Abstract
Background:
The use of prosthetic valves and intracardiac devices has steadily increased in recent years. In this group of patients with prosthetic valves or intracardiac devices, infective endocarditis could not be easily diagnosed, and in general, infective endocarditis can be missed in many patients. The purpose of this study was to evaluate the diagnostic performance of clinical, laboratory, and imaging parameters in a group of patients with pre-diagnosis of infective endocarditis.
Methods:
Ninety-four patients diagnosed with prosthetic valve or intracardiac device endocarditis during 2008-2019 were included in the study. The patients’ data were evaluated according to modified Duke criteria, and the data of the patients who were diagnosed with and without a definitive infective endocarditis were compared accordingly.
Results:
Values of procalcitonin (P < .001), leukocytes (P = .004), C-reactive protein (P < .001), sedimentation (P < .001), and maximal vegetation size (P = .012) were found to be significant in the diagnosis of IE. Criteria to determine definitive IE included a C-reactive protein level of 105 mg/dL or higher, 77% sensitivity, 75% specificity, 60% positive predictive value, and 87% negative predictive value. In particular, a C-reactive protein level of ≥105 mg/dL was found to positively indicate the diagnosis of definitive infective endocarditis by 10 times (odds ratio = 10; 95% CI: 3.6-27.8, P < .001). In a multiple logistic regression analysis, the C-reactive protein level was found to be the best independent predictor of definitive infective endocarditis in this population.
Conclusion:
In cases of prosthetic valve and intracardiac devices endocarditis where pre-diagnosis is difficult to confirm, measuring C-reactive protein levels is a reliable, strong, and simple parameter for definitive infective endocarditis diagnosis.
Keywords: C-reactive protein, echocardiography, infective endocarditis, intra-cardiac devices, prosthetic valves
Highlights
Simple, inexpensive, accessible markers are required in the diagnosis of prosthetic valve and intracardiac device endocarditis, which are difficult to diagnose.
Procalcitonin, leukocyte, C-reactive protein (CRP), and sedimentation values are significant in the diagnosis of infective endocarditis.
A CRP value of ≥105 mg/dL is a strong indicator for the diagnosis of infective endocarditis.
Introduction
Prosthetic valves (PVs) and intracardiac devices (IDs) are increasingly being used to treat heart valve diseases and arrhythmia. Unfortunately, these treatment methods have led to a rising number of complications. Of the patients treated with these methods, 1-6% develop infective endocarditis (IE), which can be fatal.1,2
In patients with PVs or IDs, IE diagnosis is more difficult than in patients without them.3 The Duke criteria with a sensitivity of 70-80% for the diagnosis of native valve endocarditis have lower diagnostic accuracy in PV and ID endocarditis.2 Similarly, the sensitivity of transesophageal echocardiography (TEE)—one of the most commonly used imaging methods for diagnosing endocarditis—is low for the patients with PVs and IDs and often results in false positive and false-negative diagnoses.4
Current guidelines recommend multi-slice computed tomography (MSCT), leukocyte-labeled single-photon emission tomography (SPECT), or fluorodeoxyglucose positron emission tomography (FDG-PET) for patients with PVs and IDs.5 However, these scans are complex and costly. Thus, testing methods are needed that are not only easily accessible and simple to perform but are also able to generate results quickly. Our study will evaluate the diagnostic performances of clinical, laboratory, and imaging methods, as well as investigate their value when diagnosing patients with definitive IE who have PVs or IDs.
Methods
Patient Population
We retrospectively reviewed the cases of 94 patients with PV or ID endocarditis diagnoses from a single medical center from 2008 to 2019.
Inclusion criteria for the study were determined as:
Being over 18 years old,
Having TEE with a preliminary diagnosis of PV or ID endocarditis,
Continuing all treatment and follow-up at our center in the post-diagnosis period, and
Having patients with access to all necessary data for the study was determined as.
