Abstract
Background:
Considering that ectopic fat accumulation in various organs, especially the heart and liver, is a cardiometabolic risk factor, the need for easily accessible markers of ectopic fat accumulation is inevitable. The main starting point of the study is based on the hypothesis of predicting cardiovascular disease risk through the link that can be established between the liver–spleen ratio, which is one of the strong indicators of hepatosteatosis, and epicardial adipose tissue volume.
Methods:
This was a retrospective study. The records of 283 consecutive patients who underwent coronary computed tomography angiography in our Radiology Department were reviewed retrospectively from our hospital’s system. All patients’ epicardial adipose tissue volume and liver–spleen ratio were calculated using appropriate criteria on non-contrast computed tomography images. Additionally, the Coronary Artery Disease–Reporting and Data System was calculated on contrast computed tomography images. The participating patients were divided into groups according to the liver–spleen ratio and Coronary Artery Disease–Reporting and Data System score.
Results:
We found that while there was a negative correlation between the liver–spleen ratio and epicardial adipose tissue volume in the hepatosteatosis group, this relationship was not observed in the non-steatosis group. In addition, we observed that the family history of cardiovascular disease and the frequency of cardiovascular disease were higher in the hepatosteatosis group than in the other group, and there was a correlation between cardiovascular disease and the liver–spleen ratio. Also, we found that age and liver–spleen ratio values were found to be independent predictors of coronary artery disease.
Conclusion:
In our study, we found that the frequency of cardiovascular disease was lower in patients with a high liver–spleen ratio. We also demonstrated in the study that the liver–spleen ratio, which indicates a low level of epicardial adipose tissue volume accumulation, is an independent predictor of cardiovascular disease. In addition, the use of liver–spleen ratio, which is more valuable than liver attenuation in predicting hepatic steatosis, may be more useful in evaluating the risk of hepatosteatosis-related cardiovascular disease.
Keywords: Liver–spleen ratio, hepatosteatosis, epicardial adipose tissue, cardiovascular disease
Highlights
Epicardial adipose tissue (EAT) volume was higher in the steatosis group than in the non-steatosis group, and there is a negative correlation between liver–spleen (L/S) ratio and EAT volume in the hepatosteatosis group.
The family history of cardiovascular disease (CVD) and CVD frequency is higher in the hepatosteatosis group than in the other group.
Liver–spleen ratio value was found to be an independent predictor of coronary artery disease.
There is no relationship between the L/S ratio and EAT volume in the group without steatosis. This situation was attributed to the change in properties with the increase in EAT accumulation.
Due to various limitations in the evaluation of hepatosteatosis by liver attenuation, it may be more beneficial to use the L/S ratio, especially in determining the risk of cardiovascular disease.
Introduction
Epicardial adipose tissue (EAT) is an accumulation of adipose tissue located between the outer wall of the myocardium and the visceral layer of the pericardium. Epicardial adipose tissue volume accounts for 15-20% of average heart volume, and EAT mass accounts for approximately 1% of total adiposite tissue (AT) mass.1 Age, waist circumference, ethnicity, and heart mass are independent predictors of EAT volume.1 Epicardial adipose tissue consists of adipocytes and neurohumoral, inflammatory, stroma-vascular, and immune system cells.2 EAT has a physical protective barrier role for the heart. It causes anti-inflammatory effects in periods when EAT accumulation is low and pro-inflammatory effects that cause cardio-metabolic events in later periods when accumulation increases.3
In obese individuals, visceral adipose tissue accumulation causes metabolic diseases and increased inflammatory events.4 Studies have shown that EAT volume is associated with the development of cardiovascular events, but it is not yet clear whether the role of EAT accumulation in cardiovascular disease (CVD) is independent of other visceral adipose tissue deposits.5,6
Although it is not a measurement method recommended by the guidelines in EAT measurement, transthoracic echocardiography methods are frequently preferred methods.7 Echocardiography is the most commonly used method for EAT measurement because it is easily accessible, non-invasive, and cost-effective, but this method has some limitations. The lack of equal intensity of EAT in all areas of the heart and the fact that the experience of the echocardiographer changes the measurement results are the most significant limitations of EAT measurement by echocardiography. The volume of epicardial adipose tissue is the most consistent measurement method for determining the amount of EAT.7 EAT volume can also measure with computed tomography (CT) and magnetic resonance (MR), and both methods provide a more accurate and volumetric measurement of epicardial fat tissue than transthoracic echocardiography. Although both methods are more sensitive and specific, they are more expensive and laborious than echocardiography.7 It is still unclear which EAT measurement method best reflects metabolic risk.
