further functionalized with a matrix metalloproteinase 2 (MMP-2)-cleavable poly(ethylene glycol) (PEG) corona and a tumour-targeting peptide (CRGDK). Moreover, the nanovesicle was loaded with IR-1061 fluorescence dye for a second near-infrared (NIR-II) fluorescence imaging and 1061 nm laser-triggered payload release. JQ1 is a potent inhibitor of bromodomain-containing protein 4 (BRD4), which can abolish IFN-ɤ-inducible PD-L1 expression [75, 76]. The glutathione (GSH)-activatable JQ1 prodrug was developed as a component of the lipid shell for tumour-targeted PD-L1 inhibition (Fig. 2a). The nanovesicles remarkably elongated blood circulation and the tumour-specific distribution of Doxy and JQ1 (Fig. 2b). Codelivery of Doxy and JQ1 with the nanovesicles efficiently elicited MHC-I expression and reduced PD-L1 expression on the tumour cells, thereby eliciting a robust T-cell antitumor response (Fig. 2c–e).
Fig. 2. Schematic demonstration of a tumour-targeted multifunctional prodrug nanovesicle (termed GPR@Doxy/JQ1) for inhibiting MHC-I autophagy and PD-L1-based immune evasion of tumour cells.
a Chemical structure of the main components integrated into the nanovesicles. b Systemic codelivery of Doxy and JQ1 with the nanovesicles for tumour-specific autophagy inhibition and PD-L1 downregulation. c Flow cytometric quantification of Doxy-mediated MHC-I restoration. d PD-L1 expression on the surface of 4T1 tumours in vivo. e Growth curves of the 4T1 breast tumours after various treatments (G1, PBS; G2, GPR@Doxy+Laser; G3, GPR@JQ1 + Laser; G4, GPR@Doxy/JQ1 + Laser) (***P < 0.001, ****P < 0.0001). Adapted with permission from [74]. Copyright (2021) John Wiley & Sons, Inc.
It was recently revealed that metformin (Met), a well-known drug for the clinical treatment of diabetes, may promote antitumor immunity by degrading PD-L1. Therefore, it became a promising strategy to deliver Met to the tumour site for PD-L1 downregulation. To this end, Hu et al. designed a nanoparticle by conjugating Met with the photosensitizer Ce6 via an MMP-2-cleavable peptide spacer, which synergistically performed photodynamic therapy (PDT) and downregulated PD-L1 [77]. Apart from PD-L1 inhibition, Met has been identified to inhibit tumour growth through M2 to M1 phenotype repolarization of tumour-associated macrophages (TAMs) [78, 79]. M1-type TAMs have been identified to promote the intratumoural infiltration of CD8+ T cells and suppress immunosuppressive regulatory T cells (Tregs) [80, 81]. Wei et al. therefore engineered a Met-loaded macrophage nanoparticle (Met@MP) to remodel the TME and boost the antitumor activity of αPD-1 [82]. The macrophage-derived nanoparticles displayed an active tumour-targeting capacity, elongated blood circulation stability, high biocompatibility, and low immunogenicity and were further modified with mannose to target M2-type TAMs. Met@MP dramatically increased TAM-mediated proinflammatory cytokine secretion and elicited an antitumor immune response, verifying the potential of Met@Man-MPs for cancer immunotherapy.
Along with chemical inhibitors, peptide antagonists of the PD-1/PD-L1 axis were also developed for anti-PD-1/PD-L1 therapy. For example, Yu et al. codelivered anti-PD-L1 peptide (dPPA) and thioketal bond-linker paclitaxel dimer (PXTK) to synergistically activate T cells and generate antitumor immunogenicity [83]. Moreover, an anti-PD-1 peptide (AUNP-12) was combined with photothermal therapy to elicit robust antitumor immune responses against melanoma [84].
