Abstract
Study Objectives:
Nocturnal symptoms are very common in asthma, which is associated with worse sleep quality. The nocturnal oxygen saturation may be decreased in asthma; however, whether this association is dependent on nocturnal asthma symptoms, lung function, coexisting obstructive sleep apnea (OSA), or other asthma-related comorbidities is unknown. The objective of this study was to examine the effects of asthma, OSA, lung function, airway symptoms, and asthma-related comorbidities on the nocturnal oxygen saturation in a cross-sectional community-based population study.
Methods:
In total, 395 women and 392 men underwent overnight polysomnography, performed spirometry, and completed questionnaires on airway symptoms and asthma-related comorbidities.
Results:
Participants with asthma (n = 88) had a lower nocturnal oxygen saturation than those without asthma (93.8% vs 94.3%, P = .01) also after adjusting for comorbidity, age, body mass index, and smoking status (coefficient −0.38; CI −0.67, −0.10; P < .01). The nocturnal oxygen saturation was lower among participants with wheezing, nocturnal chest tightness, fixed airflow limitation, gastroesophageal reflux, obesity, and OSA than in those without these conditions. The associations between lower oxygen saturation and wheezing, forced expiratory volume in 1 second, gastroesophageal reflux, and apnea-hypopnea index were significant also after adjusting for age, sex, body mass index, and smoking status. Participants with both wheezing and OSA had a significantly lower nocturnal oxygen saturation (92.5 ± 0.5%) than participants with wheezing only (94.3 ± 0.3%) and OSA only (93.6 ± 0.2% %) (P < .01).
Conclusions:
Participants with asthma displayed a lower mean oxygen saturation during sleep, which could not be explained only by coexisting OSA or obesity. Also, asthma symptoms and lung function were associated with lower nocturnal oxygen saturation. The lower oxygen saturation seen in asthma is hence multifactorial in origin and is a result of the combination of symptoms, lung function, and comorbidity.
Citation:
Sundbom F, Janson C, Ljunggren M, Lindberg E. Asthma and asthma-related comorbidity: effects on nocturnal oxygen saturation. J Clin Sleep Med. 2022;18(11):2635–2641.
Keywords: hypoxemia, OSA, asthma-related comorbidity, polysomnography, lung function
BRIEF SUMMARY
Current Knowledge/Study Rationale: The nocturnal oxygen saturation is decreased in severe asthma, and we have previously demonstrated an association between asthma and lower nocturnal oxygen saturation in a community-based population. The aim of this study was to examine the impact of asthma, lung function, airway symptoms, obstructive sleep apnea, and other asthma-related comorbidities on the nocturnal oxygen saturation.
Study Impact: Asthma was associated with a lower nocturnal oxygen saturation, despite the same level of apnea-hypopnea index in participants with and without asthma, and the lower oxygen saturation with asthma could not be explained only by comorbidity in obstructive sleep apnea or obesity. This underlines that not only the frequency of apneas or hypopneas but also the mean oxygen saturation during sleep should be considered in evaluation of obstructive sleep apnea in asthma patients.
INTRODUCTION
Patients with asthma have an increased prevalence of obstructive sleep apnea (OSA) and vice versa.1 OSA is commonly considered an important asthma-related comorbidity, and there is an association between OSA and worse asthma control.2 A meta-analysis has demonstrated that as many as 49.5% of asthma patients fulfill the criteria for OSA based on the apnea-hypopnea index (AHI), while only a few studies have focused on oximetric parameters.3 Asthma is also associated with worse self-reported sleep quality, especially in the presence of asthma-related comorbidities.4 Moreover, nocturnal symptoms are common, both in patients with asthma that have or do not have OSA.5
The nocturnal oxygen saturation is decreased in severe asthma,6 and we have previously demonstrated an association between asthma and lower nocturnal oxygen saturation in a community-based population.7 However, it is not fully known to which extent this association is dependent on nocturnal asthma symptoms, lung function, coexisting OSA, and other asthma-related comorbidities, including obesity and nasal congestion.