By screening archived patient records, demographic characteristics (age, gender, height, weight, body surface area), presence of additional diseases (diabetes, hypertension, coronary artery disease, hyperlipidemia), clinical features (presence of fever), echocardiographic parameters (replacement valve type, vegetation size, shape in TEE), laboratory parameters (sedimentation, C-reactive protein (CRP), leucocyte, procalcitonin, D-Dimer), blood culture results (presence and type of breeding microorganisms), and FDG-PET results if applied to the patient (presence of involvement), were recorded.
The data from the patients who were admitted to the medical center with a pre-diagnosis of IE were evaluated according to the modified Duke criteria specified in the current guidelines.5 Definitive IE was defined by the presence of 2 majors or, 1 major and 3 minor criteria, or 5 minor criteria. Patients who did not meet the definition were labeled as having “nondefinitive IE” and were excluded.
This study was approved by the Local Ethics Committee (decision number: I6-310-19; decision date: January 8, 2020) and was performed in accordance with the principles outlined in the Declaration of Helsinki.
Statistical Analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) (ver. 20.0 for Windows, SPSS Inc., Chicago, Ill, USA). The groups were compared in terms of the data obtained. The normality of distribution was investigated with the Kolmogorov–Smirnov test.
The normally distributed continuous data were presented as mean ± standard deviation and tested with an independent samples t-test. Nonnormally distributed continuous data were presented with median and interquartile range (IQR) and tested with Mann–Whitney U test.
The categorical data were presented as frequency and percentages and tested with a chi-squared test. Prognostic factors’ effects were tested using multiple logistic regression analysis. A receiver operating characteristic (ROC) curve was drawn to show the sensitivity and specificity of the diagnostically significant parameters. A value of P < .05 was considered statistically significant.
Results
Of those involved in the study, 34 were female and 60 were male. The mean age of the patients was 62.06 ± 13.12 years. Twenty-nine percent had diabetes, 44% had atherosclerotic heart disease, 70% had hypertension, and 39% had hyperlipidemia (n = 37). Of the patients, 26.6% had suspected PV endocarditis (25 patients), and 73.4% had a preliminary diagnosis of ID endocarditis (69 patients). From the TEE findings, 10.64% had linear moving structures. In addition, 21.28% showed vegetation growth of 0-5 mm, and 36.17% showed vegetation growth of 6-10 mm (see Table 1). Of the blood cultures, 67.02% did not indicate bacterial reproduction, although 12.77% showed Staphylococcus aureus and 8.51% showed Enterococcus faecalis infections.
Table 1.
Transesophageal Echocardiography Findings of the Group Pre-Diagnosed with Endocarditis
| Patient (n = 94) | ||
|---|---|---|
| Vegetation | ||
|
10 (10.64%) | |
|
21 (21.28%) | |
|
33 (36.17%) | |
|
11 (11.70%) | |
|
13 (13.83%) | |
| ≥21 mm | 2 (2.13%) | |
| Apse | 4 (4.26%) | |
| Dehiscence | 1 | |
| Paravalvular leak | Mild | 2 |
| Moderate | 2 | |
| Severe | 1 | |
According to the modified Duke criteria, 31 patients (32%) were diagnosed with definitive IE, all of whom demonstrated 2 major and 1 minor criteria. About 38% of these patients had PV endocarditis; the others had ID endocarditis. Positron emission tomography was not performed in the vast majority of patients (64 patients, 68.08%) included in the study. It was determined that 80.65% (n = 25) of the patients with definitive IE did not have PET scans. Of the patients with definitive IE who underwent PET scans, 1 had an abscess (3.23%), 2 had pacemaker pocket infections (6.45%), and 3 showed no vegetation growth (9.68%). While bacterial reproduction was detected in the blood cultures of all patients diagnosed with definitive IE, S. aureus (38.71%) and E. faecalis (25.81%) were the most common.