NDDS-delivering nucleic acids for PD-1/PD-L1 blockade therapy
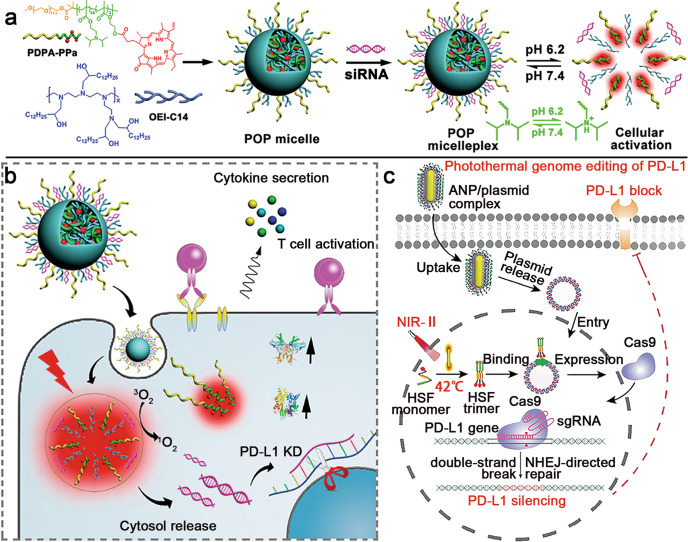
In recent years, transcriptional regulation and gene editing have been explored to boost anti-PD-1/PD-L1 therapy by remodelling the TME and overcoming immune evasion [85, 86]. For instance, Wang et al. engineered acid-activatable micelleplexes loaded with small interfering RNA of PD-L1 (siPD-L1) to knockdown PD-L1 expression on the surface of tumour cells (Fig. 3a, b) [55]. The micelleplexes were constructed with the ionizable diblock copolymer PDPA and amphiphilic oligoethylenimine (OEI-C14), which displayed high lysosome escape efficiency for the cytosolic delivery of siPD-L1. To further prompt the antitumor effect, the photosensitizer pheophorbide A (PPa) was covalently loaded in the micelleplexes to combine PD-L1 silencing and PDT. Upon cellular uptake by the tumour cells, the nanoparticles dissociated in acidic lysosomal vesicles for cytosolic release of siPD-L1. When exposed to 671 nm laser irradiation, PPa generated cytotoxic reactive oxygen species (ROS) to elicit immunogenic cell death (ICD) of the tumour cells, which recruited antigen presenting cells (APCs) for antigen engulfment in the tumour and subsequently prompted intratumoural infiltration of CTLs. Moreover, siPD-L1 silenced PD-L1 expression in tumour cells to relieve immune evasion. Similarly, positively charged liposomes were also developed as siPD-1 carriers to silence PD-1 expression in T cells [87]. Together, NDDS-based PD-1/PD-L1 downregulation significantly improved the antitumor efficacy.
Fig. 3. Nucleic acid-loaded NDDSs for anti-PD-1/PD-L1 therapy.
a Preparation of siRNA and PPa-coloaded micelleplexes. b Mechanism of micelleplex-enhanced antitumor efficacy via RNAi of PD-L1 and PDT. Adapted with permission from [55]. Copyright (2016) American Chemical Society. c Schematic illustration of the ANP/plasmid nanoparticles for photothermal genome editing of PD-L1. Adapted with permission from [88]. Copyright (2021) John Wiley & Sons, Inc.
In addition to RNA interference-based PD-L1 regulation, clustered, regularly interspaced, short palindromic repeats (CRISPR) and CRISPR-associated protein (Cas) systems have been extensively investigated for targeted genome editing. For example, Tang et al. reported a photothermal nanosystem (termed ANP/plasmid) for PD-L1 ablation to potentiate ICB therapy (Fig. 3c) [88]. The ANP/plasmid nanoparticles were constructed with a guanidinium-based cationic supramolecular layer, Au nanorod core, and CRISPR/Cas9 plasmid. The nanoparticles can be actively internalized by tumour cells through the cationic guanidyl ligand. Upon NIR-II laser irradiation, the gold nanorods induced mild hyperthermia (~42 °C) at the tumour site. Afterwards, the heat-inducible promoter (HSP)-Cas9 plasmid started transcription and performed precise PD-L1 editing in the tumour cells, which subsequently activated tumour-infiltrating T cells. The PD-L1 genome silencing strategy provides an appealing method for long-term anti-PD-1/PD-L1 therapy.
Recently, a polyaptamer hydrogel was exploited to block the PD-1/PD-L1 interaction [89]. The hydrogels were composed of PD-1-binding DNA aptamers and Cas9/sgRNA-cleavable DNA linkers, which can be intratumorally injected into B16F10 melanoma tumour-bearing mice. The Cas9/sgRNA could precisely excise DNA linkers, leading to the in situ release of DNA aptamers, which blocked PD-1 on tumour-infiltrating T cells and thereby promoted their antitumor activity. Apart from directly targeting PD-1/PD-L1, the upstream regulators of the PD-L1 pathway have also been exploited as potential targets for PD-L1 downregulation. For example, Chen et al. developed a CRISPR-Cas-based nanosystem targeting β-catenin to restrain tumour PD-L1 expression, in which β-catenin is a key mediator of the Wnt/β-catenin signalling pathway [90]. Knockout of β-catenin collectively induced tumour cell apoptosis and impaired tumour PD-L1 expression [91]. The nanosystem was composed of a cationic CaCO3 core and protamine for CRISPR–Cas9 plasmid encapsulation. The nanosystem was functionalized with HA for binding CD44 expressed on the surface of the tumour cells modified with TAT-NLS peptide for enhanced tumour penetration, and aptamer (AS1411) was further employed for targeting the nuclei. The nanosystem showed a satisfying plasmid encapsulation ratio (> 95%) and gene transfection efficiency (10 times that of naked DNA), which dramatically suppressed PD-L1 expression to boost ICB therapy.
