Abstract
Cytokines function at the cellular, microenvironmental level, but human cytokine assessment is most commonly done at the macro level, by measuring serum cytokines. The relationships between serum and cellular cytokines, if there are any, are undefined. In a study of hospitalized patients in Malawi, we compared cytometrically assessed, cell-specific cytokine data to serum interleukin 2 (IL-2), IL-4, IL-6, IL-8, IL-10, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) levels in 16 children and 71 (IL-2, -4, -6, -10) or 159 (IL-8, IFN-γ, and TNF-α) adults, using Wilcoxon rank sum tests and Pearson's (rp) and Spearman's (rs) rank correlations. For the entire study group, correlations between identical serum and cellular cytokines mainly involved IL-8 and IFN-γ, were few, and were weakly positive (r < 0.40). Blood culture-positive persons had the most and strongest correlations, including those between serum IL-2 levels and the percentages of lymphocytes spontaneously making IL-2 (rs = +0.74), serum IL-8 levels and the percentages of lymphocytes spontaneously making IL-8 (rp = +0.66), and serum IL-10 levels and the percentages of CD8+ T cells making TNF-α (rp = +0.89). Human immunodeficiency virus (HIV)-positive persons had the next largest number of correlations, including several serum IL-8 level correlations, correlation of serum IL-10 levels with the percentages of lymphocytes producing induced IL-10 (rs = +0.36), and correlation of serum IFN-γ levels and the percentages of lymphocytes spontaneously making both IL-6 and IFN-γ in the same cell (rp = +0.59). HIV-negative, malaria smear-positive, and pediatric patients had few significant correlations; for the second and third of these subgroups, serum IL-8 level was correlated with the percentage of CD8− T cells producing induced IL-8 (rs = +0.40 and rs = +0.56, respectively). Thus, the strength of associations between serum and cellular cytokines varied with the presence or absence of bloodstream infection, HIV status, and perhaps other factors we did not assess. These results strongly suggest that serum cytokines at best only weakly reflect peripheral blood cell cytokine production and balances.
Cytokines regulate cellular immune interactions and are produced by lymphocytes, monocytes, macrophages, and, for some cytokines, also fibroblasts, neutrophils, endothelial cells, or mast cells (for a review, see reference 8). Although cytokines function on a microenvironmental level, human cytokines are most commonly assessed at the macro level, by measuring serum and plasma levels or levels in the supernatant of in vitro-stimulated blood cells. Reasons for this approach are largely practical. Serum and plasma levels can be readily assessed by using commercially available enzyme immunoassays, while cell-specific cytokine assessment is laborious, involving in situ hybridization, cell separation followed by PCR measurement of mRNA, limiting dilution assays, plaque and enzyme-linked immunospot (ELISPOT) assays, or T-cell cloning. In recent years, improved reagents have permitted flow cytometric cell-specific cytokine assessments in which ex vivo peripheral blood cell stimulation or nonstimulation is followed by cell permeabilization, fixation, fluorescent staining, and cytokine detection (12, 15). With multiparameter flow cytometry, very specific cell populations can be identified by surface antigen staining, without cell separation or cloning, and the production of multiple cytokines by individual cells can be assessed.
Given the variety of techniques now used for cytokine assessments, an obvious and pressing question is how the results of these techniques compare to one another. Yet, comparisons of various cytokine assessment techniques have involved only a few cytokines and tend to be qualitative rather than quantitative. In several studies, indirect comparisons were made, or could be made by the reader, between serum or plasma cytokine levels and cytokine mRNA or protein levels in the supernatant of stimulated whole blood cell cultures (2, 10, 19, 27), between serum or plasma and monocytic intracellular interleukin 6 (IL-6) production (24), among all three measures (serum or plasma, supernatant, and intracellular) of gamma interferon (IFN-γ) production (5), and between ELISPOT cytokine results and supernatant levels or intracellular IFN-γ production (16).
In the early publications on intracellular cytokine staining, supernatant and intracellular levels of two or three cytokines were directly compared. As expected, when cells were treated in culture with monensin or brefeldin A for short periods, supernatant cytokine levels decreased and intracellular cytokine staining increased (7, 15, 16). For five patients with helminthic infections and one healthy individual, supernatant and cellular IL-4 and IL-5 were highly correlated but IFN-γ levels were not (7). In another study of 13 children with respiratory syncytial virus infection and 2 control children, supernatant and intracellular IFN-γ results on acute or convalescent blood samples showed 90% negative-positive concordance, based on positivity in either CD4+ or CD8+ cells (5). In one study of three healthy tetanus toxoid-primed individuals, ELISPOT IFN-γ results were insignificantly correlated with intracellular staining but significantly correlated with culture supernatant levels (16).