The definitive IE and nondefinitive IE groups’ demographics were similar. Both groups comprised about 33% diabetic women, about 50% coronary artery disease patients, and roughly 66% hypertension patients (see Table 2). When comparing groups, procalcitonin (P < .001), CRP (P < .001), sedimentation (P < .001), leukocyte (P = .004), and maximal vegetation size (P = .012) values were significantly higher in the group diagnosed with definite IE. D-dimer levels were similar in both groups (see Table 3).
Table 2.
Comparison of Clinical Characteristics of the Group Diagnosed with and Without Definitive IE According to Modified Duke criteria
| Definitive IE PV Endocarditis (48%, n = 12) ID Endocarditis (27.54%, n = 19) |
Excluded PV Endocarditis (12%, n = 3) ID Endocarditis (33.33%, n = 23) |
P | |
|---|---|---|---|
| Age Median (IQR) |
62.00 (20) | 66.00 (18) | .682 |
| Female Sex (%) | 32.3% | 38.1% | .580 |
| BSA (m2) mean ± SD |
1.89±0.18 | 1.87±0.17 | .596 |
| DM (%) | 32.3% | 28.6% | .713 |
| CHD (%) | 45.2% | 44.4% | .948 |
| HT (%) | 74.2% | 68.3% | .554 |
| HL (%) | 35.5% | 41.3% | .589 |
BSA, body surface area; CHD, coronary heart disease; DM, diabetes mellitus; HL, hyperlipidemia; HT, hypertension; ID, intracardiac device; PV, prosthetic valve; SD, standard deviation.
Table 3.
Comparison of Biochemical Parameters of the Group Diagnosed with and Without Definitive IE According to Modified Duke criteria
| Definitive IE PV Endocarditis (48%, n = 12) ID Endocarditis (27.54%, n = 19) |
Excluded PV Endocarditis (12%, n = 3) ID Endocarditis (33.33%, n = 23) |
P | |
|---|---|---|---|
| Procalcitonin Median (IQR) |
1.20 (4.68) | 0.10 (0.05) | <.001 |
| Leukocytes Median (IQR) |
13.64 (8.28) | 8.33 (4.66) | .004 |
| CRP Median (IQR) |
156.00 (89.00) | 34.00 (94.70) | <.001 |
| D-Dimer Median (IQR) |
367.000 (1081.00) | 234.00 (274.00) | .053 |
| Erythrocyte sedimentation rate Median (IQR) | 60.00 (59) | 19.00 (39) | <.001 |
| Maximal vegetation size, mm Median (IQR) |
10.00 (13) | 7.00 (8) | .012 |
IQR, interquartile range; IE, infective endocarditis.
The parameters that were significant in the diagnosis of definitive IE in the single-variable analyses (procalcitonin, CRP, sedimentation, leukocyte, and maximal vegetation size) were evaluated via a multiple logistics regression analysis. According to Wald statistics, high CRP levels were identified as the best independent parameter for definitive IE diagnosis (Table 4).
Table 4.
Multiple Logistics Regression Analysis
| B | Wald | df | Sig. | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|
| Leukocytes | −0.007 | 0.008 | 1 | 0.928 | 0.994 | 0.862-1.145 |
| Procalcitonin |
0.068 | 0.570 | 1 | 0.450 | 1.070 | 0.897-1.276 |
| Maximal vegetation size |
0.067 | 2.382 | 1 | 0.123 | 1.069 | 0.982-1.164 |
| Erythrocyte sedimentation rate | 0.018 | 3.528 | 1 | 0.060 | 1.018 | 0.999-1.038 |
| CRP | 0.010 | 5.193 | 1 | 0.023 | 1.010 | 1.001-1.019 |
CRP, C-reactive protein.