Nocturnal hypoxemia is also present in sleep-related breathing disorders, of which OSA is the most common. OSA severity is usually based on the AHI, which represents the number of respiratory events (apneas and hypopneas) per hour of sleep, but AHI does not reflect the degree of desaturation or the duration of apneas or hypopneas.8,9 Measures such as mean oxygen saturation, lowest oxygen saturation, sleeping time with oxygen saturation <90% (TST90), and hypoxic burden (respiratory event-associated area under the desaturation curve) have been tested as markers of chronic intermittent hypoxemia. Moreover, several adverse effects of OSA correlate to these oximetric parameters rather than to AHI.10–15 Hence, oximetric parameters have a value as markers of chronic intermittent hypoxemia, which is the main pathophysiological consequence of sleep apnea. The importance of nocturnal hypoxemia is further stressed by data showing that mean oxygen saturation is a well-known predictor of mortality and quality of life in chronic obstructive pulmonary disease (COPD).16,17
The aim of this study was to examine the effects of asthma, lung function, airway symptoms, OSA, and asthma-related comorbidities on the nocturnal oxygen saturation in a community-based population.
METHODS
Study populations
In this investigation, we used cross-sectional data from 2 cohort studies, Sleep and Health in Women (SHE) and Men in Uppsala: A Study of Sleep, Apnea and Cardiometabolic Health (MUSTACHE) (Figure S1 (51.2KB, pdf) in the supplemental material).
The SHE study was a population-based prospective cohort study based on 10,000 randomly selected women in Uppsala, Sweden who were first investigated by a postal questionnaire with 7,051 respondents in 2000.18 In phase I of the study, 400 participants with an oversampling of habitual snorers were examined with whole-night polysomnography, anthropometric measures, blood samples, and questionnaires from 2001 to 2004.19 During 2012–2014, 273 of the women who participated in phase II and 127 women randomly selected from the original cohort (total n = 400) underwent a new polysomnography, had anthropometric measurements taken, answered questionnaires, and underwent spirometry.20
MUSTACHE is a cohort study comprising 400 men in Uppsala, Sweden. All participants were matched 1:1 for age and body mass index (BMI), with the women participating in the SHE study at the 10-year follow-up. Of the male participants, 341 were recruited from men who had participated in the population-based cohort study Epi-Health.21 To fulfill the age and BMI matching criteria, an additional 59 men were recruited via local announcements. All participants were examined with whole-night polysomnography, anthropometric measures, blood samples, questionnaires, and spirometry in 2016–2019.
For variables used in this study, identical protocols were used for all women and men. Only participants with completed questionnaire data on asthma (n = 787) were included in the analyses (Figure S1 (51.2KB, pdf) ). Characteristics of the participants are presented in Table S1 (51.2KB, pdf) in the supplemental material.
Written informed consent was obtained from all participants in both studies, and the studies were approved by the ethics committee at the medical faculty at Uppsala University (Dnr 2011/244 and Dnr 2016/029).
Polysomnography
Polysomnography recording was performed in the homes of the participants using a portable sleep recorder (Embla, Flaga, Iceland). The recordings included electromyograms, electroencephalograms, electro-oculograms, electrocardiograms, respiratory effort from piezo-electric belts, 2 airflow leads, a body-position lead, and a piezo-vibration sensor for snoring. Oxygen saturation was assessed with finger pulse oximetry (Embla A10 Flex Sensor).
Sleep staging and respiratory and arousal analysis were performed and checked by a licensed sleep technician. An obstructive apnea was defined as the cessation of airflow in both nasal pressure and oronasal thermistor for at least 10 seconds, despite continuing abdominal and thoracic movements. An obstructive hypopnea was defined as a 50% reduction from baseline in both nasal pressure and oronasal thermistor for at least 10 seconds, accompanied by continuing abdominal and thoracic movements in combination with an arousal or an oxygen desaturation ≥ 3%, corresponding to the AASM 2007 alternative definition. With this definition the AHI will be lower in comparison to the AASM 2012 definition, using a 30% flow reduction.22 The definition was set when the SHE study was initiated, and it was consistently used in the SHE follow-up study in 2012–2014 and in the MUSTACHE study to allow comparisons over time and between the SHE and MUSTACHE populations. Apnea-hypopnea index was defined as the mean number of apneas and hypopneas per hour of sleep.20 Obstructive sleep apnea was considered with an AHI ≥ 15 events/h, regardless of symptoms or multimorbidity.7 Oxygen desaturation index (ODI) was defined as the mean number of desaturations of ≥ 3% per hour of sleep. TST90 was defined as the percentage of time spent with oxygen saturation below 90%. Nocturnal oxygen saturation was defined as the mean saturation during sleep based on total sleep time.