We assessed cytokines in patients enrolled in a study of the causes and immune parameters of bloodstream infections in persons in Malawi. These patients all were acutely ill, and the rate of bacteremia or mycobacteremia was high (14, 14a). Human immunodeficiency virus (HIV) infection is endemic in Malawi, and the HIV seropositivity rate was also high in this study group. Thus, this study included a high proportion of persons with chronic and acute systemic illnesses, an ideal group in which to assess relationships between peripheral blood cytokine production and serum cytokine levels. Therefore, we examined both serum and cell-specific cytokines in a large number of these patients and compared the results quantitatively, as well as qualitatively. We evaluated a wide panel of type 1 (IL-2, IFN-γ, tumor necrosis factor alpha [TNF-α]), type 2 (IL-4, IL-6, IL-10), proinflammatory (TNF-α, IL-8) and anti-inflammatory (IL-4, IL-10) cytokines. We did three additional analyses to further refine these comparisons. First, we not only examined data from this entire study group of acutely ill patients, but we also did separate analyses for HIV-seropositive (HIV+) and HIV-seronegative persons, persons with positive blood cultures, and persons with positive malaria smears. Since HIV+, blood culture-positive, and malaria smear-positive persons have organisms in their bloodstream, it seemed more reasonable to assess their peripheral blood cytokine parameters than to assess these parameters for healthy persons or persons with localized diseases. Second, we not only compared serum cytokines levels to levels of cell-specific cytokines produced following ex vivo stimulation (the cell-specific cytokine measurement usually reported in the literature), but we also compared serum cytokines to cell-specific cytokine production without ex vivo stimulation. We felt this was worth doing since these sorts of patients might be expected to have in vivo-stimulated cells and, indeed, we have noticed that the peripheral blood cells of some of the patients in this study produced cytokines without in vitro stimulation (unpublished data). Third, since cytokines interact with cells to balance type 1 and type 2 and proinflammatory and anti-inflammatory cytokine activities (see, for example, references 1, 2, 7, 8, 11, 18, 23, 25, and 29), we examined whether there were any relationships between serum cytokines and nonidentical cytokine production by peripheral blood cells.
MATERIALS AND METHODS
Patients.
During three periods in 1997 and 1998, including both the wet and dry seasons, we enrolled 161 febrile (oral temperature, >38°C) adults (≥13 years old) and 148 children (<13 years old) admitted to the Lilongwe Central Hospital, Lilongwe Malawi, into a study of the immune determinants of bloodstream infections. Nonfebrile children admitted to the hospital during the enrollment period were included in the study because infected infants often present with low or normal body temperatures. This group represents a random subset of all patients admitted to the hospital during those time periods. All patients had peripheral blood drawn at admission for culture and cytometric assessment of cell surface antigens and intracellular cytokines, with the cytometric monoclonal antibody panel varying slightly between study phases (Table 1). Patients with sera obtained and stored at the time of admission also had serum cytokine levels assessed. Serum volumes were limited; therefore, cytokine testing was prioritized. Highest priority was given to assessment of IFN-γ and TNF-α, because of the high rates of mycobacterial infection and HIV in this population and the importance of these cytokines in those infections (14), and assessment of IL-8, because these patients were all acutely ill, with systemic symptoms of an inflammatory process. One hundred fifty-nine adults and 16 children had their serum cytokine levels assessed. Of these, 115 (66%) were HIV+, 45 (26%) were blood culture-positive, and 32 (18%) had positive malaria smears.
TABLE 1.
Analysis panel for flow cytometric evaluation of cell-specific cytokine production
| Cytokine or cell antigen specificity of monoclonal antibody, with the following conjugate:
| |||
|---|---|---|---|
| FITC | PE | PerCP | APC |
| IL-12 | IL-4 | CD3 | CD19 |
| CD69 | IL-10 | CD3 | IL-2a |
| IFN-γ | IL-10 | CD3 | IL-2a |
| Antimicrotubulin | IL-8 | CD8 | CD3 |
| CD8 | IL-6 | CD3 | IFN-γ |
| TNF-α | CD16/56 | CD3 | IFN-γ |
| TNF-α | CD8 | CD3 | IFN-γ |
One or the other of these tubes was run, depending upon study phase. For no participant were both these tubes done.
The study protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) and the Malawian Health Sciences Research Committee; informed consent was obtained from all participants and/or their guardians.
Laboratory procedures. (i) HIV.
Each participant was tested for HIV antibody at study enrollment.
(ii) Blood cultures.
Blood cultures were performed as described previously (14). BACTEC MYCO/F LYTIC bottles (Becton Dickinson Microbiology Systems, Cockeysville, Md.) were incubated at 35°C for 7 days and examined each day and over 4 to 6 weeks thereafter. These culture techniques readily detect pathogenic bacteria, fungi, and mycobacterium species (14). For the 45 bacteremic persons discussed herein, organisms recovered included gram-positive cocci (n = 13), salmonellae (n = 17), gram-negative rods (n = 4), candida (n = 3), and/or mycobacteria (n = 10); two persons had dual infections.
(iii) Stimulation of cellular cytokines.
Blood was prepared for cytokine stimulation as described previously (14) and either stimulated for 5 h at 37°C with phorbol 12-myristate 13-acetate (PMA) (200 ng/ml; Sigma Chemical Co., St. Louis, Mo.) and ionomycin (4 μg/ml) (Sigma) in the presence of brefeldin A (40 μg/ml; Sigma) and RPMI 1640 with l-glutamine (induced or stimulated cytokine expression) or retained in identical media without PMA and ionomycin but with brefeldin A (spontaneous or unstimulated cytokine expression). No serum was added to the cultures. After washing, the red blood cells were lysed and lymphocytes were permeabilized and fixed using Ortho Permeafix (Ortho Diagnostic Systems, Inc., Raritan, N.J.). After processing, samples were shipped at 4 to 8°C to CDC for further analysis.
(iv) Flow cytometric reagents.
