Abstract
Background:
Nonbacterial thrombotic endocarditis is characterized by the presence of organized thrombi on cardiac valves, often associated with hypercoagulable states. There is a paucity of data regarding the predictors of mortality in patients with nonbacterial thrombotic endocarditis. Our primary aim was to identify predictors of in-hospital mortality in patients with nonbacterial thrombotic endocarditis.
Methods:
A systematic literature review of all published cases and case series was performed until May 2018 according to Preferred Reporting Items for Systematic Review and Meta-analyses statement guidelines. We applied random forest machine learning model to identify predictors of in-patient mortality in patients with nonbacterial thrombotic endocarditis.
Results:
Our search generated a total of 163 patients (mean age, 46 ± 17 years; women, 69%) with newly diagnosed nonbacterial thrombotic endocarditis. The in-hospital mortality rate in the study cohort was 30%. Among the patients who died in the hospital, initial presentation of pulmonary embolism (12.2 vs. 2.6%), splenic (38.7 vs. 10.5%), and renal (40.8 vs. 9.6%) infarcts were higher compared to patients alive at the time of discharge. Higher rates of malignancy (71.4 vs. 39.4%, P = .0003) and lower rates of antiphospholipid syndrome (8.1 vs. 48.2%, P = .0001) were noted in deceased patients. Random forest machine learning analysis showed that older age, presence of antiphospholipid syndrome, splenic infarct, renal infarct, peripheral thromboembolism, pulmonary embolism, myocardial infarction, and mitral valve regurgitation were significantly associated with increased risk of in-hospital mortality.
Conclusion:
Patients admitted with nonbacterial thrombotic endocarditis have a high rate of in-hospital mortality. Factors including older age, presence of antiphospholipid syndrome, splenic/renal infarct, lower limb thromboembolism, pulmonary embolism, myocardial infarction, and mitral valve regurgitation were significantly associated with increased risk of in-hospital mortality in patients with nonbacterial thrombotic endocarditis.
Keywords: Antiphospholipid syndrome, Libman-Sacks endocarditis, marantic endocarditis, nonbacterial thrombotic endocarditis, systemic lupus erythematosus
Highlights
In-patient mortality rate in the study cohort due to nonbacterial thrombotic endocarditis was 30%.
Random forest regression analysis showed that older age, presence of antiphospholipid syndrome, splenic infarct, renal infarct, peripheral thromboembolism, pulmonary embolism, myocardial infarction, and mitral valve regurgitation were associated with increased risk of in-hospital mortality.
The most common clinical presentation was stroke (54%).
Left-sided cardiac valves were more commonly involved than the right.
Nonbacterial thrombotic endocarditis vegetations were found using transthoracic echocardiography in 44 (27%) patients and using transesophageal echocardiography in 67 (41.1%) patients and in 24 patients, vegetations were found only during autopsy.
Among the case reports where anticoagulation use was reported (~67%), the most used anticoagulat at time of discharge was warfarin.
Introduction
Nonbacterial thrombotic endocarditis (NBTE) is a rare condition characterized by the development of sterile thrombotic vegetation(s) on cardiac valves often associated with hypercoagulable states such as malignancy (approximately 80% cases) and chronic inflammatory conditions including systemic lupus erythematosus (SLE) and antiphospholipid syndrome and myeloproliferative disorders.1-3
Sterile vegetations of NBTE are formed when there is a valvular endothelial cell injury in the setting of hypercoagulable state. The valvular endothelial injury results in local aggregation of platelets, migration of inflammatory mononuclear cells, and deposition of immune complexes forming a thrombus interwoven with fibrin.4-8 There are unique triggers for endothelial damage, depending on the disease state. In malignancy, antigen-presenting cells including macrophages interact with malignant cells to release cytokines including tumor necrosis factor and interleukins, which damage the endothelium.9,10 In SLE, high blood flow turbulence along with chronic deposition of immunoglobulins and complement factors on the endothelial lining of the cardiac valve causes immune-mediated endothelial injury.1,9 In antiphospholipid syndrome (APS), endothelial damage occurs due to autoantibodies against phospholipids in the endothelial cell membrane.1,9,11
Although many cases and case series have been published on NBTE, there is a paucity of data on the risk factors associated with in-hospital mortality, management, follow-up management, and prognosis of NBTE. Therefore, we performed a systematic review of the existing literature with the aim to identify the risk factors of in-patient mortality and provide an overview of the use of anticoagulants in NBTE patients.