Spirometry
Spirometry was performed in a seated position with an NDD Easy One spirometer (NDD Medizintechnik AG, Zurich, Switzerland). The participants underwent 3 technically acceptable trials, and the highest values for forced expiratory volume (FVC) and forced expiratory volume in 1 second (FEV1) were recorded according to the American Thoracic Society/European Respiratory Society recommendations.23
Definitions
Asthma was defined as a positive answer to either the question “Have you had an asthma-attack in the last 12 months?” and/or the question “Are you currently taking any medicine, including inhalers, aerosols or tablets, for asthma?”7 Questions on asthma-related symptoms, with response options yes or no, included wheezing (wheezing in the chest at any time in the last 12 months), nocturnal chest tightness (waking up with chest tightness at any time in the last 12 months), and nocturnal breathlessness (being woken up by shortness of breath at any time in the last 12 months).24 An exacerbation was defined as a self-reported asthma-attack during the last 12 months. Fixed airway obstruction was defined as FEV1/FVC < 0.7.
Rhinitis was defined as a positive answer to the question “Do you suffer from hay fever or any other nasal allergy?”7 Participants answering “Often” or “Very often” to the question “Do you suffer from nasal congestion at night?” were defined as having nasal obstruction at night.25
Participants answering “Rarely” or more often to the question “How often, during the last month, have you had heartburn or acid reflux after going to bed?” were defined as having gastroesophageal reflux.
Obesity was defined as a BMI of > 30 kg/m2 based on measured height and weight.
Current smoking was defined as a positive answer to the question “Have you ever smoked daily for at least six months?” and a positive answer to the question “Do you smoke now?”. Participants with a positive answer to the first question and a negative to the latter were defined as ex-smokers.
Statistical analyses
All the statistical analyses were conducted using STATA 16 (STATA Corp, College Station, TX). Univariate analyses were performed with the Chi-square test and unpaired t test. Arithmetic mean values were calculated for normally distributed variables, and geometric mean values were calculated for nonnormally distributed variables (AHI, ODI, and TST90). Unpaired t test was used for P-values. Multiple linear regression was used to analyze the association between nocturnal oxygen saturation and airway symptoms, lung function, and asthma-related comorbidity, with adjustments for age, sex, and smoking status.
RESULTS
Of the total study population, 88 (11.2%) had asthma. Characteristics of the participants with and without asthma are presented in Table 1. Participants with asthma had a higher prevalence of wheezing, nocturnal respiratory symptoms, rhinitis, and obesity than those without asthma. FEV1 was significantly lower among participants with asthma, and airway obstruction was more common.
Table 1.
Characteristics of participants with and without asthma.
| No Asthma (n = 699) | Asthma (n = 88) | P | |
|---|---|---|---|
| Women (%) | 48.5% | 63.6% | < .01 |
| Age | 59.4 ± 11.2 | 59.1 ± 10.9 | .87 |
| BMI | 26.3 ± 4.5 | 28.5 ± 5.4 | < .01 |
| Smoker (%) | 6.0% | 9.1% | .05 |
| Ex-smoker (%) | 35.1% | 45.5% | .05 |
| FVC (% predicted)* | 109.1 ± 17.1 | 107.2 ± 18.6 | .32 |
| FEV1 (% predicted)* | 99.6 ± 15.1 | 90.8 ± 19.6 | < .01 |
| FEV1/FVC < 0.7* | 11.6% | 23.9% | < .01 |
| FEV1 < 80% predicted* | 8.9% | 25.9% | < .01 |
| Wheezing | 13.1% | 63.6% | < .01 |
| Nocturnal chest tightness | 9.2% | 28.4% | < .01 |
| Nocturnal breathlessness | 7.6% | 17.1% | < .01 |
| Rhinitis | 23.5% | 55.8% | < .01 |
| Nasal obstruction at night | 4.6% | 13.8% | < .01 |
| Nocturnal reflux | 10.5% | 12.5% | .57 |
| Obesity | 18.3% | 36.4% | < .01 |
*Spirometry data available from 779 individuals. BMI = body mass index, FVC = forced expiratory volume, FEV1 = forced expiratory volume in 1 second.
Participants with asthma had a lower nocturnal oxygen saturation than those without asthma, as well as a tendency toward longer TST90. There was no difference in prevalence of OSA or severe OSA, and no differences in AHI or ODI (Table 2).
Table 2.