Hepatosteatosis is the accumulation of fatty tissue in and around the liver and is the most common liver disease. Hepatosteatosis is estimated to affect 25% of the general population.8,9 Hepatosteatosis, a type of visceral adipocyte tissue (VAT) deposition, correlates significantly with all components of the metabolic syndrome, regardless of body mass index (BMI), and can be considered the hepatic expression of the metabolic syndrome.10,11 Hepatic steatosis is also known to be associated with increased cardiovascular risk in CVD and diabetic individuals.12,13 Ultrasonography, CT scans, and magnetic resonance imaging (MRI) are the most common methods for diagnosing hepatosteatosis, but liver biopsy is the gold standard for diagnosis.14,15 On CT, hepatosteatosis is diagnosed with a hypodense image, which occurs due to lower than expected liver attenuation. One of the most critical limitations of this method is that additional deposits such as iron or copper in the liver and inflammatory events can cause changes in liver density.16,17 Liver–spleen (L/S) attenuation ratio is valuable in the diagnosis of hepatosteatosis because of its advantages, such as being in the same cross-section and not being affected by most systemic diseases.15,18 The accepted value for the L/S ratio in the detection of steatosis involving more than 30% of the liver parenchyma is approximately 1.1-1.2.18
The relationship between EAT and hepatosteatosis was firstly demonstrated in 2014. Iacobellis et al. revealed that obese individuals with steatohepatitis have higher EAT, epicardial fat is a good predictor of hepatic steatosis in obese persons; furthermore, echocardiographic epicardial fat measurement predicts ultrasound-measured hepatic steatosis better than BMI or waist circumference.19 Ectopic fat accumulation in and around critical organs, especially the heart and liver, is a cardiometabolic risk factor. Given this result, the need for easily accessible markers of ectopic fat accumulation is also inevitable. While hepatosteatosis is a traditional and established pattern of organ-specific fat deposition, recent research and clinical interest have focused on the heart's fat infiltration. Liver and cardiac steatosis may co-exist and influence each other as previously described.20,21
Defining the relationship between EAT and hepatosteatosis and revealing it with simple radiological measurement methods will provide a more accurate association of the cardiometabolic risks attributed to both adipose tissue accumulations and a more accurate interpretation of the relationship between the 2 visceral adipose tissue accumulations. While the measurement of EAT volume with CT stands out as the most essential method for the determination of the amount of EAT,7 the determination of the L/S ratio is one of the measurement methods with proven effectiveness in detecting hepatosteatosis.18,22 Based on this idea, we aimed to reveal the L/S ratio and EAT volume and their relationship with CVD by using the liver spleen density measurement technique with CT.
Methods
The study protocol was approved by the Medical Ethics Committee of our hospital (Ethics Committee number: 14567952-050/460; March 25, 2020). Written informed consent was obtained from all subjects.
The records of 283 consecutive patients who underwent coronary computed tomographic angiography in our Radiology Department between July 2012 and February 2020 were reviewed retrospectively from our hospital’s HBYS and PACS (Enlil) system. Data on CVD presence, family history of CVD, hypertension, weight, height, BMI, and biochemical blood lipid profiles were collected. Patients with a cardiac history such as coronary bypass surgery, valve replacement, pericardial effusion, volunteers with chronic liver disease, liver metastasis, or primary liver tumor, who used alcohol, and whose clinical laboratory data were insufficiently available were excluded from the study.
The patients participating in the study were divided into 2 groups according to the L/S ratio. Those with an L/S ratio above 1.2 were considered the non-steatosis group, and those below 1.2 were considered the steatosis group. In the steatosis group, those with the L/S ratio between 1.1 and 1.2 were mild steatosis; Those below 1.1 were accepted as severe steatosis group and divided into 2 groups.
Radiological Evaluation
All CT examinations were performed on the third-generation Siemens dual-energy Computed Tomography scanner (Somatom Drive; Siemens Healthcare, Forchheim, Germany). Non-contrast images were acquired using the following scanning parameters: tube voltage, 120 kV; 80 mAs tube current; high-pitch spiral acquisition mode; reconstructed slice thickness, 1.5 mm; prospectively electrocardiography-triggered; scanning range, from the tracheal carina to portal vein level of the liver. If the heart rate was > 65 beats per minute, heart rate control was achieved with a β-blocker. All reconstructions were transferred to Syngo Via workstation (Siemens Healthcare, Forchheim, Germany) to quantify EAT volume and hepatosteatosis. Measurements of EAT, hepatosteatosis and Coronary Artery Disease–Reporting and Data System (CAD-RADS) scores were evaluated by 2 radiologists (with 10 years and 6 years of cardiovascular imaging experience, respectively) blinded to the study protocol. The interobserver variability was 10%.
The data obtained from coronary computed tomography angiography (CCTA) images were evaluated for grading of stenosis severity. The CAD-RADS classification system is used for CVD presence and CAD-RADS scores of the patients were calculated according to the reference values. Table 1 shows the scoring of the scale. They range from CAD-RADS 0 (absence of atherosclerosis) to CAD-RADS5 (presence of at least 1 total occlusion).
Table 1.
Coronary Artery Disease–Reporting and Data System Score System
| Score | Stenosis | Interpretation |
|---|---|---|
| 0 | 0% | Absence of CAD |
| 1 | 1-24% | Minimal non-obstructive CAD |
| 2 | 25-49% | Mild non- obstructive CAD |
| 3 | 50-69% | Moderate stenosis |
| 4A | 70-99% | |
| 4B | Left main >50% or 3-vessel obstructive (70%) disease | Severe stenosis |
| 5 | 100% | Total coronary occlusion |
| N | Non-diagnostic study |
CAD, coronary artery disease.
On the CACS images, hepatic CT attenuation was measured by drawing 3 different regions of interest (ROI) (approximately 1.5 × 1.5 cm2) at the portal vein level of the liver (left lobe, right anterior lobe, and right posterior lobe of the liver, respectively). All ROIs were distributed in the hepatic parenchyma, and the biliary, vascular, and extrahepatic structures were excluded. Spleen attenuation was obtained by averaging 3 ROIs (approximately 1.5 × 1.5 cm2) from 3 different areas in the same section. The attenuation index-the liver-to-spleen attenuation ratio (CT L/S)- was calculated as L/S, where L is the hepatic attenuation, and S is the splenic attenuation18 (Figure 1).
Figure 1.
(A) Unenhanced transverse CT image shows 1.5-cm ROI (white circle) devoid of macroscopic vessels; ROI is placed approximately right anterior-posterior and left hepatic lobes. (B) Spleen attenuation was obtained by averaging 3 ROIs from 3 different areas in the same section. CT, computed tomography; ROI, region of interest.