The surface antigens assessed in this study were ones shown in our laboratory to be stable with this permeabilization and fixation protocol (data not shown); i.e., using these techniques, we had comparable results for the surface-related antigens when staining was done either pre- or postpermeabilization. Fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, peridinin chlorophyll protein (PerCP)-conjugated, or allophycocyanin (APC)-conjugated, murine monoclonal antibodies were obtained from the following sources: (i) Becton Dickinson Immunocytometry Systems/PharMingen (BD/PMG), San Jose, Calif. (CD8-FITC and -PE [clone SK1], CD3-PerCP and -APC [clone SK7], CD4-APC [clone SK3], CD45-FITC [clone 2D1], CD19-APC [clone SJ25C1], CD14-PE [clone MφP9], CD16-PE [clone B73.1], CD56 [clone MY31], IL-4-PE [clone 8D4-8], IL-8-PE [clone G265-8], and IL-10-PE [clone JES3-9D7]); (ii) Research and Diagnostics, Minneapolis, Minn. (IL-6-PE [clone 1927.311]); and (iii) Immune Source, Reno, Nev. (CD8-APC [clone KL.12], IL-2 APC [R-56.2], TNFα-FITC [clone DTX.34], and IFN-γ-APC [clone 13.TR]). Isotype controls were obtained from BD/PMG.
(v) Flow cytometry.
After permeabilization, fixation, and shipment to CDC, all staining was done at room temperature for 30 min in the dark. Staining was followed by a buffered saline wash. Four-color cytometry was done using a FACSort or FACSCalibur flow cytometer and CellQuest software (BD/PMG). Between 50,000 and 80,000 ungated events were collected from each tube in the panel. Lymphocytes were defined on the basis of forward and side scatter; monocytes were defined on the basis of a wide gate based on forward and side scatter of stimulated and unstimulated CD14+ cells.
(vi) Flow cytometric analytic techniques.
For each participant, cellular cytokine analyses were as follows: for all lymphocytes and CD3+ lymphocytes, all the cytokines listed in section (iv) above; for CD3+ CD8+ lymphocytes and CD3+ CD8− lymphocytes, IL-6, IL-8, IFN-γ, and TNF-α; for CD3+ CD16/56+ natural T lymphocytes (NT) and CD3− CD16/56+ natural killer lymphocytes (NK), IFN-γ, and TNF-α; for CD19+ (B) lymphocytes, IL-4; and for monocytes, IL-6, IL-8, IL-10, and TNF-α (Table 1).
(vii) Serum cytokines.
Serum samples obtained at admission were analyzed for IL-2, IL-4, IL-6, IL-8, IL-10, IFN-γ, and TNF-α by using pairs of cytokine-specific monoclonal antibodies according to the manufacturers' instructions (BD/PMG and Genzyme Diagnostics, Cambridge, Mass.) (Table 2). Each plate included a standard curve of recombinant human cytokine and known positive and negative controls. All specimens were measured in duplicate, and the means of the two values were used in all analyses. Detection limits were 7.8 pg/ml for IL-2, IL-8, and IL-10; 15.6 pg/ml for IL-4, TNF-α, and IFN-γ; and 4.7 pg/ml for IL-6. The numbers of adults with matching serum cytokines and cellular cytokines assessed were (depending upon the number of patients with samples assessed for that cell type): IL-2, n = 67; IL-4, n = 67; IL-6, n = 66 to 67; IL-8, n = 150 to 151; IL-10, n = 67; IFN-γ, n = 151-4 and TNF-α, n = 67 or n = 143 to 153. For 16 children, serum and matching cellular cytokines were assessed.
TABLE 2.
Median and ranges for serum cytokine level evaluated in Malawian patients
| Cytokine | No. of patients assessed | Concn (pg/ml)
|
|
|---|---|---|---|
| Median | Rangea | ||
| IL-2 | 87 | 9 | <8–446 |
| IL-4 | 87 | <16 | <16 |
| IL-6 | 87 | 387 | 9–124,300 |
| IL-8 | 175 | 107 | <8–4,477,000 |
| IL-10 | 87 | 39 | <8–23,000 |
| IFN-γ | 175 | 26 | <16–1,280 |
| TNF-α | 175 | <16 | <16–971 |
Detection limits for IL-2, IL-8, and IL-10, 7.8 pg/ml; for IL-4, IFN-γ, and TNF-α, 15.6 pg/ml; and for IL-6, 4.7 pg/mL.
Statistical techniques.
Analyses were done for all participants with serum cytokine levels assessed and, in addition, where noted the following subgroups: HIV+, HIV−, blood culture-positive, malaria smear-positive, and children. Comparisons of cellular parameters between those with and and without detectable serum cytokines were made using Wilcoxon rank sum tests. Pearson's (rp) and Spearman's (rs) rank correlations were computed to assess correlations between continuous serum and cellular parameters, for those with detectable serum cytokines. The significance level for all comparisons between identical serum and cellular cytokines was set at P < 0.05 (Table 3). Comparisons between nonidentical serum and cellular cytokines are described in Table 4 only if |r| ≥ 0.35 and P < 0.0001. For both types of comparisons, results are included only if the trend remained with outliers excluded and a compatible trend was found with the appropriate regression analysis. Data not provided herein did not meet these criteria. For IL-4, all serum levels were below the detection limit; therefore, trends could not be assessed. For serum TNF-α levels, 146 samples, including all the pediatric samples, were below the detection limit and 29 were above that limit.
TABLE 3.