Oxygen saturation and sleep apnea parameters in participants with and without asthma.
| No Asthma | Asthma | P | |
|---|---|---|---|
| Mean saturation (%) | 94.3 (94.2–94.4) | 93.8 (93.4–94.3) | .01 |
| TST90 (%)* | 0.5 (0.4–0.5) | 0.7 (0.4–1.2) | .05 |
| AHI > 15 events/h | 30.0% | 36.4% | .22 |
| AHI > 30 events/h | 12.1% | 12.5% | .92 |
| AHI* | 5.0 (4.4–5.7) | 5.6 (3.9–7.9) | .59 |
| ODI* | 6.0 (5.3–6.7) | 7.4 (5.5–10.1) | .11 |
*Geometric means. AHI = apnea-hypopnea index, ODI = oxygen desaturation index, TST90 = sleeping time with oxygen saturation < 90%.
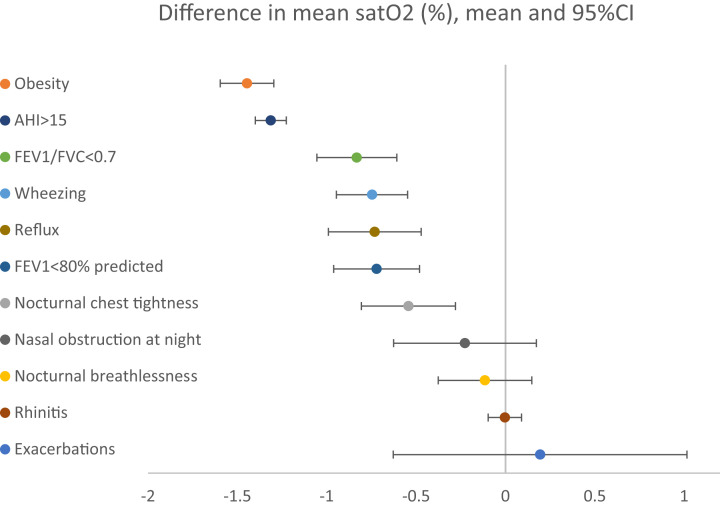
To investigate risk factors for the lower oxygen saturation seen in asthma, associations between nocturnal oxygen saturation and asthma-associated symptoms and asthma-related comorbidities were analyzed (Figure 1). The nocturnal oxygen saturation was lower among participants reporting wheezing or nocturnal chest tightness, as well as among participants with fixed airflow limitation (FEV1/FVC < 0.7). Five common asthma-related comorbidities were evaluated: rhinitis, nasal obstruction at night, gastroesophageal reflux, obesity, and OSA. Of these, the nocturnal oxygen saturation was lower with gastroesophageal reflux, obesity, or OSA. The lowest oxygen saturation was found in participants with severe OSA (AHI > 30 events/h, n = 90) (93.0% vs 94.4%, P < .01).
Figure 1. Difference in nocturnal oxygen saturation (mean and 95% CI), depending on airway symptoms, asthma-related comorbidity, and smoking.
AHI = apnea-hypopnea index, CI = confidence interval, FVC = forced expiratory volume, FEV1 = forced expiratory volume in 1 second.
Having an asthma diagnosis was associated with lower nocturnal oxygen saturation also after adjusting for comorbidity, age, BMI, and smoking status (coefficient −0.38; CI −0.67, −0.10; P < .01). Wheezing and FEV1 were chosen as markers of 2 different domains of asthma control, and further tested in a multiple linear regression model with adjustments for relevant comorbidities, age, and smoking status (Table 3). The associations between lower oxygen saturation and wheezing, FEV1, BMI, nocturnal reflux, and AHI remained significant in this analysis. The same results were seen when OSA was tested as a dichotomous variable (AHI > 15) or when ODI was used as the measure of OSA instead of AHI.
Table 3.
Multiple linear regression with mean nocturnal oxygen saturation (%) as dependent variable (adjusted r2 = 47.0%).
| Mean Oxygen Saturation | |||
|---|---|---|---|
| Coefficient | 95% CI | P | |
| Wheeze | −0.36 | −0.60, −0.13 | < .01 |
| FEV1 (% of predicted, by 10) | 0.08 | 0.02, 0.14 | < .01 |
| Female | 1.11 | 0.94, 1.29 | < .01 |
| Age (by 5) | −0.24 | −0.28, −0.20 | < .01 |
| BMI (by 5) | −0.48 | −0.58, −0.37 | < .01 |
| Nocturnal gastroesophageal reflux | −0.41 | −0.70, −0.13 | < .01 |
| Nasal obstruction at night | 0.09 | −0.29, 0.47 | .64 |
| AHI (by 10) | −0.19 | −0.26, −0.13 | < .01 |
Adjusted for smoking history and all variables in the table. AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, FEV1 = forced expiratory volume in 1 second.
