Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has influenced antibiotic consumption over a long period, with variability in trends among studies. We conducted this systematic review to explore and compare the effect of the pandemic on overall and individual antibiotic consumption in 2020 with that in 2019.
Methods
This systematic literature review was conducted using PubMed, EMBASE, and Web of Science databases. Data on antibiotic consumption in Japan was sourced from the Japan Surveillance of Antimicrobial Consumption.
Results
A total of 1,442 articles and reports were screened, and 16 eligible articles were reviewed. The included studies were conducted in Jordan, Australia, Canada, UK, Japan, Brazil, India, China, and the EU. There was no study from African and Southeast Asian Countries. Overall, antibiotic consumption in the community consistently reduced in 2020. Studies from Australia, Canada, Portugal, Spain, the UK, Japan, and the European Union reported both decreases in overall and selected individual antibiotics consumption. In contrast, hospital-based studies reported both increases and decreases. Hospital-based studies in Lebanon, Spain, Italy, India, and the UK reported an increase in antibiotic consumption in 2020. Studies reporting an interruption of antibiotic stewardship programs during the pandemic also reported increases in antibiotic consumption for hospitalized patients in 2020 compared with that in 2019.
Conclusion
Our results showed a different trend between communities and hospitals in antibiotic consumption during 2020 compared to 2019. The continuity of the antibiotic stewardship program might have influenced the antibiotic consumption trend variability among hospitals in 2020. Alongside this, the lack of information on antibiotic consumption from low-income countries and limited reports from middle-income countries revealed gaps that need to be urgently filled.
Keywords: antibiotic consumption, COVID-19, antibiotic stewardship, systematic review, hospital, community
Introduction
Antimicrobial-resistant (AMR) bacterial infections—which cause longer hospital stays and higher treatment costs and mortality rates—have been regarded as one of the biggest global health issues (1, 2). Death caused by or associated with AMR bacterial infection has been reported to be the highest in sub-Saharan African countries (1). The overuse of antibiotics can accelerate the emergence of AMR (2). Thus, a key element in AMR control is antibiotic stewardship programs developed to prevent antibiotic over prescription and overuse (3, 4).
The coronavirus disease (COVID-19) pandemic has impacted many aspects of our daily lives, including economics (5), social healthcare (6), and healthcare services (7, 8). A number of studies have reported an immediate increase in antibiotic use during the first wave of COVID-19 (9–11). This was partly because of 1) the treatment of bacterial coinfection among COVID-19 patients (10, 12) and 2) the extra workload for infectious disease professionals, resulting in the interruption of antibiotic stewardship programs (13). However, following the initial increase in antibiotic consumption, a reduction in antibiotic consumption has also been reported (14). Recent publications on yearly antibiotic consumption in 2020 provided us with an opportunity to conduct this systematic review. The COVID-19 pandemic and its potential effect on antibiotic consumption have been reported (Table 1). However, these potential factors that might affect antibiotic consumption could have an opposite effect. Therefore, this study aimed to review the current reported trend of change in antibiotic consumption from 2019 to 2020 during the COVID-19 pandemic and explore differences in the target population (hospital inpatients and community).
Table 1.
Summary of potential effect of the COVID-19 pandemic on health systems, society, and personal attitude/knowledge that could influence antibiotic consumption (increase or decrease).
| Target | Potential effect of COVID-19 on antibiotic consumption | Direction of impact | Refs. |
|---|---|---|---|
| Community | City/country lockdown, school closure, state of emergency | Decrease | (19, 31, 32) |
| Reducing people-people contact could reduce the opportunity of infectious disease transmission in society. | |||
| Individual | The hesitation of visiting hospitals | (40) | |
| - People with mild symptoms avoid visiting hospitals | Decrease | ||
| - Impatient might have more severe conditions | Increase | ||
| Individual | The general public's increased knowledge about infectious disease transmission and application of preventive measures such as hand washing, wearing masks, and maintaining social distancing. | Decrease | (41) |
| Hospitals | Interruption or reduction of antibiotic stewardship program owing to the extra workload for health professionals to react to the COVID-19 pandemic | Increase | (13, 14, 25) |
| Hospitals | Extra enhanced hand hygiene/environmental cleaning | Decrease | (42) |
Materials and methods
Systematic review
An electronic literature search of the PubMed, Web of Science, and EMBASE databases was conducted. The search terms used were (antibiotic OR antimicrobial) AND consumption AND (COVID-19 OR SARS-CoV-2). The antibiotic consumption in Japan and Japanese hospitals were identified using data from the Japan Surveillance of Antimicrobial Consumption (JSAC) database (https://amrcrc.ncgm.go.jp/surveillance/Surveillance_en.html) (15). The search was completed on the February 8, 2022. Duplicate articles were removed, after which titles and abstracts were reviewed. Non-English articles were included in the study, and non-English articles were translated into English for consideration using DeepL (DeepL.com). The entire procedure of the systematic review was conducted by two independent reviewers: MF and NNH.