NDDS-integrating anti-PD-1/PD-L1 antibodies for ICB therapy
Antibodies targeting the PD-1/PD-L1 axis have been approved for the treatment of multiple malignancies (e.g., nivolumab and pembrolizumab) [92–97]. Currently, there are over 5000 clinical trials for anti-PD-1/PD-L1 monoclonal antibody (mAb)-based monotherapy or combinational therapy [61]. Moreover, bispecific antibodies containing two antigen binding sites to simultaneously block dual immune checkpoints are being exploited as novel anti-PD-1/PD-L1 therapy strategies [61]. Nevertheless, systemic administration of anti-PD-L1 antibodies suffers from “on-target but off-tumour” effects since PD-L1 is also expressed in normal tissues, which severely hampers therapeutic efficiency and causes irAEs [98].
In recent years, various NDDSs have been engineered to improve the tumour-specific delivery of immune checkpoint antibodies [99, 100]. Yang et al. conjugated αPD-L1 with reduction-detachable glucosylated PEG chains to improve the performance of anti-PD-1/PD-L1 therapy against glioblastoma [101]. The glucosylated PEG chains could bind to glucose transporter-1 (GLUT1) overexpressed on the brain capillaries and then be detached in the reductive environment of tumours, which promoted tumour accumulation of antibodies and avoided the irAE in the healthy tissues.
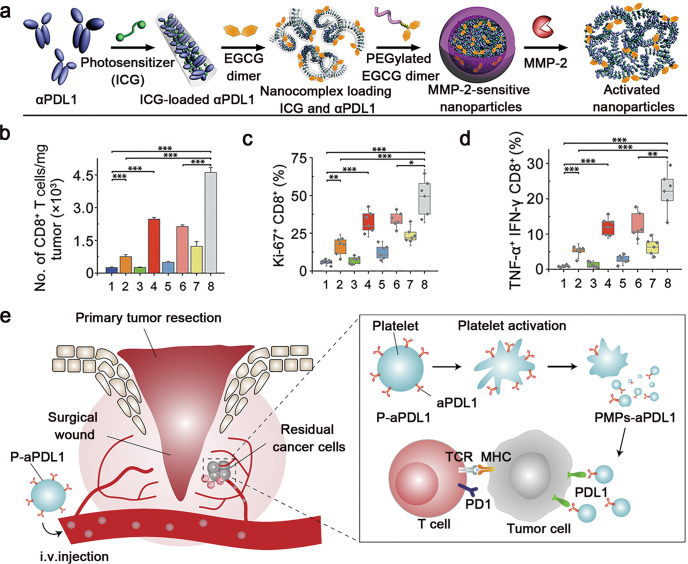
To achieve tumour-specific distribution and activation of immune checkpoint inhibitors, Wang et al. further developed an MMP-2-liable nanoparticle for tumour-targeted delivery of αPD-L1 and the photosensitizer ICG (Fig. 4a) [56]. The antibody nanoparticles with long-circulating features can only be activated in the TME with high MMP-2 expression to release αPD-L1 while preventing on-target but off-tumour effects. Moreover, the combination of PD-L1 blockade and PDT synergistically recruited tumour-infiltrating CD8+ T cells and eventually regressed tumour growth (Fig. 4b–d). Cell-based bioinspired NDDSs have also been applied for the delivery of anti-PD-1/PD-L1 mAbs. For instance, platelets conjugated with αPD-L1 on the surface (P-αPD-L1) were exploited to improve the therapeutic effect of αPD-L1 [102]. αPD-L1 was conjugated onto the plasma membrane of platelets via a maleimide linker, which improved the stability of αPD-L1 in blood circulation. Upon reaching the tumour sites, the platelets could specifically adhere to the surface of the tumour cells and be activated to generate αPD-L1-bearing platelet-derived microparticles (PMPs), thus specifically delivering αPD-L1 to the tumour (Fig. 4e). Compared to free αPD-L1, PMPs displayed increased blood circulation and increased tumour accumulation and thereby induced a robust T-cell immune response to prevent recurrence of B16F10 melanoma and 4T1 triple-negative breast tumours.
Fig. 4. Schematic illustration of the NDDS integrating anti-PD-1/PD-L1 antibodies for ICB therapy.
a Self-assembly of the S-αPD-L1/ICG@NP nanoparticles. b Quantification of the tumour-infiltrating CD8+ T cells after different treatments. c Proliferation assay of the tumour-infiltrating CD8+ T cells. d Frequency of TNF-α and IFN-γ dual-positive CD8+ T cells (1, PBS; 2, αPD-L1; 3, S-ICG@NP; 4, S-ICG@NP + laser; 5, αPD-L1/ICG@NP; 6, αPD-L1/ICG@NP + laser; 7, S-αPD-L1/ICG@NP; 8, S-αPD-L1/ICG@NP + laser) (*P < 0.05, **P < 0.01, ***P < 0.001). Adapted with permission from [56]. Copyright (2019) American Association for the Advancement of Science. e P-αPD-L1 for the treatment of residual cancer cells in the primary tumour resection sites. Adapted with permission from [102]. Copyright (2017) Springer Nature.
