Abstract
Background:
Since lobaplatin (LBP) has been approved to treat metastatic breast cancer in China, this study aimed to evaluate the safety and efficacy of LBP-based chemotherapy in clinical practice.
Methods:
This trial was a prospective, open-label, multicenter phase IV clinical trial that enrolled patients with unresectable locally advanced or recurrent/metastatic breast cancer from 34 sites between July 2013 and March 2017. Patients were treated with LBP monotherapy or in combination for four to six cycles. The primary endpoint was safety. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR).
Results:
A total of 1179 patients were analyzed; 59 (5.0%) were treated with LBP alone, 134 (11.4%) with LBP plus paclitaxel, 263 (22.3%) with LBP plus docetaxel, 237 (20.1%) with LBP plus gemcitabine, 403 (34.2%) with LBP plus vinorelbine, and 83 (7.0%) with other LBP-based regimens. The overall incidence of adverse events (AEs) was 95.2%, and 57.9% of patients had grade >3 AEs. The most common grade >3 AEs were neutropenia (43.9%), leukopenia (39.4%), anemia (17.8%), and thrombopenia (17.7%). LBP monotherapy showed the lowest incidence of grade >3 AEs (39.0%), followed by LBP plus docetaxel (52.9%), LBP plus paclitaxel (59.0%), LBP plus vinorelbine (62.5%), and LBP plus gemcitabine (62.9%). The ORR and DCR were 36.8 and 77.0%, respectively. The median PFS was 5.5 months (95% confidence interval: 5.2–5.9).
Conclusion:
LBP-based chemotherapy shows favorable efficacy in patients with advanced breast cancer, with manageable safety profile.
Trial registration:
This trial was registered with ChiCTR.org.cn, ChiCTR-ONC-13003471.
Keywords: breast cancer, chemotherapy, efficacy, lobaplatin, neoplasm metastasis, safety
Introduction
Breast cancer is the most common cancer in women worldwide and one of the leading causes of cancer-related deaths among women.1 Despite the substantial progress in patient management over the past few decades, metastatic breast cancer is still considered incurable. In the treatment of metastatic breast cancer, monotherapy is usually preferred due to a lower impact on the quality of life of patients while the combination therapy do not demonstrate an increase in overall survival (OS).2–4 Anthracyclines and taxanes are the mainstay chemotherapy agents for breast cancer, but many patients with metastatic breast cancer have already received these agents in the early course of disease. They cannot use them further due to drug resistance or cumulative toxicities.5 For these patients, no standard regimen exists and platinum-based chemotherapy is one of the several options available.6–8
The first-generation platinum drug cisplatin (DDP) exhibits severe renal, auricular, neurological, and gastrointestinal toxicities. The second-generation platinum drug carboplatin (CBP) exerts bone marrow suppression and displays high cross-resistance with DDP. These characteristics restrict their long-term clinical application and treatment effect.9
Lobaplatin (LBP) is a third-generation platinum drug discovered during the screening of platinum compounds for DDP-resistant tumors. LBP in combination with another chemotherapy agent (paclitaxel, pemetrexed, gemcitabine, vinorelbine, or docetaxel) was effective and well tolerated for patients with metastatic breast cancer.10,11
This study aimed to explore the characteristics of LBP treatment in patients with advanced breast cancer and investigate tailored regimens for specific populations. The results reflected the safety and efficacy of LBP-based chemotherapy in patients with advanced breast cancer, which might guide the rational use of LBP in clinical practice.
Methods
Trial design
This trial was a prospective, open-label, multicenter phase IV clinical trial. It was conducted in accordance with the Declarations of Helsinki and Good Clinical Practice, and was approved by the institutional review boards of the participating centers. All patients provided written informed consent. This study was registered with ChiCTR.org.cn, ChiCTR-ONC-13003470 (date of registration: August 11, 2013).