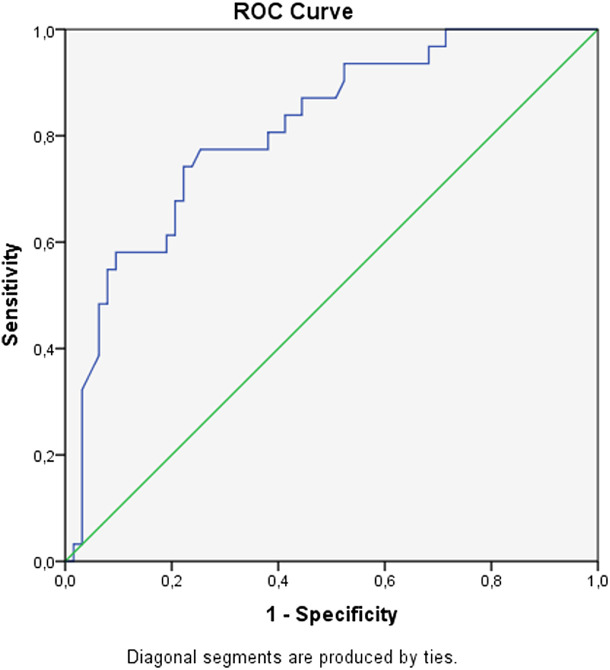
The area under the curve was calculated as 0.81 (P < .001; CI: 0.72-0.90) when the ROC curve indicated the effectiveness of CRP in diagnosing IE (see Figure 1). We found that when the CRP value reached ≥105 mg/dL, sensitivity was 77%, positive predictive value was 75%, and negative predictive value was 87%. Furthermore, a CRP value of ≥105 mg/dL increases the risk of developing IE by 10 times (odds ratio = 10.0; CI: 3.6-27.8; P < .001).
Figure 1.
Receiver-operating characteristic curve in predicting definitive IE diagnosis of CRP (AUC = 0.81; P < .001; CI: 0.72-0.90) (sensitivity 77%, specificity 75%, PPV 60%, NPV 87%, if CRP value is 105 and above). CRP, C-reactive protein; AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Discussion
With increased interventional therapies, the characteristics of the patients of IE are also changed.6 Prosthetic valves and IDs are increasingly encountered in patients with endocarditis,6 but unfortunately, the diagnostic values of the Duke criteria and TEE for this group of patients are quite low.5 Furthermore, advanced imaging methods, while useful, are expensive, time-consuming, and very difficult to perform. The need for fast, economical, and accessible methods is great. In this study, we found 1 such method.
C-reactive protein is one of the oldest known acute phase reactants produced in the liver.7 Levels rise from acute diagnoses, such as infection, sepsis, and trauma, as well as from chronic diseases, such as atherosclerosis.8 Although CRP takes part in limiting inflammation with complement activation in acute infections, high levels of CRP can predict poor prognosis for many diseases.8,9
Numerous studies related to high CRP levels have been performed with IE patients. For example, high levels were found to be associated with septic embolism and death in those with native left-valve endocarditis.10 High CRP levels were also found to be associated with in-hospital death and stroke in PV endocarditis patients.11 In addition, serial CRP measurements were found valuable in determining the treatment response of IE patients.12
There have also been studies investigating the diagnostic value of CRP levels in IE patients.13 One study suggested that high CRP levels support the diagnosis of valve IE in patients.14 There is also data that indicates high CRP levels are more specific to staphylococcal endocarditis in patients diagnosed with certain types of IE.15 However, studies investigating the diagnostic value of CRP were mostly focused on left-heart endocarditis.16 Because of the exclusion of right-heart and device-related endocarditis, using CRP to diagnosis PV and ID endocarditis has been difficult.17 However, because of this study, the prognostic value of CRP for patients with PV or ID endocarditis has been proven.
Transthoracic echocardiography imaging has both low sensitivity and specificity in the diagnosis of PV and ID endocarditis.18 Transthoracic echocardiography is more sensitive—but more invasive—than TTE.19 In our study, maximal vegetation size measured by TEE was significantly higher in the group diagnosed with definitive IE. However, this variable was not statistically significant in the multivariate analysis. This can be explained by the reduction of the specialization of TEE in this group of patients by fibrillar structures formed by time on electrodes and mixed with vegetation.