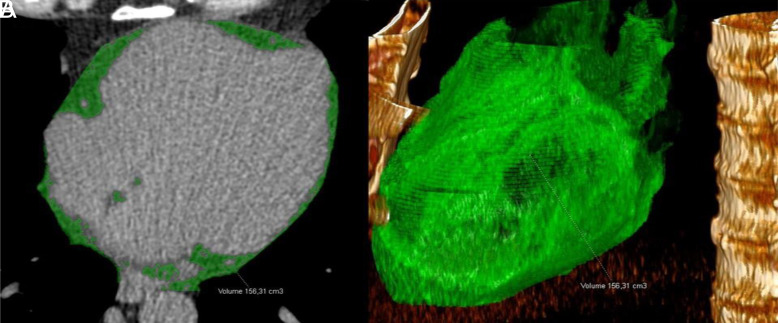
Semi-automatic quantification of EAT volume in non-contrast CT images. All data sets were checked for coverage of the entire epicardial sac. First, the reader identified the upper and lower limits of the pericardial sac as the bifurcation of the pulmonary trunk and, respectively, the slice caudal to the posterior descending artery. Next, the contour of the pericardial sac is automatically traced and adjusted by the reader, if necessary. The EAT volume (green) (in mL) is automatically calculated by the inclusion of all contiguous 3D voxels with CT attenuations between the specified upper threshold (here −40 HU) and the lower threshold of −200 HU (Figures 2 and 3).
Figure 2.
(A-D) The epicardial adipose tissue area (green) was determined semiautomatically on an unenhanced transverse CT image. CT, computed tomography.
Figure 3.
(A) The epicardial adipose tissue area (green) was determined by tracing a single region of interest on an unenhanced transverse CT image. Fat voxels were identified by using a threshold attenuation range from −200 HU to −40 HU. (B) EAT volume (cm3) was automatically calculated as the sum of the fat areas for the whole heart.
Statistical Analysis
The data obtained were evaluated using the Statistical Package for Social Sciences for Windows 21.0 (SPSS Inc., Chicago, Ill, USA) statistical program. Descriptive statistics were determined for each variable. Data were expressed as mean ± standard deviation or median and interquartile range. A statistically significant difference between the groups was determined by the χ2 test for categorical variables. Nonparametric statistics (Mann–Whitney U test) and parametric statistics (independent sample t-test) were all used for continuous variables. Associations between the variables were explored using Spearman’s rho test. Binary logistic regression analysis was performed to determine independent predictors for CAD. Factors with a P value of <.2 were included in the univariate analysis in the regression test, while those that were significant in the univariate analysis were included in the multivariable evaluation. If the P value is less than .05; was considered statistically significant.
Results
A total of 236 patients, 108 women and 128 men were included in the study. There were 154 patients without hepatosteatosis and 82 patients in the hepatosteatosis group. There was no significant difference between the 2 groups in total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol, and triglyceride levels (P > .05) (Table 2).
Table 2.
Demographic and Biochemical Characteristics of the Group with And Without Hepatosteatosis
| Non-Hepatosteatosis Group (n = 154) | Hepatosteatosis Group (n = 182) | P | |
|---|---|---|---|
| Age | 43.6 ± 11.16 | 56.25 ± 10.74 | <.001 |
| Gender (F/M) | 73/80 | 35/48 | .258 |
| Total cholesterol (mg/dL) | 201.12 ± 45.51 | 200.75 ± 47.27 | .960 |
| HDL cholesterol (mg/dL) | 45.96 ± 11.97 | 44.11 ± 11.23 | .328 |
| LDL cholesterol (mg/dL) | 123 (52.75) | 121.5 (40.33) | .565 |
| Triglyceride (mg/dL) | 135.5 (100.75) | 145.5 (99) | .516 |
| BMI (kg/m2) | 25.93 ± 2.96 | 30.62 ± 5.03 | <.001 |
| HT (mm Hg) | 61 (39.8%) | 61 (73.4%) | <.001 |
| CVD | 5 (3.2%) | 46 (55.4%) | <.001 |
| CVD family history | 57 (37.2%) | 50 (60.2%) | .001 |
| Liver attenuation (HU) | 62.05 ± 6.15 | 50.32 ± 7.93 | <.001 |
| L/S ratio | 1.32 ± 0.9 | 1.01 ± 0.13 | <.001 |
| EAT volume (mm3) | 69.03 (30.95) | 112.24 (44) | <.001 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index; HT, hypertension; CVD, cardiovascular disease; L/S, liver–spleen ratio; EAT volume, epicardial adipose tissue volume.
The mean age of the hepatosteatosis group (56.25 ± 10.74) was significantly higher than the group without hepatosteatosis (43.6 ± 11.16) (P < .001). When the BMI of the 2 groups was compared, it was found that the hepatosteatosis group (30.62 ± 5.03) was significantly higher than the other group (25.93 ± 2.96) (P < .001).
When the 2 groups were evaluated in terms of atherosclerotic heart disease, the family history of CVD and the frequency of CVD were significantly higher in the hepatosteatosis group than in the other group (P < .001). The incidence of HT in the hepatosteatosis group was also more frequent than in the other group (P < .001).
Eighty-two patients with hepatosteatosis were divided into 2 groups mild and severe steatosis. There were 24 patients with mild steatosis and 58 patients with severe steatosis. There was no significant difference between the 2 groups regarding age, gender variables, and total cholesterol, HDL, LDL, triglyceride, and BMI levels. While there was no significant difference in terms of family history of CVD in the 2 groups evaluated for atherosclerotic heart disease, it was found that the patient group with severe steatosis had a higher history of CAD (P < .001). When the epicardial adipose tissue volume was examined, the EAT volume was significantly higher in patients with severe steatosis (P = .023) (Table 3).