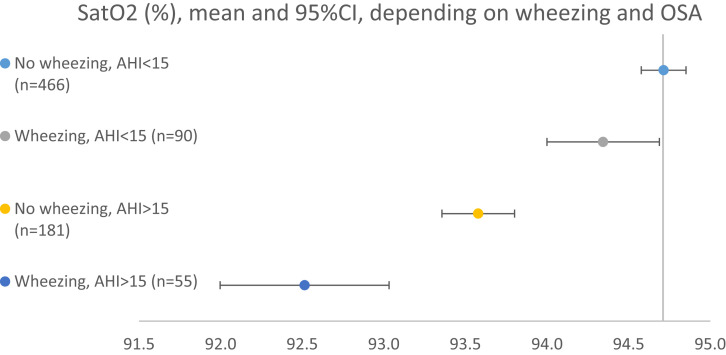
The population was divided into 4 groups depending on the presence of wheezing and/or OSA. The nocturnal oxygen saturation was highest in the group without wheezing or OSA (94.7 ± 0.1%) (Figure 2). The group with both wheezing and OSA had a significantly lower nocturnal oxygen saturation (92.5 ± 0.5%) than the groups with wheezing only (94.3 ± 0.3%, P < .01) and OSA only (93.6 ± 0.2%, P < .01). The associations remained significant also after adjusting for BMI, FEV1, sex, and age. TST90 was significantly higher in both groups with AHI > 15 events/h than in the group without wheezing or OSA. Further, TST90 was significantly higher in the group with wheezing and OSA than in the group with OSA only (6.2% vs 2.3%, P < .01). Differences in AHI (32.0 vs 27.2, P > .99) or ODI (27.4 vs 21.3, P > .99) between these groups did not reach statistical significance.
Figure 2. Mean nocturnal saturation (%) depending on wheezing and obstructive sleep apnea.
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea.
DISCUSSION
Asthma was associated with lower mean oxygen saturation during sleep. The association was not only due to coexisting OSA and obesity but also to asthma symptoms and FEV1. These findings confirm our previous results, and add new information on the role of lung function and asthma control.7 Wheezing and airflow obstruction were independently associated with lower nocturnal oxygen saturation, while exacerbation history was not. The nocturnal oxygen saturation was lowest among participants with both wheezing and OSA.
There are not many community-based polysomnography studies of asthma, as most tend to focus on smaller, selected populations of severe asthma.6 A large number of unselected asthma patients were included in the European Sleep Apnea Database (ESADA), which contains data on a very large number of patients referred for sleep studies. A cross-sectional ESADA study of 16,236 patients, of which 4.8% had self-reported asthma, showed no difference in OSA severity or nocturnal hypoxemia between patients with asthma and patients without asthma. The cited study benefits from the large study population, but it is limited by referral bias and does not consider asthma control or lung function.26
Asthma control encompasses 2 domains: the level of clinical asthma control (ie, symptoms) and the risk of future adverse events, including exacerbations and loss of lung function.27 Hence, self-reported wheezing, exacerbation history, and FEV1 were chosen as markers of the different domains of asthma. Both wheezing and FEV1 were independently associated with lower mean oxygen saturation during sleep, while only a few of the participants in the study had a positive exacerbation history. Having an asthma diagnosis was not associated per se with a lower nocturnal oxygen saturation after adjusting for wheezing or lung function. This implies that the level of achieved asthma control is of much greater importance than an “asthma label” in itself for nocturnal oxygen saturation. We have shown similar results previously for the association between asthma and insomnia symptoms.4 For FEV1, it has been demonstrated that lung function decline is more rapid in patients with high OSA risk, and that this effect is especially prominent in those with asthma.28 Apparently, nocturnal hypoxemia in asthma is a result of the combination of symptoms, lung function, and comorbidity. Although statistically significant, the difference in mean oxygen saturation between groups was small. It could be speculated that the difference may be more pronounced in a population with more severe asthma. As the number of participants with severe asthma was limited in the present study, this could not be further analyzed.
