Version Changes
Revised. Amendments from Version 1
The following preliminary phytochemical study of the Bidens pilosa L. and Croton floccosus plants provided important information on the content of secondary metabolites and response to the DPPH radical reported for the first time in Ecuador, which may be future use for medicinal application. The changes that were made in relation to the previous version were updating of the bibliography in the Phytochemical screening section, and comments were also added in the Determination of total phenols and flavonoids and Vegetal material sections. Lastly, Figure 1 was added. map with the collection points of the studied species
Abstract
Background: Given the chemical richness of medicinal plants ( Bidens pilosa L. and Croton floccosus) in Ecuador, they are considered the natural source of numerous medicines.
Methods: The leaves were dried at 40°C and 50°C and the extracts were characterized by means of phytochemical screening, verifying the presence of secondary metabolites such as alkaloids, reducing sugars, phenols, flavonoids, tannins and saponins. Three extraction processes were carried out, with two solvents of different polarities: hexane and ethanol. The extraction methods that were applied to the leaves of the plants were Soxhlet, ultrasonic bath and maceration, the latter two at room temperature and Soxhlet at the boiling temperature of the solvent. Determination of the total content of phenols and flavonoids is carried out using the Follin-Ciocalteau colorimetric reaction, Quercetin standard, Aluminum Chloride solution measured with a UV-Vis spectrophotometer. The antioxidant activity was performed with the DPPH radical and measured with the same equipment.
Results: The highest content of total phenols obtained by employing the Soxhlet method for extraction when the material was dried at 50°C was 48.609 ± 0.370 mg GAE/g of dry sample for Bidens pilosa L. while in the case of Croton floccosus it was 128.212 ± 0.601 mg GAE/g of dry sample obtained from the extraction by means of maceration. Finally, the antioxidant activity against the 1.1-diphenyl-2-picryl-hydrazyl radical was determined, and it was found that the Bidens pilosa L. species performed better and responded better to the test, with an IC 50 value of 239.33 µg/mL, than Croton floccosus (IC 50 of 644.125 µg/mL).
Conclusions: The following preliminary phytochemical study of the Bidens pilosa L. and Croton floccosus plants provided important information on the content of secondary metabolites and response to the DPPH radical reported for the first time in Ecuador, which may be future use for medicinal application.
Keywords: extraction, phytochemical study, phenols, flavonoids, Bidens pilosa L, Croton floccosus, Asteraceae, Euphorbiaceae
Introduction
The biodiversity of Ecuador ranks sixth worldwide for the number of species. 1 Many species comprise the Croton genus in this country, and 39 have been recognized. 2 Of these, 13 are considered native, 3 and the others have been documented in the last 15 years. 4 , 5 Many species of this genus have been used in traditional medicine by the country’s indigenous population. Antimicrobial, 6 antioxidant, 7 anti-inflammatory 8 and anticancer 9 , 10 biological activity studies have been carried out on them in America, Asia and Africa.
Brian A. Smith found a new species of Croton (Euphorbiaceae) on the western slopes of the Ecuadorian Andes in 2006, Croton floccosus. 11 This is described as a medium-sized tree with grayish external and internal bark with a reddish exudate, along with simple leaves (4–15 × 2.5–8 cm) and alternate elliptic-oval unisexual flowers and tricoco-type fruits with persistent styles. This plant is commonly found next to streams and disturbed sites in the provinces of Pichincha and Imbabura in Ecuador.
Some 230 to 240 species of Bidens have been described, 12 , 13 including Bidens pilosa, which was identified in 1753 by Carl Linnaeus. This is a representative perennial herb, distributed globally in temperate and tropical regions, which has traditionally been used in food and medicine without having any obvious adverse effects. About 116 publications on this species have documented its medical use, 14 and phytochemical studies show that it contains a high number of flavonoids and polyines, which have anticancer, 15 anti-inflammatory, 16 antibacterial 17 biological activity, as well as antifungal, 18 antimalarial, 19 and antioxidant 20 , 21 properties.
Bidens pilosa is an erect, strongly branched plant with a strong aromatic odor. It has opposite leaves and a long petiole, whose limbs are generally deeply divided into 3 to 5 segments giving it the appearance of a compound leaf. It also has white ray flowers and numerous yellow tubular flowers. 13
Although this species has been studied to a greater extent than Croton floccosus, we have not found phytochemical studies of these plants in Ecuador, which is why it has important research potential that may allow us to transform traditional knowledge into scientific knowledge.
In the following work, a preliminary phytochemical study of the leaves of the species Bidens pilosa and Croton floccosus was carried out using the Tukey test, 22 which is employed in ANOVA in order to compare the means of the values obtained. This study joins the recently published work by Ruiz-Reyes et al. on Melampodium divaricatum and Zanthoxylum sprucei plants, 23 in an attempt to discover which active compounds are present and if they have the potential to be used as medicinal plants.
Methods
Vegetal material
The fresh leaves of the Bidens pilosa L. and Croton floccosus species were collected in January 2021 from the Botanical Garden at the Technical University of Manabí (UTM), Portoviejo, in the Manabí province in western Ecuador. The leaves that present better conservation in their structure are chosen to clean them of dust and branches, then the petiole of the leaf blade is extracted. The studied leaves were collected from various plants and then mixed to create the sample for analysis. The botanical identification was carried out by the botanists in charge of the herbarium in the botanical garden at the UTM. The vouchers of the specimens were deposited with the following codes Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae). It is shown in the following map of the botanical garden. The collection points of the studied species. For croton floccosus it was two points while for bidens pilosa it was three points. The coordinates of the studied species are placed on the map Figure 1.
Figure 1. Map with the collection points of the studied species.
Image obtained from Google Maps.
Chemicals and reagents
The chemicals and reagents employed in this study were: Folin-Ciocalteu reagent, 1.1-diphenyl-2-picryl-hydrazyl (DPPH), sodium nitrite, aluminum chloride, sodium hydroxide, sodium carbonate, catechin, quercetin, trolox, methanol, and ethanol. All the reagents and solvents were supplied by Sigma-Aldrich and are of an appropriate analytical grade for study.