A study was considered eligible if it met all the following inclusion criteria: 1) involved human participants; 2) reported annual overall antibiotic consumption in 2019 and 2020; 3) quantified antibiotic consumption at a hospital or hospital department or area or country level; and 4) controlled number of inpatients or admissions when they reported hospital-based consumption. A study was considered ineligible if it met any of the following exclusion criteria: 1) only involved non-human subjects; 2) only reported quantified antibiotic consumption among specially selected study participants based on their health conditions (e.g., COVID-19, HIV, diabetes); 3) indirect measurements to quantify antibiotic consumption (e.g., questionnaire, detection in the wastewater); 4) failed to report the measuring unit of antibiotic consumption; 5) hospital-based study that did not control the number of patients when they reported the quantity of antibiotic consumption; and 6) failed to report the quantity of total antibiotic consumption, 7) case reports, review, opinion, and/or study protocol.
Antibiotic consumption, prescription, and dispensation
The most accurate index that reflected antibiotic use among the targeted populations was antibiotic consumption. However, some studies used antibiotic prescription or dispensation as a proxy of consumption because of the difficulty in accurately measuring antibiotic consumption at the community level. Therefore, community-based studies reporting antibiotic prescriptions and/or dispensations were also included.
Study period
The main objective of this study was to explore trends in antibiotic consumption over a long period during the COVID-19 pandemic. Therefore, studies that reported the annual consumption of antibiotics in both 2019 and 2020 were initially considered in this review. In addition, studies that reported a quasi-annual consumption (i.e., antibiotic consumption up to October 2020) were included in this review. Studies that exclusively focused on antibiotic consumption during the first wave of the COVID-19 pandemic were excluded.
Measurement of change in antibiotic consumption quantity
Several studies reported the statistical significance of the change in the quantity of antibiotic consumption during the study years. In this case, their categorization of the trend of this change was followed, namely an increase, no significant change, or a decrease. In contrast, some studies reported only the total quantity of annual antibiotic consumption without any statistical analysis. In this case, their categorization of the trend was followed, namely a ≥10% change between 2019 and 2020. These studies were classified into increase or decrease categories. The remaining studies were classified as the no-change category.
Quality assurance of studies
Quality assurance of the studies was conducted using a graded scale. The seven criteria were considered in the graded scale: 1) source of antibiotic consumption data was described; 2) antibiotics considered in the study were reported; 3) study period was reported, 4) pre-COVID-19 pandemic antibiotic consumption quantity was reported; 5) antibiotic consumption quantity during the COVID-19 pandemic (or in 2020) was reported; 6) antibiotic consumption quantity was controlled by the number of inpatients and/or inhabitants; and 7) antibiotic consumption quantity was controlled by the number of days of treatment. The scores of the seven criteria were added, and the quality of the study was categorized as follows: low, 0–2; medium, 3–5; and high, 6–7. Based on these criteria, there were no low-quality studies (Supplementary material 1).
Results
Systematic review results
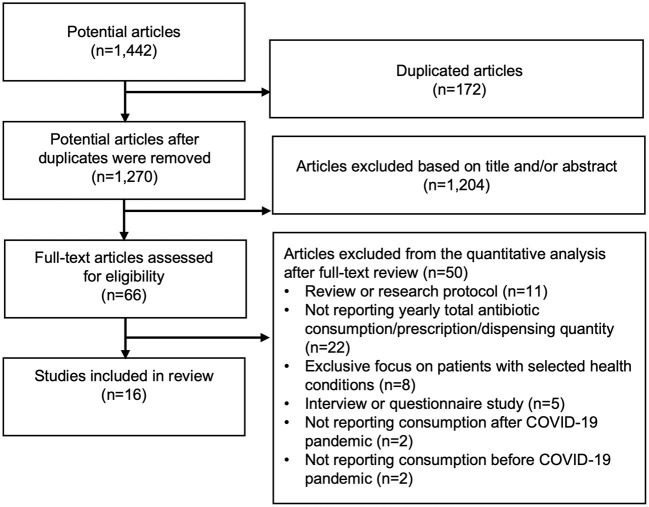
Through an electronic literature search, 1,441 articles were identified. In addition, one article was identified from the JSAC database. After excluding 172 duplicated articles, 1,270 articles were reviewed and 1,204 articles were excluded by titles and abstracts because they were clearly not relate to our current study. Full text of remaining 66 articles were reviewed, and finally, 16 articles were included in this systematic review (13–28) (Figure 1). Eight studies reported only hospital-based results (13, 14, 23–28), nine reported only community-level antibiotic consumption (14–22), and one [conducted in England (14)] reported antibiotic consumption in both hospitals and communities (Tables 2, 3). Although we did not limit the studies to the year 2020, we could not find any study that reported antibiotic consumption in 2021 through the systematic review.