αPD-1 is delivered to reverse the immune inhibiting state of T cells and other immune cells. αPD-1 platelet-conjugated stem cells (S-P-αPD-1) were generated to augment antileukaemia efficacy [103]. The αPD-1 platelets were modified with PEG4-N-hydroxysuccinimidyl ester (DBCO-PEG4-NHS ester), and the haematopoietic stem cells were modified with an azide. Then, they were integrated through a click reaction. The systems facilitated the bone marrow-targeting transportation of αPD-1 and released αPD-1 in situ through platelet activation. The in vivo treatment with S-P-αPD-1 showed 80% survival of C1498 leukaemia-bearing mice on the 80th day after tumour cell injection, validating the significant antileukaemia efficacy of S-P-αPD-1.
Anti-PD-1/PD-L1 antibodies have also been exploited for generating tumour-targeted NDDSs for the potentiation of immunotherapy. For instance, Schmid et al. engineered T-cell-targeting nanoparticles by conjugating the F(ab’)2 PD-1 antibody to the surface of PEGylated poly(lactic-co-glycolic acid) (PLGA) nanoparticles [104]. The nanoparticles displayed satisfying T-cell binding affinity and T-cell activation efficiency. The authors further utilized the nanoparticles to deliver a transforming growth factor β (TGF-β) inhibitor and a Toll-like receptor (TLR7/8) agonist, which significantly increased tumour-infiltrating CD8+ T cells and prolonged the survival of the tumour-bearing mice.
Cellular vesicles integrating PD-1/PD-L1 proteins to boost ICB therapy
Bioengineering strategies hold great prospects in the field of antitumor immunotherapy, in which engineered cellular vesicles (CVs) are utilized as biomedicine carriers since they hold outstanding advantages in terms of stability, biocompatibility, and cellular characteristics [105]. Given that most immune checkpoints are membrane proteins, CVs functionalized with immune checkpoint ligands/receptors may establish a Trojan horse strategy that disrupts immunosuppressive cellular interactions [106].
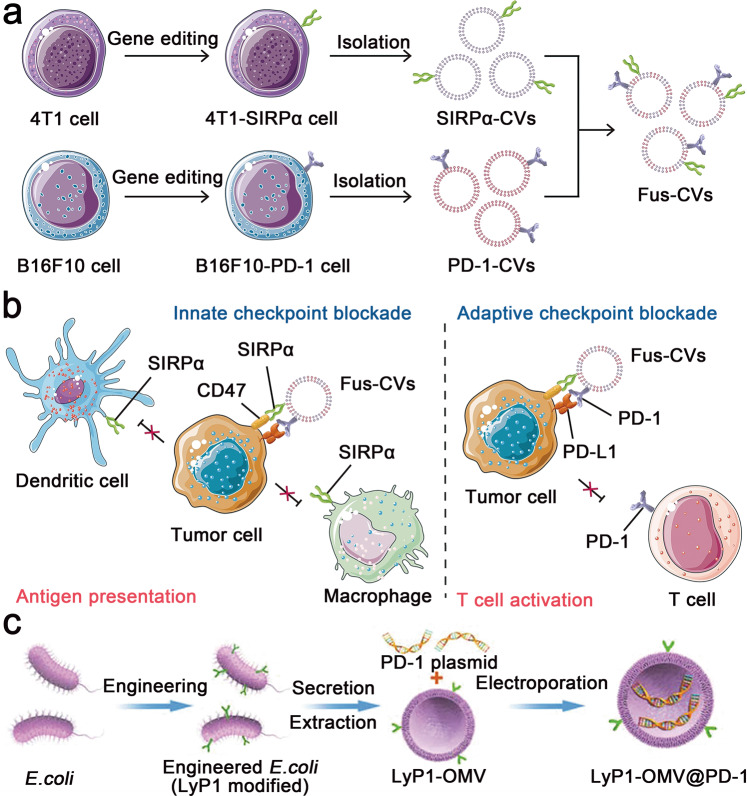
Apart from the adaptive immune effects exerted by T lymphocytes, innate immune effects, including phagocytosis and antigen presentation by macrophages, also play an important role in the antitumor immune process. CD47 is an innate immune checkpoint that is highly expressed on the surface of tumour cells and prevents macrophage phagocytosis by binding to its receptor signal regulatory protein alpha (SIRPα) on the surface of macrophages [44]. Meng et al. reported fused cellular vesicles (Fus-CVs) to concurrently block PD-L1 and CD47 for enhanced antitumor immunity (Fig. 5a, b) [58]. The Fus-CVs were prepared by the following steps. First, tumour cell lines overexpressing PD-1 and SIRPα were constructed by genome editing. Then, single CV components were isolated and fused into Fus-CVs containing both PD-1 and SIRPα. Fus-CVs ensured the concurrent blockade of tumour-overexpressing PD-L1 and CD47 through bispecific targeting effects, which significantly promoted the immune response of T cells in the B16F10 melanoma tumour model. Moreover, Fus-CVs remarkably elongated the survival rate of 4T1 breast tumour-bearing mice by inhibiting postoperative metastasis of the tumour cells.