Methods
We performed a systematic review of all the case reports and case series of NBTE published from January 1975 to May 2018 in accordance with Preferred Reporting Items for Systematic Review and Meta-analyses guidelines (Figure 1). Pubmed, Cochrane, Embase, Scopus, and Web of Science databases were searched for cases and case series published up to May 2018 using keywords “nonbacterial thrombotic endocarditis,” “noninfective endocarditis,” “Libman-Sacks endocarditis,” “NBTE,” and “anticoagulants.” Additionally, we manually searched independent journals for published case reports on NBTE. Inclusion criteria were (1) clinically diagnosed cases during hospitalization for NBTE-predisposing conditions with or without supporting echocardiographic evidence. (2) Asymptomatic cases with supportive echocardiographic findings and multiple negative blood cultures. (3) Cases diagnosed by histological specimens of the thrombotic valves. (4) Cases diagnosed on autopsy. (5) Among all the cases in each case series, only cases diagnosed as NBTE clinically with supporting evidence on histological biopsy or on autopsy at death were included. The following studies were excluded: studies other than case reports, previous literature reviews or systematic reviews, complications other than NBTE, non-English language literature, data that could not be extracted, duplicated studies, abstract-only articles (conference, letters, commentaries), articles without available full-text, theses, books, review editorials, and author responses.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-analyses Statement.
Data were extracted by 2 authors (NV and PA) and were reviewed by a third author (RA) for inclusion or if there were any doubts. When disagreement occurred, a consensus decision was made following discussion with a senior reviewer (FM). We extracted all case reports and relevant cases from the case series into a dataset. The data extraction form was developed by 2 authors (NV and PA) based on a pilot review and extraction. The extracted data included the author, year of publication, journal, patient demographics, relevant medical history, comorbidities, current anticoagulation, initial presentation and hospital course, relevant laboratory studies and diagnostic tests, medications used for treatment, surgical intervention, outcome of each patient, mortality, autopsy findings if available, follow-up tests, embolic events, and complications.
The statistical analyses were performed using several R packages under R (v3.5.1). First, the data were summarized with package dlookr (v0.3.0), which reports the summary and the missing percentage of each variable. Variables with greater than 5% missing percentages were excluded. Then, similarities between each variable pair were calculated using Pearson correlation coefficient. A variable was excluded when its average similarity was ≥ 95% against all other pair-mates. Finally, the remaining variables were sent to machine-learning methods, including random forest and logistic regression. The random forest model is applied using package random Forest (v4.6).
Ethical review and approval were waived for this study, as our study is a systematic review of published case reports and involves no interaction with human subjects and access to any subject identifiers.
Results
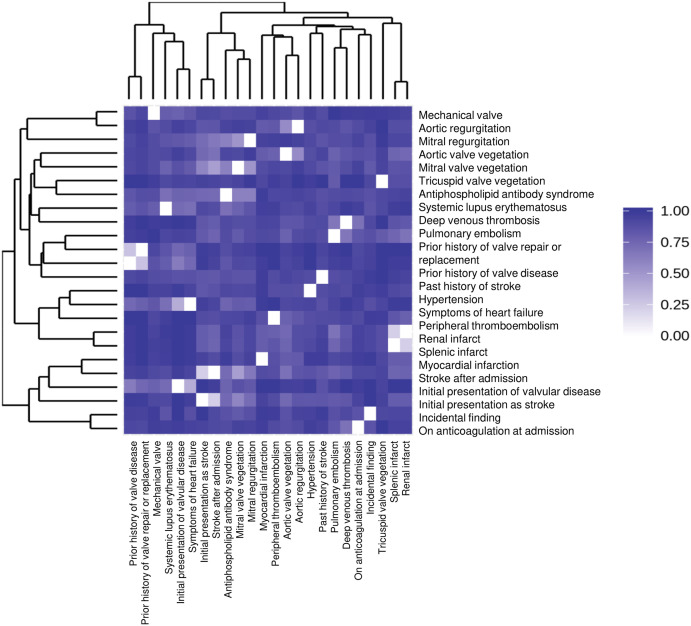