The role of AHI as the single measure of OSA severity has been questioned, both because of its poor correlation to daytime symptoms and because of its limited value as a marker of chronic intermittent hypoxemia.29,30 AHI is a measure of the frequency of apneas and hypopneas, rather than a measure of the nocturnal hypoxemic stress that leads to vascular endothelium damage and systemic inflammation in OSA.12–14 In the present study, nocturnal oxygen saturation was chosen as the primary outcome measure. The clinical relevance of mean oxygen saturation is established in COPD, where uncorrected hypoxemia is associated with increased systemic inflammation and cardiovascular mortality.17 Nocturnal desaturation is also common in the absence of daytime hypoxemia in COPD, and there is an association between sleep-related hypoxemia and brain atrophy, which is over-represented in COPD.31,32 Several oximetric parameters have been investigated, and adverse effects of OSA often correlate to these measures rather than to AHI. For example, more severe hypoxemia is associated with increased inflammation and vascular risk in patients with similar AHI.14 TST90, but not AHI, has been identified as a predictor of all-cause mortality in heart failure patients.12 In another study, mean oxygen desaturation and TST90 were associated with cardiovascular disease progression, while there was no association to AHI.13 Hypoxic burden, but not AHI, has also been pointed out as a predictor of cardiovascular disease-related mortality.15 Further, CPAP treatment of moderate-to-severe OSA, as based on AHI, did not prevent cardiovascular events in a large cohort of patients with established cardiovascular disease.10
In the present study, participants with asthma had a lower nocturnal oxygen saturation and a higher TST90 than participants without asthma, while there was no difference in AHI or ODI. Our interpretation of these results is that OSA patients with asthma may experience longer or more severe desaturations than patients without asthma, despite the same AHI. Mean oxygen saturation has previously been pointed out as a reliable marker of sleep-related hypoxemia, while TST90 often has a positive-skewed distribution, with many observations close to 0%.32 This distribution of TST90 was seen also in the present study. The small variance may explain that the association between asthma and TST90 was weaker than the association between asthma and nocturnal oxygen saturation, and TST90 may thereby be a less useful measure for most patients. As discussed, AHI is not a perfect measure of chronic intermittent hypoxemia, but neither is nocturnal oxygen saturation or TST90 when used as single parameters.16 A precision medicine approach to OSA, considering several parameters, is likely beneficial, particularly in patients with coexisting airway disease.33 Further research is needed to define associations between oximetric parameters and clinical consequences, and it is still not known which oximetric parameter best identifies a different hypoxemic OSA phenotype.16
The present study benefits from a large community-based sample, standardized polysomnography and spirometry procedures, and well-validated questionnaires. There was an over-sampling of habitual snorers in the female population. The prevalence of OSA and asthma may thereby not reflect the prevalence in the entire population, but this would not interfere with the results on differences between groups or associations. Another limitation is the absence of a validated questionnaire for asthma control, such as the Asthma Control Test or Asthma Control Questionnaire. Asthma was defined using self-reported asthma attacks and current medication. This definition was also used in the major multinational studies on asthma prevalence in adults, The European Community Respiratory Health Survey (ECRHS) and The Global Allergy and Asthma European Network (GA2LEN).34,35 The definition is considered robust, although healthy participants as well as COPD patients could be mislabeled as having asthma.