Participants
From July 30, 2013 to March 15, 2017, patients were enrolled from 34 sites in China. The eligible patients were females aged ⩾18 years; had histologically confirmed unresectable locally advanced or recurrent/metastatic breast cancer; had at least one measurable lesion according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; had a life expectancy ⩾3 months; had adequate hematological (absolute neutrophil count ⩾1.5 × 109/L, platelet count ⩾100 × 109/L, and hemoglobin ⩾90 g/L), hepatic [serum total bilirubin and conjugated bilirubin lower than or equal to the upper limit of normal (ULN) × 1.5, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ⩽ULN × 2.5 (⩽ULN × 5 for patients with liver metastasis)], and renal [creatinine ⩽ULN and creatinine clearance rate ⩾60 mL/min (Cockcroft–Gault formula)] function; had no plans to prepare for pregnancy during the study period; and were not breastfeeding. Either patients with or without prior treatment with anthracyclines and/or taxanes could be enrolled. Patients with active central nervous system disease or previous resistance to platinum were allowed to be enrolled. Patients with previous allergies to platinum compounds, coagulation dysfunction, or those who were considered unsuitable by investigators were excluded. The molecular subtype of breast cancer was extracted from previous pathological records, or determined using biopsy tissues by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Human epidermal growth factor receptor 2 (HER2)-positive disease was defined as IHC score 3+, or 2+ with gene amplification by FISH.
Procedures
Patients were treated with LBP-based chemotherapy, including but not limited to LBP alone, LBP plus vinorelbine, LBP plus paclitaxel, LBP plus docetaxel, and LBP plus gemcitabine.12 Investigators could choose one of the LBP-based regimens at their discretion. The dose of LBP was 50 mg/m2 when used alone and 30 mg/m2 when used in combination. Treatment was given for 4–6 cycles, and every 21 days were deemed as a cycle. If disease progression did not occur after 6 cycles, patients would receive tailored maintenance treatment regimens or discontinue treatment at investigators’ discretion. When grade ⩾3 adverse events (AEs) occurred, the necessity of dose reduction or discontinuation was judged by the investigators. Routine supportive care and other concomitant anticancer therapies (such as anti-HER2 therapy, endocrine therapy, radiotherapy, and surgery) were allowed during the treatment period.
Physical examination, electrocardiogram, tumor marker tests, and urinalysis were performed within 1 week before each cycle. Blood routine test and blood chemistry were performed at least once per week. Spiral computed tomography scan or magnetic resonance imaging was used for tumor assessment by investigators according to RECIST 1.1 at baseline (within 2 weeks before treatment), every two cycles during the treatment period, and every 3 months after treatment completion or discontinuation until disease progression or patient withdrawal from study. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Antiemetic drugs were allowed for the prophylaxis and treatment of gastrointestinal toxicity, while granulocyte colony-stimulating factor, thrombopoietin, and interleukin-11 were only allowed for the treatment of hematological toxicity.
Endpoints
The primary endpoint was safety (incidence and severity of AEs). Secondary endpoints were progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). PFS was defined as the time from the initiation of treatment to disease progression or death from any cause, whichever occurred first. ORR was calculated as the proportion of patients with complete response (CR) or partial response (PR). DCR was calculated as the proportion of patients with CR, PR, or stable disease.
Statistical analysis
According to the requirements of Chinese registration regulations, the number of valid patients in phase IV clinical trials should be more than 2000. Hence, two phase IV studies of LBP in small-cell lung cancer and metastatic breast cancer were conducted simultaneously, and the number of valid patients in each study was planned to be at least 1000. Considering the 20% dropout rate, the total number of patients in this study was approximately 1200.
Safety analysis was performed in the safety set, defined as all patients who received at least one dose of the study drug and had safety assessment. Baseline characteristics were summarized in the full analysis set, defined as all patients who received at least one dose of study drug. Efficacy was analyzed in the efficacy-evaluable set, defined as all patients who received at least one dose of study drug and had available efficacy evaluation data.
Categorical variables were presented as frequency (percentage) and analyzed using the chi-square test. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. They were presented as mean ± standard deviation (SD) if normally distributed or median (range) if not normally distributed. PFS was estimated using the Kaplan–Meier method, and the 95% confidence interval (CI) of PFS was estimated using the Brookmeyer–Crowley method. Statistical analyses were done with SAS 9.4 (SAS Institute, Cary, NY, USA).