Using the retrieval strategy described above, a total of 141 case reports and 10 case series were included in this systematic review, generating a total of 163 patients with newly diagnosed NBTE (Figure 1). The basic characteristics of these patients are described in detail in Table 1. The mean age of the patient cohort was 46 ± 17 years, and the majority were women (69%). The similarities between each variable pair were calculated using Pearson correlation coefficient and were shown in Figure 2. The most common presentation was stroke (54%). Shortness of breath or symptoms of heart failure was the initial complaint in 25 patients (15.3%) and chest pain in 19 patients (11.7%). Symptoms of acute limb ischemia were the reason for initial presentation in 9 patients, followed by pulmonary emboli in 6 patients. Nonbacterial thrombotic endocarditis was diagnosed in 6 patients who were asymptomatic at presentation and presented to the hospital for evaluation of cancer or autoimmune disease. Three patients presented with a murmur, 1 each presented with ischemic bowel symptoms, seizure, paranoid behavior, hemoptysis, and fever. In 2 patients, the reason for initial presentation was not mentioned. In our patient cohort, we found past medical history significant for malignancy (46.6%), APS (36.2%), and SLE (22%). Among patients with malignancy (n = 76), lung cancer (23.6%) was the most common, followed by ovarian cancer (15.8%) and pancreatic cancer (13.3%). During the hospital course, stroke occurred in 70% of patients in the cohort, splenic and renal infarcts in 19%, heart failure in 18%, and myocardial infarction in 14.7%. Cardiac vegetations were found using transthoracic echocardiography (TTE) (42.9%), transesophageal echocardiography (TEE) (58.3%), and with autopsy (20.2%). The most commonly involved cardiac valves were mitral valve (66.7%), followed by aortic valve (35%) and tricuspid valve (9.2%), and the least common was pulmonary valve.
Table 1.
Demographic and Clinical Characteristics of NBTE Patients
| Patients, n | 163 |
|---|---|
| Clinical data, n (%) | |
| Gender | |
| Male | 50 (30.7) |
| Female | 113 (69.3) |
| Age, years | |
| Mean ± SD | 46 ± 17 |
| 11-20 | 12 (7.4) |
| 21-30 | 25 (15.3) |
| 31-40 | 24 (14.7) |
| 41-50 | 32 (19.6) |
| 51-60 | 30 (18.4) |
| 61-70 | 27 (16.6) |
| 71-80 | 11 (6.7) |
| Associated comorbidities: n (%) | |
| Malignancy | 76 (46.6) |
| APS | 59 (36.2) |
| SLE | 36 (22) |
| Past hospitalizations: n (%) | |
| Pulmonary embolism | 36 (22) |
| Deep venous thrombosis | 24 (14.7) |
| Stroke | 22 (13.5) |
| Valvular disease | 14 (8.5) |
| Clinical presentations at diagnosis, n (%) | |
| Stroke (including TIA’s) | 88 (54) |
| Dyspnea | 25 (15.3) |
| Chest pain | 19 (11.6) |
| Thromboembolism | 16 (9.8) |
| Hospital course findings: n (%) | |
| Stroke (including TIA’s) | 114 (69.9) |
| Splenic infarcts | 31 (19) |
| Renal infarcts | 31 (19) |
| Heart failure | 30 (18.4) |
| Myocardial ischemia/infarction | 24 (14.7) |
| Location of vegetations: n (%) | |
| Mitral valve alone | 92 (56.4) |
| Aortic valve alone | 42 (25.8) |
| Mitral + aortic | 10 (6.1) |
| Mitral + aortic + tricuspid | 11 (6.7) |
| Tricuspid + mitral | 4 (2.4) |
| Tricuspid + aortic | 1 (0.6) |
| Tricuspid + pulmonary | 1 (0.6) |
| Pulmonary + aortic | 1 (0.6) |
| Vegetations found by n (%) | |
| TTE | 44 (27) |
| TEE | 67 (41.1) |
| TTE and TEE (both) | 28 (17.2) |
| Autopsy alone | 24 (14.7) |
| Associated valve abnormality: n (%) | |
| MR | 55 (33.7) |
| AR | 28 (17.2) |
| MS | 09 (5.5) |
| AS | 07 (4.3) |
| TR | 07 (4.3) |
| TS | 01 (0.6) |
| Surgical procedure: n (%) | |
| Mechanical valve replacement | 19 (11.6) |
| Bioprosthetic valve replacement | 14 (8.5) |
| Surgical resection of vegetations | 12 (7.3) |
| Patients discharged: n (%) | |
| Warfarin alone | 58 (50.9) |
| LMWH alone | 23 (20.2) |
| Heparin alone | 16 (14) |
| Mortality: | |
| In-hospital mortality | 49 (30) |
| Deaths during follow-up | 08 (7) |
SD, standard deviation; NBTE, nonbacterial thrombotic endocarditis; APS, antiphospholipid syndrome; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography; TIA, transient ischemic attack; MR, Mitral Regurgitation; AR, Aortic regurgitation; MS, Mitral stenosis; AS, Aortic stenosis; TR, Tricuspid regurgitation; TS, Tricuspid stenosis; LMWH, Low molecular weight heparin.