CONCLUSIONS
In conclusion, asthma was associated with a lower nocturnal oxygen saturation, despite the same level of AHI in participants with and without asthma. The lower oxygen saturation with asthma could not be explained only by comorbidity in OSA or obesity, but it was also independently associated with FEV1 and wheezing. The findings have a high clinical relevance and underline that not only the frequency of apneas or hypopneas but also the mean oxygen saturation during sleep should be considered in evaluation of OSA in asthma patients.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. Fredrik Sundbom has received grants from the Bror Hjerpstedt Foundation (Bror Hjerpstedts Stiftelse), the Uppsala County Association Against Heart and Lung Diseases (Uppsala läns förening mot hjärt- och lungsjukdomar), and the Swedish Heart-Lung Foundation (Hjärt-lungfonden). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the Bror Hjerpstedt Foundation (Bror Hjerpstedts Stiftelse), the Uppsala County Association Against Heart and Lung Diseases (Uppsala läns förening mot hjärt- och lungsjukdomar) and the Swedish Heart-Lung Foundation (Hjärt-lungfonden).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- COPD
chronic obstructive airway disease
- FEV1
forced expiratory volume in 1 second
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- TST90
sleeping time with oxygen saturation <90%
REFERENCES
- 1. Prasad B , Nyenhuis SM , Imayama I , Siddiqi A , Teodorescu M . Asthma and obstructive sleep apnea overlap: what has the evidence taught us? Am J Respir Crit Care Med. 2020. ; 201 ( 11 ): 1345 – 1357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies SE , Bishopp A , Wharton S , Turner AM , Mansur AH . The association between asthma and obstructive sleep apnea (OSA): a systematic review . J Asthma. 2018. ; 56 ( 2 ): 118 – 129 . . [DOI] [PubMed] [Google Scholar]
- 3. Kong DL , Qin Z , Shen H , Jin HY , Wang W , Wang ZF . Association of obstructive sleep apnea with asthma: a meta-analysis . Sci Rep. 2017. ; 7 ( 1 ): 4088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sundbom F , Malinovschi A , Lindberg E , Almqvist C , Janson C . Insomnia symptoms and asthma control-Interrelations and importance of comorbidities . Clin Exp Allergy. 2020. ; 50 ( 2 ): 170 – 177 . [DOI] [PubMed] [Google Scholar]
- 5. Greenberg H , Cohen RI . Nocturnal asthma . Curr Opin Pulm Med. 2012. ; 18 ( 1 ): 57 – 62 . [DOI] [PubMed] [Google Scholar]
- 6. Julien JY , Martin JG , Ernst P , et al . Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma . J Allergy Clin Immunol. 2009. ; 124 ( 2 ): 371 – 376 . [DOI] [PubMed] [Google Scholar]
- 7. Sundbom F , Janson C , Malinovschi A , Lindberg E . Effects of coexisting asthma and obstructive sleep apnea on sleep architecture, oxygen saturation, and systemic inflammation in women . J Clin Sleep Med. 2018. ; 14 ( 2 ): 253 – 259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry RB , Gamaldo CE , Harding SM , et al . AASM Scoring Manual Version 2.2 Updates: new chapters for scoring infant sleep staging and home sleep apnea testing . J Clin Sleep Med. 2015. ; 11 ( 11 ): 1253 – 1254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 10. McEvoy RD , Antic NA , Heeley E , et al. SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea . N Engl J Med. 2016. ; 375 ( 10 ): 919 – 931 . [DOI] [PubMed] [Google Scholar]
- 11. Kulkas A , Tiihonen P , Julkunen P , Mervaala E , Töyräs J . Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index . Med Biol Eng Comput. 2013. ; 51 ( 6 ): 697 – 708 . [DOI] [PubMed] [Google Scholar]
- 12. Oldenburg O , Wellmann B , Buchholz A , et al . Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients . Eur Heart J. 2016. ; 37 ( 21 ): 1695 – 1703 . [DOI] [PubMed] [Google Scholar]
- 13. Seo MY , Lee SH , Hong SD , Chung SK , Kim HY . Hypoxemia during sleep and the progression of coronary artery calcium . Cardiovasc Toxicol. 2021. ; 21 ( 1 ): 42 – 48 . [DOI] [PubMed] [Google Scholar]
- 14. Yilmaz Avci A , Avci S , Lakadamyali H , Can U . Hypoxia and inflammation indicate significant differences in the severity of obstructive sleep apnea within similar apnea-hypopnea index groups . Sleep Breath. 2017. ; 21 ( 3 ): 703 – 711 . [DOI] [PubMed] [Google Scholar]
- 15. Azarbarzin A , Sands SA , Stone KL , et al . The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study . Eur Heart J. 2019. ; 40 ( 14 ): 1149 – 1157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Labarca G , Gower J , Lamperti L , Dreyse J , Jorquera J . Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach . Sleep Breath. 2020. ; 24 ( 2 ): 751 – 760 . [DOI] [PubMed] [Google Scholar]