Results
Characteristics of the participants
From July 30, 2013 to March 15, 2017, a total of 1181 patients were enrolled; 1179 patients received LBP-based chemotherapy and were included in the safety and full analysis sets (Figure 1). The mean age was 50.2 ± 9.4 years, and 449 (38.1%) patients were premenopausal women. The majority (94.9%) of patients had an ECOG performance status of 0 or 1. There were 235 (19.9%) patients with unresectable locally advanced breast cancer and 944 (80.1%) with recurrent/metastatic breast cancer; 341 (28.9%) patients had hormone receptor (HR)-positive, HER2-negative breast cancer, 436 (37.0%) had HER2-positive breast cancer, 252 (21.4%) had triple-negative breast cancer (TNBC), and 150 (12.7%) had unknown molecular subtype. Regarding treatment, 59 (5.0%) patients were treated with LBP alone, 134 (11.4%) with LBP plus paclitaxel, 263 (22.3%) with LBP plus docetaxel, 237 (20.1%) with LBP plus gemcitabine, 403 (34.2%) with LBP plus vinorelbine, and 83 (7.0%) with other LBP-based regimens (Table 1). Of 341 patients with HR-positive, HER2-negative breast cancer, 5 (1.5%) received concomitant endocrine therapy. Of 436 patients with HER2-positive breast cancer, 17 (3.9%) received concomitant anti-HER2 therapy (trastuzumab for all these 17 patients). Notably, 242 (20.5%) patients received <4 cycles of LBP-based chemotherapy because of patient refusal [98 (8.3%)], loss to follow-up [54 (4.6%)], switching to radiotherapy, surgery, or systemic therapy without LBP [36 (3.1%)], no clinical benefit [17 (1.4%)], intolerable toxicity [15 (1.3%)], death [7 (0.6%)], allergy to study drug [2 (0.2%)], or unknown reasons [13 (1.1%)]. Before enrollment, 141 (12.0%) patients were pretreated with taxanes, 136 (11.5%) with anthracyclines, 753 (63.9%) with both anthracyclines and taxanes, and 149 (12.6%) had no previous use of anthracyclines or taxanes. Of 436 patients with HER2-positive breast cancer, 25 (5.7%) had previous anti-HER2 therapy.
Figure 1.
Patient flowchart.
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; LBP, lobaplatin.
Table 1.
Baseline characteristics.
| Characteristics | Patients (n = 1179) |
|---|---|
| Age (years) | 50.2 ± 9.4 |
| <65 | 1102 (93.5) |
| ⩾65 | 65 (5.5) |
| Missing | 12 (1.0) |
| Menopause | |
| No | 449 (38.1) |
| Yes | 714 (60.6) |
| Missing | 16 (1.4) |
| Smoking history | |
| No | 1141 (96.8) |
| Yes | 22 (1.9) |
| Missing | 16 (1.4) |
| ECOG performance status | |
| 0 | 319 (27.1) |
| 1 | 800 (67.9) |
| 2 | 37 (3.1) |
| Missing | 23 (2.0) |
| Disease status | |
| Unresectable locally advanced | 235 (19.9) |
| Recurrent/metastatic | 944 (80.1) |
| Molecular subtype | |
| HR positive, HER2 negative | 341 (28.9) |
| HER2 positive | 436 (37.0) |
| Triple negative | 252 (21.4) |
| Missing | 150 (12.7) |
| Chemotherapy regimen | |
| LBP alone | 59 (5.0) |
| LBP + paclitaxel | 134 (11.4) |
| LBP + docetaxel | 263 (22.3) |
| LBP + gemcitabine | 237 (20.1) |
| LBP + vinorelbine | 403 (34.2) |
| Other LBP-based regimensa | 83 (7.0) |
| Treatment cycle | |
| 0 | 3 (0.3) |
| 1–3 | 239 (20.3) |
| 4 | 404 (34.3) |
| 5 | 262 (22.2) |
| 6 | 206 (17.5) |
| >6 | 63 (5.3) |
| Missing | 2 (0.2) |
Data were expressed as mean ± SD or n (%).