Figure 2.
Similarities between each variable pair using pearson correlation coefficient.
Among the patients discharged on anticoagulation (n = 118), the most used anticoagulant was warfarin (56.8%) followed by LMWH (20.3%) and unfractionated heparin (13.5%). Factor Xa inhibitor use was reported in 2 case reports and direct thrombin inhibitor use was reported in 1 case report. Surgical intervention with mechanical valve replacement (11.7%) was the most common, followed by bioprosthetic valve replacement (8.5%) and surgical resection of vegetations (7.3%).
The in-hospital mortality rate was 30%. The following diagnoses were more common among patients who expired in the hospital compared to patients who were discharged from the hospital: initial presentation of pulmonary embolism (PE) (12.2 vs. 2.6%, P = .022), splenic infarct (38.7 vs. 10.5%, P = .0001), and renal infarct (40.8 vs. 9.6%, P = .0001). Although a higher percentage of stroke and myocardial infarction (MI) was noted in the deceased patient population compared to the population alive at discharge, they were statistically non-significant: stroke (61% vs. 51%, P = .24) and MI (12.2% vs. 8.7%, P = .57). Higher rates of malignancy (71.4 vs. 39.4%, P = .0003) and lower rates of APS (8.1 vs. 48.2%, P = .0001) were noted in deceased patients.
Random forest regression analysis showed that the top 10 predictors of in-hospital mortality were older age, presence of APS, splenic infarct, renal infarct, peripheral thromboembolism, PE, MI, and mitral valve regurgitation (Figure 3).
Figure 3.
Top 10 predictors of in-hospital mortality in NBTE patients using random forest regression analysis.
Discussion
This is the first study to look into risk factors associated with in-patient mortality in patients with NBTE. Our analysis of 163 patients with newly diagnosed NBTE revealed the following: (1) inpatient mortality rate was 30%. (2) Random forest regression analysis showed that older age, presence of APS, splenic infarct, renal infarct, peripheral thromboembolism, PE, MI, and mitral valve regurgitation were associated with increased risk of in-hospital mortality. (3) The most common clinical presentation was stroke (54%). (4) Left-sided cardiac valves were more commonly involved than the right. (5) Nonbacterial thrombotic endocarditis vegetations were found using TTE in 44 (27%) patients, TEE in 67 (41.1%) patients, and in 24 patients, vegetations were found only during autopsy. (6) Among the case reports where anticoagulation use was reported (~67%), most used anticoagulation at time of discharge was warfarin.
