Abstract
Aims: Achilles tendon (AT) xanthomas are a specific physical finding of familial hypercholesterolemia (FH) and AT thickness has been used for its diagnosis and evaluation of its severity. Recently, we reported that the AT of FH patients was softer than that of non-FH patients and the combined use of a cut-off value for AT softness with that for AT thickness improved diagnostic accuracy. However, an association between AT softness and severity of atherosclerosis has not been reported. Accordingly, the present study aimed to investigate whether AT softness was associated with carotid atherosclerosis and presence of atherosclerotic cardiovascular disease (ASCVD) in FH.
Methods: The AT of 176 genetically diagnosed FH patients and 98 non-FH patients was examined to measure AT thickness and the elasticity index (EI) as an indicator for assessing AT softness using ultrasonography.
Results: Increased age was associated with AT softness, and overweight was negatively related to AT softness. There were significant inverse correlations between EI and maximum and mean intima-media thickness (IMT) within the common carotid artery only among FH patients. In multiple linear regression analysis, although the relationship between EI and mean IMT was attenuated, the association between EI and maximum IMT remained robust. In logistic regression analysis adjusted for age, sex and traditional cardiovascular risk factors (smoking history, presence of hypertension, presence of diabetes mellitus, overweight, LDL-cholesterol, HDL-cholesterol, and Log triglycerides), EI was associated with presence of ASCVD (Odds ratio per 1-SD increase, 0.37; 95% CI, 0.15 – 0.86;P=0.0252).
Conclusion: The degree of lipid deposition in the AT of FH patients could be assessed by its thickness as well as its softness. AT softness is not only useful in diagnosing FH but is also associated with the severity of carotid atherosclerosis and presence of ASCVD. In addition, these findings suggest that AT softness would be helpful in risk assessment for FH patients.
Keywords: Familial hypercholesterolemia, Achilles tendon softness, Ultrasound elastography, Achilles tendon tissue characterization, Atherosclerotic cardiovascular disease
See editorial vol. 29: 1568-1570
Introduction
Familial hypercholesterolemia (FH) is an autosomal dominant hereditary disease due to gene mutations in the low-density lipoprotein (LDL) receptor pathway. FH is characterized by a high plasma LDL cholesterol (LDL-C) concentration, Achilles tendon (AT) thickening, and premature atherosclerotic cardiovascular disease (ASCVD) 1) . Although the frequency of heterozygous FH is high (1 in 200-300) 2) , the diagnosis rate is still low 3) . As we and others have reported that FH is much more common among patients with early-onset acute coronary syndrome 4 - 6) , earlier diagnosis and appropriate treatment for FH is a clinically important issue to be addressed 7) .
AT thickening is the most specific physical finding in the diagnosis of FH. In Japan, radiography has been used for measuring AT thickness 8 - 10) , and ultrasonography has also been used as an imaging modality for its evaluation 11 , 12) . Recently, we set a cut-off value for AT thickness measured by ultrasonography for the diagnosis of FH in Japanese patients and demonstrated that AT thickness measured in this way was not only a diagnostic criterion for FH but also an important predictor for the presence of CAD in FH patients 12) .
On the other hand, there is a challenge in diagnosing patients whose AT thickness is slightly below or borderline with respect to the cut-off value, especially in young adults and women. Therefore, we previously focused on the presence of lipid deposition in the AT of such patients and evaluated AT softness using elastography in 115 FH and 77 non-FH patients to determine a cut-off value for elasticity index (EI) 13) . We also reported that the AT in FH is softer than that in non-FH, and that the thicker the tendon, the softer it is. Moreover, the diagnostic accuracy of FH using combinative assessments of AT thickness and softness resulted in a higher detection rate than with thickness alone (from 72% to 82%) 13) . Therefore, in this previous paper, we concluded that measuring EI in addition to AT thickness may be beneficial for suspected FH patients whose AT thickness is borderline or falls short of the cut-off value due to lipid-lowering treatment or younger age. In addition, based on a comparison of the EI of the AT in 94 FH patients and 38 healthy control patients in a pilot study, Zhang et al. reported that elastography may be effective in diagnosing FH 14) . However, a clear relationship between AT softness and severity of atherosclerosis had not been demonstrated in FH patients.
Although carotid atherosclerosis and coronary artery disease are not necessarily specific to FH, lipid deposition in the AT and atherosclerosis have similar mechanisms 15) . Since AT softness was associated with AT thickness in our previous study, we hypothesized that AT softness is related to the severity of atherosclerosis. The purpose of this study was to determine whether AT softness is associated with the severity of carotid atherosclerosis and presence of ASCVD.