Extraction
The plant material was dried in a tray dryer (universal oven UF110, Memmert) at temperatures of 40°C and 50°C. A reference weight was taken every two hours until it remained constant. The sample was pulverized using a blade mill (grinder) with a sieve so that a more uniform size of the material can be obtained, in order to fractionate the plant tissue and produce a better extraction. The time used for grinding was 5 minutes. The extracts of the crude plants were obtained using the ultrasonic bath, maceration and Soxhlet extraction methods. In the case of Soxhlet extraction, 10 g of plant material were used and placed in the equipment extractor cartridge with 250 mL of ethanol. Between 8 and 10 extractions were performed at the boiling temperature of the solvent, and the solvent was eventually evaporated in order to obtain the extracts. With regard to ultrasonic bath extraction, 10 g of finely divided plant material was placed in a 250 mL beaker containing 100 mL of ethanol. The mixture was sonicated at 50 W for 1 h in an ultrasonic bath and subsequently filtered. The solvent was then evaporated in a vacuum to obtain the corresponding extracts for analysis. In the case of extraction with maceration, 10 g of finely divided plant material was placed in a 250 mL beaker with 100 mL of ethanol. The mixture was macerated for 72 hours and stirred from time to time. It was subsequently filtered and the solvent was evaporated in a vacuum to obtain the corresponding extracts for analysis.
Phytochemical screening
The crude extracts were evaluated phytochemically to determine the presence of chemical constituents using standard procedures, following the same methodology described by Ruiz-Reyes 23 , 24 for the determination of metabolites: Alkaloids: Each extract (10 mg) was dissolved in 2 mL of 5% hydrochloric acid and, after mixing and filtering, three aliquots were taken. Drops of Wagner, Mayer, Bouchardat, and Dragendorff reagents were added to each one. A red-brown precipitate (Wagner), a yellowish white precipitate (Mayer), a brown precipitate (Bouchardat), and an orange-red precipitate (Dragendorff) indicated the presence of these metabolites. Flavonoids (Shinoda test): 1 ml of absolute ethanol and three drops of concentrated hydrochloric acid were added to 10 drops of the extract diluted in isopropyl alcohol. The formation of a red color indicated the presence of aurones and chalcones while the formation of an orange, red, or magenta color formation indicated the presence of isoflavones, flavonols, and flavonoids, respectively. Saponins (with sodium bicarbonate): 1 mL of distilled water and one drop of saturated sodium bicarbonate solution were added to five drops of the extract dissolved in isopropyl alcohol (20 mg/mL) in a test tube and shaken vigorously for three minutes. The formation of honeycomb shaped foam indicated the presence of saponins, and tannins: 10 mg of each extract was dissolved in 1 mL of ethanol and extract with 3 mL of boiling distilled water for 15 minutes. Once it had been allowed to stand at room temperature, 0.2 mL of 10% sodium chloride solution was added to the mixture and filtered. In addition, four drops of 10% ferric chloride solution were added. The precipitation observed was indicative of the presence of tannins. For reducing sugars, 200 μL of the extract were dissolved in a tube, Benedict’s reagent was added until the extract turned bluish, it was then heated in a water bath at a temperature of 60°C. It was considered positive if it turned reddish brown. 25
Determination of total phenols and flavonoids
Determination of the total content of phenols in the extracts was carried out using the Follin-Ciocalteau reaction and by employing gallic acid as the reference phenolic compound. The gallic acid calibration curve was prepared by weighing 2 mg of gallic acid and making it up to a volume of 10 mL with distilled water, which is the stock solution at a concentration of 0.2 mg/mL. Aliquots of 50 μL were subsequently taken, after which 200 μL of Follin-Ciocalteau reagent and 2 mL of 7% Na 2CO 3 were added, and the solution was made up to 5 mL. After the absence of light for 30 minutes and at room temperature, we measured the absorbance at 725 nm using the prepared solution as a blank without the analyte. Total flavonoid content was determined using quercetin as standard. For the preparation of the Quercetin standard, 2 mg of the Quercetin standard was weighed and dissolved with 70% methanol in a 10 mL volumetric flask, which is considered the mother solution with a concentration of 0.2 mg/mL. The aluminum chloride solution was subsequently prepared by weighing 500 mg of AlCl 3 and dissolving it with a solution of 25 mL 5% Acetic Acid in methanol, which led to a solution with a concentration of 20 mg/mL and aluminum chloride. In order to perform the calibration curve with the Quercetin standard, aliquots of 5, 10, 15, 20, 25, 30, 35, 40 μL of the standard solution were taken, and made up to 1 mL with methanol (70%), after which 1 mL of the AlCl 3 solution was added. The same solution was used as a blank without adding the standard compound. After waiting 15 min for the reaction, the absorbance of the solution was measured at a wavelength of 430 nm in the UV-Vis spectrophotometer. The preparation of the extracts was carried out by taking 5 mg of the sample, which was dissolved in 5 mL of 70% aqueous methanol. The determination procedure for total flavonoids was carried out by taking 200 μL aliquots of different extracts of the sample in triplicate and making them up with 1 mL of methanol (70%), after which 1 mL of the AlCl3 solution was added. After waiting 15 min, the absorbance of the solution was measured at a wavelength of 430 nm in the UV-Vis spectrophotometer. Both methodologies were described by Ruiz-Reyes. 23 , 26 – 28
Test with the radical DPPH
The antioxidant activity was determined using a spectrophotometer and the DPPH molecule as a reagent to generate the free radical following the methodology described by the author himself and other authors. 23 , 29 DPPH reagent preparation: 0.02 g of the DPPH reagent was weighed out and made up to volume with 100 mL of methanol in a flask. This was then homogenized and left to react for 24 hours in an amber bottle in the dark.
-
•
Wavelength determination
2.5 mL of the prepared DPPH reagent was taken and 15 mL ethanol added. A scan was carried out in the spectrophotometer. A maximum absorbance of 0.750 ± 0.05 should appear at a wavelength of 517 nm.
-
•
Blank preparation
Methanol (15 mL) was added and a scan was performed in the spectrophotometer to verify that it did not have absorbance in the working wavelength range in which the maximum of the sample is found.
-
•
Sample preparation
We took 3.0 mL of the DPPH reagent and 100, 200, 300, 400, 500 μL of the extract added. Once the sample and the blank were prepared, the absorbance reading was performed in the Thermo Scientific Genesys 180 (UV-Visible) spectrophotometer at 517 nM every 10 minutes.
Results and discussion
Analysis of extraction methods (yield)
A multifactorial statistical analysis of variance (ANOVA) was carried out for the different species studied, with a maximum order of interaction of 2 for the yield values. This made it possible to determine which of the extraction factors evaluated had a statistically significant effect on the yield. Table 1 presents a summary of the multifactorial arrangement used for the processes of obtaining extracts from Bidens pilosa L. and Croton floccosus at drying temperatures of 40 and 50°C.