Figure 1.
A systematic review flow diagram. Diagram of the number of articles and reports identified and examined at each stage of the review. A total of 16 articles published from 2021 to 2022 met all inclusion criteria and were included in the review.
Table 2.
Antibiotic consumption trend at hospital levels showing the overall trend and individually reported antibiotics.
| Authors | Country | Source | Overall trend | Unit | Antibiotics |
|---|---|---|---|---|---|
| Chamieh et al. (24) | Lebanon | Saint George Hospital University Medical Center | Increased by 20% | DDD/1,000 PD | Carbapenem |
| Grau et al. (25) | Spain | Acute care hospitals affiliated with the VINCat program | Increased P < 0.001 |
DDD/100 PD | Cephalosporins Carbapenems |
| Other cephalosporins and penems | |||||
| Macrolides | |||||
| Penicillins | |||||
| Aminoglycoside antibacterials | |||||
| Quinolone antibacterials | |||||
| Macera et al. (13) | Italy | The University Hospital luigi Vanvitelli in Naples | Increased Surgical ward | DDD/100 PD | III/IV Generation Cephalosporins (Surgical) |
| Aminopenicillins/BLI (Surgical) | |||||
| Metronidazole (Surgical) | |||||
| Vancomycin | |||||
| Linezolid | |||||
| Tigecycline | |||||
| Cefazolin | |||||
| Carbapenems | |||||
| Piperacillin/tazobactam | |||||
| Fluoroquinolones (ICU) | |||||
| III/IV Generation Cephalosporins (ICU) | |||||
| Padhan et al. (26) | India | AIIMS Raipur | Increased (p < 0.0001) |
DDD/100 PD | Piperacilli + Tazobactam |
| Ceftriaxone | |||||
| Azithromycin | |||||
| Paracetamol | |||||
| Dexamethasone | |||||
| Ciprofloxacin | |||||
| Diclofenac | |||||
| Hydrocortisone | |||||
| Meropenem | |||||
| Metronidazole | |||||
| Andrews et al. (14) | UK | The ePACT2 from the NHS Business Services Authority | Increased by 12% | DDD/1,000 admissions | NA |
| Silva et al. (27) | Brazil | A tertiary hospital in Rio de Janeiro | Stable (p = 0.068) | DDD/BD | Polymyxin B |
| Polymyxin E | |||||
| Daptomycin | |||||
| Ciprofloxacin | |||||
| Amikacin | |||||
| Linezolid | |||||
| Ceftazidime/avibactam | |||||
| Ceftozolone/tazobactam | |||||
| Tigecycline | |||||
| Azithromycin | |||||
| Cefepime, Ceftazidime, Ceftriaxone, Cefuroxime | |||||
| Ertapenem | |||||
| Levofloxacin | |||||
| Meropenem | |||||
| Moxifloxacin | |||||
| Piperacilin/tazobactam | |||||
| Teicoplanin | |||||
| Vancomycin | |||||
| Ampicillin, Ampicillin/sulbactam | |||||
| Amoxicillin and clavulanate | |||||
| Liu et al. (23) | China | A Tertiary teaching hospital in Shanghai | Decreased by >10% | DDD/100 PD | NA |
| Murgadella-Sancho et al. (28) | Spain | Moises Broggi Hospital | Decreased (p = 0.045) | DDD/100 BD | NA |
Overall trend and individual antibiotics: text in red represents a significant increase in 2020 compared to 2019 and/or an increase >10%. Similarly, text in #3bc1cd represents a significant decrease and/or a decrease >10%. Text in black represents antibiotics that did not show statistically significant change and/or reported < 10% change.
DDD, defined daily dose; PD, patient days.
Table 3.