Fig. 5. Schematic illustration of the CVs for blocking immune checkpoints and achieving tumour-targeted therapeutic delivery.
a Procedure to prepare Fus-CVs. b Mechanistic illustration of the Fus-CVs coblocking PD-L1 and CD47 of the tumour cells. Adapted with permission from [58]. Copyright (2021) John Wiley & Sons, Inc. c Procedure to prepare LyP1-OMV@PD-1. Adapted with permission from [109]. Copyright (2022) John Wiley & Sons, Inc.
Furthermore, the engineered CVs can perform tumour-targeted delivery of immunotherapeutics through ligand–receptor interactions. Indoleamine 2,3-dioxygenase 1 (IDO-1) is an immunosuppressive molecule overexpressed by tumour cells and dendritic cells (DCs) that can inhibit CTLs and recruit Tregs [107]. To circumvent IDO-1-induced immune resistance, Zhang et al. encapsulated the IDO-1 inhibitor 1-methyl-tryptophan (1-MT) into PD-1-presenting CVs to concurrently block the PD-1/PD-L1 axis and overcome the immunosuppressive effects of IDO-1 [108]. An in vitro study demonstrated that the PD-1 CVs colocalized with PD-L1 on the membrane of B16F10 tumour cells. Furthermore, fluorescently labelled CVs can be efficiently internalized by marrow-derived DCs for the intracellular delivery of 1-MT and subsequently inhibit IDO-1 activity in DCs.
The CVs have also recently been explored as nanovectors for gene delivery. For example, Pan et al. developed a bacterial outer membrane-based vesicle (OMV) integrating a PD-1 plasmid to block PD-L1 on the surface of tumour cells (Fig. 5c) [109]. Escherichia coli was genetically engineered to express the tumour-targeting peptide LyP1 on the surface of the membrane. Then, the bacterial membrane was extracted, and the PD-1 plasmid was loaded into the OMV through electroporation to form LyP1-OMV@PD-1. Upon systemic administration, LyP1-OMV@PD-1 specifically delivered the PD-1 plasmid into the tumour cells via LyP1-mediated endocytosis, leading to PD-1 expression by the tumour cells. The immunofluorescence assay showed significantly elevated expression of PD-1 in 4T1 cells after LyP1-OMV@PD-1 treatment. PD-1 could interact with the tumour-originating PD-L1 for self-blockade of the tumour PD-L1.
Conclusion and perspectives
In summary, we overviewed recent advances in NDDSs for anti-PD-1/PD-L1 immunotherapy. The NDDSs exploited thus far can be catalogued into micellar nanoparticles, nanovesicles, micelleplexes and cellular vesicles. These NDDSs are rationally engineered to target the PD-1/PD-L1 axis by delivering various immunotherapeutics, including small molecule antagonists, nucleic acids, antibodies and PD-1/PD-L1 proteins. The NDDSs display elongated blood circulation and a controllable payload release profile at the tumour site by minimizing the nonspecific distribution of the immunotherapeutics. Furthermore, NDDSs can be readily engineered to integrate multiple immunotherapeutics for combinatory immunotherapy. Overall, NDDSs are promising platforms to circumvent the challenges of current anti-PD-1/PD-L1 therapy.
Nevertheless, several challenges remain to be addressed before the clinical application of NDDS-based anti-PD-1/PD-L1 therapy. First, it is crucial to thoroughly evaluate the systematic immune response to NDDSs since some of their ingredients may induce unexpected immune toxicity. Second, standardized fabrication procedures are essential for scale-up production and batch quality control of NDDSs. Furthermore, clinically relevant cell culture or animal models, for example, organ-on-a-chip, patient-derived cell lines and humanized animal models, should be employed for evaluation of the antitumor immune response. Last but not least, compared to the complicated formulation of NDDSs, well-established nanoformulations (e.g., liposomes and micellar nanoparticles) might represent promising candidates for the clinical translation of nanomedicine-based anti-PD-1/PD-L1 therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51873228, 32050410287) and the International Cooperation Project of Science and Technology Commission of Shanghai Municipality (20430711800).
Competing interests
The authors declare no competing interests.
Contributor Information
Lu-jia Huang, Email: huanglujia@simm.ac.cn.
Wu-jun Xu, Email: wujun.xu@uef.fi.
Hai-jun Yu, Email: hjyu@simm.ac.cn.