The mean age of our cohort was 46 ± 17 years affecting patients from the third through eighth decade of life which is consistent with previous studies.5,6-13 However, in this systematic review, 69% of patients were females, whereas previous studies reported equal proportion of males and females.12-20
In our cohort population, malignancy was seen in 46.6%, APS in 36.2%, and SLE in 22% of patients. Many prior studies have reported the association between NBTE and chronic hypercoagulable states such as malignancy, as well as autoimmune inflammatory disorders like SLE and APS.1,2,7,17,20-22 Malignancy is the most common disease state in which NBTE is seen (80% of cases).5,23,24
The incidence of NBTE with concurrent malignant neoplasm in our cohort is 46.6% which is close to the incidence reported by MacDonald and Robbins12 of 34.6%12 and 42.4% reported by Biller et al.20 Three studies reported an associated cancer incidence of 75%, 62.5%, and 85%, which could be due to differences in patient characteristics.13,19,25 In this systematic review, the most common malignancy was lung cancer (23.6%) followed by ovarian cancer (15.8%) and pancreatic cancer (13.2%) as shown in Table 2. This is similar to prior studies which showed lung cancer to be the most common in NBTE patients with malignancy.13,17,19,20
Table 2.
Relative Incidence Malignancy Type in NBTE
| Lung cancer | 18 (23.6%) |
| Ovarian cancer | 12 (15.8%) |
| Pancreatic cancer | 10 (13.1%) |
| Metastatic cancer of unknown primary site | 7 (9.2%) |
| Endometrial cancer | 6 (7.9%) |
| Gastric cancer | 5 (6.6%) |
NBTE, nonbacterial thrombotic endocarditis.
In our cohort, the most common initial presentation was stroke in 88 (54%) patients which is similar to prior studies.9,22,26,27 Many prior studies have shown that the incidence of cerebral ischemia is higher in NBTE patients28,29 and is more common in NBTE than with infective endocarditis (33% vs 19%).3 Twenty-five (15.3%) patients were admitted for shortness of breath or symptoms of heart failure and 19 (11.7%) patients were admitted for chest pain. Symptoms of acute limb ischemia were the reason for initial presentation in 9 patients and pulmonary emboli in 6 patients.
During the hospital course, 114 (69.9%) patients developed stroke episodes, 31 (19%) patients developed splenic and renal infarcts, 30 (18.5%) patients developed symptoms and signs of heart failure, 24 (15%) patients developed myocardial infarction, 20 (12%) patients developed acute limb ischemia, and 9 patients developed pulmonary emboli. Most of these hospital course findings were due to delay in diagnosis and initiation of antithrombotic therapy. This data emphasize that high clinical suspicion is critical to make the antemortem diagnosis of NBTE, and early diagnosis and treatment are essential to prevent mortality and morbidity in NBTE patients.
Unlike infective endocarditis, NBTE vegetations are sterile, friable, and more prone to embolization. These patients typically present with systemic or pulmonary embolization. Nonbacterial thrombotic endocarditis accounts for approximately 20%-25% of cerebrovascular accidents in cancer patients and is discovered on autopsy in 1.2% of patients.21 High suspicion, early recognition, and appropriate treatment are paramount to prevent valve damage and embolization complications.
Clinical suspicion of NBTE should be considered in patients with embolic phenomena with underlying cancer and connective tissue disease, especially SLE and APS, and in patients with presumed infective endocarditis who do not respond to antibiotics.28,30 Prior to the use of echocardiography, the diagnosis of NBTE was dependent on postmortem findings. Echocardiographic identification of thrombotic vegetations on the heart valves and negative blood cultures are needed for definitive diagnosis of NBTE.9,30
Majority of patients had NBTE vegetations on the mitral valve (72.4%, n = 118) followed by the aortic valve (40.5%, n = 66). Isolated involvement of the mitral valve was seen in 92 (56.5%) patients, and isolated aortic valve involvement was seen in 42 (25.8%) patients. Coexisting mitral and aortic valve vegetations were seen in 10 (6.1%) patients and 4 patients showed coexisting tricuspid and mitral valve vegetations. Right-sided NBTE is uncommon and was seen in 19 patients (11.6%). One case each of tricuspid and aortic valve, tricuspid and pulmonary valve, and pulmonary and aortic valve was found. There were 11 (6.75%) cases of triple valve involvement—tricuspid, mitral, and aortic valve. One case of 4 valve involvement was reported. In a recent systematic review by Patel and Elzweig31 similar findings of NBTE most commonly affecting the mitral valve (61%) and then aortic valve (36%) were reported. A total of 86% cases of isolated valve, 10% cases of 2 valves, and 3% cases of 3 or more valve involvement were seen.