Procalcitonin is released in response to endotoxins and systemic mediators released during bacterial infections.20 In studies investigating the relationship between procalcitonin and IE etiology, the results showed that high procalcitonin levels were related to infections of S. aureus or gram-negative bacteria.20,21 In our study, the diagnostic value of high procalcitonin levels was determined to be significant in single-variable analysis, but it was not in the multivariate analysis.22
Several studies have shown a correlation between D-dimer levels and in-hospital complications and death in patients with endocarditis.23,24 However, our study showed no additional diagnostic contribution in PV and ID endocarditis.
Study Limitations
Our research was limited by its retrospective nature, small sample size, and inability to confirm IE diagnosis via MSCT, leukocyte-labeled SPECT, or FDG-PET.
Conclusion
The frequency of PV and ID endocarditis is increasing, yet it is difficult to diagnose. Guidelines recommend the use of expensive imaging methods that cannot be performed easily or quickly. To our knowledge, this was the first study to demonstrate the value of CRP levels in diagnosing PV and ID endocarditis. We showed that a high CRP level greatly increases the probability of diagnosis for patients with PVs and IDs and that measuring CRP is reliable, simple, and easily accessible.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Ankara University (approval number: İ6-310-19).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.H.; Design – M.H., D.M.G.U.; Supervision – M.K., H.G.; Fundings – None; Materials –M.H., V.Ö.B.; Data collection &/or processing – M.H., V.Ö.B., D.M.G.U.; Analysis &/or interpretation: V.Ö.B, M.K.; Literature search – M.H., V.Ö.B., C.G.T.C; Writing – V.Ö.B., M.H., D.M.G.U.; Critical review – E.T., D.M.G.U., M.K.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This study received no funding.
References
- 1. Tarakji KG, Chan EJ, Cantillon DJ.et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes [presentation]. Heart Rhythm. 2010;7(8):1043 1047. 10.1016/j.hrthm.2010.05.016) [DOI] [PubMed] [Google Scholar]
- 2. Habib G, Thuny F, Avierinos JF. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis. 2008;50(4):274 281. 10.1016/j.pcad.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 3. Baddour LM. Cardiac device infection--or not. Circulation. 2010;121(15):1686 1687. 10.1161/CIR.0b013e3181de0334) [DOI] [PubMed] [Google Scholar]
- 4. San Román JA, Vilacosta I, Zamorano JL, Almería C, Sánchez-Harguindey L. Transesophageal echocardiography in right-sided endocarditis. J Am Coll Cardiol. 1993;21(5):1226 1230. 10.1016/0735-1097(93)90250-5) [DOI] [PubMed] [Google Scholar]
- 5. Habib G, Lancellotti P, Antunes MJ.et al. ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075 3128. 10.1093/eurheartj/ehv319) [DOI] [PubMed] [Google Scholar]
- 6. Hoen B, Alla F, Selton-Suty C.et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288(1):75 81. 10.1001/jama.288.1.75) [DOI] [PubMed] [Google Scholar]
- 7. Hung SK, Lan HM, Han ST, Wu CC, Chen KF. Current evidence and limitation of biomarkers for detecting sepsis and systemic infection. Biomedicines. 2020;8(11):494. 10.3390/biomedicines8110494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agrawal A. CRP after 2004. Mol Immunol. 2005;42(8):927 930. 10.1016/j.molimm.2004.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gershov D, Kim S, Brot N, Elkon KB. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192(9):1353 1364. 10.1084/jem.192.9.1353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verhagen DW, Hermanides J, Korevaar JC.et al. Prognostic value of serial C-reactive protein measurements in left-sided native valve endocarditis. Arch Intern Med. 2008;168(3):302 307. 10.1001/archinternmed.2007.73) [DOI] [PubMed] [Google Scholar]