Significant correlations between serum cytokine levels and matching intracellular cytokinesa
| Cytokine | Cell type | Stimulation statusb | Coefficient | P | Testc | No. of patients assessed |
|---|---|---|---|---|---|---|
| IL-2 | None | NAd | NA | NA | NA | 44 |
| IL-4 | NA | NA | NA | NA | NA | 0 |
| IL-6 | CD3+ CD8+ cells | U | +0.36 | <.001 | P | 82 |
| CD3+ CD8+ cells | S | +0.26 | 0.017 | P | 82 | |
| IL-8 | Lymphocytes | U | +0.21 | 0.017 | Sp | 128 |
| CD3+ cells | U | +0.22 | 0.012 | Sp | 128 | |
| CD3+ CD8+ cells | U | +0.33 | <.001 | Sp | 128 | |
| Monocytes | U | +0.30 | <.001 | Sp | 128 | |
| CD3+ cells | S | +0.23 | 0.009 | Sp | 127 | |
| CD3+ CD8+ cells | S | +0.39 | <.001 | Sp | 127 | |
| IL-10 | Lymphocytes | S | +0.30 | 0.018 | Sp | 61 |
| IFN-γ | Lymphocytes | U | +0.30 | 0.003 | P | 95 |
| CD3+ cells | U | +0.35 | <.001 | Sp | 95 | |
| CD3+ CD8+ cells | S | +0.25 | 0.020 | P | 85 | |
| CD3+ CD8− cells | S | +0.25 | 0.020 | P | 85 | |
| TNF-α | None | NA | NA | NA | NA | 29 |
Correlations between percentages of cells making cytokine and serum cytokine levels, including only those with detectable titers. Listed only if trend remained with outliers excluded. All values for serum IL-4 levels were nondetectable (<15.6 pg/ml); therefore, trends could not be assessed.
Unstimulated cells (U) and cells stimulated with PMA and ionomycin (S) (both with brefeldin A).
Abbreviations: P, Pearson; Sp, Spearman.
NA, nonapplicable.
TABLE 4.
Correlation coefficients of ≥0.35 between serum cytokine levels and nonmatching intracellular cytokinesa
| Serum cytokine | Cell type | Intracellular cytokine(s) | Stimulation statusb | Coefficient | Testc | No. |
|---|---|---|---|---|---|---|
| IL-2 | None | NAd | NA | NA | NA | 44 |
| IL-4 | NA | NA | NA | NA | NA | 0 |
| IL-6 | None | NA | NA | NA | NA | 87 |
| IL-8 | Lymphocytes | IFN-γ | S | −0.39 | Sp | 128 |
| Lymphocytes | IFN-γ and TNF-α | S | −0.42 | Sp | 128 | |
| Lymphocytes | IFN-γ and IL-6 | S | −0.39 | Sp | 124 | |
| Lymphocytes | IL-2 and IL-10 | U | −0.41 | Sp | 127 | |
| CD3+ cells | IL-2 | S | −0.35 | Sp | 128 | |
| CD3+ cells | IFN-γ | S | −0.49 | Sp | 128 | |
| CD3+ cells | IFN-γ and TNF-α | S | −0.37 | Sp | 127 | |
| CD3+ CD8+ cells | IFN-γ | S | −0.39 | Sp | 120 | |
| CD3+ CD8− cells | IFN-γ | S | −0.37 | Sp | 120 | |
| IL-10 | None | NA | NA | NA | NA | 83 |
| IFN-γ | Lymphocytes | IFN-γ and IL-6 | U | +0.48 | P | 91 |
| TNF-α | None | NA | NA | NA | NA | 83 |
Correlations between percentages of cells making cytokine and serum cytokine levels. Included only if trend remained with outliers excluded. All values for serum IL-4 levels were nondetectable (below 15.6 pg/ml); therefore, trends could not be assessed.
Unstimulated cells (U) and cells stimulated with PMA and ionomycin (S) (both with brefeldin A).
All P values <0.0001 Abbreviations: P, Pearson; Sp, Spearman.
NA, nonapplicable.
RESULTS
Significant relationships between detectability of serum cytokines and matching cellular cytokine.
We first assessed cellular cytokine differences between those having and those not having the same cytokine detectable in serum. For IL-2, detectability in serum was significantly, positively associated with higher percentages of lymphocytes making IL-2 with or without stimulation. For stimulated cells, the medians were 0.9% for those with nondetectable serum levels versus 1.5% for those with detectable serum levels, P = 0.032; for unstimulated cells, the medians were 0.3% versus 0.6%; P = 0.028. These IL-2 relationships were due to HIV-seropositive (HIV+)(n = 26, 21), blood culture-positive (n = 10, 9), and pediatric (n = 12, 4) participants; when HIV+ and HIV-negative participants were analyzed separately, it was not present in the latter group (n = 12, 22), nor was it present in those with malaria (n = 9, 17). All participants tested had detectable serum IL-6 levels. The percentages of CD3+ CD8+ cells and monocytes making IL-8 with stimulation were the only cellular parameters significantly associated with detectable serum IL-8, but the directions were opposite to one another (medians 1.5% for those with nondetectable levels versus 2.4%, P = 0.031 for those with detectable levels and 47.2% vs 35.8%, P = 0.022, respectively). These IL-8 relationships were present in all subgroups except the pediatric; all children had measurable levels of IL-8 in their sera. No cellular parameters were significantly associated with the presence or absence of detectable IL-10 in the serum. For all patients combined, the presence of detectable serum IFN-γ was significantly associated with the percentages of NT cells spontaneously making IFN-γ; however, this association was due to and present in only the HIV+ subgroup (medians 1.0% vs. 1.3%, P = 0.027). There were no other significant relationships between serum and cellular IFN-γ findings in any subgroup. The children and all but three HIV-negative persons had nondetectable serum levels of TNF-α. For those with a positive malaria smear, detectable serum TNF-α was associated with higher percents of CD3+ CD8− lymphocytes making induced TNF-α (medians, 9.7 versus 25.4%; P = 0.036).
Significant correlations between serum cytokines and matching cellular cytokines.