Table 3.
Evaluation of Patient Groups According to The Severity of Hepatosteatosis
| Mild Steatosis (n = 24) | Severe Steatosis (n = 58) | P | |
|---|---|---|---|
| Age | 53.44 ± 11.67 | 57.46 ± 10.19 | .118 |
| Gender (F/M) | 30.52 ± 4.92 | 30.67 ± 5.11 | .899 |
| Total Cholesterol (mg/dL) | 13/12 | 22/36 | .134 |
| HDL Cholesterol (mg/dL) | 206.53 ± 34.9 | 198.38 ± 51.66 | .542 |
| LDL Cholesterol (mg/dL) | 45.97 ± 11.12 | 43.35 ± 11.31 | .410 |
| Triglyceride (mg/dL) | 131.03 ± 29.35 | 120.65 ± 42.59 | .349 |
| BMI (kg/m2) | 157.55 (57.55) | 144.5 (148) | .213 |
| HT (mm Hg) | 15 (60%) | 46 (79.3%) | .102 |
| CVD | 6 (24%) | 40 (68.9%) | <.001 |
| CVD family history | 12 (48%) | 38 (65.5%) | .150 |
| Liver attenuation (HU) | 54.72 ± 5.49 | 48.43 ± 8.1 | .001 |
| L/S ratio | 1.15 ± 0.3 | 0.95 ± 0.11 | <.001 |
| EAT volume (mm3) | 93.85 (43) | 112.11 (42) | .023 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index; HT, hypertension; CVD, cardiovascular disease; L/S, liver–spleen ratio; EAT volume, epicardial adipose tissue volume.
The groups with and without hepatosteatosis were also evaluated separately in terms of L/S ratio and EAT volume. In the group with hepatosteatosis L/S ratio was inversely correlated with EAT volume (rs: −0.241 P = .028). No significant correlation was found between EAT volume and L/S ratio in the group without hepatosteatosis (P = .675).
The patients were divided into 2 groups in terms of CVD according to the CAD-RADS score. 185 patients with a CAD-RADS score of 0 were included in the non-CVD group, and 51 patients with a CAD-RADS score of ≥1 were included in the CVD group. We also performed binomial logistic regression analysis to define variables that are independently associated with CAD (Table 4). Age, L/S ratio, EAT volume, and BMI were included in this model. As a result of our multivariable analysis, age and L/S ratio values were found to be independent predictors of CAD (Table 4).
Table 4.
Binary Logistic Regression Analysis of Cardiovascular Disease
| Parameters | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.88 (0.85-0.92) |
<.001 | 0.91 (0.87-0.95) |
<.001 |
| L/S ratio | 32765.44 (1909.77-562 146.08) |
<.001 | 6312.15 (337.44-118 072.95) |
<.001 |
| EAT volume (mm3) | 0.96 (0.95-0.97) |
<.001 | 0.98 (0.97-1.00) |
.119 |
| BMI (kg/m2) | 0.83 (0.77-0.90) |
<.001 | 1.09 (0.96-1.23) |
.131 |
L/S ratio, liver–spleen ratio; EAT volume, epicardial adipose tissue volume; BMI, body mass index.
Discussion
In our study, in which the relationship between EAT and CVD of patients diagnosed with hepatosteatosis according to the L/S ratio, the following results were obtained was evaluated: (1) EAT volume was higher in the steatosis group than in the non-steatosis group; (2) There is a negative correlation between the L/S ratio and EAT volume in the hepatosteatosis group; (3) The family history of CVD and CVD frequency is higher in the hepatosteatosis group than in the other group; (4) L/S ratio value was found to be an independent predictor of CAD. Other aspects of our study worthy of attention are as follows. The use of the CT method, which provides high reproducibility, for epicardial fat measurements. In the EAT measurement, EAT volume was measured rather than the thickness of EAT, which is not equal in all regions of the heart, and the L/S ratio, which is more valuable than liver attenuation, was used in predicting liver steatosis.
In our study, EAT volume was nearly 2 times higher in the patient group with hepatosteatosis than in patients without steatosis. Many studies have shown that the amount of EAT is higher in patients with hepatosteatosis.19,21,23–25 Our EAT volume measurements with the CT method are consistent with the data of similar studies in the literature when evaluated by taking into account the local distribution of EAT, regional variability, and the measurements of patients with different ethnic origins.26–28 Pathogenetic processes in which insulin resistance, oxidative stress, and inflammation play a role-play a role in the relationship between fatty liver and the amount of EAT.29–31
These processes cause liver and cardiac adiposity to be seen together with the increase of visceral adipocyte tissue. Our finding that the patient group with hepatosteatosis had much higher EAT volume measurements than the other group in our study is consistent with similar data in the literature.