Antibiotic consumption trend at regional or national levels showing the overall trend and individually reported antibiotics.
| Author | Country | Source | Overall trend | Unit | Antibiotics |
|---|---|---|---|---|---|
| Al-Azzam et al. (16) | Jordan | Jordan food and drug administration (JFDA) | Stable (28.4–>26.8 DDD/1,000 ID) | DDD/1,000 ID | Beta-lactamase resistant penicillin Third-generation cephalosporins |
| Fourth-generation cephalosporins | |||||
| Carbapenems sulfonamides with trimethoprim | |||||
| Macrolides | |||||
| Lincosamides | |||||
| Combinations of antibacterials | |||||
| Tetracyclines | |||||
| Other aminoglycosides | |||||
| Fluoroquinolones | |||||
| Combinations of penicillin | |||||
| Second-generation cephalosporins | |||||
| Glycopeptide antibacterials | |||||
| Other cephalosporins and penems | |||||
| Penicillin with extended-spectrum | |||||
| Gillies et al. (19) | Australia | National claims data Australia | Decreased by 36.4% | Dispensing/1,000 inhabitants | Trimethoprim Flucloxacillin |
| Metronidazole | |||||
| Cefalexin | |||||
| Amoxicillin | |||||
| Amoxicillin with clavulanic acid | |||||
| Doxycycline | |||||
| Roxithromycin | |||||
| Clarithromycin | |||||
| Phenoxymethylpenicillin | |||||
| Knight et al. (20) | Canada | National antibiotic dispensing data from IQVIA's CompuScript database | Decreased by >10% | Dispensing/1,000 inhabitants (mean) | NA |
| Silva et al. (17) | Portugal | The Portuguese National Health System (NHS) | Decreased by >10% | Prescription DDD/1,000 inhabitants | Third-generation cephalosporins |
| Fluoroquinolones | |||||
| Clarithromycin | |||||
| Rojas-Garcia and Antonanzas (21) | Spain | The Department of Pharmaceutical and Health Products of La Rioja | Decreased by >10% | Prescription DDD/1,000 inhabitants | Doxycycline |
| Amoxicillin and beta-lactamase inhibitor Amoxicillin |
|||||
| Cefuroxime | |||||
| Azithromycin | |||||
| Levofloxacin | |||||
| Nicieza García et al. (18) | Spain | The Health Service of the Principality of Asturias | Decrease by 24% (p < 0.001) | DDD/1,000 insured adult population | NA |
| Andrews et al. (14) | UK | The ePACT2 from the NHS Business Services Authority | Decreased by >10% | Prescription items/1,000 population | NA |
| JSAC (15) | Japan | The National Database (NDB) of Health Insurance Claims and Specific Health Checkups of Japan | Decreased by 21% | DDD/1,000 ID | B-lactam antibacterials, Penicillins |
| Quinolones | |||||
| Macrolides, Lincosamides, and Streptogramins | |||||
| Other B-lactam antibacterials | |||||
| Hogberg et al. (22) | EU (29 countries) | European Surveillance of Antimicrobial Consumption Network (ESAC-Net) | Decreased by >10% | DDD/1,000 ID | Tetracyclines |
| Sulfonamides and trimethoprim | |||||
| Beta-lactams, penicillins | |||||
| Other beta-lactam antibacterials | |||||
| Macrolides, lincosamides, and streptogramins | |||||
| Quinolones |
Overall trend and individual antibiotics: text in red represents a significant increase in 2020 compared to 2019 and/or an increase >10%. Similarly, text in #3bc1cd represents a significant decrease and/or a decrease >10%. Text in black represents antibiotics that did not show statistically significant change and/or reported < 10% change.
DDD, defined daily dose; ID, inhabitants per day.
These hospital-based studies were conducted in Lebanon, Spain, Italy, India, Brazil, China, and the UK (13, 14, 23–28). Community antibiotic consumption studies were conducted in Jordan, Australia, Canada, Portugal, Spain, the UK, Japan, and the EU (14–22). A study in the EU reported the total antibiotic consumption of 29 countries and the antibiotic consumption of 27 individual countries (including Austria, Belgium, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, and Sweden) (22). Notably, all community/national-level studies reported a decrease in overall antibiotic consumption in 2020 compared with that in 2019 (before the COVID-19 pandemic). All community-based studies, except one conducted in Jordan (16), reported that the individual or group antibiotic consumption decreased in addition to the reduction in overall antibiotic consumption. In contrast, hospital-based studies have reported an overall increase and decrease in antibiotic consumption in 2020.
Similarly, Andrews et al. reported a decrease in antibiotic prescriptions in the community in 2020 and an increase in hospital admissions in England (14). They also reported a decrease of more than 10% in total antibiotic consumption at the community level from January to October 2020 compared with that in the same months in 2019. However, the consumption at hospitals in DDDs/1,000 admissions increased by 12% during the same study period in 2020 compared with that in 2019.
Reported interruption of antibiotic stewardship program in 2020
The interruption or reduction of antibiotic stewardship programs during the COVID-19 pandemic has been reported by Grau et al. (25), Macera et al. (25), Andrews et al. (14), and Murgadella-Sancho et al. (28). These studies, except for Murgadella-Sancho et al., reported an increase in antibiotic consumption in 2020. Only one study from China reported that the continuous implementation of antibiotic stewardship programs since 2019 throughout 2020 resulted in the reduction of overall antibiotic consumption in 2020 (23).