Next, we assessed the relationships between the actual serum cytokine levels and the percentages of cells making that cytokine. Correlations between matching serum and cellular cytokines were weak but in a positive direction (Table 3).
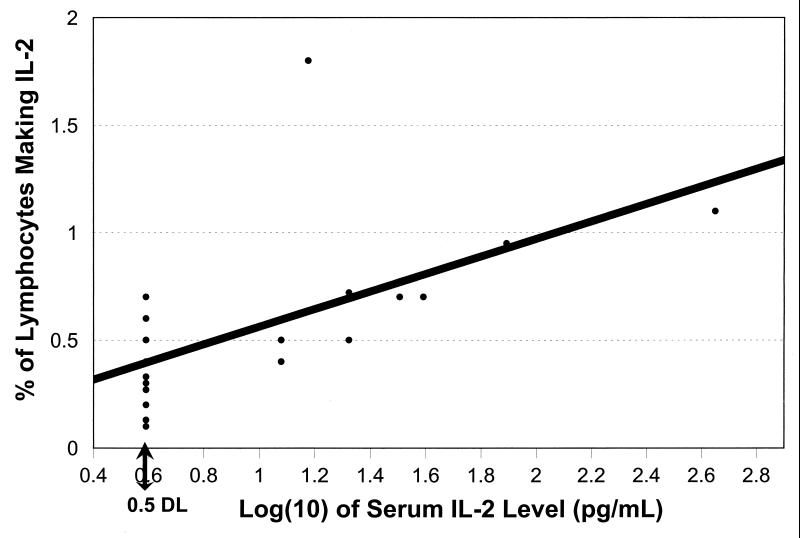
We also examined correlations for the following five subgroups: HIV+, HIV-negative, blood culture-positive, malaria-positive, and pediatric participants. The only notable deviations from Table 3 were as follows. Only nine blood culture-positive persons had measurable serum IL-2. If nondetectable levels were set at half the detection limit for the assay, the correlation between serum IL-2 levels and the percentages of lymphocytes spontaneously producing IL-2 was high (Fig. 1). This was not the case for other subgroups; for HIV-negative participants, serum IL-2 levels were in fact negatively, not positively, correlated with the percentages of CD3+ cells making induced IL-2 (rs = −0.43; P = 0.044; n = 22). For those with positive blood cultures, serum IL-8 levels were positively correlated with the percentages of lymphocytes and monocytes spontaneously making IL-8 (rp = +0.66, P < 0.001, and rs = +0.38, P = 0.020, respectively [n = 37]) and the percentages of CD3+ CD8+ lymphocytes making induced IL-8 (rs = +0.50; P = 0.001). For HIV+ persons, all the IL-8 correlations listed in Table 3 were significant; for HIV-negative persons, serum IL-8 levels were correlated with only the percents of CD3+ CD8+ cells making IL-8 with stimulation. For malaria smear-positive and pediatric patients, serum IL-8 was correlated with the percents of CD3+ CD8− lymphocytes making IL-8 with induction (rs = +0.40, P = 0.041, n = 27, and rs = +0.56, P = 0.024, n = 16, respectively). For children, there also was a significant correlation between the percentages of monocytes making induced IL-8 (rs = +0.55, P = 0.028). The significant IL-6 correlations for the entire study group were secondary to a small number of HIV+ and of malaria smear-positive individuals who had relatively high levels of both serum and cellular parameters. Similarly, the IL-10 finding for the entire study group was largely due to the HIV+ subgroup (rs = +0.36, P = 0.036, n = 34). For both HIV+ and blood culture-positive patients, cellular production of IFN-γ by a variety of cell types was significantly correlated with serum IFN-γ levels, including CD3+ cells (HIV+); all, CD3+ CD8−, and NT lymphocytes (blood culture positive); and CD3+ CD8− and NK cells (both). Almost all the patients with detectable serum TNF-γ levels were HIV+ (n = 26 for HIV+, n = 3 for HIV negative, n = 6 for blood culture positive, n = 5 for malaria smear positive, and n = 0 for children). Therefore, subgroup analyses could only be done for HIV+ persons, for whom no significant serum-cellular relationships were found.
FIG. 1.
Serum IL-2 levels, by the percentage of lymphocytes spontaneously making IL-2 (blood culture-positive patients). Some points have been shifted slightly in order to be seen. Ten patients had serum IL-2 levels below the detection limit of this assay. For graphing purposes, their levels were recorded as half the detection limit (0.5 DL). If all samples were included in the analysis, rs = +0.74, n = 19; if only detectable samples were included, rs = +0.58, n = 9.
Significant correlations between serum cytokines and nonmatching cellular cytokines.