Other LBP-based regimens included LBP plus etoposide, LBP plus S-1, LBP plus irinotecan, LBP plus epirubicin, LBP plus pirarubicin, LBP plus doxorubicin, LBP plus cyclophosphamide, and LBP plus ifosfamide.
ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; LBP, lobaplatin.
Safety
The incidence of any grade AEs and grade ⩾3 AEs was 95.2% and 57.9% in the safety set, respectively. Table 2 summarizes the incidence and severity of common AEs (incidence > 3%).Hematological toxicities showed the highest incidence of any grade AEs (leukopenia: 80.1%; neutropenia: 75.7%; anemia: 67.6%; and thrombopenia: 51.2%), followed by gastrointestinal toxicities (nausea: 30.6%; vomiting: 15.8%) and liver toxicities (elevated AST: 21.9%; elevated ALT: 20.4%). Regarding grade ⩾3 AEs, neutropenia (43.9%) and leukopenia (39.4%) were the most common events. Prophylactic antiemetic drugs were used in 947 (80.3%) patients. No treatment-related deaths occurred.
Table 2.
Incidence and severity of AEs (n = 1179).
| MedDRA classification system preferred term | All grades | Severity | ||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | ||
| Leukopenia | 944 (80.1) | 99 (8.4) | 380 (32.2) | 372 (31.6) | 93 (7.9) | 0 |
| Neutropenia | 893 (75.7) | 109 (9.2) | 267 (22.6) | 292 (24.8) | 225 (19.1) | 0 |
| Anemia | 797 (67.6) | 144 (12.2) | 443 (37.6) | 171 (14.5) | 39 (3.3) | 0 |
| Thrombopenia | 604 (51.2) | 200 (17.0) | 195 (16.5) | 142 (12.0) | 67 (5.7) | 0 |
| Nausea | 361 (30.6) | 295 (25.0) | 60 (5.1) | 6 (0.5) | 0 | 0 |
| AST increased | 258 (21.9) | 194 (16.5) | 50 (4.2) | 10 (0.8) | 4 (0.3) | 0 |
| ALT increased | 241 (20.4) | 180 (15.3) | 52 (4.4) | 8 (0.7) | 1 (0.1) | 0 |
| Fatigue | 228 (19.3) | 185 (15.7) | 43 (3.6) | 0 | 0 | 0 |
| Vomiting | 186 (15.8) | 118 (10.0) | 45 (3.8) | 23 (2.0) | 0 | 0 |
| Pain | 155 (13.1) | 102 (8.7) | 42 (3.6) | 11 (0.9) | 0 | 0 |
| Loss of appetite | 141 (12.0) | 117 (9.9) | 24 (2.0) | 0 | 0 | 0 |
| Blood glucose increased | 83 (7.0) | 60 (5.1) | 21 (1.8) | 2 (0.2) | 0 | 0 |
| Hypocalcemia | 75 (6.5) | 58 (4.9) | 16 (1.4) | 2 (0.2) | 1 (0.1) | 0 |
| Albumin decreased | 76 (6.4) | 72 (6.1) | 4 (0.3) | 0 | 0 | 0 |
| Fever | 75 (6.4) | 58 (4.9) | 13 (1.1) | 1 (0.1) | 3 (0.3) | 0 |
| Anorexia and bulimia syndrome | 61 (5.2) | 56 (4.7) | 5 (0.4) | 0 | 0 | 0 |
| Hypoalbuminemia | 61 (5.2) | 50 (4.2) | 11 (0.9) | 0 | 0 | 0 |
| Blood bilirubin increased | 59 (5.0) | 29 (2.5) | 23 (2.0) | 6 (0.5) | 1 (0.1) | 0 |
| Hypokalemia | 54 (4.6) | 43 (3.6) | 2 (0.2) | 9 (0.8) | 0 | 0 |
| Cough | 52 (4.4) | 40 (3.4) | 12 (1.0) | 0 | 0 | 0 |
| Blood uric acid increased | 50 (4.2) | 49 (4.2) | 1 (0.1) | 0 | 0 | 0 |
| Hyponatremia | 41 (3.5) | 32 (2.7) | 1 (0.1) | 7 (0.6) | 1 (0.1) | 0 |
| Hemoglobin decreased | 38 (3.2) | 12 (1.0) | 20 (1.7) | 6 (0.5) | 0 | 0 |
| Insomnia | 37 (3.1) | 32 (2.7) | 5 (0.4) | 0 | 0 | 0 |
Data were expressed as n (%).