Table 1. Yields of Bidens pilosa and Croton floccosus at drying temperatures of 40 and 50°C.
| Bidens pilosa L. | Croton floccosus | ||||||
|---|---|---|---|---|---|---|---|
| A: Drying temperature (°C) | B: Extraction process | C: Solvent | Yield (%) | A: Drying temperature (°C) | B: Extraction process | C: Solvent | Yield(%) |
| 40 | Soxhlet | Ethanol | 22.04 | 40 | Soxhlet | Ethanol | 38.08 |
| Maceration | 24.96 | Maceration | 12.5 | ||||
| Ultrasonic bath | 37.95 | Ultrasonic bath | 33.8 | ||||
| Soxhlet | Hexane | 13.26 | Soxhlet | Hexane | 3.57 | ||
| Maceration | 0.96 | Maceration | 4.2 | ||||
| Ultrasonic bath | 4.77 | Ultrasonic bath | 4.48 | ||||
| 50 | Soxhlet | Ethanol | 55.55 | 50 | Soxhlet | Ethanol | 50.63 |
| Maceration | 23.46 | Maceration | 14.26 | ||||
| Ultrasonic bath | 54.52 | Ultrasonic bath | 33.48 | ||||
| Soxhlet | Hexane | 6.24 | Soxhlet | Hexane | 0.79 | ||
| Maceration | 3.5 | Maceration | 3.2 | ||||
| Ultrasonic bath | 3.5 | Ultrasonic bath | 0.7 | ||||
Table 2 shows the analysis of variance for the extraction performance in the case of Bidens pilosa L., for which it was determined that there is a statistically significant difference in the treatments. The contribution of each factor was measured by eliminating the effects of the other factors. The P-values test the statistical significance of each of the factors. Since the P-value of factor C (solvent) is less than 0.05, this factor has a statistically significant effect on performance at a confidence level of 95.0%.
Table 2. Analysis of variance for extraction yields of Bidens pilosa L.
| Source | Sum of squares | Gl | Middle square | F-Reason | P-Value |
|---|---|---|---|---|---|
| MAIN EFFECTS | |||||
| A: Drying temperature | 152.867 | 1 | 152.867 | 1.23 | 0.3827 |
| B: Extraction process | 354.869 | 2 | 177.434 | 1.43 | 0.4117 |
| C: Solvent | 2890.76 | 1 | 2890.76 | 23.28 | 0.0404 |
| INTERACTIONS | |||||
| AB | 81.3555 | 2 | 40.6778 | 0.33 | 0.7532 |
| AC | 245.979 | 1 | 245.979 | 1.98 | 0.2946 |
| BC | 208.387 | 2 | 104.194 | 0.84 | 0.5437 |
| WASTE | 248.338 | 2 | 124.169 | ||
| TOTAL (CORRECTED) | 4182.55 | 11 |
Figure 2 shows the influence of the factors of the experimental design with respect to the extraction yield for the extracts obtained from Bidens pilosa L. A notable decrease in yields is observed when the extraction process is carried out using hexane. Although it is not statistically significant, it is necessary to mention that there was a better yield in the extraction processes in which leaves dried at 50°C were used. The process that obtained the best yields was the Soxhlet extraction.
Figure 2. ANOVA graph for the influence of factors on the extraction performance of Bidens pilosa L.
For each significant factor, multiple range tests provide information regarding which means were significantly different from others. Tukey’s honestly significant difference (HSD) method was selected for this test because it allows the comparison of individual means from an analysis of variance of several samples subjected to different treatments, thus widening the intervals in order to allow multiple comparisons between all pairs of means.
This test showed that there was no significant difference between the means for the drying temperatures of the leaves of Bidens pilosa L., as is noted in Figure 3. It is necessary to mention that although there was no statistically significant difference between the temperatures used for drying, the extraction processes during which 50°C was used led to higher yield values.
Figure 3. Comparison of Tukey's means for yields of Bidens pilosa L. extracts according to drying temperature.
The multiple range test carried out for Bidens pilosa L. also showed that there was no difference between the extraction process methods evaluated, as is noted in Figure 4. It is, however, necessary to mention that the extraction with an ultrasonic bath and Soxhlet led to higher yields than the maceration process, despite the fact that they are not statistically different.
Figure 4. Comparison of Tukey means for yields of Bidens pilosa L. extracts according to the extraction process.
Furthermore, Table 3 shows the analysis of variance for the extraction performance of Croton floccosus, which determined that there is a statistically significant difference in the treatments. The contribution of each factor is measured by eliminating the effects of the other factors. The P-values test the statistical significance of each of the factors. Since the P-value of factor C (solvent) is less than 0.05, this factor has a statistically significant effect on the extraction yield for Croton floccosus at a confidence level of 95.0%. It is also necessary to highlight that the interaction between factor B (extraction process) and C (solvent) has a significant effect on the extraction process.
Table 3. Analysis of variance for extraction yields of Croton floccosus.
| Source | Sum of squares | Gl | Middle square | F-Reason | P-Value |
|---|---|---|---|---|---|
| MAIN EFFECTS | |||||
| A: Drying temperature | 3.44541 | 1 | 3.44541 | 0.28 | 0.6517 |
| B: Extraction process | 446.838 | 2 | 223.419 | 17.91 | 0.0529 |
| C: Solvent | 2291.08 | 1 | 2291.08 | 183.66 | 0.0054 |
| INTERACTIONS | |||||
| AB | 24.7647 | 2 | 12.3824 | 0.99 | 0.5019 |
| AC | 38.7002 | 1 | 38.7002 | 3.10 | 0.2202 |
| BC | 545.456 | 2 | 272.728 | 21.86 | 0.0437 |
| WASTE | 24.9493 | 2 | 12.4747 | ||
| TOTAL (CORRECTED) | 3375.23 | 11 |
Figure 5 shows the influence of the factors of the experimental design on the extraction yield. A notable decrease in yields is observed when hexane is used in the extraction process. It will also be observed that there is a difference with respect to the yields obtained as regards the interaction between the extraction method and the solvent (B and C), showing that the highest yields are obtained with the Soxhlet extraction processes with ethanol at both drying temperatures.
Figure 5. ANOVA graph for the influence of factors on the extraction yield of Croton floccosus.
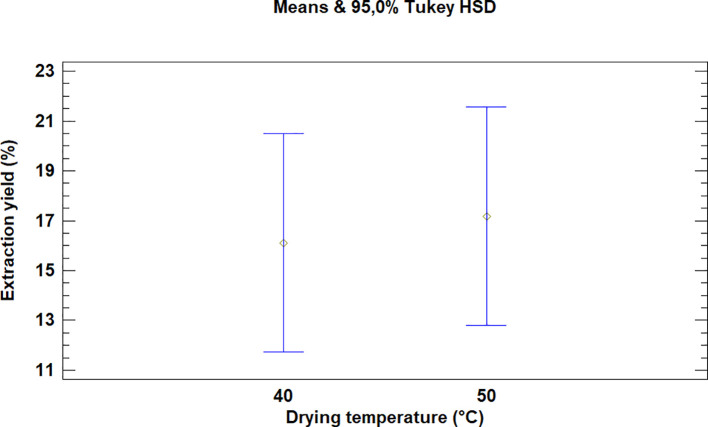
As described above for the temperatures selected in order to dry Bidens pilosa L., there is no significant difference between the means obtained for the drying temperatures of Croton floccosus leaves ( Figure 6). However, it is necessary to mention that, although there is no statistically significant difference, the extraction processes during which the drying temperature of the material was 50°C obtained better extraction yields, as occurred with the Bidens pilosa L.