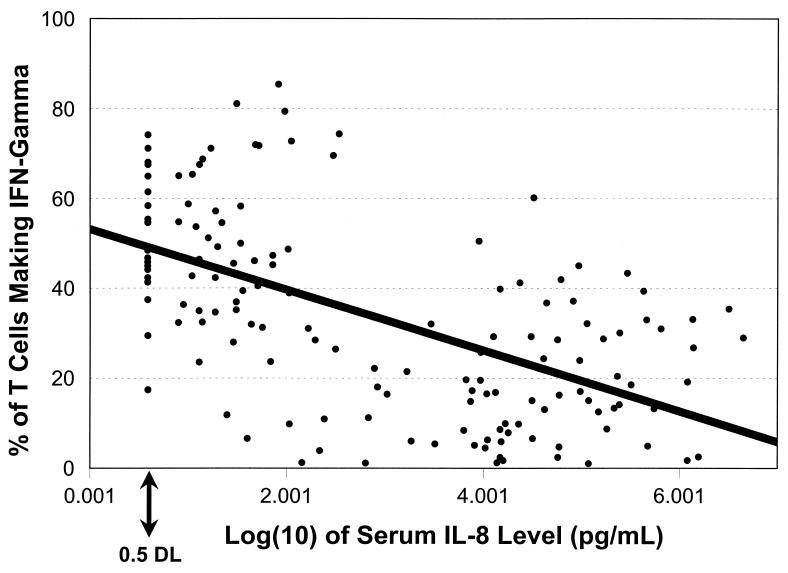
We last sought evidence for interactions between serum cytokines and cellular cytokine production by assessing correlations between nonidentical serum and cellular cytokines. For all subjects combined, the only significant correlations were between serum IL-8 and various cellular cytokine parameters and between serum IFN-γ and the percentages of lymphocytes spontaneously producing both IL-6 and IFN-γ in the same cell (Table 4). These correlations tended to be stronger than those between identical serum and cellular cytokines. All the correlations between serum IL-8 and non-IL-8 cellular parameters were inverse (e.g., Fig. 2).
FIG. 2.
Serum IL-8 levels, by the percentage of T cells making induced IFN-γ (all participants). For graphing purposes, samples with nondetectable serum IL-8 levels were recorded as being half the detection limit (0.5 DL). rs = −0.49, n = 128, using detectable samples only.
On subgroup analyses, the only notable findings were as follows. Blood culture-positive participants had the largest number of serum-cell correlations (6), followed by HIV+ persons (4) and HIV-negative persons (1). There were no significant correlations in those with malaria or the children, with the caveat that no child had detectable serum TNF-α levels and only four children had detectable serum IL-2 levels. For the blood culture-positive persons, all but two correlations were with more complex cellular variables. There were significant correlations between serum IL-2 and the ratios of the percentages of CD3+ cells spontaneously making IL-10 to the percentages making TNF-α (rp = +1.00; P < 0.0001) or IL-2 (rp = +0.91; P < 0.001)(n = 9); between serum IL-8 and the percentages of CD3+ cells making induced IFN-γ (rs = −0.66), between serum IL-8 and the percentages of lymphocytes and of CD3+ cells making both IFN-γ and TNF-α in the same cell with stimulation (rs = −0.62 and rs = −0.59, respectively)(P < 0.0001 and n = 37 for all); and between serum IL-10 and the percentages of CD8+ T cells spontaneously making TNF-α (rp = +0.89; P < 0.0001; n = 14). HIV+ participants had the same IL-8 correlations as did the blood culture-positive persons (rs = −0.54, rs = −0.43, and rs = −0.43, respectively)(P < 0.0001, n = 80 for all). In addition, in HIV+ persons serum IFN-γ levels were correlated with the percentages of lymphocytes spontaneously making both IL-6 and IFN-γ in the same cell (rp = +0.59; P < 0.0001; n = 57). For HIV-negative persons, serum IL-8 levels were significantly associated with the percentages of lymphocytes spontaneously making both IL-2 and IL-10 in the same cell (rs = −0.54; P < 0.0001; n = 46).
DISCUSSION
The role of cytokines in infection or various physiologic states has usually been inferred from measurements of serum or plasma cytokine levels. This approach has a number of positive aspects, not the least of which is the feasibility of assaying many samples quickly. It is plausible that these levels reflect the systemic effects of cytokines (e.g., the interactions among cytokines, hormones, and the hypothalamic axis) and cytokine production by both immune and nonblood cells (e.g., epithelial cells and fibroblasts), although these are not usually given as the rationale for this approach. Serum and plasma cytokine measurements have provided statistically significant, albeit variable, results in a number of clinical studies concerning infection (for IL-6 and IL-8, see reference 5), severity of sepsis-related illness (for an IL-6 review, see reference 9; for IL-1b, TNF-α, and TNF receptors, see reference 6), injury (for IL-6 and IL-8, see reference 20; for an IL-6 review, see reference 4), HIV infection (for TNF-α and receptors, see references 1 and 10), hypothalamic-endocrine-cytokine interactions (for a review, see reference 25), exercise, (for IL-6, see reference 24; for IL-1 and IL-2, see reference 22), and the effects of surgery (for IL-6, see reference 3).
Despite the large amount of research involving serum and plasma cytokine assessments, this approach does have a number of limitations. Serum and plasma cytokine levels can be affected by receptor binding, temperature degradation, urinary excretion, and cytokine breakdown within reacting cells. Since cytokines are released in a paracrine manner, the levels may vary widely depending upon when the subject's blood sample is drawn. Further, a major function of most cytokines is to act in intercellular communication; cytokine levels in serum and plasma provide at best an indirect measure of this key cytokine function.
Cell-specific cytokine assessment, using cloning, cell separation, or multiparameter cytometry, was essential for the development of the elegant concepts of a cytokine network and type 1-type 2 cytokine balance (18, 29) as well as the development of current hypotheses about the cytokine network's role in allergy (21), cancer, transplantation, pregnancy (for a review, see reference 23), and infectious diseases (for a review, see reference 11). Cytometric, cellular cytokine assessment is laborious and requires reagents and techniques that only recently have been refined (12), but a number of investigators have already used cytometric techniques to examine the role of cell-specific cytokines in HIV infection (13, 14, 28), allergy (21), exercise (24; for a review, see reference 19), and surgery (for IL-2 and IFN-γ, see reference 3).