- 17. Kent BD , Mitchell PD , McNicholas WT . Hypoxemia in patients with COPD: cause, effects, and disease progression . Int J Chron Obstruct Pulmon Dis. 2011. ; 6 : 199 – 208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svensson M , Lindberg E , Naessen T , Janson C . Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study . Chest. 2006. ; 129 ( 4 ): 933 – 941 . [DOI] [PubMed] [Google Scholar]
- 19. Theorell-Haglöw J , Berne C , Janson C , Lindberg E . Obstructive sleep apnoea is associated with decreased insulin sensitivity in females . Eur Respir J. 2008. ; 31 ( 5 ): 1054 – 1060 . [DOI] [PubMed] [Google Scholar]
- 20. Ljunggren M , Theorell-Haglöw J , Freyhult E , et al . Association between proteomics and obstructive sleep apnea phenotypes in a community-based cohort of women . J Sleep Res. 2020. ; 29 ( 4 ): e13041 . [DOI] [PubMed] [Google Scholar]
- 21. Lind L , Elmståhl S , Bergman E , et al . EpiHealth: a large population-based cohort study for investigation of gene-lifestyle interactions in the pathogenesis of common diseases . Eur J Epidemiol. 2013. ; 28 ( 2 ): 189 – 197 . [DOI] [PubMed] [Google Scholar]
- 22. BaHammam AS , Obeidat A , Barataman K , Bahammam SA , Olaish AH , Sharif MM . A comparison between the AASM 2012 and 2007 definitions for detecting hypopnea . Sleep Breath . 2014. ; 18 ( 4 ): 767 – 773 . [DOI] [PubMed] [Google Scholar]
- 23. Graham BL , Steenbruggen I , Miller MR , et al . Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement . Am J Respir Crit Care Med. 2019. ; 200 ( 8 ): e70 – e88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sundbom F , Lindberg E , Bjerg A , et al . Asthma symptoms and nasal congestion as independent risk factors for insomnia in a general population: results from the GA(2)LEN survey . Allergy. 2013. ; 68 ( 2 ): 213 – 219 . [DOI] [PubMed] [Google Scholar]
- 25. Bengtsson C , Jonsson L , Holmstrom M , Svensson M , Theorell-Haglow J , Lindberg E . Impact of nasal obstruction on sleep quality: a community-based study of women . Eur Arch Otorhinolaryngol. 2015. ; 272 ( 1 ): 97 – 103 . [DOI] [PubMed] [Google Scholar]
- 26. Bonsignore MR , Pepin JL , Anttalainen U , et al. ESADA Study Group . Clinical presentation of patients with suspected obstructive sleep apnea and self-reported physician-diagnosed asthma in the ESADA cohort . J Sleep Res. 2018. ; 27 ( 6 ): e12729 . [DOI] [PubMed] [Google Scholar]
- 27. Reddel HK , Taylor DR , Bateman ED , et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice . Am J Respir Crit Care Med. 2009. ; 180 ( 1 ): 59 – 99 . [DOI] [PubMed] [Google Scholar]
- 28. Emilsson OI , Sundbom F , Ljunggren M , et al . Association between lung function decline and obstructive sleep apnoea: the ALEC study . Sleep Breath. 2021. ; 25 ( 2 ): 587 – 596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Randerath W , Bassetti CL , Bonsignore MR , et al . Challenges and perspectives in obstructive sleep apnoea: report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society . Eur Respir J. 2018. ; 52 ( 3 ): 1702616 . [DOI] [PubMed] [Google Scholar]
- 30. Arnardottir ES , Bjornsdottir E , Olafsdottir KA , Benediktsdottir B , Gislason T . Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms . Eur Respir J. 2016. ; 47 ( 1 ): 194 – 202 . [DOI] [PubMed] [Google Scholar]
- 31. Lewis CA , Fergusson W , Eaton T , Zeng I , Kolbe J . Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep . Thorax. 2009. ; 64 ( 2 ): 133 – 138 . [DOI] [PubMed] [Google Scholar]
- 32. Marchi NA , Ramponi C , Hirotsu C , et al . Mean oxygen saturation during sleep is related to specific brain atrophy pattern . Ann Neurol. 2020. ; 87 ( 6 ): 921 – 930 . [DOI] [PubMed] [Google Scholar]
- 33. Martinez-Garcia MA , Campos-Rodriguez F , Barbé F , Gozal D , Agustí A . Precision medicine in obstructive sleep apnoea . Lancet Respir Med. 2019. ; 7 ( 5 ): 456 – 464 . [DOI] [PubMed] [Google Scholar]
- 34. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS) . Eur Respir J. 1996. ; 9 ( 4 ): 687 – 695 . [DOI] [PubMed] [Google Scholar]
- 35. Bjerg A , Ekerljung L , Middelveld R , et al . Increased prevalence of symptoms of rhinitis but not of asthma between 1990 and 2008 in Swedish adults: comparisons of the ECRHS and GALEN surveys . PloS One. 2011. ; 6 ( 2 ): e16082 . [DOI] [PMC free article] [PubMed] [Google Scholar]