Our study found a negative correlation between the L/S ratio and EAT volume in the hepatosteatosis group. The L/S ratio is a measurement method with proven effectiveness in detecting hepatosteatosis radiologically and histologically.18,22,32,33 Determination of steatosis with the L/S ratio is more valuable than using only liver attenuation in the diagnosis of hepatosteatosis due to its advantages, such as having the liver and spleen in the same cross-section and not being affected by most systemic diseases.15,18 The accepted reference value for the L/S ratio in detecting severe steatosis involving more than 30% of the liver parenchyma is 1.1.18 We interpreted our data by considering the reference ratio of 1.1 in the differentiation of mild and severe hepatosteatosis.18 In the light of the literature, we accepted that patients with L/S ratio above 1.2 do not have hepatosteatosis, those between 1.2 and 1.1 have mild hepatosteatosis, and those below 1.1 have severe hepatosteatosis.18,34 We found that the severity of hepatosteatosis, which can be calculated according to liver attenuation, increased with the decrease in the L/S ratio. There was no relationship between the L/S ratio and EAT volume in the group without steatosis. There was a negative correlation between EAT volume and the L/S ratio in the steatosis group. The most important reason for this difference between the L/S ratio and EAT volume between groups with and without steatosis is the change in inflammatory properties due to the change in the amount of accumulation of EAT. This adipose tissue shows anti-inflammatory properties in the early stages when EAT volume accumulation is low; when the accumulation increases, its properties change and inflammatory molecules are released.3,35 EAT volume, whose inflammatory properties increase, causes an increase in visceral adipose tissue accumulations over time.
The family history of CVD and the frequency of CVD were higher in the hepatosteatosis group than in the other group. As a result of our multivariable analysis, age and L/S ratio values were found to be independent predictors of CAD. As the L/S ratio decreases, EAT volume and incidence of CVD increase. The increase in the frequency of CVD is an important known consequence of increased visceral adipose tissue and secondary hepatic and epicardial adipose tissue.36–38 In explaining the role of hepatosteatosis and increase in the amount of EAT in the development of CVD, there are pathogenetic processes in which insulin resistance, oxidative stress, and inflammation play a role.29–31 EAT, which is found in low amounts in healthy people, has beneficial effects on both immunological and coronary blood flow and has cardioprotective properties with the functions of using and storing free fatty acids. Under conditions of low oxidative stress, normal epicardial adipocytes secrete adiponectin, that is, anti-inflammatory properties, which protect cardiomyocytes from hypertrophic stimuli, minimize inflammation and fibrosis in the coronary arteries and myocardium, thereby reducing the likelihood of adverse clinical events.39,40 In physiological conditions, EAT carries out tasks such as removing and storing excess free fatty acids that are toxic to the myocardium, while it is involved in the increased supply of free fatty acids required for the myocardium with increasing ischemia.38 With the changing biological structure of EAT, the release of adiponectin from EAT to the systemic circulation decreases, and instead, pro-inflammatory adipocytokines such as leptin, adipsin, TNF-α, IL-1β, IL-6, IL-18, and resistin are released. It causes a systemic inflammatory response. Systemic inflammation also triggers a positive feedback mechanism that causes epicardial adipose tissue to accumulate. 35 In many previous studies conducted by some researchers in our group, it was concluded that the relationship between EAT and CVD frequency was the result of concomitant inflammatory events.25,41-45 Studies in subjects with CVD or at high risk of CVD have shown that pro-inflammatory cytokines are released from EAT rather than from subcutaneous adipose tissue.2 It means that EAT is more closely associated with cardiovascular events than the amount of total body adipose tissue. In a meta-analysis evaluating more than 40,000 patients, EAT was also an independent risk factor for CVD, and EAT volume correlates with coronary artery calcification and myocardial ischemia.46 In recent studies, it has been concluded that EAT is an independent predictor of coronary events and left ventricular dysfunction, but visceral adipocyte tissue accumulation is not an independent risk factor for cardiovascular events.47–50 In patients with CVD, pro-inflammatory adipokine production of epicardial adipose tissue is considerably higher than subcutaneous adipose tissue.35 It indicates that epicardial adipose tissue is a better predictor of CVD than subcutaneous fat deposition.
In our study, the mean BMI was higher in the hepatosteatosis group compared to the other group and also, BMI was an independent predictor for CVD. There is a close relationship between BMI and visceral adipose tissue; as the weight gain increases, the amount of visceral adipose tissue, liver steatosis, and epicardial adipose tissue also increases.51,52 Inflammation caused by increased adipose tissue also causes an increase in local adipose tissue accumulations with positive feedback. Our data showing BMI is higher in patients with hepatosteatosis, and the increase in the frequency of concomitant CVDs supports these pathophysiological processes and similar data in the literature. Compared with BMI and waist circumference, EAT thickness is more valuable in demonstrating hepatosteatosis.19 The patient population of most of the studies investigating the relationship between hepatosteatosis and EAT consists of obese individuals.19,39,53 In obese individuals, like many visceral adipocyte tissue accumulations, EAT also increases, and the biological structure and anti-inflammatory properties of EAT also change.39 In obese individuals, EAT has inflammatory properties rather than anti-inflammatory properties.2 Although obesity is a severe risk factor for CVD, the correlation of epicardial fat with the risk of CVD and the development of high-risk obstructive plaque is independent of obesity.54–56 Only 23.7% (n=56) of our patient group are obese individuals. Considering the studies in the literature showing that EAT thickness is higher in obese patients with steatosis than in obese patients without steatosis and studies showing that the role of EAT in the development of CVD is independent of VAT, our study shows that the relationship between EAT and hepatosteatosis cannot be explained only by obesity or VAT accumulations.19