Economic levels of countries where a study was conducted
The economies of countries were categorized according to the World Bank country and lending groups (29). Hospital-based studies have been conducted in high-income economies (Spain, Italy, and the UK), upper-middle-income economies (Lebanon and China), and lower-middle-income economies (India and Brazil) (13, 14, 23–28). All community-based studies were conducted in high-income countries, except Jordan, an upper-middle-income economy (14–22). Neither hospital nor community-based studies have been conducted in low-income economies.
Discussion
Our systematic review results revealed a consistent decrease in community antibiotic consumption (14–22). In contrast, more studies reported an increase in overall hospital antibiotic consumption in 2020 compared with that in 2019 (13, 14, 23–28). Multiple hospital-based studies reported the interruption of antibiotic stewardship programs in 2020 because of the allocation of infection control specialists for COVID-19, which resulted in increased overall antibiotic consumption (13, 14, 25). However, only one study reported that the continuous antibiotic stewardship program that was started from 2019 throughout 2020, resulted in a decrease in antibiotic consumption in 2020, despite a transient increase during the first wave of COVID-19 (23). Furthermore, one study from Spain reported a continuous antibiotic stewardship program during the pandemic with some amendments to react to the change in human resource allocation (28). The study reported a decrease in antibiotic consumption in 2020, showing the possibility of continuous antibiotic consumption control while fighting against the pandemic. This review highlights the importance of antibiotic stewardship programs even during the COVID-19 pandemic.
Decrease in antibiotic consumption in the community
All the studies identified in our systematic review reported a decrease in antibiotic consumption at the national level (14–22). The decrease in antibiotic consumption in the community could reflect 1) a decrease in antibiotic use for respiratory infection, which reflects the effectiveness of personal infection protection efforts of individuals in the community; 2) the hesitancy of visiting hospitals when people had no/light/mild symptoms owing to the fear of contracting COVID-19 (30); 3) reduced social activities observed through national infection control in many countries, e.g., city or country lockdown, which decreases the likelihood of getting an infection when amidst other people (31, 32); and 4) increased awareness and infection prevention activities among the public (e.g., enhanced hand hygiene, universal wearing of masks, and social distancing).
Increased public awareness regarding infectious disease control was a positive side effect of the COVID-19 pandemic. However, regardless of the benefits of strict regulations such as city/country lockdown and state of emergency in COVID-19 control, the pandemic has affected the economy (33) and mental health (34) of the nation. Therefore, such strict infection control measures cannot last for a long time. Antibiotic consumption must be continuously and carefully monitored once social restrictions are lifted.
Decrease/increase in antibiotic consumption in hospitals
Contrary to the consistent decrease in overall antibiotic consumption in the community, there was a different trend in antibiotic consumption in hospitals (13, 14, 23–28). An increase was reported in hospitals in Spain, Italy, India, and the UK. However, a decrease was reported in hospitals in China and Spain. This variability in antibiotic consumption in 2020 compared with that in 2019 could be caused by 1) the levels of antibiotic stewardship program implementation during the COVID-19 pandemic; 2) the reluctance of people to visit hospitals during the COVID-19 pandemic (30) hence, those hospitalized were likely to have more severe symptoms than that noted in the regular year; and 3) the use of antibiotics to treat COVID-19 patients (12, 35).
One study from Spain reported an interrupted antibiotic stewardship program in 66 acute care hospitals and increased antibiotic consumption (25). In contrast, another study from Spain reported that a continuous antibiotic stewardship program with necessary amendment to the reaction to the COVID-19 pandemic caused a decrease in antibiotic consumption (28). Similarly, Liu et al. also reported that the implementation of an antibiotic stewardship program from 2019 to 2020 resulted in a successful reduction of overall antibiotic consumption by 2020 (23). These studies highlight the benefit of a continuous antibiotic stewardship program even with some amendments.
Lack of reports from low-income countries
Our review results showed that the studies were mostly published in high-income countries. Only one community-based study was reported from a middle-income country (Jordan) (16) and three hospital-based studies from India (26), Brazil (27), and China (23). Furthermore, no studies have been conducted in low-income countries. Sub-Saharan Africa has been reported to have the highest rate of death associated with AMR (1). Moreover, the antibiotic market is expected to grow rapidly in middle- and low-income countries (36). An interview-based study in Nigeria reported up to a 6-fold increase in antibiotic use among local pharmacists during the first wave of COVID-19 in 2020 (37). There have been reports concerning the inappropriate and increased use of antibiotics during the COVID-19 pandemic in low- and middle-income countries, such as Zimbabwe (38), Malawi, and Uganda (39). This includes antibiotics used in the hospital and at home (over-the-counter drugs without a prescription). Although these reports are highly concerning, we could not identify any quantitative studies or governmental reports that contained the quantity of antibiotics consumed in these countries. More information on antibiotic consumption in middle- and low-income countries is urgently needed.