In this systematic review, NBTE vegetations were found using TTE in 44 (27%) patients, TEE in 67 (41.1%) patients, and both TTE and TEE in 28 (17.2%) patients. In 24 patients, vegetations were found only at autopsy. In the published literature, NBTE vegetations were first reported in 1976—visualized by M-mode echocardiography.32 Transesophageal echocardiography is found to be more sensitive than TTE for the detection of valvular lesions, particularly for vegetations smaller than 5 mm.30,33,34
In this systematic review, anticoagulation therapy was started with warfarin (n = 58), LMWH (n = 23), and heparin (n = 16). Concurrent antiplatelet therapy was used in 4 patients treated with warfarin and 1 patient on LMWH. Direct oral anticoagulants (DOACs) were rarely used (direct thrombin inhibitor, n = 1 and factor Xa inhibitor, n = 1). In 1 patient, acenocoumarol (a coumadin derivative) was used. The American College of Chest Physician Guidelines recommends the use of long-term anticoagulation regardless of evidence of emboli.35 Heparin has been shown to reduce the incidence of thromboembolic events in prior studies, especially in patients with malignancy.36,37
Surgical procedures with mechanical valve replacement were done in 19 (11%) cases and bioprosthetic valve replacement in 14 (8.5%), and surgical resection of vegetations with valve-sparing was done in 12 (7.5%) cases. A study done in infective endocarditis group compared valve repair with valve replacement which showed better 5-year survival estimates compared to valve replacement (91.6% vs. 70%, P = .08).38 The 2017 American Heart Association / American College of Cardiology (AHA/ACC) focused update of the 2014 guideline for the management of patients with valvular heart diseases recommends operation without delay may be considered in patients who have suffered a stroke but have no evidence of intracranial hemorrhage or extensive neurological damage.39
Despite therapy, the prognosis of NBTE is poor with morbidity from neurocognitive dysfunction and mortality due to either recurrent embolization or from underlying advanced malignancy. In this systematic review, we noted an in-patient mortality rate of 30%. Among the 49 patients who died in the hospital, 28 died before the diagnosis of NBTE. The following diagnoses were more common among patients who expired in the hospital compared to patients who were discharged from the hospital: initial presentation of stroke (61 vs. 50.8%), MI (12.2 vs. 8.7%), PE (12.2 vs. 2.6%), splenic infarct (38.7 vs. 10.5%), and renal infarct (40.8 vs. 9.6%). Higher rates of malignancy (71.4 vs. 39.4%) and lower rates of APS (8.1 vs. 48.2%) were noted in deceased patients. Random forest regression analysis showed that age, presence of APS, splenic infarct, renal infarct, peripheral thromboembolism, PE, MI, and mitral valve regurgitation were significantly associated with increased risk of in-hospital mortality (Figure 3).
Additional research must be done to understand the predictors of mortality and evaluate the efficacy of newer anticoagulants in reducing the severity of NBTE and its complications. A global registry may be one such mechanism, in this uncommon condition, to allow for a better study of much larger cohort of patients.
Study Limitations
Our review has certain limitations. First, all inherent limitations of retrospective studies apply to our analysis. Second, publication bias might have affected our results since our analysis was based on all published case series and case reports and we limited this to English literature. Third, we excluded few cases as they were lacking comprehensive clinical data further limiting the scope of interpretation.
Footnotes
Ethics Committee Approval: Ethical review and approval were waived for this study, as our study is a systematic review of published case reports and involves no interaction with human subjects and access to any subject identifiers.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – F.M., R.A.; Design – F.M., R.A. P.A.; Supervision – F.M., R.A.; Funding – Not needed.; Materials – N.R.V., B.K.K.; Data Collection and/or Processing – N.R.V., P.A., B.K.K.; Analysis and/or Interpretation – P.W., N.R.V.; Literature Review – N.R.V., B.K.K.; Writing – N.R.V., B.K.K., P.A.; Critical Review – F.M., R.A., T.B., C.J.C., A.R.F.
Acknowledgments: Assistance provided by Mayo Clinic librarian Ms. Almader-Douglas, Diana, M.L.S., AHIP is greatly appreciated.