References
- 1.Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541:321–30.. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 3.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68.. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 5.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–47. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews MC, Wargo JA. Cancer evolution during immunotherapy. Cell. 2017;171:740–2. doi: 10.1016/j.cell.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 9.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intlekofer AM, Thompson CB. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–5. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67.. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 15.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52.. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu QR, Sun F, Li TL, Zhou MX, Ye JY, Ji AY, et al. Engineering oxaliplatin prodrug nanoparticles for second near-infrared fluorescence imaging-guided immunotherapy of colorectal cancer. Small. 2021;17:11. doi: 10.1002/smll.202007882. [DOI] [PubMed] [Google Scholar]
- 21.Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22:124–38. doi: 10.1038/s41577-021-00568-1. [DOI] [PubMed] [Google Scholar]
- 22.Li ZM, Liu YL, Fang XD, Shu ZB. Nanomaterials enhance the immunomodulatory effect of molecular targeted therapy. Int J Nanomed. 2021;16:1631–61. doi: 10.2147/IJN.S290346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:9. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou FQ, Gao J, Xu ZA, Li TL, Gao A, Sun F, et al. Overcoming immune resistance by sequential prodrug nanovesicles for promoting chemoimmunotherapy of cancer. Nano Today. 2021;36:12. doi: 10.1016/j.nantod.2020.101025. [DOI] [Google Scholar]
- 25.Sun B, Hyun H, Li LT, Wang AZ. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta Pharmacol Sin. 2020;41:970–85.. doi: 10.1038/s41401-020-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi FL, Wang MF, Li BZ, Lu ZF, Nie GJ, Li SP. Reversal of the immunosuppressive tumor microenvironment by nanoparticle-based activation of immune-associated cells. Acta Pharmacol Sin. 2020;41:895–901. doi: 10.1038/s41401-020-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeed M, Chen FM, Ye JY, Shi Y, Lammers T, De Geest BG, et al. From design to clinic: Engineered nanobiomaterials for immune normalization therapy of cancer. Adv Mater. 2021;33:2008094. doi: 10.1002/adma.202008094. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23.. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–47.. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Herck S, De Geest BG. Nanomedicine-mediated alteration of the pharmacokinetic profile of small molecule cancer immunotherapeutics. Acta Pharmacol Sin. 2020;41:881–94.. doi: 10.1038/s41401-020-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang WQ, Jin YL, Liu X, Chen FM, Zheng XH, Liu TQ, et al. Endogenous stimuli-activatable nanomedicine for immune theranostics for cancer. Adv Funct Mater. 2021;31:2100386. doi: 10.1002/adfm.202100386. [DOI] [Google Scholar]
- 32.Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater. 2019;31:1803322. doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 33.Yu HJ, De Geest BG. Nanomedicine and cancer immunotherapy. Acta Pharmacol Sin. 2020;41:879–80. doi: 10.1038/s41401-020-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg MS. Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell. 2015;161:201–4. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Wang WQ, Pei Q, Lord MS, Yu HJ. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol Sin. 2020;41:986–94.. doi: 10.1038/s41401-020-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Wang F, Hu C, Zhou Y, Gao H, Hu J. The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharm Sin B. 2020;10:2037–53.. doi: 10.1016/j.apsb.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Q, Bai X, Sofias AM, van der Meel R, Ruiz-Hernandez E, Storm G, et al. Cancer nanomedicine meets immunotherapy: opportunities and challenges. Acta Pharmacol Sin. 2020;41:954–8. doi: 10.1038/s41401-020-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin WM, Li YW, Gu YQ, Luo M. Nanoengineered targeting strategy for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:902–10. doi: 10.1038/s41401-020-0417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanif S, Muhammad P, Chesworth R, Rehman FU, Qian RJ, Zheng M, et al. Nanomedicine-based immunotherapy for central nervous system disorders. Acta Pharmacol Sin. 2020;41:936–53. doi: 10.1038/s41401-020-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang TT, Wang DG, Liu JP, Feng B, Zhou FY, Zhang HW, et al. Acidity-triggered ligand-presenting nanoparticles to overcome sequential drug delivery barriers to tumors. Nano Lett. 2017;17:5429–36. doi: 10.1021/acs.nanolett.7b02031. [DOI] [PubMed] [Google Scholar]
- 41.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–46. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: Progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou MJ, Liu X, Chen FM, Yang LL, Yuan MJ, Fu DY, et al. Stimuli-activatable nanomaterials for phototherapy of cancer. Biomed Mater. 2021;16:042008. doi: 10.1088/1748-605X/abfa6e. [DOI] [PubMed] [Google Scholar]
- 44.Zhou FY, Feng B, Yu HJ, Wang DG, Wang TT, Ma YT, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31:1805888. doi: 10.1002/adma.201805888. [DOI] [PubMed] [Google Scholar]