Limitations
This review excluded studies that exclusively focused on patients with specific comorbidities, including COVID-19. However, the magnitude of the effect of antibiotic consumption on COVID-19 patients could not be distinguished from hospital- or department-level antibiotic consumption, even though this effects the overall antibiotic consumption trend. National-level studies (e.g., national antibiotic consumption in Japan) included both outpatient and inpatient antibiotic consumption. Thus, although the main interest was antibiotic use in the community, this report included inpatient antibiotic use. Because of the type of data, we could not distinguish between these data in some community- and national-level studies. In addition, the variability of the size of the targeted population could not be directly considered in the current study.
Conclusion
During the COVID-19 pandemic, all community- and national-level studies reported an overall decrease in antibiotic consumption. This could be because of the reduction in infectious disease cases induced by social and individual control of infection. In contrast, hospital inpatients' antibiotic consumption was reported to decrease or increase depending on the hospital. This could be because of the increase in the ratio of more severe patients during the pandemic and the interruption of antibiotic stewardship programs. Thus, hospitals that reported an interruption of antibiotic stewardship programs in 2020 also reported an increase in antibiotic consumption. Our results highlight the importance of continuing antibiotic stewardship programs even while implementing amendments to manage to the pandemic. In addition, the review showed a research output gap between low- and middle-income countries. More studies evaluating the effect of the COVID-19 pandemic on antibiotic consumption should be conducted in these countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF, DL, and OO: initial conception and design of the study. MF and N-HN: systematic review and data extraction. MF, N-HN, DL, SF, and OO: contribution to drafting manuscript editing/reviewing. All authors approved the final version of the manuscript.
Funding
MF was supported by the Japan Agency for Medical Research and Development (project code: 22fk0108630h0001) and the University of Tsukuba's review paper-editing project. The funders do not play a role in this review results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Christine Chang, University of Tsukuba, for her support in data collection. We would also like to thank all members of the Laboratory of Regenerative Medicine and Stem Cell Biology for their continuous support.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.946077/full#supplementary-material
References
- 1.Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Fact Seets: Antibiotic Resistance. Geneva: World Health Organization (2020). Available online at: https://www.who.int/health-topics/antimicrobial-resistance (accessed Feburary 27, 2022).
- 3.CDC. Core Elements of Antibiotic Stewardship: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Atlanta, GA: CDC (2021). Available online at: https://www.cdc.gov/antibiotic-use/core-elements/index.html (accessed Feburary 27, 2022).
- 4.Majumder MAA, Rahman S, Cohall D, Bharatha A, Singh K, Haque M, et al. Antimicrobial stewardship: fighting antimicrobial resistance and protecting global public health. Infect Drug Resist. (2020) 13:4713–38. 10.2147/IDR.S290835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow P, van Schalkwyk MC, McKee M, Labonte R, Stuckler D. Covid-19 and the collapse of global trade: building an effective public health response. Lancet Planet Health. (2021) 5:e102–7. 10.1016/S2542-5196(20)30291-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flor LS, Friedman J, Spencer CN, Cagney J, Arrieta A, Herbert ME, et al. Quantifying the effects of the Covid-19 pandemic on gender equality on health, social, and economic indicators: a comprehensive review of data from March, 2020, to September, 2021. Lancet. (2022) 399:2381–97. 10.1016/S0140-6736(22)00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahriani M, Anwar S, Yufika A, Bakhtiar B, Wardani E, Winardi W, et al. Disruption of childhood vaccination during the Covid-19 pandemic in Indonesia. Narra J. (2021) 1:e7. 10.52225/narraj.v1i1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of Covid-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. (2021) 11:e045343. 10.1136/bmjopen-2020-045343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro-Lopes A, Correia S, Leal C, Resende I, Soares P, Azevedo A, et al. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the Covid-19 pandemic. Antibiotics. (2021) 10:778. 10.3390/antibiotics10070778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Zorn B. Antibiotic use in the Covid-19 crisis in Spain. Clin Microbiol Infect. (2021) 27:646–7. 10.1016/j.cmi.2020.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tejedor Prado P, Lázaro Cebas A, Sánchez Artola B, Palencia Herrejon E, Aznar Cano E, Salso Ortiz S, et al. Coronavirus first wave effect on antibiotic consumption and antimicrobial resistance. Eur J Hosp Pharm. (2021) 28(SUPPL 1):A38. 10.1136/ejhpharm-2021-eahpconf.7828850992 [DOI] [Google Scholar]