Declaration of Interests: The authors report no financial relationships or conflicts of interest regarding the content herein.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1. Hojnik M, George J, Ziporen L, Shoenfeld Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996;93(8):1579 1587. 10.1161/01.cir.93.8.1579) [DOI] [PubMed] [Google Scholar]
- 2. Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120(7):636 642. 10.1016/j.amjmed.2007.01.024) [DOI] [PubMed] [Google Scholar]
- 3. Bathina JD, Daher IN, Plana JC, Durand JB, Yusuf SW. Acute myocardial infarction associated with nonbacterial thrombotic endocarditis. Tex Heart Inst J. 2010;37(2):208 212. [PMC free article] [PubMed] [Google Scholar]
- 4. Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clin Proc. 2001;76(12):1204 1212. 10.4065/76.12.1204) [DOI] [PubMed] [Google Scholar]
- 5. Mazokopakis EE, Syros PK, Starakis IK. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets. 2010;10(2):84 86. 10.2174/187152910791292484) [DOI] [PubMed] [Google Scholar]
- 6. Aryana A, Esterbrooks DJ, Morris PC. Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. J Gen Intern Med. 2006;21(12):C12 C15. 10.1111/j.1525-1497.2006.00614.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12(5):518 523. 10.1634/theoncologist.12-5-518) [DOI] [PubMed] [Google Scholar]
- 8. Ferrans VJ, Rodríguez ER. Cardiovascular lesions in collagen-vascular diseases. Heart Vessels Suppl. 1985;1:256 261. 10.1007/BF02072405) [DOI] [PubMed] [Google Scholar]
- 9. Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev. 2016;24(5):244 247. 10.1097/CRD.0000000000000106) [DOI] [PubMed] [Google Scholar]
- 10. Smeglin A, Ansari M, Skali H, Oo TH, Maysky M. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol. 2008;26(8):1383 1385. 10.1200/JCO.2007.12.9148) [DOI] [PubMed] [Google Scholar]
- 11. Bhimani AA, Hoit BD. Extensive nonbacterial thrombotic endocarditis isolated to the tricuspid valve in primary antiphospholipid syndrome. J Am Soc Echocardiogr. 2010;23(1):107.e5 107.e6. 10.1016/j.echo.2009.07.019) [DOI] [PubMed] [Google Scholar]
- 12. Macdonald RA, Robbins SL. The significance of nonbacterial thrombotic endocarditis: an autopsy and clinical study of 78 cases. Ann Intern Med. 1957;46(2):255 273. 10.7326/0003-4819-46-2-255) [DOI] [PubMed] [Google Scholar]
- 13. Barron KD, Siqueira E, Hirano A. Cerebral embolism caused by nonbacterial thrombotic endocarditis. Neurology. 1960;10:391 397. 10.1212/wnl.10.4.391) [DOI] [PubMed] [Google Scholar]
- 14. Eliakim M, Pinchas S. Degenerative verrucous endocardiosis. A clinical-pathological study of 45 cases with reference to a protracted form of the disease. Isr J Med Sci. 1966;2(1):42 51 [PubMed] [Google Scholar]
- 15. Rohner RF, Prior JT, Sipple JH. Mucinous malignancies, venous thrombosis and terminal endocarditis with emboli. A syndrome. Cancer. 1966;19(12):1805 1812. [DOI] [PubMed] [Google Scholar]
- 16. Guinn GA, Ayala A, Liddicoat J. Clinical and therapeutic considerations in nonbacterial thrombotic endocarditis. Chest. 1973;64(1):26 28. 10.1378/chest.64.1.26) [DOI] [PubMed] [Google Scholar]
- 17. Rosen P, Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am J Med. 1973;54(1):23 29. 10.1016/0002-9343(73)90079-x) [DOI] [PubMed] [Google Scholar]
- 18. Deppisch LM, Fayemi AO. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J. 1976;92(6):723 729. 10.1016/s0002-8703(76)80008-7) [DOI] [PubMed] [Google Scholar]
- 19. Kooiker JC, MacLean JM, Sumi SM. Cerebral embolism, marantic endocarditis, and cancer. Arch Neurol. 1976;33(4):260 264. 10.1001/archneur.1976.00500040044006) [DOI] [PubMed] [Google Scholar]