- 45.Hou B, Zhou L, Wang H, Saeed M, Wang DG, Xu Z, et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32:1907210. doi: 10.1002/adma.201907210. [DOI] [PubMed] [Google Scholar]
- 46.Zhao ZH, Wang WQ, Li CX, Zhang YQ, Yu TR, Wu RF, et al. Reactive oxygen species-activatable liposomes regulating hypoxic tumor microenvironment for synergistic photo/chemodynamic therapies. Adv Funct Mater. 2019;29:1905013. doi: 10.1002/adfm.201905013. [DOI] [Google Scholar]
- 47.Peng S, Xiao F, Chen M, Gao H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci. 2022;9:2103836. doi: 10.1002/advs.202103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan AT, Pulukuri AJ, Davaritouchaee M, Abbasi A, Hendricksen AT, Opp LK, et al. Comparing the immunogenicity of glycosidase-directed resiquimod prodrugs mediated by cancer cell metabolism. Acta Pharmacol Sin. 2020;41:995–1004. doi: 10.1038/s41401-020-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang F, Shi K, Jia YP, Hao Y, Peng JR, Qian ZY. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:911–27. doi: 10.1038/s41401-020-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong XF, Sun X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:928–35. doi: 10.1038/s41401-020-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J, Wang WQ, Yu HJ. Acid-activatable polymeric drug delivery systems for cancer therapy. Acta Polym Sin. 2019;50:1156–66. [Google Scholar]
- 52.Zhou FY, Feng B, Wang TT, Wang DG, Meng QS, Zeng JF, et al. Programmed multiresponsive vesicles for enhanced tumor penetration and combination therapy of triple-negative breast cancer. Adv Funct Mater. 2017;27:1606530. doi: 10.1002/adfm.201606530. [DOI] [Google Scholar]
- 53.Koshy ST, Mooney DJ. Biomaterials for enhancing anti-cancer immunity. Curr Opin Biotechnol. 2016;40:1–8. doi: 10.1016/j.copbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu T, Nie W, Hong Z, He Y, Chen J, Mi X, et al. Synergy of immunostimulatory genetherapy with immune checkpoint blockade motivates immune response to eliminate cancer. Adv Funct Mater. 2021;31:2100715. doi: 10.1002/adfm.202100715. [DOI] [Google Scholar]
- 55.Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, et al. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016;16:5503–13. doi: 10.1021/acs.nanolett.6b01994. [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Wang T, Yu H, Feng B, Zhou L, Zhou F, et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci Immunol. 2019;4:eaau6584. doi: 10.1126/sciimmunol.aau6584. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Xie L, Li B, Sang W, Yan J, Li J, et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat Commun. 2021;12:5733. doi: 10.1038/s41467-021-25990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng QF, Zhao Y, Dong C, Liu L, Pan Y, Lai J, et al. Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew Chem Int Ed. 2021;60:26320–6. doi: 10.1002/anie.202108342. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Zhao Z, Qiu N, Zhou Q, Wang G, Jiang H, et al. Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy. Nat Commun. 2021;12:2425. doi: 10.1038/s41467-021-22407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Q, Jiang L, Li SC, He QJ, Yang B, Cao J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol Sin. 2021;42:1–9. doi: 10.1038/s41401-020-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upadhaya S, Neftelinov ST, Hodge J, Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Disco. 2022 doi: 10.1038/d41573-022-00030-4. [DOI] [PubMed] [Google Scholar]
- 62.Feng B, Niu ZF, Hou B, Zhou L, Li YP, Yu HJ. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of paclitaxel nanoparticles. Adv Funct Mater. 2020;30:1906605. doi: 10.1002/adfm.201906605. [DOI] [Google Scholar]
- 63.Feng B, Zhou FY, Hou B, Wang DG, Wang TT, Fu YL, et al. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater. 2018;30:1803001. doi: 10.1002/adma.201803001. [DOI] [PubMed] [Google Scholar]
- 64.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–27.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song RD, Li TL, Ye JY, Sun F, Hou B, Saeed M, et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv Mater. 2021;33:2101155. doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]
- 69.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–5. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16:1014–24. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 72.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou F, Gao J, Tang Y, Zou Z, Jiao S, Zhou Z, et al. Engineering chameleon prodrug nanovesicles to increase antigen presentation and inhibit PD-L1 expression for circumventing immune resistance of cancer. Adv Mater. 2021;33:2102668. doi: 10.1002/adma.202102668. [DOI] [PubMed] [Google Scholar]
- 75.Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–37. doi: 10.1016/j.celrep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hogg SJ, Vervoort SJ, Deswal S, Ott CJ, Li J, Cluse LA, et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017;18:2162–74. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu C, He X, Chen Y, Yang X, Qin L, Lei T, et al. Metformin mediated PD‐L1 downregulation in combination with photodynamic‐immunotherapy for treatment of breast cancer. Adv Funct Mater. 2021;31:2007149. doi: 10.1002/adfm.202007149. [DOI] [Google Scholar]