Methods
Patients
Five hundred forty-eight consecutive patients who visited National Cerebral and Cardiovascular Center Hospital to undergo carotid ultrasonography and consented to AT ultrasonography were initially enrolled in this cross-sectional study. They consisted of 450 clinically diagnosed FH patients and 98 consecutive non-FH patients who have diabetes, dyslipidemia not caused by FH, and/or hypertension. Among the FH patients, those with calcification in the AT (N=35), and those with a history of AT injury or damage (N=2) were excluded from the study. DNA analysis was then performed for the LDL receptor (LDLR) gene and/or proprotein convertase subtilisin/kexin 9 (PCSK9) gene as previously reported 16) in 217 patients with clinically diagnosed FH. Among them, true homozygotes or compound heterozygotes for pathogenic LDLR mutations (N=9) and patients with no pathogenic mutations (N=228) were excluded. Thus, patients were defined as FH (N=176) if they had a pathogenic mutation of the LDLR and/or PCSK9 gene in the present study. As a result, the final total number of subjects enrolled by the present study was 274 (176 FH patients and 98 non-FH patients).
This study was performed in conformity with the Helsinki Declaration and approved by the ethics committee of National Cerebral and Cardiovascular Center (Approval No. M17-056 and M25-112). Written informed consent was obtained from all subjects following explanation of the purpose, method, and risks of the study.
Ultrasonography of Carotid Artery
Concurrent with AT measurements, the intima-media thickness (IMT) was also measured as previously reported 12) , using the same measurement equipment. Briefly, the location of the maximum IMT within the common carotid artery was extracted and brought to the center of the screen, and then 3 points, the point itself and 2 points, at 1 cm on both sides, were measured, defining the mean value of the 3 points as the mean IMT. In the event maximum IMT could not be extracted and brought to the center of the screen, 3 points in total including maximum IMT as the starting point were measured to calculate the mean IMT, with a 1 cm interval between points. The mean of the values for the left and right carotid arteries was used as the measurement result for mean IMT, while the greater of the values for the left and right was used for maximum IMT.
Measurement of Achilles Tendon Thickness
Measurement of AT thickness using ultrasonography was performed as previously reported 12) , with blinding to FH or non-FH status. Assessments were carried out by an ultrasound examiner certified by the Japan Society of Ultrasonics in Medicine.
Measurement of Elasticity Index
Carotid IMT, EI and AT thickness were measured concurrently using the same equipment (LOGIQ S8). Measurement of EI was performed as previously reported 13) . The elastography measurement conditions were as follows. Frequency: 6.3 MHz, Line density: 4, soft compress: 5, Hard compress: 7, Velocity: 0.7 cm/s, Noise Reject: 7, Frame Reject: 6. Ankles were flexed at 90° with the upper abdomen positioned on the bed. Observing the guide bar on the screen indicating the degree of compression of the probe, compression was manually applied to the skin with the probe and then relaxed, while referring to previous measurement methods 17 , 18) . We measured the EI when the degree of strain became stable during repeated compression and relaxation. It was calculated as the ratio of the average value of the EI and that in a selected region, within the region of interest (ROI). The ROI was selected so that its upper end was immediately below the probe including the gel pad and the lower end just above the tibial bone. Horizontally, we selected the ROI so that it included the whole image of the AT, traced the cross section of the AT and measured EI ( Fig.1(a) ) . At each examination, a color map from blue (hardest tissue) to red (softest tissue) was produced. The normal ultrasound image of the AT exhibits a multiple linear hyperechoic structure called fibrillar patterns and the AT area is uniformly blue. In FH, as the AT thickens along with lipid accumulation, the fibrillar patterns are no longer observed, and spotty, non-uniform sonolucent areas of local lipid deposition are generally seen.
Fig.1. Quantitative ultrasound images obtained during elastography of Achilles tendon of patients with familial hypercholesterolemia.
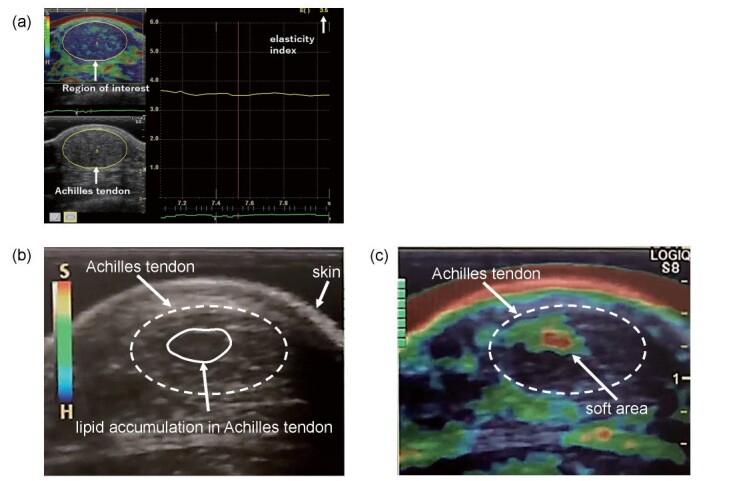
(a) Elastogram of a 44-year-old male patient with familial hypercholesterolemia. His AT thickness was 14.8 mm and EI was 3.5. Region of interest for Achilles tendon is defined as ellipse surrounded by yellow line.
(b) Horizontal sonogram of 24-year-old man with familial hypercholesterolemia. Ellipse surrounded by dotted line indicates Achilles tendon. Low-echoic lesions in Achilles tendon suggest lipid accumulation. His AT thickness was 8.7 mm and EI was 4.0.