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The subgroup analyses showed that the incidence of any grade AEs and grade ⩾3 AEs was similar among different molecular subtypes of breast cancer (HR-positive, HER2-negative breast cancer: 94.7% and 57.2%; HER2-positive breast cancer: 95.6% and 56.2%; TNBC: 94.4% and 62.3%). LBP monotherapy (83.1% and 39.0%) showed the lowest incidence of any grade AEs and grade ⩾3 AEs, followed by LBP plus docetaxel (93.5% and 52.9%), LBP plus paclitaxel (96.3% and 59.0%), LBP plus vinorelbine (96.0% and 62.5%), and LBP plus gemcitabine (98.3% and 62.9%) (Supplemental Table S1).
In the SS, dose reductions of LBP and its chemotherapy partner due to AEs occurred in 6.2% and 5.9% of patients, respectively. Discontinuation of the whole LBP-based regimen due to AEs was observed in 5.1% of patients, while 0.9% of patients only discontinued the chemotherapy partner of LBP due to AEs. Specifically, patients treated with LBP plus taxanes (docetaxel or paclitaxel) had the lowest dose reduction and discontinuation rates of LBP (3.8% and 2.0%) (Supplemental Table S2).
Efficacy
There were 1023 patients with available tumor evaluation data. In all, 26 patients achieved CR, and 350 achieved PR; the ORR was 36.8%, and DCR was 77.0%. ORR and DCR in different subgroups are shown in Table 3. A low ORR was observed in patients with brain metastasis (17.0%) or ECOG performance status 2 (24.1%). Patients with unresectable locally advanced breast cancer had an ORR of 48.3% and DCR of 82.9%, while those with recurrent/metastatic breast cancer had an ORR of 33.9% and DCR of 75.6%. Different molecular subtypes achieved similar ORR and DCR (HR-positive, HER2-negative breast cancer: 36.4% and 79.8%; HER2-positive breast cancer: 37.0% and 74.2%; TNBC: 36.5% and 74.9%). Among the different chemotherapy regimens, LBP plus taxanes showed the highest ORR and DCR (LBP plus docetaxel: 51.7% and 84.6%; LBP plus paclitaxel: 49.6% and 85.6%), followed by LBP plus gemcitabine (29.4% and 72.1%), LBP plus vinorelbine (28.9% and 76.3%), and LBP alone (26.9% and 63.5%).
Table 3.
ORR and DCR in different subgroups.
| Subgroup | ORR (%) | DCR (%) |
|---|---|---|
| Metastatic sites | ||
| Lymph nodes (n = 614) | 39.3 | 77.2 |
| Bone (n = 401) | 34.9 | 74.6 |
| Lung (n = 493) | 33.7 | 76.1 |
| Liver (n = 363) | 31.1 | 68.6 |
| Brain (n = 47) | 17.0 | 76.6 |
| Number of metastasis | ||
| 1 (n = 189) | 46.0 | 84.1 |
| 2 (n = 329) | 36.8 | 74.5 |
| ⩾3 (n = 481) | 33.5 | 75.3 |
| ECOG performance status | ||
| 0 (n = 275) | 33.8 | 75.3 |
| 1 (n = 699) | 38.1 | 78.0 |
| 2 (n = 29) | 24.1 | 69.0 |
| Disease status | ||
| Unresectable locally advanced (n = 205) | 48.3 | 82.9 |
| Recurrent/metastatic (n = 818) | 33.9 | 75.6 |
| Molecular subtype | ||
| HR positive, HER2 negative (n = 302) | 36.4 | 79.8 |
| HER2-positive (n = 376) | 37.0 | 74.2 |
| Triple negative (n = 211) | 36.5 | 74.9 |
| Menopause | ||
| No (n = 384) | 39.6 | 77.1 |
| Yes (n = 623) | 34.7 | 77.0 |
| Age (years) | ||
| <65 (n = 957) | 36.5 | 76.5 |
| ⩾65 (n = 56) | 39.3 | 83.9 |
| Chemotherapy regimens | ||
| LBP alone (n = 52) | 26.9 | 63.5 |
| LBP + paclitaxel (n = 125) | 49.6 | 85.6 |
| LBP + docetaxel (n = 240) | 51.7 | 84.6 |
| LBP + gemcitabine (n = 201) | 29.4 | 72.1 |
| LBP + vinorelbine (n = 342) | 28.9 | 76.3 |
DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; LBP, lobaplatin; ORR, objective response rate.