There was no significant difference in total cholesterol, HDL, LDL cholesterol, and triglyceride levels between groups with and without hepatosteatosis in the study. Another known traditional risk factor for CVD is dyslipidemia. While there was no difference in cholesterol levels between the 2 groups in our study, the significant difference in cardiovascular events may raise the idea that CVDs may be associated with the EAT relationship independent of hyperlipidemia. In studies examining the relationship between cholesterol levels, CVH, and EAT, EAT was found to be inversely correlated with HDL cholesterol and positively correlated with LDL cholesterol and triglyceride.25,46,49,57,58
Among the exciting aspects of our study are (1) the use of the CT method, which provides high reproducibility for epicardial fat measurements; (2) more consistent measurements can be made by measuring the volume of EAT, which is not found in equal amounts in each area on the heart, rather than the thickness, (3) the liver-spleen attenuation ratio can be measured in hepatosteatosis; (4) Finally, by comparing the L/S ratio with EAT, factors that may cause an increase in EAT volume, such as visceral adipose tissue accumulation caused by structural causes, can be ignored. Although it is not a measurement method recommended by the guidelines in EAT measurement, transthoracic echocardiography is often the preferred method because it is easily accessible, non-invasive, and low cost. This method has some limitations. The biggest limitations of EAT measurement with echocardiography are that EAT is not equal in all areas of the heart, the experience of the echocardiographer changes the measurement results, and the difficulty of the procedure in obese individuals. Epicardial adipose tissue volume can also be measured with CT and MR, and both methods provide a more accurate and volumetric measurement of epicardial fat tissue than transthoracic echocardiography. CT and MR imaging are more sensitive and specific than echocardiography, but they are more expensive and laborious.7 In studies comparing volume and thickness measurements of EAT from different cross-sectional areas, EAT volume was found to be correlated with intra-abdominal visceral adipose tissue deposition compared to other measurements.26 In line with all this information, the measurement of EAT volume stands out as the best method to determine the amount of EAT.7,37,59,60 In the meta-analysis of 9 studies published by Nerlekar et al.37 EAT volume was found to be more valuable in CVD risk assessment than other EAT measurement methods.37 In most studies examining the relationship between EAT and hepatosteatosis, liver attenuation is used to diagnose hepatosteatosis. Non-contrast CT is the most effective method for demonstrating fatty liver. In contrast-enhanced CT, attenuation is affected by contrast agent concentration, volume, rate of administration, and timing of measurements.33 The spleen is unaffected by most common pathological processes and is located on the same CT scan as the liver, facilitating liver and spleen measurements. In diagnosing hepatosteatosis with computed tomography, a more hypodense image is obtained due to the decrease in liver attenuation compared to expected. The average liver density is 50-57 HU on non-contrast computed tomography and 40-48 HU in the spleen. Liver density below 48 HU also suggests fatty liver.17 In the light of these data, we would like to state that in determining the presence and severity of hepatosteatosis with liver density, iron accumulation in the liver or inflammatory events may cause a change in liver density,16 therefore, the risk of error may increase by determining steatosis only by density measurement. Our study aimed to eliminate the effect of possible iron accumulation in the liver and spleen or density changes caused by the inflammatory disease using the L/S ratio.
In the light of these data, we would like to state that in determining the presence and severity of hepatosteatosis with liver density, iron accumulation in the liver or inflammatory events may cause a change in liver density. Therefore, the risk of error may increase by determining steatosis only by density measurement. Our study aimed to eliminate the effect of possible iron accumulation in the liver and spleen or density changes caused by the inflammatory disease using the L/S ratio. Our study has 2 important limitations. One of them, the inability to perform pathological evaluation, which is the gold standard method in diagnosing hepatosteatosis. Second, when patients with CVD were distributed according to the CAD-RADS score, it was not possible to make subgroup analyzes because the number of patients was low.
Conclusion
In conclusion, we showed in the study that the L/S ratio was an independent predictor for CVD, EAT volume was negatively correlated with the L/S ratio, and the frequency of CVD is much lower in patients with a high L/S ratio. Use of L/S ratio, which is more valuable than liver attenuation in predicting hepatic steatosis, may be more useful in evaluating the risk of hepatosteatosis-related CVD.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Necmettin Erbakan University (approval number: 14567952-050/460).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – N.P., A.L.S., K.T.; Design – N.P., İ.B., A.L.S., K.T.; Supervision – N.P., H.Ö., P.D.Y., A.L.S.; Funding – None; Materials – H.Ö., C.K., P.D.Y., A.L.S.; Data Collection and/or Processing – N.P., H.Ö., İ.B., C.K.; Analysis and/or Interpretation – İ.B., K.T.; Literature Review - H.Ö., İ.B.; Writing – N.P., H.Ö., İ.B., K.T.; Critical Review – P.D.Y., K.T.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This study received no funding.