(c) Elastogram of 24-year-old man with familial hypercholesterolemia (same patient as in Fig. 1(b)). A color map from blue (hardest tissue) to red (softest tissue) was produced in the strain elastogram. Red (softest tissue) was observed in the low-echoic area in B-mode (Fig. 1(b)).
In Fig.1 , the large low echoic area ( Fig.1(b) ) and large red area fringed with yellow and green colors ( Fig.1(c) ) observed in the AT of a male patient suggest large lipid deposition.
Clinical and Laboratory Characteristics
Hypertension was defined as use of anti-hypertensive drugs or blood pressure more than 140 mmHg systolic or more than 90 mmHg diastolic, or both, at the time of AT ultrasonography. Fasting blood glucose and lipid profile were measured by standard methods. Diabetes was defined as history of diabetes, use of any anti-diabetic medication or presence of a fasting blood glucose ≥ 126 mg/dl. Overweight was defined as a body mass index (BMI) ≥ 25 kg/m2. Patients with current smoking or past smoking were defined as subjects with smoking history. ASCVD was defined as presence of any of the following: (1) myocardial infarction proven by electrocardiogram abnormalities and enzyme changes, (2) diagnosis of angina pectoris with significant stenosis ≥ 75% on cardio-angiogram, (3) coronary bypass surgery or percutaneous coronary interventions, and (4) symptomatic ischemic stroke.
Statistical Analyses
Single regression analysis was used to analyze relationships between AT measurements and carotid IMT, while Pearson’s correlation coefficients were used for the correlation analysis. The t-test or Mann-Whitney test was used to compare continuous variables between two groups, while ANOVA was used for comparisons among multiple groups. Pearson’s chi-squared test was used for comparison of categorical variables. In the present study, the statistical significance level was set at p<0.05 and JMP (version14.0) was used for the analyses.
Results
Clinical Characteristics of Study Subjects
The clinical characteristics of the study subjects are shown in Table 1 . The age of patients ranged from 20 to 87 years in the FH group and from 22 to 82 years in the non-FH group. BMI, triglycerides (TG), and high-density lipoprotein (HDL) cholesterol were significantly higher in the non-FH group than in the FH group. On the other hand, LDL-C levels, prevalence of ASCVD and mean IMT in carotid arteries were significantly greater in the FH group compared to the non-FH group. The maximum IMT in the carotid artery was comparable between FH and non-FH. Since the mean age was significantly higher in non-FH, the above results suggested that the speed of carotid atherosclerosis progression was faster in FH.
Table 1. Clinical characteristics of patients with FH and non-FH patients.
| FH (N = 176) | Non-FH (N = 98) | p value | |
|---|---|---|---|
| Female sex, n (%) | 106 (60) | 59 (60) | 0.968 |
| Age, years | 50 ± 17 | 58 ± 15 | <0.001 |
| Body mass index, kg/m2 | 22.4 ± 3.1 | 23.2 ± 3.2 | 0.037 |
| Total cholesterol, mg/dl | 228 ± 65 | 213 ± 51 | 0.060 |
| LDL-cholesterol, mg/dl | 154 ± 59 | 127 ± 44 | <0.001 |
| HDL-cholesterol, mg/dl | 54 ± 15 | 58 ± 14 | 0.032 |
| Triglycerides, mg/dl | 83 (61 – 122) | 116 (81 – 176) | 0.005 |
| Overweight, n (%) | 33 (19) | 29 (30) | 0.042 |
| Hypertension, n (%) | 43 (24) | 36 (37) | 0.017 |
| Diabetes mellitus, n (%) | 21 (12) | 28 (29) | 0.002 |
| Smoking history, n (%) | 74 (42) | 30 (31) | 0.084 |
| ASCVD, n (%) | 42 (24) | 4 (4) | <0.001 |
| mean IMT, mm | 0.8 (0.6 – 1.1) | 0.7 (0.6-0.9) | 0.046 |
| maximum IMT, mm | 0.9 (0.6 – 1.4) | 0.9 (0.7 – 1.3) | 0.084 |
Values are presented as mean±standard deviation or median (interquartile range 25% - 75%) or number. FH, familial hypercholesterolemia; LDL, low-density lipoprotein; HDL, high density lipoprotein; ASCVD, atherosclerotic cardiovascular disease, and IMT, intima-media thickness.
Table 2 shows mean values of AT thickness and EI in both the FH and non-FH groups. AT thickness was greater and EI was lower in the FH group compared to the non-FH group, indicating that the AT of FH patients was thicker and softer than that of non-FH patients. The AT of males was significantly thicker than that of females for both the FH (P=0.048) and non-FH (P=0.004) groups. However, the mean EI was similar in men and women in both groups (male vs. female, P=0.196 in FH group and P=0.725 in non-FH group).