Figure 6. Comparison of Tukey means for yields of Croton floccosus extracts according to drying temperature.
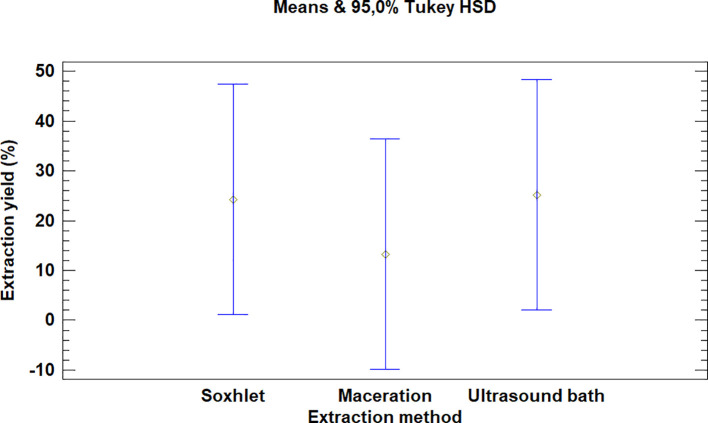
It was also noted that the multiple range test carried out for Croton floccosus made it possible to identify that there was a difference between the Soxhlet and maceration process extraction methods ( Figure 7). The Soxhlet extraction process method obtains higher yields than the maceration and ultrasonic bath extraction methods.
Figure 7. Comparison of Tukey means for yields of Croton floccosus extracts according to the extraction method.
Analysis for solvents
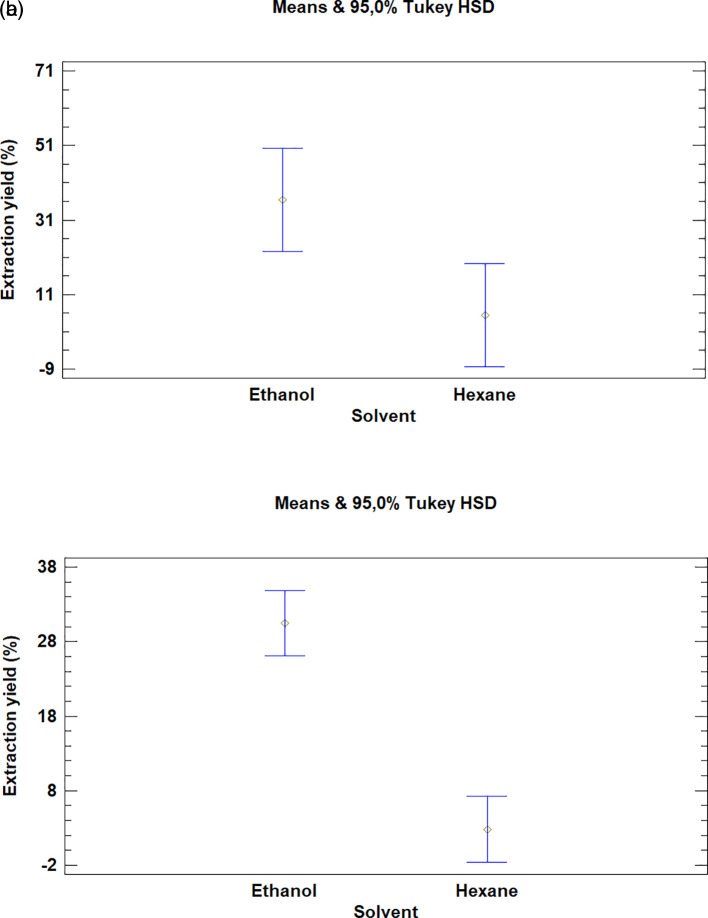
Finally, in the case of both plants, the multiple range test showed that there was a difference between the solvents used in the extraction ( Figure 8). The processes in which hexane was used obtained a low yield when compared to extractions with ethanol.
Figure 8. Comparison of Tukey means for yields of extracts of Bidens pilosa L. and Croton floccosus according to the solvent used.
Data obtained from phytochemical tests
Table 4 details the qualitative results of the phytochemical tests performed on the ethanolic extracts of Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae), obtained with Soxhlet extraction. There is a low presence of flavonoids and saponins, and a relatively abundant presence of phenols and tannins for both species. In addition, a relatively abundant presence of alkaloids is detected for the species Croton floccosus and no reducing sugars are detected, while a low presence of reducing sugars is detected for the species Bidens pilosa L.
Table 4. Results of the phytochemical tests carried out on the ethanolic extracts of the species Bidens pilosa L. and Croton floccosus obtained with Soxhlet extraction.
| Alkaloids | Reducing sugars | Phenols | Flavonoids | Tannins | Saponins | |
|---|---|---|---|---|---|---|
| Ethanolic extract of Bidens pilosa L. obtained by Soxhlet | + | + | + + | + | + + | + |
| Ethanol extract of Croton floccosus obtained by Soxhlet | + + | - | + + | + | + + | + |
The equivalent mg of gallic acid corresponding to each gram of dry sample of Bidens pilosa L. were obtained, as shown in ( Table 5). As will be noted, Tukey’s HSD test for the phenolic content of the different samples made it possible to determine that there are significant differences among them (p<0.05). Samples that share the same letter are homogeneous.
Table 5. Total phenolic content corresponding to the equivalent of gallic acid.
| Sample | mg gallic acid equivalent/g dry sample | Average ± D. standard | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Soxhlet at 40°C | 38.546 | 39.036 | 39.444 | 39.009 ± 0.450 a |
| Soxhlet at 50°C | 48.192 | 48.738 | 48.898 | 48.609 ± 0.370 b |
| Ultrasonic bath at 40°C | 30.73 | 30.35 | 29.734 | 30.271 ± 0.503 c |
| Ultrasonic bath at 50°C | 40.256 | 40.302 | 40.546 | 40.368 ± 0.156 d |
| Maceration at 40°C | 26.446 | 27.092 | 27.566 | 27.035 ± 0.562 e |
| Maceration at 50°C | 29.746 | 30.428 | 30.748 | 30.307 ± 0.512 c |
Values are the mean of triplicate determinations. Different lowercase letters indicate statistical difference p<0.05.