References
- 1. De Feyter PJ. Epicardial adipose tissue: an emerging role for the development of coronary atherosclerosis. Clin Cardiol. 2011;34(3):143 144. 10.1002/clc.20893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazurek T, Zhang L, Zalewski A.et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460 2466. 10.1161/01.CIR.0000099542.57313.C5) [DOI] [PubMed] [Google Scholar]
- 3. Le Jemtel TH, Samson R, Milligan G, Jaiswal A, Oparil S. Visceral adipose tissue accumulation and residual cardiovascular risk. Curr Hypertens Rep. 2018;20(9):77. 10.1007/s11906-018-0880-0) [DOI] [PubMed] [Google Scholar]
- 4. Neeland IJ, Ayers CR, Rohatgi AK.et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21:439 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11(6):363 371. 10.1038/nrendo.2015.58) [DOI] [PubMed] [Google Scholar]
- 6. Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12(1):31 42. 10.1089/met.2013.0107) [DOI] [PubMed] [Google Scholar]
- 7. Sarin S, Wenger C, Marwaha A.et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102(6):767 771. 10.1016/j.amjcard.2008.04.058) [DOI] [PubMed] [Google Scholar]
- 8. Altin C, Yilmaz M, Ozsoy HM.et al. Assessment of epicardial fat and carotid intima media thickness in gestational hypertension. J Obstet Gynaecol Res. 2018;44(6):1072 1079. 10.1111/jog.13631) [DOI] [PubMed] [Google Scholar]
- 9. Tomic D, Kemp W. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. 2018. Available at: ingentaconnect.com [DOI] [PubMed] [Google Scholar]
- 10. Smits MM, Ioannou GN, Boyko EJ, Utzschneider KM. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol. 2013;28(4):664 670. 10.1111/jgh.12106) [DOI] [PubMed] [Google Scholar]
- 11. Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(1):27 38. 10.1161/ATVBAHA.107.147538) [DOI] [PubMed] [Google Scholar]
- 12. Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6:33386. 10.1038/srep33386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou YY, Zhou XD, Wu SJ.et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(6):631 636. 10.1097/MEG.0000000000001075) [DOI] [PubMed] [Google Scholar]
- 14. Brunt EM, Tiniakos DG. Pathology of steatohepatitis. Baillieres Best Pract Res Clin Gastroenterol. 2002;16(5):691 707. 10.1053/bega.2002.0326) [DOI] [PubMed] [Google Scholar]
- 15. Johnston RJ, Stamm ER, Lewin JM, Hendrick RE, Archer PG. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: limitations of liver-minus-spleen attenuation difference measurements. Abdom Imaging. 1998;23(4):409 415. 10.1007/s002619900370) [DOI] [PubMed] [Google Scholar]
- 16. Mills SR, Doppman JL, Nienhuis AW. Computed tomography in the diagnosis of disorders of excessive iron storage of the liver. J Comput Assist Tomogr. 1977;1(1):101 104. 10.1097/00004728-197701000-00012) [DOI] [PubMed] [Google Scholar]
- 17. Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137(3):727 729. 10.1148/radiology.137.3.6934563) [DOI] [PubMed] [Google Scholar]
- 18. Iwasaki M, Takada Y, Hayashi M.et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78(10):1501 1505. 10.1097/01.tp.0000140499.23683.0d) [DOI] [PubMed] [Google Scholar]
- 19. Iacobellis G, Barbarini G, Letizia C, Barbaro G. Epicardial fat thickness and nonalcoholic fatty liver disease in obese subjects. Obesity (Silver Spring). 2014;22(2):332 336. 10.1002/oby.20624) [DOI] [PubMed] [Google Scholar]
- 20. Iacobellis G, Pellicelli AM, Grisorio B.et al. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring). 2008;16(1):179 183. 10.1038/oby.2007.50) [DOI] [PubMed] [Google Scholar]
- 21. Turan Y. The nonalcoholic fatty liver disease fibrosis score is related to epicardial fat thickness and complexity of coronary artery disease. Angiology. 2020;71(1):77 82. 10.1177/0003319719844933) [DOI] [PubMed] [Google Scholar]
- 22. Ma X, Holalkere NS, Kambadakone R A, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. RadioGraphics. 2009;29(5):1253 1277. 10.1148/rg.295085186) [DOI] [PubMed] [Google Scholar]
- 23. Iozzo P. Myocardial, perivascular,and epicardial fat. Diabetes Care. 2011;34(suppl 2):S371 S379. 10.2337/dc11-s250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tonbul HZ, Turkmen K, Kayıkcıoglu H, Ozbek O, Kayrak M, Biyik Z. Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail. 2011;33(8):770 775. 10.3109/0886022X.2011.599913) [DOI] [PubMed] [Google Scholar]
- 25. Akbas EM, Demirtas L, Ozcicek A.et al. Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Exp Med. 2014;7(7):1794 1801. [PMC free article] [PubMed] [Google Scholar]
- 26. Wang TD, Lee WJ, Shih FY.et al. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab. 2009;94(2):662 669. 10.1210/jc.2008-0834) [DOI] [PubMed] [Google Scholar]
- 27. Gorter PM, van Lindert AS, de Vos AM.et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896 903. 10.1016/j.atherosclerosis.2007.08.016) [DOI] [PubMed] [Google Scholar]
- 28. Lin A, Wong ND, Razipour A.et al. Metabolic syndrome, fatty liver, and artificial intelligence-based epicardial adipose tissue measures predict long-term risk of cardiac events: a prospective study. Cardiovasc Diabetol. 2021;20(1):27. 10.1186/s12933-021-01220-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golabi P, Otgonsuren M, Avila de L, Sayiner M. Components of metabolic syndrome increase the risk of mortality in Nonalcoholic Fatty Liver Disease (NAFLD). 2018.. Available at: ncbi.nlm.nih.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Apostolopoulou M, Gordillo R, Koliaki C. et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Am Diabetes Assoc. 2018;41(6):1235 1243. [DOI] [PubMed] [Google Scholar]
- 31. Kankaanpää M, Lehto HR, Pärkkä JP.et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91(11):4689 4695. 10.1210/jc.2006-0584) [DOI] [PubMed] [Google Scholar]
- 32. Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol. 2003;15(5):539 543. 10.1097/01.meg.0000059112.41030.2e) [DOI] [PubMed] [Google Scholar]