Table 2. Mean values of Achilles tendon thickness and elasticity index.
| FH | Non-FH | p value | |
|---|---|---|---|
| N (Male/Female) | 176 (70/106) | 98 (39/59) | |
| EI | |||
| Total | 4.4 ± 0.6 | 5.0 ± 0.3 | <0.001 |
| Male | 4.3 ± 0.6 | 5.0 ± 0.3 | <0.001 |
| Female | 4.5 ± 0.6 | 5.0 ± 0.3 | <0.001 |
| AT thickness, mm | |||
| Total | 7.9 ± 3.6 | 4.9 ± 0.5 | <0.001 |
| Male | 8.7 ± 4.6 | 5.1 ± 0.4 | <0.001 |
| Female | 7.3 ± 2.6 | 4.8 ± 0.5 | <0.001 |
Values are presented as mean±standard deviation.
FH, familial hypercholesterolemia; AT, Achilles tendon; and EI, elasticity index.
Determinants of AT Softness in FH Patients
We conducted a linear regression analysis with traditional cardiovascular risk factors (age, sex, overweight (BMI >25), LDL-cholesterol, HDL-cholesterol, Log triglycerides, and smoking history) to find determinants of the EI among the FH group. As shown in Table 3 , increased age was associated with AT softness (β=-0.007; 95% CI, -0.014 to -0.0009; P=0.0243). Interestingly, overweight was negatively related to AT softness (β=0.28; 95% CI, 0.038 to 0.52; P=0.0237).
Table 3. Multiple linear regression analysis for independent determinants of EI in FH patients (n = 176) .
| beta coefficient | 95% CI | P value | |
|---|---|---|---|
| Age | -0.073 | -0.014 – -0.00095 | 0.0243 |
| Female sex | -0.015 | -0.13 – 0.10 | 0.7994 |
| Overweight | 0.28 | 0.038 – 0.52 | 0.0237 |
| Smoking history | -0.096 | -0.20 – 0.012 | 0.0824 |
| Hypertension | -0.11 | -0.34 – 0.12 | 0.3398 |
| Diabetes mellitus | 0.14 | -0.16 – 0.44 | 0.3656 |
| LDL-cholesterol | -0.000065 | -0.0017 – 0.0017 | 0.9453 |
| HDL-cholesterol | 0.0034 | -0.0041 – 0.011 | 0.3676 |
| Log Triglycerides | 0.085 | -0.10 – 0.27 | 0.3797 |
EI, elasticity index; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein.
Association between AT Softness and Carotid IMT
Fig.2 shows the correlations between EI and maximum IMT in common carotid arteries among non-FH patients ((b)) and among FH patients ((d)), alongside correlations of EI with AT thickness (ATT) in non-FH patients (a) and FH patients (c). There were significant inverse correlations between EI and maximum IMT (r=-0.38, P<0.001) and between EI and mean IMT (r=-0.22, P=0.014) in common carotid arteries only among FH patients.
Fig.2. Correlation between elasticity index and Achilles tendon thickness ((a), (c)) as well as carotid atherosclerosis ((b), (d)).
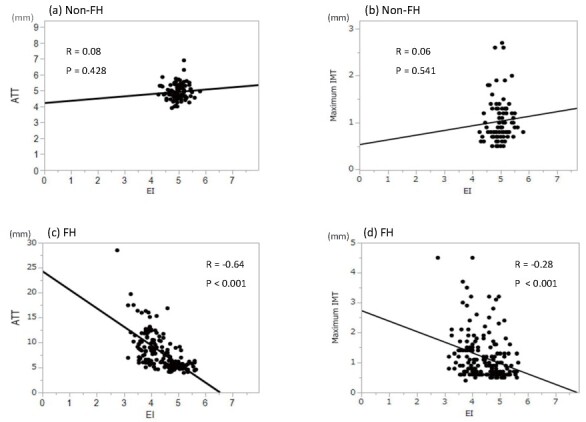
Scatter plots show associations between EI and AT thickness among non-FH patients (a) and among FH patients (c). In addition, they show associations between EI and carotid atherosclerosis among non-FH patients (b) and among FH patients (d).
FH, familial hypercholesterolemia; AT, Achilles tendon; EI, elasticity index; max-BI, maximum IMT within the bifurcation area to the internal carotid artery.
A multivariate linear regression analysis adjusted for age, sex and traditional cardiovascular risk factors (smoking history [current and past], presence of hypertension, presence of diabetes mellitus, overweight, LDL-cholesterol, HDL-cholesterol, and Log triglycerides) also showed a negative association between EI and maximum IMT ( Table 4 ) . On the other hand, the negative associations between EI and mean IMT were attenuated in multivariate analyses ( Supplemental Table 1 ) .
Table 4. Multiple linear regression analysis for independent determinants of maximum IMT in common carotid arteries among FH patients (n = 176) .
| beta coefficient | 95% CI | P value | |
|---|---|---|---|
| Age | 0.022 | 0.016 – 0.028 | <0.0001 |
| Female sex | 0.097 | -0.016 – 0.21 | 0.0916 |
| Body mass index | 0.011 | -0.019 – 0.043 | 0.4580 |
| Smoking history | 0.043 | -0.063 – 0.15 | 0.4289 |
| Hypertension | 0.11 | -0.12 – 0.33 | 0.3416 |
| Diabetes mellitus | 0.057 | -0.24 – 0.36 | 0.7089 |
| LDL-cholesterol | -0.00039 | -0.0021 – 0.0013 | 0.6453 |
| HDL-cholesterol | -0.0073 | -0.015 – 0.000039 | 0.0512 |
| Log Triglycerides | -0.0069 | -0.19 – 0.18 | 0.9423 |
| Elasticity index | -0.16 | -0.31 – -0.010 | 0.0366 |
IMT, intima-media thickness; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein.