The basis for assessing cytokines at a cellular level is both simple and logical: cytokines function at that level. However, the assessment itself is not simple, logically requiring cells from the site of disease activity. Obtaining these cells is often a difficult process for both the investigator and the study participant. In the Malawian study described herein as well as in a similar study in Thailand (13), we examined cell-specific cytokine profiles in the peripheral blood cells of patients with or without a variety of subsequently documented bloodstream infections. All these patients had systemic symptoms at the time of blood collection; most had HIV infection, a chronic, systemic viral infection. Thus, this was an optimal patient group in which to compare cytokine production by peripheral blood cells and serum and cellular cytokine levels: both might be expected to reflect the immune response to a systemic disease process(es).
Two underlying goals of the Malawian and Thai studies were to evaluate whether cellular cytokine assessment would be feasible in a field setting and if these assessments would be useful in examining the immune response to bloodstream infections and/or predicting morbidity and mortality in various subgroups of patients. Obviously, usefulness needed to be weighed against the labor and cost involved in applying this technique in the setting of a developing country. In Malawi, we obtained serum samples to address a third goal: to compare the relative usefulness of serum and cellular cytokine assessments in achieving our second goal above.
In the Thai study, we had found that HIV-related mortality was inversely related to the percents of NT cells making TNF-α (13). Blood culture-positivity and the causative organism were associated with the percents of CD3+ CD8+ cells making IL-8 and making IFN-γ (13). In the Malawian study, mortality was again related to cytokine production by NT cells (14). In further analyses of the Malawi data, we have found the production of various, specific cytokines by various, specific cell types to be associated with iron deficiency, vitamin A deficiency, human herpesvirus type 8 seropositivity, Mycobacterium bovis BCG vaccine scarring, or malaria. Of these conditions, serum cytokine patterns were associated with only malaria parasitemia (14a, 14b; S. M. DeSantis, C.-P. Pau, L. K. Archibald, O. C. Nwanyanwu, P. N. Kazembe, H. Dobbie, W. R. Jarvis, and J. Jason, submitted for publication; J. Jason, L. K. Archibald, O. C. Nwanyanwu, P. N. Kazembe, J. A. Chatt, E. Norton, H. Dobbie, and W. R. Jarvis, submitted for publication; J. Jason, L. K. Archibald, O. C. Nwanyanwu, A. L. Sowell, I. Buchanan, J. Larned, M. Bell, P. N. Kazembe, H. Dobbie, and W. R. Jarvis, submitted for publication). Thus, in a practical sense, we have found strong evidence for the importance of cellular cytokine assessment in various disease states and only weak evidence for the importance of serum cytokine assessment.
It has been debated whether there are any relationships between cellular and serum or plasma cytokines (for example, see the Cytometry Newsletter published by Purdue University Cytometry Laboratories, Purdue, Ind. [for more information, contact cytometry@flowcyt.cyto.purdue.edu]). There are some data on this issue, but the numbers of samples and cytokines assessed in each study are quite limited. Therefore, we examined this question using a large panel of monoclonal antibodies to cell surface antigens and intracellular cytokines, reagents to seven serum cytokines, and a sizable number of samples. Further, these samples were from persons with systemic symptoms and many had detectable levels of serum cytokines.
We found that the relationships between identical serum and cellular cytokines were weak but in a positive direction. Several relationships were, arguably, unexpected. IL-6 is produced by several types of nonblood cells as well as blood cells, thus serum levels might not necessarily reflect immune cell production. Yet, serum IL-6 levels were correlated with the percent of CD8+ lymphocytes making IL-6. Elevated serum TNF-α levels are reported in both sepsis and advanced HIV infection, yet there were no significant correlations between serum and cellular TNF-α parameters.
IFN-γ is a type 1 cytokine associated with cellular immunity; IFN-γ correlations were stronger and more numerous in the blood culture- and HIV+ subgroups. Similarly, IL-2 is a type 1 cytokine inducing T-cell proliferation and cellular immunity. IL-2 correlations were present in, and IL-8 correlations were stronger in, patients with positive blood cultures. These patients also had a number of correlations between serum IL-2, a type 1 cytokine, and proinflammatory and type 2 cellular cytokines; between serum IL-10, a type 2, anti-inflammatory cytokine, and cellular TNF-α, a proinflammatory cytokine; and between serum IL-8, a proinflammatory cytokine, and cellular type 1 and proinflammatory cytokines. Other subgroups, especially HIV+ persons, had similar relationships suggestive of interregulation between serum and cellular cytokines but these were fewer in number and weaker in strength than those in the blood culture-positive patients. These correlations may reflect systemic pro- and anti-inflammatory cytokine balances, catecholamine influences (17), or lymphocyte-epithelial interactions (26).
In summary, serum cytokine levels in our study participants only weakly reflected the percents of immune cells producing that cytokine. Further, the strengths of these serum-cell associations varied with the presence/absence of bloodstream infections and HIV status. Serum cytokines appear to only poorly, at best, reflect peripheral blood cellular cytokine production.
ACKNOWLEDGMENTS
We thank the nursing and medical staff of Lilongwe Central Hospital and the patients and their parents, who so generously cooperated in this study.