- 20. Biller J, Challa VR, Toole JF, Howard VJ. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol. 1982;39(2):95 98. 10.1001/archneur.1982.00510140029007) [DOI] [PubMed] [Google Scholar]
- 21. Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007;32(5):696 701. 10.1016/j.ejcts.2007.07.029) [DOI] [PubMed] [Google Scholar]
- 22. Markides V, Nihoyannopoulos P. Non-bacterial thrombotic endocarditis. Eur J Echocardiogr. 2000;1(4):291 294. 10.1053/euje.2000.0027) [DOI] [PubMed] [Google Scholar]
- 23. González Quintela A, Candela MJ, Vidal C, Román J, Aramburo P. Non-bacterial thrombotic endocarditis in cancer patients. Acta Cardiol. 1991;46(1):1 9. [PubMed] [Google Scholar]
- 24. Wada H, Sase T, Yamaguchi M. Hypercoagulant states in malignant lymphoma. Exp Oncol. 2005;27(3):179 185. [PubMed] [Google Scholar]
- 25. Chino F, Kodama A, Otake M, Dock DS. Nonbacterial thrombotic endocarditis in a Japanese autopsy sample. A review of eighty cases. Am Heart J. 1975;90(2):190 198. 10.1016/0002-8703(75)90119-2) [DOI] [PubMed] [Google Scholar]
- 26. Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987;113(3):773 784. 10.1016/0002-8703(87)90719-8) [DOI] [PubMed] [Google Scholar]
- 27. Waller BF, Knapp WS, Edwards JE. Marantic valvular vegetations. Circulation. 1973;48(3):644 650. 10.1161/01.cir.48.3.644) [DOI] [PubMed] [Google Scholar]
- 28. Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Med (Baltim). 1985;64(1):16 35. 10.1097/00005792-198501000-00002) [DOI] [PubMed] [Google Scholar]
- 29. Joshi SB, Richards MJ, Holt DQ, Yan BP, Aggarwal A. Marantic endocarditis presenting as recurrent arterial embolisation. Int J Cardiol. 2009;132(1):e14 e16. 10.1016/j.ijcard.2007.07.106) [DOI] [PubMed] [Google Scholar]
- 30. Dutta T, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97(6):894 898. 10.1016/j.amjcard.2005.09.140) [DOI] [PubMed] [Google Scholar]
- 31. Patel MJ, Elzweig J. Non-bacterial thrombotic endocarditis: a rare presentation and literature review. BMJ Case Rep. 2020;13(12). 10.1136/bcr-2020-238585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Estevez CM, Corya BC. Serial echocardiographic abnormalities in nonbacterial thrombotic endocarditis of the mitral valve. Chest. 1976;69(6):801 804. 10.1378/chest.69.6.801) [DOI] [PubMed] [Google Scholar]
- 33. de Bruijn SF, Agema WR, Lammers GJ.et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37(10):2531 2534. 10.1161/01.STR.0000241064.46659.69) [DOI] [PubMed] [Google Scholar]
- 34. Roldan CA. Diagnostic value of transesophageal echocardiography in Libman-Sacks endocarditis. Minerva Cardioangiol. 2009;57(4):467 481. [PubMed] [Google Scholar]
- 35. Lansberg MG, O'Donnell MJ, Khatri P.et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e601S e636S. 10.1378/chest.11-2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau's syndrome. Devastating coagulopathy in the absence of heparin. Am J Med. 1985;79(4):423 430. 10.1016/0002-9343(85)90028-2) [DOI] [PubMed] [Google Scholar]
- 37. Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med. 1987;83(4):746 756. 10.1016/0002-9343(87)90908-9) [DOI] [PubMed] [Google Scholar]
- 38. Tepsuwan T, Rimsukcharoenchai C, Tantraworasin A.et al. Comparison between mitral valve repair and replacement in active infective endocarditis. Gen Thorac Cardiovasc Surg. 2019;67(12):1030 1037. 10.1007/s11748-019-01132-4) [DOI] [PubMed] [Google Scholar]
- 39. Nishimura RA, Otto CM, Bonow RO.et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135(25):e1159 e1195. 10.1161/CIR.0000000000000503) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a