Supplemental Table 1. Multiple linear regression analysis for independent determinants of mean IMT in common carotid arteries among FH patients (n = 176) .
| beta coefficient | 95% CI | P value | |
|---|---|---|---|
| Age | 0.013 | 0.0098 – 0.016 | <0.0001 |
| Female sex | 0.035 | -0.024 – 0.095 | 0.2425 |
| Body mass index | 0.015 | -0.0019 – 0.031 | 0.0827 |
| Smoking history | 0.019 | -0.036 – 0.075 | 0.4948 |
| Hypertension | 0.016 | -0.10 – 0.13 | 0.7866 |
| Diabetes mellitus | 0.076 | -0.082 – 0.23 | 0.3423 |
| LDL-cholesterol | 0.00019 | -0.00069 – 0.0011 | 0.6453 |
| HDL-cholesterol | -0.0051 | -0.0089 – 0.0012 | 0.0099 |
| Log Triglycerides | -0.0071 | -0.11 – 0.092 | 0.8871 |
| Elasticity index | -0.041 | -0.12 – 0.037 | 0.3050 |
IMT, intima-media thickness; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein.
In a logistic regression analysis adjusted for age, sex and traditional cardiovascular risk factors (smoking history [current and past], presence of hypertension, presence of diabetes mellitus, overweight, LDL-cholesterol, HDL-cholesterol, and Log triglycerides), EI was associated with presence of ASCVD (Odds ratio per 1-SD increase, 0.37; 95% CI, 0.15 – 0.86; P=0.0252) ( Table 5 ) , suggesting that EI is associated with both subclinical carotid vascular disease and ASCVD in FH. Among non-FH, no relationship between EI and ASCVD was found in a univariate analysis (4.86±0.10 for ASCVD (+) and 4.98±0.29 for ASCVD (-), P=0.4142). Since there were only a small number of patients with ASCVD in non-FH (N=4), we were unable to provide results for multivariate analysis. However, the EI values of all four patients with ASCVD were greater than the cutoff value (4.7 for both sexes) set in our previous paper 13) .
Table 5. Multiple logistic regression analysis for independent determinants of presence of ASCVD in FH patients (n = 176) .
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | 1.05 | 1.02 – 1.10 | 0.0027 |
| Female sex | 0.47 | 0.12 – 1.70 | 0.2497 |
| Overweight | 0.91 | 0.26 – 3.02 | 0.8736 |
| Smoking history | 1.90 | 0.55 – 6.54 | 0.3048 |
| Hypertension | 2.91 | 1.06 – 8.25 | 0.0382 |
| Diabetes mellitus | 0.73 | 0.19 – 2.68 | 0.6422 |
| LDL-cholesterol | 0.98 | 0.97 – 0.99 | 0.0033 |
| HDL-cholesterol | 0.94 | 0.89 – 0.98 | 0.0035 |
| Log Triglycerides | 1.70 | 0.64 – 4.75 | 0.2825 |
| Elasticity index | 0.37 | 0.15 – 0.86 | 0.0252 |
ASCVD, atherosclerotic cardiovascular disease; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein.
Discussion
In this study, we found that AT softness evaluated by elastography was associated with the severity of subclinical carotid vascular disease (increase in IMT) and presence of ASCVD in FH patients. Since we previously reported that the use of AT softness together with AT thickness improves the diagnostic accuracy of FH, AT softness may be a new tool not only for diagnosing FH but also for assessing cardiovascular risk in FH.
In FH patients, increased age was independently associated with AT softness ( Table 3 ) . It was also independently associated with AT thickness (β=0.052; 95% CI, 0.016 to 0.087; P=0.0047, Supplemental Table 2 ) in multivariate analysis. In addition, AT thickness was associated with AT softness in univariate analysis ( Fig.2(C) ) and in multivariate analysis (β=-0.11; 95% CI, -0.13 to -0.08; P<0.0001, Supplemental Table 3 ). Therefore, we speculate that the association between age and EI mirrors that between age and AT thickness.
Supplemental Table 2. Multiple linear regression analysis for independent determinants of AT thickness in FH patients (n = 176) .
| beta coefficient | 95% CI | P value | |
|---|---|---|---|
| Age | 0.052 | 0.016 – 0.087 | 0.0047 |
| Female sex | 0.31 | -0.35 – 0.97 | 0.3484 |
| Overweight | -0.78 | -2.2 – 0.60 | 0.2673 |
| Smoking history | 0.51 | -0.10 – 1.12 | 0.1027 |
| Hypertension | 0.44 | -084 – 1.72 | 0.5009 |
| Diabetes mellitus | -0.79 | -2.49 – 0.91 | 0.3597 |
| LDL-cholesterol | 0.0039 | -0.0058 – 0.014 | 0.4273 |
| HDL-cholesterol | -0.041 | -0.083 – 0.0012 | 0.0567 |
| Log Triglycerides | -0.47 | -1.54 – 0.61 | 0.3931 |
AT, Achilles tendon; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein.