- 78.Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6:36441–55. doi: 10.18632/oncotarget.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiang CF, Chao TT, Su YF, Hsu CC, Chien CY, Chiu KC, et al. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget. 2017;8:20706–18. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao W, Yurdagul A, Jr., Kong N, Li W, Wang X, Doran AC, et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci Transl Med. 2020;12:eaay1063. doi: 10.1126/scitranslmed.aay1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19:228–49. doi: 10.1038/s41569-021-00629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei ZH, Zhang XQ, Yong TY, Bie NN, Zhan GT, Li X, et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12:20. doi: 10.1038/s41467-020-20723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu W, He X, Yang Z, Yang X, Xiao W, Liu R, et al. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 84.Chen WF, Guo ZF, Zhu YN, Qiao N, Zhang ZR, Sun X. Combination of bacterial-photothermal therapy with an anti-PD-1 peptide depot for enhanced immunity against advanced cancer. Adv Funct Mater. 2020;30:13. [Google Scholar]
- 85.Wang WQ, Saeed M, Zhou Y, Yang LL, Wang DG, Yu HJ. Non-viral gene delivery for cancer immunotherapy. J Gene Med. 2019;21:11. doi: 10.1002/jgm.3092. [DOI] [PubMed] [Google Scholar]
- 86.Gao J, Zhang HW, Zhou FQ, Hou B, Chen MW, Xie ZG, et al. Acid-activatible micelleplex delivering siRNA-PD-L1 for improved cancer immunotherapy of CDK4/6 inhibition. Chin Chem Lett. 2021;32:1929–36. doi: 10.1016/j.cclet.2020.12.009. [DOI] [Google Scholar]
- 87.Barati M, Mirzavi F, Nikpoor AR, Sankian M, Namdar Ahmadabad H, Soleimani A, et al. Enhanced antitumor immune response in melanoma tumor model by anti-PD-1 small interference RNA encapsulated in nanoliposomes. Cancer Gene Ther. 2021 doi: 10.1038/s41417-021-00367-9. [DOI] [PubMed] [Google Scholar]
- 88.Tang H, Xu X, Chen Y, Xin H, Wan T, Li B, et al. Reprogramming the tumor microenvironment through second-near-infrared-window photothermal genome editing of PD-L1 mediated by supramolecular gold nanorods for enhanced cancer immunotherapy. Adv Mater. 2021;33:2006003. doi: 10.1002/adma.202006003. [DOI] [PubMed] [Google Scholar]
- 89.Lee J, Le QV, Yang G, Oh YK. Cas9-edited immune checkpoint blockade PD-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials. 2019;218:9. doi: 10.1016/j.biomaterials.2019.119359. [DOI] [PubMed] [Google Scholar]
- 90.He XY, Ren XH, Peng Y, Zhang JP, Ai SL, Liu BY, et al. Aptamer/Peptide-functionalized genome-editing system for effective immune restoration through reversal of PD-L1-mediated cancer immunosuppression. Adv Mater. 2020;32:2000208. doi: 10.1002/adma.202000208. [DOI] [PubMed] [Google Scholar]
- 91.Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39:648–58. doi: 10.1016/j.tips.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilkinson E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015;16:e9. doi: 10.1016/S1470-2045(14)71129-5. [DOI] [PubMed] [Google Scholar]
- 94.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 95.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 96.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 97.O’Sullivan Coyne G, Madan RA, Gulley JL. Nivolumab: Promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol. 2014;32:986–8. doi: 10.1200/JCO.2013.54.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang L, Chen F, Lai Y, Xu Z, Yu H. Engineering nanorobots for tumor-targeting drug delivery: From dynamic control to stimuli-responsive strategy. Chembiochem. 2021;22:3369–80. doi: 10.1002/cbic.202100347. [DOI] [PubMed] [Google Scholar]
- 100.Wang WQ, Jin YL, Xu ZA, Liu X, Bajwa SZ, Khan WS, et al. Stimuli-activatable nanomedicines for chemodynamic therapy of cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:14. doi: 10.1002/wnan.1614. [DOI] [PubMed] [Google Scholar]
- 101.Yang T, Mochida Y, Liu X, Zhou H, Xie J, Anraku Y, et al. Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma. Nat Biomed Eng. 2021;5:1274–87. doi: 10.1038/s41551-021-00803-z. [DOI] [PubMed] [Google Scholar]
- 102.Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng. 2017;1:0011. doi: 10.1038/s41551-016-0011. [DOI] [Google Scholar]
- 103.Hu QY, Sun WJ, Wang JQ, Ruan HT, Zhang XD, Ye YQ, et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng. 2018;2:831–40. doi: 10.1038/s41551-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1747. doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–50. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y, et al. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020;14:16698–711. doi: 10.1021/acsnano.0c03776. [DOI] [PubMed] [Google Scholar]
- 107.Gao A, Chen BF, Gao J, Zhou FQ, Saeed M, Hou B, et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353–62. doi: 10.1021/acs.nanolett.9b04012. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y, et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater. 2018;30:1707112. doi: 10.1002/adma.201707112. [DOI] [PubMed] [Google Scholar]
- 109.Pan J, Li X, Shao B, Xu F, Huang X, Guo X, et al. Self-Blockade of PD-L1 with bacteria-derived outer-membrane vesicle for enhanced cancer immunotherapy. Adv Mater. 2022;34:2106307. doi: 10.1002/adma.202106307. [DOI] [PubMed] [Google Scholar]
- 110.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng B, Xiao Y, Xue M, Cao H, Chen J. Recent advances in the development of PD-L1 modulators: Degraders, downregulators, and covalent inhibitors. J Med Chem. 2020;63:15389–98. doi: 10.1021/acs.jmedchem.0c01362. [DOI] [PubMed] [Google Scholar]