Based on data from Ruiz Reyes et al. 30
As can be seen in Figure 9, the phenolic content for the extracts has values of 27–49 mg GAE/g of dry sample for the different processes employed to obtain ethanolic extracts of Bidens pilosa L. with leaves dried at 40°C and 50°C. Those with the lowest value correspond to the extracts obtained using the maceration method and those with the highest value correspond to the Soxhlet extraction process, with a value of 48.609 ± 0.370 mg GAE/g of dry sample of the extract obtained from the material dried at 50°C. In their research, Singh et al. 31 obtained methanolic extracts from leaves of Bidens pilosa L. dried at 30°C with a total phenolic content corresponding to 72 mg GAE/g of dry extract. This value is approximately 32% higher than that obtained in the present investigation, but it is necessary to mention that the extraction carried out by Singh et al. was carried out using an extraction process involving broken evaporation at a reduced pressure. In their investigation, meanwhile, Falowo et al. 32 obtained phenolic content for the extracts of B. pilosa corresponding to 75.9 mg GAE/g of dry extract when an exhaustive maceration was carried out with an ethanol-water solution (7:3) with shaking at room temperature for two days.
Figure 9. Phenolic content of the different ethanolic extracts of Bidens pilosa L.

The equivalent mg of gallic acid corresponding to each gram of dry Croton floccosus sample were obtained in the same way. These values are presented in Table 6. It was noted that Tukey’s HSD test for the phenolic content of the different samples determined that there is a significant difference among them (p <0.05) Samples that shared the same letter are homogeneous.
Table 6. Total phenolic content corresponding to the equivalent of gallic acid.
| Sample | mg gallic acid equivalent per g of dry sample | Average ± D. standard | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Soxhlet at 40°C | 124.146 | 125.562 | 125.55 | 125.086 ± 0.814 a |
| Soxhlet at 50°C | 89.86 | 89.746 | 89.9 | 89.835 ± 0.080 b |
| Ultrasonic bath at 40°C | 42.614 | 42.964 | 42.988 | 42.855± 0.209 c |
| Ultrasonic bath at 50°C | 80.576 | 80.618 | 80.82 | 80.671 ± 0.130 d |
| Maceration at 40°C | 121.91 | 122.284 | 121.704 | 121.966 ± 0.294 e |
| Maceration at 50°C | 127.874 | 127.856 | 128.906 | 128.212 ± 0.601 f |
Values are the mean of triplicate determinations. Different lowercase letters indicate statistical difference p<0.05.
Based on data from Ruiz Reyes et al. 30
A range of 42–129 mg GAE/g of dry sample were obtained from the different processes employed to obtain ethanolic extracts of Croton floccosus, as can be seen in Figure 10. Those with the lowest value correspond to the extracts obtained using the ultrasonic bath method, and those with the highest value correspond to the extraction process carried out using maceration, with a value of 128.212 ± 0.601 mg GAE/g of dry sample of the extract obtained from the material dried at 50°C. This contrasts strongly with the values obtained for Bidens pilosa L., since the extracts obtained by means of maceration had the lowest total phenolic content. Taking into account that this species is considered endemic to Ecuador, Pazmiño, 33 in his research, obtained ethanolic extracts from the freeze-dried “latex” of Croton floccosus, which had a total phenolic content corresponding to 271.212 ± 3.1728 mg GAE/g of dry extract. This value is approximately 53% higher than that obtained in the present investigation, and it is necessary to mention that the values presented by this author correspond to those of an extract obtained from the “latex” of this species.
Figure 10. Phenolic content of the different ethanolic extracts of Croton floccosus.
Content of total flavonoid tests
The equivalent mg of quercetin corresponding to each gram of dry sample of Bidens pilosa L. were obtained, as shown in Table 7. Tukey’s HSD test for the flavonoid content of the different samples determined that there is a significant difference among them (p<0.05). Samples that shared the same letter are homogeneous.
Table 7. Total flavonoid content corresponding to quercetin equivalents.
| Sample | mg quercetin equivalent/g dry sample | Average ± D. standard | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Soxhlet at 40°C | 17.851 | 17.809 | 17.725 | 17.795 ± 0.0644 a |
| Soxhlet at 50°C | 16.879 | 16.815 | 16.853 | 16.849 ± 0.0322 b |
| Ultrasonic bath at 40°C | 17.643 | 17.705 | 17.648 | 17.665 ± 0.0042 a |
| Ultrasonic bath at 50°C | 16.583 | 16.590 | 16.582 | 16.585 ± 0.0218 b |
| Maceration at 40°C | 13.966 | 14.009 | 13.982 | 13.986 ± 0.0218 c |
| Maceration at 50°C | 12.374 | 12.849 | 12.440 | 12.554 ± 0.2573 d |
Values are the mean of triplicate determinations. Different lowercase letters indicate statistical difference p<0.05.
Based on data from Ruiz Reyes et al. 30
A range of 12–18 mg QE/g of dry sample were obtained from the different processes employed to obtain ethanolic extracts of Bidens pilosa L., as can be seen in Figure 11. Those with the lowest value correspond to the extracts obtained using the maceration method, and those with the highest value correspond to the extraction process carried out with Soxhlet and the ultrasonic bath. There is statistical homogeneity between the values obtained for both processes, with a value of 17.795 ± 0.0644 and 17.665 ± 0.0042a mg QE/g of dry sample of the extract obtained from the corresponding material dried at 40°C. In their study, Cortes-Rojas et al. 34 determined the flavonoid content of Bidens pilosa L. extracts by employing dynamic maceration, Soxhlet, ultrasound and microwave, obtaining corresponding values of 21.988 ± 0.127; 19.623 ± 0.100; 16.482 ± 0.180 and 11.590 ± 0.042 mg QE/g dry sample. These values coincide with those obtained in the present study, with the exception of those for dynamic maceration and microwave.
Figure 11. Total flavonoid content of the different ethanolic extracts of Bidens pilosa.

The equivalent mg of quercetin corresponding to each gram of dry Croton floccosus sample were obtained in the same way, as shown in Table 8. Tukey’s HSD test for the flavonoid content of the different samples determined that there is a significant difference among them (p <0.05). Samples that shared the same letter are homogeneous.