Supplemental Table 3. Multiple linear regression analysis for independent determinants of EI in FH patients (n = 176) .
| beta coefficient | 95% CI | P value | |
|---|---|---|---|
| Age | -0.0017 | -0.0069 – 0.0034 | 0.5096 |
| Female sex | 0.018 | -0.075 – 0.11 | 0.6970 |
| Overweight | 0.20 | 0.0033 – 0.39 | 0.0463 |
| Smoking history | -0.042 | -0.13 – 0.046 | 0.3489 |
| Hypertension | -0.063 | -0.24 – 0.12 | 0.4931 |
| Diabetes mellitus | 0.054 | -0.19 – 0.29 | 0.6603 |
| LDL-cholesterol | 0.00036 | -0.0010 – 0.0017 | 0.6084 |
| HDL-cholesterol | -0.00097 | -0.0070 – 0.0051 | 0.7538 |
| Log Triglycerides | 0.035 | -0.12 – 0.19 | 0.6501 |
| AT thickness | -0.11 | -0.13 – 0.08 | <0.0001 |
EI, elasticity index; FH, familial hypercholesterolemia; LDL, low-density lipoprotein; and HDL, high density lipoprotein; AT, Achilles tendon.
Interestingly, overweight was negatively associated with AT softness in FH ( Table 3 ) . In overweight patients, the AT is located deeper below the skin due to thicker subcutaneous fat. Therefore, in these patients, we speculate that the AT may be less susceptible to strain caused by the probe as it is farther from the skin. Sun et al. reported that the thicker the subcutaneous fat, the more excellent was the reproducibility of shear wave elastography-based quantitative assessment for skin elasticity 19) . In other words, thin patients may be more susceptible to strain from the probe. Although there is no concern about inter-observer reproducibility because only one ultrasound examiner measured the EI in this study, further studies are needed to examine whether EI values should be corrected for subcutaneous fat thickness or BMI. Furthermore, as the mean BMI of non-FH patients in this study was larger than that of FH patients ( Table 1 ) , the possibility of overestimation of the EI in non-FH patients should be considered. However, the association between EI and BMI in non-FH patients was not significant in either univariate (r=0.05, P=0.5970) or multivariate analysis (β=-0.0012; 95% CI, -0.023 to -0.021; P=0.9165).
A significant relationship between AT softness and the severity of carotid IMT was observed only in the FH group ( Fig.2 (d) ) . In addition, softer AT was associated with increased risk of ASCVD even after adjusting for traditional cardiovascular risk factors in FH patients ( Table 5 ) . Furthermore, we previously reported that AT thickness was positively related to the severity of carotid IMT only in FH 12) and several other studies demonstrated that AT thickness was associated with presence of ASCVD 20 - 23) . Since EI was negatively associated with AT thickness in FH, we speculate that common mechanisms underlie the development of both xanthomas and atherosclerosis and that the EI may reflect lipid accumulation not only in the AT but also in blood vessels.
Another interesting finding of the current study was that AT softness was associated with maximal IMT, but not with mean IMT in multivariate analyses ( Table 4 , Supplemental Table 1 ) . In patients with type 2 diabetes, maximum IMT has been reported to be more important in relation to presence of ASCVD than mean IMT 24 , 25) . In addition, the results of a recent study showed that, compared with mean IMT, maximum IMT was also more strongly associated with ASCVD in FH patients 22) . Therefore, we speculated that AT softness was more strongly associated with maximum IMT than mean IMT due to the greater importance of the former in the relationship with ASCVD. In addition, since the maximum IMT in this study included plaque, we believe that examining the relationship between the characteristics and location of carotid plaque and the AT softness is an interesting issue for future research.
Study Limitations
This study has several limitations. First, a single ultrasound examiner carried out the measurements using the same acoustic coupler and ultrasound machine, which may have affected the reproducibility of the results. Nevertheless, in the clinical setting, we believe that it is useful to measure carotid IMT, AT thickness and EI using the same equipment because this enables us to diagnose FH and assess the risk of atherosclerosis at the same time. Second, since the design of this study was a cross-sectional nature, it was not possible to show a causal relationship between the changes in the EI and the development of atherosclerosis. Changes in Achilles tendon thickness and EI in individuals and how EI is altered by lipid-lowering therapy are interesting topics for future research. Third, we excluded patients who had calcification in the AT based on the presence of an acoustic shadow so effects of acoustic shadows on the EI should be investigated in the future. Forth, our data were obtained in a Japanese population making it necessary to investigate the EI in other ethnic groups in the future. Finally, LDL-C levels before starting lipid-lowering treatment and the length of time that patients had remained untreated were not evaluated. Therefore, as a determinant of EI, the influence of LDL-C levels may have been underestimated because current levels do not reflect those before starting treatment or the length of time that patients went untreated. However, as we speculate that EI may be affected by the magnitude and duration of high LDL-C levels, our findings suggest that early diagnosis using AT thickness and EI as well as adequate treatment are important for preventing the development of atherosclerosis in FH patients.