- 33. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdallaet al SA. Comparison of CT methods for determining the fat content of the liver. 2012;188:1307 1312. [DOI] [PubMed] [Google Scholar]
- 34. Park SH, Kim PN, Kim KW.et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105 112. 10.1148/radiol.2391050361) [DOI] [PubMed] [Google Scholar]
- 35. Cheng KH, Chu CS, Lee KT. et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond). 2008;32(2):268-274. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 36. Aprigliano G, Scuteri L, Iafelice I.et al. Epicardial adipose tissue thickness and acute coronary syndrome: a matter of how much or how? Int J Cardiol. 2015;199:8 9. 10.1016/j.ijcard.2015.06.168) [DOI] [PubMed] [Google Scholar]
- 37. Nerlekar N, Brown AJ, Muthalaly RG.et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(8). 10.1161/JAHA.117.006379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang TD, Lee WJ, Shih FY.et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010;213(1):279 287. 10.1016/j.atherosclerosis.2010.07.055) [DOI] [PubMed] [Google Scholar]
- 39. Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40(7):442 445. 10.1055/s-2008-1062724) [DOI] [PubMed] [Google Scholar]
- 40. Iacobellis G, di Gioia CRT, Cotesta D, et al. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm Metab Res. 2009;41(3):227 231. 10.1055/s-0028-1100412) [DOI] [PubMed] [Google Scholar]
- 41. Aydin H, Toprak A, Deyneli O.et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8(3):229 234. 10.1089/met.2009.0080) [DOI] [PubMed] [Google Scholar]
- 42. Akbas EM, Hamur H, Demirtas L.et al. Predictors of epicardial adipose tissue in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6:55. 10.1186/1758-5996-6-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozcicek A, Ozcicek F, Yildiz G.et al. Neutrophil-to-lymphocyte ratio as a possible indicator of epicardial adipose tissue in patients undergoing hemodialysis. Arch Med Sci. 2017;13(1):118-123. 10.5114/aoms.2015.50784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turkmen K, Tonbul HZ, Erdur FM.et al. Peri-aortic fat tissue and malnutrition-inflammation-atherosclerosis/calcification syndrome in end-stage renal disease patients. Int Urol Nephrol. 2013;45(3):857 867. 10.1007/s11255-012-0286-x) [DOI] [PubMed] [Google Scholar]
- 45. Karatas A, Canakci E, Bektas O.et al. Relationship of epicardial fat tissue thickness with oxidant biomarkers in chronic kidney disease. Bratisl Lek Listy. 2018;119(9):566 571. 10.4149/BLL_2018_102) [DOI] [PubMed] [Google Scholar]
- 46. Saritas T, Reinartz SD, Nadal J.et al. Epicardial fat, cardiovascular risk factors and calcifications in patients with chronic kidney disease. Clin Kidney J. 2020;13(4):571-579. 10.1093/ckj/sfz030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin HH, Lee JK, Yang CY, Lien YC, Huang JW, Wu CK. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol. 2013;12:127. 10.1186/1475-2840-12-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oikawa M, Owada T, Yamauchi H.et al. Epicardial adipose tissue reflects the presence of coronary artery disease: comparison with abdominal visceral adipose tissue. BioMed Res Int. 2015;2015:483982. 10.1155/2015/483982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D’Marco LG, Bellasi A, Kim S, Chen Z, Block GA, Raggi P. Epicardial adipose tissue predicts mortality in incident hemodialysis patients: a substudy of the Renagel in New Dialysis trial. Nephrol Dial Transplant. 2013;28(10):2586 2595. 10.1093/ndt/gft264) [DOI] [PubMed] [Google Scholar]
- 50. Versteylen MO, Takx RAP, Joosen IAPG.et al. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. Eur Heart J Cardiovasc Imaging. 2012;13(6):517 523. 10.1093/ehjci/jes024) [DOI] [PubMed] [Google Scholar]
- 51. Iacobellis G, Ribaudo MC, Assael F.et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163 5168. 10.1210/jc.2003-030698) [DOI] [PubMed] [Google Scholar]
- 52. Song DK, Hong YS, Lee H, Oh JY, Sung YA, Kim Y. Increased epicardial adipose tissue thickness in type 2 diabetes mellitus and Obesity. Diabetes Metab J. 2015;39(5):405 413. 10.4093/dmj.2015.39.5.405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311 9; quiz 1417. 10.1016/j.echo.2009.10.013) [DOI] [PubMed] [Google Scholar]
- 54. Silaghi A, Piercecchi-Marti MD, Grino M.et al. Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring). 2008;16(11):2424 2430. 10.1038/oby.2008.379) [DOI] [PubMed] [Google Scholar]
- 55. Yerramasu A, Dey D, Venuraju S.et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220(1):223 230. 10.1016/j.atherosclerosis.2011.09.041) [DOI] [PubMed] [Google Scholar]
- 56. Nakanishi K, Fukuda S, Tanaka A.et al. Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non-obese subjects with coronary artery disease. Atherosclerosis. 2014;237(1):353 360. 10.1016/j.atherosclerosis.2014.09.015) [DOI] [PubMed] [Google Scholar]
- 57. Baloglu I, Turkmen K, Selcuk NY.et al. The relationship between visceral adiposity index and epicardial adipose tissue in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2021;129(5):390 395. 10.1055/a-0892-4290) [DOI] [PubMed] [Google Scholar]
- 58. Wilund KR, Tomayko EJ, Wu PT.et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant. 2010;25(8):2695 2701. 10.1093/ndt/gfq106) [DOI] [PubMed] [Google Scholar]
- 59. Topuz M, Dogan A, Celik A, Can C, Ter IO-C. Investigation of the relationship between non-alcoholic fatty liver disease and coronary artery disease. 2014. Available at: apbs.mersin.edu.tr [DOI] [PubMed] [Google Scholar]
- 60. Cho KI, Jo EA, Cho SH, Kim BH. The influence of epicardial fat and nonalcoholic fatty liver disease on heart rate recovery in metabolic syndrome. Metab Syndr Relat Disord. 2017;15(5):226 232. 10.1089/met.2016.0132) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a