Table 8. Total flavonoid content corresponding to quercetin equivalents.
| Sample | mg quercetin equivalent per g of dry sample | Average ± D. standard | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Soxhlet at 40°C | 34.117 | 34.209 | 34.091 | 34.139 ± 0.06224 a |
| Soxhlet at 50°C | 13.079 | 13.103 | 13.183 | 13.122 ± 0.05441 b |
| Ultrasonic bath at 40°C | 7.458 | 7.193 | 7.201 | 7.284 ± 0.15096 c |
| Ultrasonic bath at 50°C | 12.494 | 12.520 | 12.515 | 12.509 ± 0.01360 d |
| Maceration at 40°C | 22.201 | 22.281 | 22.329 | 22.270 ± 0.06461 e |
| Maceration at 50°C | 27.315 | 27.025 | 26.952 | 27.097 ± 0.1984 f |
Values are the mean of triplicate determinations. Different lowercase letters indicate statistical difference p<0.05.
Based on data from Ruiz Reyes et al. 30
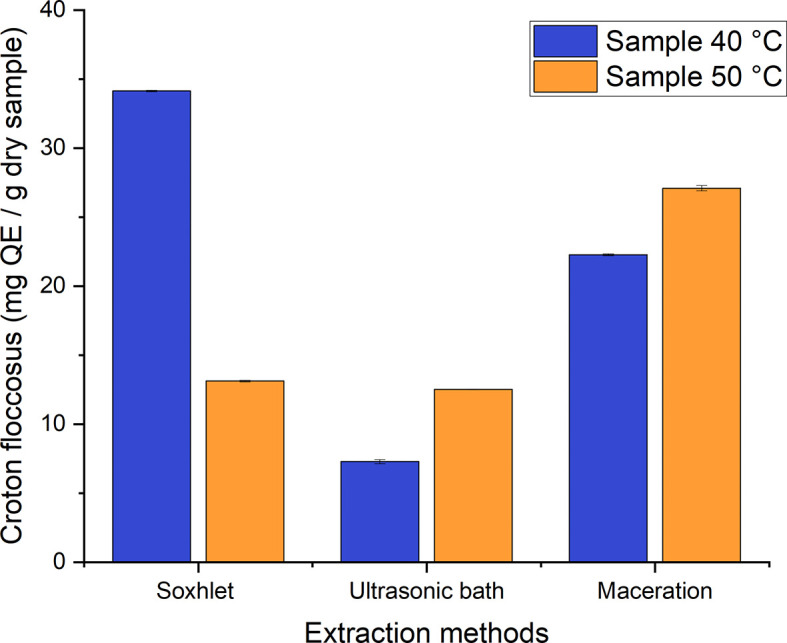
The established range of total flavonoids comprises values between 7–35 mg QE/g of dry samples for the different processes used to obtain ethanolic extracts of Croton floccosus ( Figure 12). As can be seen, there is a great difference between the different treatments. That with the highest flavonoid content corresponds to the Soxhlet extraction process of the material dried at 40°C, with a value of 34.139 ± 0.06224 mg QE/g, while that with the lowest value corresponds to the extracts obtained by employing the ultrasonic bath method at the same temperature, with a value of 7.284 ± 0.15096 mg QE/g of dry sample.
Figure 12. Total flavonoid content of the different ethanolic extracts of Croton floccosus.
Antioxidant capacity (DPPH)
The in vitro antioxidant activity was performed by employing the 2.2-diphenyl-1-picryl-hydracil radical scavenging method (DPPH assay). All the antioxidant activity values for the ethanolic extracts of both species were obtained from the Trolox equivalent calibration curve (TEAC). The established range for the Trolox equivalent antioxidant activity (TEAC) for Bidens pilosa L. is 74–78 μmol Trolox equivalent (TE)/g of dry samples for the different processes used to obtain ethanolic extracts with leaves dried at 40°C and 50°C ( Figure 13). The means of the values were compared using the Tukey test, which showed that there is no marked difference among the processes studied with regard to the TEAC. It was, therefore, established that the drying temperatures do not directly influence the TEAC values for the maceration, ultrasonic bath and Soxhlet extraction processes.
Figure 13. Antioxidant activity equivalent to Trolox of the different extracts of Bidens pilosa L.
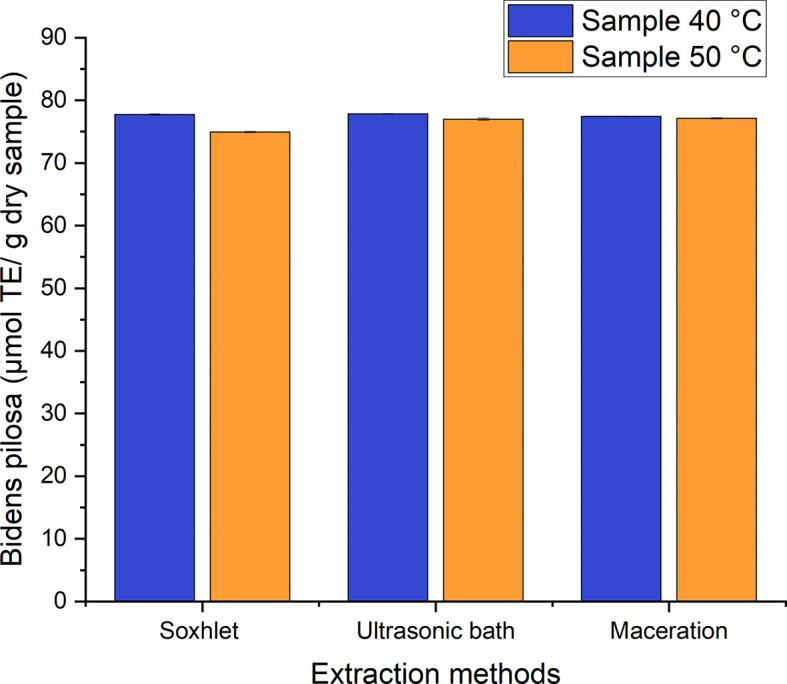
Considering that there was no marked difference among the processes, we then proceeded to establish the mean radical inhibition coefficient (IC50) of the process that attained the highest antioxidant activity value equivalent to Trolox, Soxhlet extraction of the material dried at 50°C. Table 9 shows the inhibition coefficient (IC50) determined by the DPPH method performed on the extract of Bidens pilosa L. and Trolox (standard). The difference between the extract and the standard used in the previous determinations is compared.
Table 9. Comparison of inhibition coefficients for Bidens pilosa L. and Trolox.
| Sample | IC 50 (μg/mL) |
|---|---|
| Bidens pilosa L. ethanolic extract | 239.333 |
| Trolox | 2.599 |
The values reported in the present study are lower than those reported by Cortés-Rojas et al., 34 who obtained an IC50 for the DPPH radical of 35.35 μg/mL for Bidens pilosa L. extracts from flowers and leaves collected in Brazil using dynamic maceration at 45°C and with 200 rpm of agitation. Furthermore, Singh et al. 31 obtained an IC50 value of 80.45 μg/mL for extracts from the leaves of the same species collected in India using maceration with methanol for 48 h. We are, therefore, of the opinion that the differences between our IC50 values and those reported are related to the time of year and the country in which the plant was collected, along with the conditions under which the plant material was prepared and the method used to obtain the extract of secondary metabolites.