Conclusions
The present study demonstrated that AT softness was associated with atherosclerosis among FH patients.
Acknowledgement
We express our special thanks to Mr. Naotaka Ohta, Mr. Suguru Yamamoto and Ms. Rieko Isoda for their outstanding technical assistance in genetic testing. We sincerely thank Mr. Kenji Furuta and Ms. Chizuru Fuke for excellent assistance with the sonographic studies.
Funding
This study was supported by a grant from Health, Labor and Welfare Sciences Research Grants for Research on Rare and Intractable Diseases (Committee on Primary Dyslipidemia, 21FC1009) and one from the Ministry of Education, Culture, Sports, Science and Technology: KAKENHI Grant Number 19H00455.
Conflicts of Interest
Masahito Michikura is the representative director of Kansai Ultrasound Service Co. Ltd. Masatsune Ogura receives lecture fees from Amgen, Astellas Pharma Inc, and Kowa. Mika Hori, Kota Matsuki, Hisashi Makino, and Kiminori Hosoda has nothing to disclose. Mariko Harada-Shiba holds stocks of Liid Pharma, receives lecture fees from Amgen, Astellas Pharma Inc, Sanofi, receives scholarship grants from Aegerion, Recordati, and Kaneka.
References
- 1).Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD and Wierzbicki AS: Familial hypercholesterolaemia. Nat Rev Dis Primers, 2017; 3: 17093 [DOI] [PubMed] [Google Scholar]
- 2).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J and Hokuriku FHSG: Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 3).Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR and Tybjaerg-Hansen A: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Harada-Shiba M, Ako J, Arai H, Hirayama A, Murakami Y, Nohara A, Ozaki A, Uno K and Nakamura M: Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis, 2018; 277: 362-368 [DOI] [PubMed] [Google Scholar]
- 5).Ohmura H, Fukushima Y, Mizuno A, Niwa K, Kobayashi Y, Ebina T, Kimura K, Ishibashi S, Daida H, Research Committee on Primary Hyperlipidemia of the Ministry of H and Welfare of J: Estimated Prevalence of Heterozygous Familial Hypercholesterolemia in Patients With Acute Coronary Syndrome. Int Heart J, 2017; 58: 88-94 [DOI] [PubMed] [Google Scholar]
- 6).Nanchen D, Gencer B, Auer R, Raber L, Stefanini GG, Klingenberg R, Schmied CM, Cornuz J, Muller O, Vogt P, Juni P, Matter CM, Windecker S, Luscher TF, Mach F and Rodondi N: Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J, 2016: 36: 2438-2445 [DOI] [PubMed] [Google Scholar]
- 7).Representatives of the Global Familial Hypercholesterolemia C, Wilemon KA, Patel J, Aguilar-Salinas C, Ahmed CD, Alkhnifsawi M, Almahmeed W, Alonso R, Al-Rasadi K, Badimon L, Bernal LM, Bogsrud MP, Braun LT, Brunham L, Catapano AL, Cillikova K, Corral P, Cuevas R, Defesche JC, Descamps OS, de Ferranti S, Eisele JL, Elikir G, Folco E, Freiberger T, Fuggetta F, Gaspar IM, Gesztes AG, Groselj U, Hamilton-Craig I, Hanauer-Mader G, Harada-Shiba M, Hastings G, Hovingh GK, Izar MC, Jamison A, Karlsson GN, Kayikcioglu M, Koob S, Koseki M, Lane S, Lima-Martinez MM, Lopez G, Martinez TL, Marais D, Marion L, Mata P, Maurina I, Maxwell D, Mehta R, Mensah GA, Miserez AR, Neely D, Nicholls SJ, Nohara A, Nordestgaard BG, Ose L, Pallidis A, Pang J, Payne J, Peterson AL, Popescu MP, Puri R, Ray KK, Reda A, Sampietro T, Santos RD, Schalkers I, Schreier L, Shapiro MD, Sijbrands E, Soffer D, Stefanutti C, Stoll M, Sy RG, Tamayo ML, Tilney MK, Tokgozoglu L, Tomlinson B, Vallejo-Vaz AJ, Vazquez-Cardenas A, de Luca PV, Wald DS, Watts GF, Wenger NK, Wolf M, Wood D, Zegerius A, Gaziano TA and Gidding SS: Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol, 2020; 5: 217-229 [DOI] [PubMed] [Google Scholar]
- 8).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K and Working Group by Japan Atherosclerosis Society for Making Guidance of Familial H: Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Mabuchi H, Ito S, Haba T, Ueda K and Ueda R: Discrimination of familial hypercholesterolemia and secondary hypercholesterolemia by Achilles’ tendon thickness. Atherosclerosis, 1977; 28: 61-68 [DOI] [PubMed] [Google Scholar]