- 12.Khan S, Hasan SS, Bond SE, Conway BR, Aldeyab MA. Antimicrobial consumption in patients with COVID-19: a systematic review and meta-analysis. Exp Rev Anti Infect Ther. (2022) 20:749–72. 10.1080/14787210.2022.2011719 [DOI] [PubMed] [Google Scholar]
- 13.Macera M, Onorato L, Calo F, Monari C, Annibale R, Signoriello G, et al. The impact of the Sars-Cov2 pandemic on a persuasive educational antimicrobial stewardship program in a university hospital in Southern Italy: a pre-post study. Antibiotics. (2021) 10:1405. 10.3390/antibiotics10111405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews A, Budd EL, Hendrick A, Ashiru-Oredope D, Beech E, Hopkins S, et al. Surveillance of antibacterial usage during the Covid-19 pandemic in England, 2020. Antibiotics. (2021) 10:841. 10.3390/antibiotics10070841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Japan Surveillance of Antimicrobial Consumption (JSAC) . Surveillance of Antimicrobial Consumption by Prefecture and Age Category, Based on Data from the Ndb. (2022) 21st Jan. 2022. Report No.: Contract No.: 25th Feb (2022). [Google Scholar]
- 16.Al-Azzam S, Mhaidat NM, Banat HA, Alfaour M, Ahmad DS, Muller A, et al. An assessment of the impact of coronavirus disease (Covid-19) pandemic on national antimicrobial consumption in Jordan. Antibiotics. (2021) 10:690. 10.3390/antibiotics10060690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva TM, Estrela M, Gomes ER, Pineiro-Lamas M, Figueiras A, Roque F, et al. The impact of the Covid-19 pandemic on antibiotic prescribing trends in outpatient care: a nationwide, quasi-experimental approach. Antibiotics. (2021) 10:1040. 10.3390/antibiotics10091040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicieza García ML, Pérez Solís P, Gómez de Oña C, Suárez Gil P, Rolle Sóñora V, Suárez Mier B. Antibiotic consumption in primary care in the adult population of asturias during 2014–2020 period. Atencion Primaria. (2022) 54:102261. 10.1016/j.aprim.2021.102261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies MB, Burgner DP, Ivancic L, Nassar N, Miller JE, Sullivan SG, et al. Changes in antibiotic prescribing following COVID-19 restrictions: lessons for post-pandemic antibiotic stewardship. Br J Clin Pharmacol. (2022) 88:1143–51. 10.1111/bcp.15000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight BD, Shurgold J, Smith G, MacFadden DR, Schwartz KL, Daneman N, et al. The impact of COVID-19 on community antibiotic use in Canada: an ecological study. Clin Microbiol Infect. (2022) 28:426–32. 10.1016/j.cmi.2021.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas-Garcia P, Antonanzas F. Analysis of the prescription of antibiotics during the implementation of Covid-19 personal protection measures in a regional health system. Clin Outcomes Res. (2021) 13:927–36. 10.2147/CEOR.S337621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogberg LD, Vlahovic-Palcevski V, Pereira C, Weist K, Monnet DL, Grp ES-NS. Decrease in community antibiotic consumption during the Covid-19 pandemic, Eu/Eea, 2020. Eurosurveillance. (2021) 26:2101020. 10.2807/1560-7917.ES.2021.26.46.2101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YX, Liang C, Yang Y, Le KJ, Zhang ZL, Gu ZC, et al. Reduction in antimicrobial use associated with a multifaceted antimicrobial stewardship programme in a tertiary teaching hospital in Shanghai: a segmented regression analysis. Ann Palliat Med. (2021) 10:7360. 10.21037/apm-21-700 [DOI] [PubMed] [Google Scholar]
- 24.Chamieh A, Zgheib R, El-Sawalhi S, Yammine L, El-Hajj G, Zmerli O, et al. Trends of multidrug-resistant pathogens, difficult to treat bloodstream infections, and antimicrobial consumption at a tertiary care center in lebanon from 2015–2020: Covid-19 aftermath. Antibiotics. (2021) 10:1016. 10.3390/antibiotics10081016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grau S, Hernandez S, Echeverria-Esnal D, Almendral A, Ferrer R, Limon E, et al. Antimicrobial consumption among 66 acute care hospitals in catalonia: impact of the Covid-19 pandemic. Antibiotics. (2021) 10:943. 10.3390/antibiotics10080943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padhan S. T P, Chandrakar R, Galhotra A, Borkar NB. Assessment of the impact of Covid-19 on drug store management in a tertiary care teaching hospital of central India. Cureus. (2021) 13:e19723. 10.7759/cureus.19723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva ARO, Salgado DR, Lopes LPN, Castanheira D, Emmerick ICM, Lima EC. Increased use of antibiotics in the intensive care unit during coronavirus disease (Covid-19) pandemic in a Brazilian hospital. Front pharmacol. (2021) 12:778386. 10.3389/fphar.2021.778386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murgadella-Sancho A, Coloma-Conde A, Oriol-Bermúdez I. Impact of the strategies implemented by an antimicrobial stewardship program on the antibiotic consumption in the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. (2022) 43:1292–3. 10.1017/ice.2021.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The WORLD BANK. World Bank Country and Lending Groups. Washington, DC: The WORLD BANK. (2022). Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed Feburary 25, 2022).