The established range for the Trolox equivalent antioxidant activity (TEAC) for Croton floccosus is 22–27 μmol TE/g of dry samples for the different processes used to obtain ethanolic extracts with leaves dried at 40°C and 50°C ( Figure 14). The means of the values were compared using the Tukey test. No marked difference among the processes studied was found for the TEAC, and there was statistical homogeneity among them. It was, therefore, established that, as with Bidens pilosa L., there is no marked difference in the antioxidant activity values obtained using the different extraction processes carried out in the present research.
Figure 14. Antioxidant activity equivalent to Trolox of Croton floccosus.

As with the previous process, there is no marked difference among the methods, and we, therefore, proceeded to establish the mean radical inhibition coefficient (IC50) of the process that obtained the highest value of antioxidant activity equivalent to Trolox, which was the ultrasound of the material dried at 40°C.
Table 10 shows the inhibition coefficient (IC50) determined by the DPPH method performed on the extract of Croton floccosus and Trolox (standard). The difference between the extract and the standard used in the previous determinations is compared.
Table 10. Comparison of inhibition coefficients for Croton floccosus and Trolox.
| Sample | IC 50 (μg/mL) |
|---|---|
| Croton floccosus ethanolic extract | 644.125 |
| Trolox | 2.599 |
It is necessary to mention that, since Croton floccosus is a species that is endemic to Ecuador, there are only a few studies referring to this species. In his study, M.E. Flores 35 obtained IC50 values of 91.16 μg/mL for extracts obtained from the leaves of Croton floccosus collected during the flowering period of this species using a maceration process in which ethanol was employed as a solvent. This, therefore, made it possible to establish what is described by Altamirano 36 in his research work on Croton species, which states that the collection carried out to obtain extracts must take place at the beginning of flowering because it is the moment at which these plants contain the greatest amount of active substances. This may, therefore, have influenced the properties of the extract, as may the conditions under which the material was prepared, the extraction and the storage of the extracts.
Conclusions
The use of phytochemical tests to identify metabolites, along with their qualitative characterization show that the plants studied have a great variety of chemical compounds, which contain flavonoids, phenols, reducing sugars, saponins and tannins in the case of Bidens pilosa L., and alkaloids, phenols, flavonoids, tannins and saponins in that of Croton floccosus. It has been shown that the content of total phenols for Bidens pilosa L. had the highest yield of phenolic compounds when the Soxhlet extraction method was used with the plant material dried at 50°C, with a value corresponding to 48.609 ± 0.370 mg GAE/g of dry sample. With regard to the content of flavonoids, a maximum value of 17.795 ± 0.0644 mg QE/g of dry sample was obtained, corresponding to the Soxhlet extraction process method at 40°C. In the case of Croton floccosus, the maximum reported value of total phenols corresponds to the maceration extraction process when the material had been dried at 50°C, with a value of 128.212 ± 0.601 mg GAE/g of dry sample. With regard to the content of total flavonoids, the best results corresponded to the extraction process carried out using Soxhlet extraction with the plant material dried at 40°C, with a value of 34.139 ± 0.06224 mg QE/g dry sample. Finally, both plant species showed antioxidant potential, although it is necessary to establish that the Bidens pilosa L. species responded better to the test established, with an IC50 of 239.33 μg/mL when compared to Croton floccosus, which attained an IC50 of 644.125 μg/mL.
Data availability
Underlying data
Open Science Framework: Underlying data for ‘Phytochemical study of the plant species Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae)’, https://doi.org/10.17605/OSF.IO/VNPMJ. 30
This project contains the following underlying data:
-
•
Data file: Antioxidant activity DPPH.xlsx
-
•
Data file: Flavonoids.xlsx
-
•
Data file: Phenols.xlsx
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
The authors acknowledge the support of the Universidad Técnica de Manabí (UTM) in the Convocation Project approved in 2018 with the “Tamizaje fitoquímico y determinación de la actividad antioxidante y antibacterial de 8 familias de plantas medicinales que se encuentran en la provincia de Manabí”.
Funding Statement
This research was developed with the funds received by the 2018 Universidad Técnica de Manabí Call Project.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Mittermeier RA: Primate diversity and the tropical forest. Washington, DC Washington, DC: National Academy Press;1988. [Google Scholar]
- 2. Jørgensen PMlLo-YnSMBG: Catalogue of the vascular plants of Ecuador = Catologo de las plantas vasculares del Ecuador. St. Louis, Mo: Missouri Botanical Garden Press;1999. [Google Scholar]
- 3. Riina R, Vigo MA, Ceron CE: Croton condorensis: an enigmatic new species of Euphorbiaceae from southern Ecuador. Phytotaxa. 2014;164(2):154–158. 10.11646/phytotaxa.164.2.10 [DOI] [Google Scholar]
- 4. Riina R, Cumbicus N, Feio AC, et al. : A new species of dragon’s blood Croton (Euphorbiaceae) from South America with singular inflorescences. Webbia. 2015;70(1):187–192. 10.1080/00837792.2015.1020129 [DOI] [Google Scholar]
- 5. Riina R, Berry PE: Two new South American species of Croton (Euphorbiaceae) and their phylogenetic affinities. Anales del Jardín Botánico de Madrid. Consejo Superior de Investigaciones Científicas;2010;67(1):23–27. [Google Scholar]
- 6. Obey JK, Wright A, Orjala J, et al. : Antimicrobial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. J. Pathog. 2016:1–5. 10.1155/2016/1453428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nath R, Roy S, De B, et al. : Anticancer and antioxidant activity of croton: a review. Int. J. Pharm. Pharm. Sci. 2013;5(2):63–70. [Google Scholar]