- 10).Tada H, Hori M, Matsuki K, Ogura M, Nohara A, Kawashiri M, and Harada-Shiba M: Achilles Tendon Thickness Assessed by X-ray Predicting a Pathogenic Mutation in Familial Hypercholesterolemia Gene. J Atheroscler Thromb, 2022; 29: 816-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP and Elisaf MS: Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest, 2005; 35: 236-244 [DOI] [PubMed] [Google Scholar]
- 12).Michikura M, Ogura M, Yamamoto M, Sekimoto M, Fuke C, Hori M, Arai K, Kihara S, Hosoda K, Yanagi K and Harada-Shiba M: Achilles Tendon Ultrasonography for Diagnosis of Familial Hypercholesterolemia Among Japanese Subjects. Circ J, 2017; 81: 1879-1885 [DOI] [PubMed] [Google Scholar]
- 13).Michikura M, Ogura M, Hori M, Furuta K, Hosoda K and Harada-Shiba M: Achilles Tendon Softness as a New Tool for Diagnosing Familial Hypercholesterolemia. JACC Cardiovasc Imaging, 2021; 17: 1483-1485 [DOI] [PubMed] [Google Scholar]
- 14).Zhang L, Yong Q, Pu T, Zheng C, Wang M, Shi S and Li L: Grayscale ultrasonic and shear wave elastographic characteristics of the Achilles’ tendon in patients with familial hypercholesterolemia: A pilot study. Eur J Radiol, 2018; 109: 1-7 [DOI] [PubMed] [Google Scholar]
- 15).Sugiyama N, Marcovina S, Gown AM, Seftel H, Joffe B and Chait A: Immunohistochemical distribution of lipoprotein epitopes in xanthomata from patients with familial hypercholesterolemia. Am J Pathol, 1992; 141: 99-106 [PMC free article] [PubMed] [Google Scholar]
- 16).Hori M, Ohta N, Takahashi A, Masuda H, Isoda R, Yamamoto S, Son C, Ogura M, Hosoda K, Miyamoto Y and Harada-Shiba M: Impact of LDLR and PCSK9 pathogenic variants in Japanese heterozygous familial hypercholesterolemia patients. Atherosclerosis, 2019; 289: 101-108 [DOI] [PubMed] [Google Scholar]
- 17).Schneebeli A, Del Grande F, Vincenzo G, et al.: Real-time ultrasound elastography using an external reference material: test-retest reliability of healthy Achilles tendons. Skeletal Radiol, 2016; 45(8): 1045-1052 [DOI] [PubMed] [Google Scholar]
- 18).Foure A: New Imaging Methods for Non-invasive Assessment of Mechanical, Structural, and Biochemical Properties of Human Achilles Tendon: A Mini Review. Front Physiol, 2016; 7: 324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Sun Y, Ma C, Liang X, Wang R, Fu Y, Wang S, Cui L and Zhang C: Reproducibility analysis on shear wave elastography (SWE)-based quantitative assessment for skin elasticity. Medicine (Baltimore), 2017; 96: e6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Sugisawa T, Okamura T, Makino H, Watanabe M, Kishimoto I, Miyamoto Y, Iwamoto N, Yamamoto A, Yokoyama S, Harada-Shiba M: Defining patients at extremely high risk for coronary artery disease in heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 369-375 [DOI] [PubMed] [Google Scholar]
- 21).Ogura M, Hori M and Harada-Shiba M: Association between cholesterol efflux capacity and atherosclerotic cardiovascular disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol, 36: 181-188 [DOI] [PubMed] [Google Scholar]
- 22).Ogura M, Harada-Shiba M, Masuda D, Arai H, Bujo H, Ishibashi S, Daida H, Koga N, Oikawa S, Yamashita S, FAME Study Group: Factors Associated with Carotid Atherosclerosis and Achilles Tendon Thickness in Japanese Patients with Familial Hypercholesterolemia: A Subanalysis of the Familial Hypercholesterolemia Expert Forum (FAME) Study. J Atheroscler Thromb, 2022; 29: 906-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H and Yamagishi M: Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J, 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 24).Irie Y, Katakami N, Kaneto H, Kasami R, Sumitsuji S, Yamasaki K, Tachibana K, Kuroda T, Sakamoto K, Umayahara Y, Ueda Y, Kosugi K and Shimomura I: Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis, 2012; 221: 438-444 [DOI] [PubMed] [Google Scholar]
- 25).Fujihara K, Suzuki H, Sato A, Ishizu T, Kodama S, Heianza Y, Saito K, Iwasaki H, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Sone H and Shimano H: Comparison of the Framingham risk score, UK Prospective Diabetes Study (UKPDS) Risk Engine, Japanese Atherosclerosis Longitudinal Study-Existing Cohorts Combine (JALS-ECC) and maximum carotid intima-media thickness for predicting coronary artery stenosis in patients with asymptomatic type 2 diabetes. J Atheroscler Thromb, 2014; 21: 799-815 [DOI] [PubMed] [Google Scholar]