- 11. Elbey MA, Kalkan ME, Akdag S.et al. Predictors of mortality in patients with prosthetic valve infective endocarditis: a nation-wide multicenter study. Cardiol J. 2013;20(3):323 328. 10.5603/CJ.2013.0079) [DOI] [PubMed] [Google Scholar]
- 12. Olaison L, Hogevik H, Alestig K. Fever, C-reactive protein, and other acute-phase reactants during treatment of infective endocarditis. Arch Intern Med. 1997;157(8):885 892. 10.1001/archinte.157.8.885) [DOI] [PubMed] [Google Scholar]
- 13. Roberts-Thomson PJ, Koh LY, Kennedy A, Smith MD, Neoh S, Turnidge J. Serological investigations in the diagnosis and management of infective endocarditis. Aust N Z J Med. 1986;16(6):761 765. 10.1111/j.1445-5994.1986.tb00032a.x) [DOI] [PubMed] [Google Scholar]
- 14. Hryniewiecki T, Rawczyńska-Englert I, Sitkiewicz D, Jabłoński D. Comparison of interleukin-6 and C-reactive protein serum concentrations assessment in diagnosis of infective endocarditis. pol Arch Med Wewn. 2002;108(4):947 952. [PubMed] [Google Scholar]
- 15. Tascini C, Aimo A, Arzilli C.et al. Procalcitonin, white blood cell count and C-reactive protein as predictors of S. aureus infection and mortality in infective endocarditis. Int J Cardiol. 2020;301:190 194. 10.1016/j.ijcard.2019.08.013) [DOI] [PubMed] [Google Scholar]
- 16. Alter P, Hoeschen J, Ritter M, Maisch B. Usefulness of cytokines interleukin-6 and interleukin-2R concentrations in diagnosing active infective endocarditis involving native valves. Am J Cardiol. 2002;89(12):1400 1404. 10.1016/s0002-9149(02)02353-6) [DOI] [PubMed] [Google Scholar]
- 17. Ribeyrolles S, Ternacle J, San S.et al. Infective endocarditis without biological inflammatory syndrome: description of a particular entity. Arch Cardiovasc Dis. 2019;112(6-7):381 389. 10.1016/j.acvd.2019.02.005) [DOI] [PubMed] [Google Scholar]
- 18. Arber N, Pras E, Copperman Y.et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine. 1994;73(6):299 305. 10.1097/00005792-199411000-00003) [DOI] [PubMed] [Google Scholar]
- 19. Cacoub P, Leprince P, Nataf P.et al. Pacemaker infective endocarditis. Am J Cardiol. 1998;82(4):480 484. 10.1016/s0002-9149(98)00365-8) [DOI] [PubMed] [Google Scholar]
- 20. Müller B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-I gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86(1):396 404. 10.1210/jcem.86.1.7089) [DOI] [PubMed] [Google Scholar]
- 21. Kocazeybek B, Küçükoğlu S, Öner YA. Procalcitonin and C-reactive protein in infective endocarditis: correlation with etiology and prognosis. Chemotherapy. 2003;49(1-2):76 84. 10.1159/000069777) [DOI] [PubMed] [Google Scholar]
- 22. Cuculi F, Toggweiler S, Auer M, der Maur ChA, Zuber M, Erne P. Serum procalcitonin has the potential to identify Staphylococcus aureus endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27(11):1145 1149. 10.1007/s10096-008-0541-3) [DOI] [PubMed] [Google Scholar]
- 23. Barış VÖ, Kılıçkap M, Göksülük H, Gerede Uludağ DMG, Erol Ç. D-dimer is a strong predictor of in-hospital mortality in patients with infective endocarditis. Anatol J Cardiol. 2019;21(3):124-133. 10.14744/AnatolJCardiol.2018.56752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turak O, Canpolat U, Özcan F.et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res. 2014;134(3):587 592. 10.1016/j.thromres.2014.06.015) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a