- 8. Suárez AI, Blanco Z, Compagnone RS, et al. : Anti-inflammatory activity of Croton cuneatus aqueous extract. J. Ethnopharmacol. 2006;105(1–2):99–101. 10.1016/j.jep.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 9. Savietto JP, et al. : Antiproliferative activity of methanol extracts of four species of Croton on different human cell lines. Rev. Bras. 2013;23:662–667. 10.1590/S0102-695X2013005000058 [DOI] [Google Scholar]
- 10. Suárez AI, et al. : Cytotoxic activity of seco-entkaurenes from Croton caracasana on human cancer cell lines. Nat. Prod. Commun. 2009;4(11):1934578X0900401117. [PubMed] [Google Scholar]
- 11. Smith BA: A new species of Croton (Euphorbiaceae) from Ecuador. Novon. 2006;16(2):272–275. 10.3417/1055-3177(2006)16[272:ANSOCE]2.0.CO;2 [DOI] [Google Scholar]
- 12. Karis P, Ryding O: Asteraceae Cladistics and Classification. Bremer K, editor. Asteraceae: cladistics & classification. Portland. USA: Timber Press;1994. [Google Scholar]
- 13. Pozharitskaya O, et al. : Anti-inflammatory activity of a HPLC-fingerprinted aqueous infusion of aerial part of Bidens tri-partita L. Phytomedicine. 2010;17(6):463–468. 10.1016/j.phymed.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 14. Lima Silva F, Fischer DCH, Fechine Tavares J, et al. : Compilation of secondary metabolites from Bidens pilosa L. Molecules. 2011;16(2):1070–1102. 10.3390/molecules16021070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumari P, et al. : A promising anticancer and antimalarial component from the leaves of Bidens pilosa. Planta Med. 2009;75(01):59–61. 10.1055/s-0028-1088362 [DOI] [PubMed] [Google Scholar]
- 16. Horiuchi M, Seyama Y: Antiinflammatory and Antiallergic Activity of Bidens pilosa L. var. radiata SCHERFF. J. Health Sci. 2006;52(6):711–717. 10.1248/jhs.52.711 [DOI] [Google Scholar]
- 17. Deba F, Xuan TD, Yasuda M, et al. : Chemical composition and antioxidant, antibacterial and antifungal ac-tivities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 2008;19(4):346–352. 10.1016/j.foodcont.2007.04.011 [DOI] [Google Scholar]
- 18. Deba F, Xuan TD, Yasuda M, et al. : Herbicidal and fungicidal activities and identification of potential phyto-toxins from Bidens pilosa L. var. radiata Scherff. Weed Biology and Management. 2007;7(2):77–83. 10.1111/j.1445-6664.2007.00239.x [DOI] [Google Scholar]
- 19. Uchôa VT, Paula RC, Krettli LG, et al. : Antimalarial activity of compounds and mixed fractions of Cecropia pachystachya. Drug Dev. Res. 2010;71(1):82–91. [Google Scholar]
- 20. Bartolome AP, Villaseñor IM, Yang WC: Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evidence-based Complementary and Alternative Medicine, Review. 2013; vol.2013. Art. no. 340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayasundera M, Florentine S, Tennakoon KU, et al. : Medicinal value of three agricultural weed species of the asteraceae family: A review. Pharmacognosy Journal, Review. 2021;13(1):264–277. 10.5530/pj.2021.13.36 [DOI] [Google Scholar]
- 22. Abdi H, Williams LJ: Tukey’s honestly significant difference (HSD) test. Encyclopedia of research design. 2010;3(1):1–5. [Google Scholar]
- 23. Ruiz-Reyes E, Moreira-Castro JM, Pin-Molina JM, et al. : PHYTOCHEMICAL STUDY OF THE PLANT SPECIES ZANTHOX-YLUM SPRUCEI (RUTACEAE) AND MELAMPODIUM DIVARICATUM (ASTERACEAE). Pharmacol. Ther. 2021;1:322–335. [Google Scholar]
- 24. María R, Shirley M, Xavier C, et al. : Fenoles totales, contenido de flavonoides y actividad antioxidante de los extractos etanólicos de plantas ecuatorianas. Revista de la Facultad de Farmacia. 2018;60(2):3–13. [Google Scholar]
- 25. Paredes ME, Morales RE, Lima WK, et al. : Morphoanatomical and phytochemical studies for the quality control of Neurolaena lobata (L.) R. Br. ex Cass.(Asteraceae). Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. 2019;18(3):277–288. [Google Scholar]
- 26. Goto T, Obara M, Aoki S, et al. : Evaluation of Polyphenolic Content and Potential Antioxidant Activity of Japanese Cultivars of Peaches, Prunes, and Plums Based on Reversed-and Normal-Phase HPLC and Principal Component Analyses. ACS Food Sci. Technol. 2021;1(10):2019–2029. [Google Scholar]
- 27. Yucailla A: Composición química y actividad antioxidante de extractos obtenida de las hojas de Mansoa alliacea. SANTA CLARA: UNIVERSIDAD CENTRAL “MARTA ABREU” DE LAS VILLAS;2018. [Google Scholar]
- 28. Camacho O, Melgarejo S: Actividad antioxidante del extracto etanólico e hidroalcohólico y determinación quí-mica de los componentes mayoritarios del fruto de Eugenia jambolana (Uva venezolana). Tesis Pregrado Programa de Farmacia. 2017.
- 29. Deng J, Cheng W, Yang G: A novel antioxidant activity index (AAU) for natural products using the DPPH assay. Food Chem. 2011;125(4):1430–1435. 10.1016/j.foodchem.2010.10.031 [DOI] [Google Scholar]
- 30. Ruiz-Reyes E, Mendoza-Cevallos MA, Polanco-Moreira AP, et al. : PHYTOCHEMICAL STUDY OF THE PLANT SPECIES BIDENS PILOSA L. (ASTERACEAE) AND CROTON FLOCCOSUS (EUPHORBIACEAE). Open Science Framework. Dataset. 2022, May 30. 10.17605/OSF.IO/VNPMJ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh G, et al. : Pharmacological potential of Bidens pilosa L. and determination of bioactive compounds using UHPLC-QqQLIT-MS/MS and GC/MS. BMC Complement. Altern. Med. 2017; Article vol.17(no.1): Art. no.492. 10.1186/s12906-017-2000-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falowo A, Muchenje V, Hugo C, et al. : In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. Cyta-J. Food. 2016;14(4):541–546. 10.1080/19476337.2016.1162847 [DOI] [Google Scholar]
- 33. Pazmiño Chancusi DA: Estudio farmacognósico de los productos naturales procesados de uso medicinal a base de sangre de drago y del látex en su forma natural. Quito: UCE;2021. [Google Scholar]
- 34. Cortés-Rojas DF, Chagas-Paula DA, Da Costa FB, et al. : Bioactive compounds in Bidens pilosa L. populations: A key step in the standardization of phytopharmaceutical preparations. Revista Brasileira de Farmacognosia, Article. 2013;23(1):28–35. 10.1590/S0102-695X2012005000100 [DOI] [Google Scholar]
- 35. Flores Herrera ME: Evaluación de la actividad antimicrobiana del extracto de corteza y hojas de Sangre de drago. Quito: UCE;2021. [Google Scholar]
- 36. Altamirano Pérez IV: Evaluación de la actividad antioxidante de cuatro especies del género Croton. Quito: UCE;2015. [Google Scholar]