- 30.Toyoda Y, Katanoda K, Ishii K, Yamamoto H, Tabuchi T. Negative impact of the Covid-19 state of emergency on breast cancer screening participation in Japan. Breast Cancer. (2021) 28:1340–5. 10.1007/s12282-021-01272-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angoulvant F, Ouldali N, Yang DD, Filser M, Gajdos V, Rybak A, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections-a time series analysis. Clin Infect Dis. (2021) 72:319–22. 10.1093/cid/ciaa710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haklai Z, Applbaum Y, Myers V, Saban M, Gordon ES, Luxenburg O, et al. The effect of the Covid-19 pandemic on non-Covid respiratory Ed visits in Israel. Am J Emerg Med. (2022) 53:215–21. 10.1016/j.ajem.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goolsbee A, Syverson C. Fear, lockdown, and diversion: comparing drivers of pandemic economic decline 2020. J Public Econ. (2021) 193:104311. 10.1016/j.jpubeco.2020.104311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams-Prassl A, Boneva T, Golin M, Rauh C. The impact of the coronavirus lockdown on mental health: evidence from the United States. Econ Policy. (2022) 37:139–55. 10.1093/epolic/eiac002 [DOI] [Google Scholar]
- 35.Van Laethem J, Wuyts S, Van Laere S, Dirkx S, Seyler L, Mertens R, et al. Antibiotic prescriptions targeting bacterial respiratory infections in admitted patients with Covid-19: a prospective observational study. Infect Dis Ther. (2021) 10:2575–91. 10.1007/s40121-021-00535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortune Business Insights,. The Global Antibiotics Market is Projected to Grow from $38.08 Billion in 2021 to $45.30 Billion in 2028 at a Cagr of 2.5% in Forecast Period, 2021–2028: Fortune Business Insights. Pune: Fortune Business Insights (2022). Available online at: https://www.fortunebusinessinsights.com/antibiotics-market-104583 (accessed Feburary 27, 2022).
- 37.Haque M, Abubakar A, Ogunleye O, Sani I, Sefah I, Kurdi A, et al. Changes in availability, utilization, and prices of medicines and protection equipment for Covid-19 in an urban population of Northern Nigeria. J Res Pharm Pract. (2021) 10:17–22. 10.4103/jrpp.JRPP_20_92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitungo I, Dzinamarira T, Nyazika TK, Herrera H, Musuka G, Murewanhema G. Inappropriate antibiotic use in Zimbabwe in the Covid-19 era: a perfect recipe for antimicrobial resistance. Antibiotics. (2022) 11:244. 10.3390/antibiotics11020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixon J, MacPherson EE, Nayiga S, Manyau S, Nabirye C, Kayendeke M, et al. Antibiotic stories: a mixed-methods, multi-country analysis of household antibiotic use in Malawi, Uganda and Zimbabwe. BMJ Glob Health. (2021) 6:e006920. 10.1136/bmjgh-2021-006920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandl C, Zimmermann ME, Günther F, Dietl A, Küchenhoff H, Loss J, et al. Changes in healthcare seeking and lifestyle in old aged individuals during Covid-19 lockdown in Germany: the population-based augur study. BMC Geriatr. (2022) 22:34. 10.1186/s12877-021-02677-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azlan AA, Hamzah MR, Sern TJ, Ayub SH, Mohamad E. Public knowledge, attitudes and practices towards Covid-19: a cross-sectional study in Malaysia. PLoS ONE. (2020) 15:e0233668. 10.1371/journal.pone.0233668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roshan R, Feroz AS, Rafique Z, Virani N. Rigorous hand hygiene practices among health care workers reduce hospital-associated infections during the Covid-19 pandemic. J Prim Care Commun Health. (2020) 11:2150132720943331. 10.1177/2150132720943331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.