REFERENCES
- 1.Aukrust P, Liabakk N-B, Müller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-α (TNFα) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and viral parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann M, Gornikiewicz A, Sautner T, Waldmann E, Weber T, Mittlböck M, Roth E, Függer R. Attenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsis. Shock. 1999;12:421–427. doi: 10.1097/00024382-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Berguer R, Bravo N, Bowyer M, Egan C, Knolmayer T, Ferrick D. Major surgery suppresses maximal production of helper T-cell type 1 cytokines without potentiating the release of helper T-cell type 2 cytokines. Arch Surg. 1999;34:540–544. doi: 10.1001/archsurg.134.5.540. [DOI] [PubMed] [Google Scholar]
- 4.Biffl W L, Moore E E, Moore F A, Peterson V M. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandenburg A H, Kleinjan A, van het Land B, Moll H A, Timmerman H H, de Swart R L, Neijens H J, Fokkens W, Osterhaus A D M E. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol. 2000;62:267–277. [PubMed] [Google Scholar]
- 6.Cain B S, Meldrum D R, Harken A H, McIntyre R C. The physiologic basis for anticytokine clinical trials in the treatment of sepsis. J Am Coll Surg. 1998;186:337–350. doi: 10.1016/s1072-7515(98)00036-2. [DOI] [PubMed] [Google Scholar]
- 7.Elson L H, Nutman T B, Metcalfe D D, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27− lymphocyte subpopulation. J Immunol. 1995;154:4294–4301. [PubMed] [Google Scholar]
- 8.Feghali C A, Wright T M. Cytokines in acute and chronic inflammation. Frontiers Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 9.Hack C E, DeGroot E R, Felt-Bersma R J F, Nuijens J H, Van Schijndel R J M S, Eerenberg-Belmer A J M, Thijs L G, Aarden L A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 10.Heijligenberg R, Romijn J A, Godfried M H, Endert E, Sauerwein H P. In vitro production of cytokines in whole blood versus plasma concentrations of cytokines in AIDS. AIDS Res Hum Retrovir. 1998;14:123–127. doi: 10.1089/aid.1998.14.123. [DOI] [PubMed] [Google Scholar]
- 11.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol. 1999;21:317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 12.Jason J, Larned J. Single-cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/s0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 13.Jason J, Archibald L K, McDonald L C, Hart W M, Rheanppumikankit S, Tansuphwaswadikul S, Byrd M G, Larned J, Han A, Green T A, Jarvis W R. Immune determinants of organism and outcome in febrile hospitalized Thai patients with bloodstream infections. Clin Diagn Lab Immunol. 1999;6:73–78. doi: 10.1128/cdli.6.1.73-78.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jason J, Buchanan I, Archibald L K, Nwanyanwu O C, Bell M, Green T A, Eick A, Han A, Razsi D, Kazembe P N, Dobbie H, Midathada M, Jarvis W R. Natural T, γδ, and NK cells in mycobacterial, salmonella, and human immunodeficiency virus infections. J Infect Dis. 2000;182:474–481. doi: 10.1086/315740. [DOI] [PubMed] [Google Scholar]
- 14a.Jason J, Archibald L, Nwanyanwu O, Bell M, Buchanan I, Larned J, Kazembe P N, Dobbie H, Parekh B, Byrd M G, Eick A, Han A, Razsi D, Jarvis W R. Cytokine correlates of malaria parasitemia. Clin Immunol. 2001;100:208–218. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- 14b.Jason, J., L. K. Archibald, O. C. Nwanyanwu, R. J. Jensen, M. Bell, I. Buchanan, J. Larned, P. N. Kazembe, H. Dobbie, and W. R. Jarvis. The effects of iron deficiency on the cytokine network: preservation of hepatic iron but not at all costs. Clin. Exp. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Kabilan L, Andersson G, Lolli F, Ekre H-P, Olsson T, Troye-Blomberg M. Detection of intracellular expression and secretion of interferon-γ at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990;20:1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- 17.Kavelaars A, van de Pol M, Zijlstra J, Heijnen C J. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77:211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T R, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 19.Northoff H, Weinstock C, Berg A. The cytokine response to strenuous exercise. Int J Sports Med. 1994;15:S167–171. doi: 10.1055/s-2007-1021132. [DOI] [PubMed] [Google Scholar]
- 20.Partrick D A, Moore F A, Moore E E, Biffl W L, Sauaia A, Barnett C C. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg. 1996;172:425–431. doi: 10.1016/s0002-9610(96)00252-8. [DOI] [PubMed] [Google Scholar]
- 21.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 22.Shephard R J, Rhind S, Shek P N. Exercise and training: influences on cytotoxicity, interleukin-1, interleukin-2, and receptor structures. Int J Sports Med. 1994;15:S154–S166. doi: 10.1055/s-2007-1021131. [DOI] [PubMed] [Google Scholar]
- 23.Shurin M R, Lu L, Kalinski P, Stewart-Akers A M, Lotze M T. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 24.Starkie R L, Angus D J, Rolland J, Hargreaves M, Febbraio M A. Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J Physiol. 2000;528:647–655. doi: 10.1111/j.1469-7793.2000.t01-1-00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbull A V, Rivier C L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Vachula M, Van Epps D E. In vitro models of lymphocyte transendothelial migration. Invasion Metastasis. 1992;12:66–81. [PubMed] [Google Scholar]
- 27.Van der Poll T, Coyle S M, Barbosa K, Braxton C C, Lowry S F. Epinephrine inhibits tumor necrosis factor-α and potentiates interleukin 10 production during human endotoxemia. J Clin Investig. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westby M, Marriott J B, Guckian M, Cookson S, Hay P, Dalgleish A G. Abnormal intracellular IL-2 and interferon-gamma (IFN-γ) production as HIV-1-associated markers of immune dysfunction. Clin Exp Immunol. 1998;111:257–263. doi: 10.1046/j.1365-2249.1998.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing Z. Current understanding of macrophage type 1 cytokine responses during intracellular infections. Histol Histopathol. 2000;15:199–205. doi: 10.14670/HH-15.199. [DOI] [PubMed] [Google Scholar]