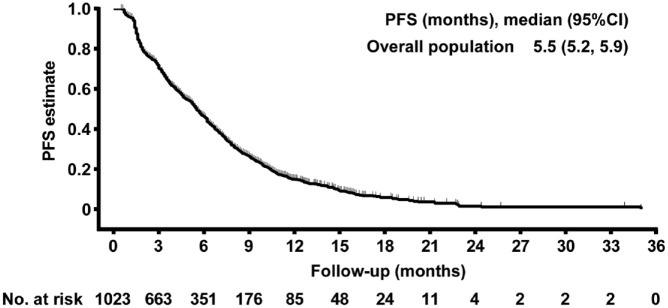
By the data cutoff date on May 15, 2019, the median PFS was 5.5 months (95% CI: 5.2–5.9) in the overall population (Figure 2). PFS in different subgroups is shown in Table 4. The median PFS for patients with metastases at lymph nodes, bone, lung, liver, and brain, was 5.7, 5.6, 5.5, 4.2, and 4.2 months, respectively. Patients with only one metastasis showed the highest median PFS (6.6 months), followed by those with two metastases (5.6 months), and those with at least three metastases (5.4 months). The median PFS was 6.4 months in patients with unresectable locally advanced breast cancer and 5.4 months in those with recurrent/metastatic breast cancer. The median PFS was the highest in patients with HR-positive, HER2-negative breast cancer (5.7 months), followed by those with HER2-positive breast cancer (5.4 months), and those with TNBC (4.6 months). Among the different chemotherapy regimens, the median PFS was 8.0 months with LBP plus paclitaxel, 7.0 months with LBP plus docetaxel, 5.1 months with LBP plus gemcitabine, 4.6 months with LBP plus vinorelbine, and 4.5 months with LBP alone.
Figure 2.
Kaplan–Meier estimates of PFS.
PFS, progression-free survival.
Table 4.
PFS in different subgroups.
| Subgroup | PFS (months), median (95% CI) |
|---|---|
| Metastatic sites | |
| Lymph nodes (n = 614) | 5.7 (5.3–6.2) |
| Bone (n = 401) | 5.6 (4.9–6.3) |
| Lung (n = 493) | 5.5 (4.9–6.1) |
| Liver (n = 363) | 4.2 (3.5–4.9) |
| Brain (n = 47) | 4.2 (3.3–5.8) |
| Number of metastasis | |
| 1 (n = 189) | 6.6 (5.2–7.4) |
| 2 (n = 329) | 5.6 (4.6–6.3) |
| ⩾3 (n = 481) | 5.4 (4.6–5.7) |
| ECOG performance status | |
| 0 (n = 275) | 5.7 (4.8–6.7) |
| 1 (n = 699) | 5.4 (4.8–6.0) |
| 2 (n = 29) | 5.9 (3.0–6.5) |
| Disease status | |
| Unresectable locally advanced (n = 205) | 6.4 (5.4–7.7) |
| Recurrent/metastatic (n = 818) | 5.4 (4.8–5.7) |
| Molecular subtype | |
| HR positive, HER2 negative (n = 302) | 5.7 (5.1–6.6) |
| HER2 positive (n = 376) | 5.4 (4.6–6.1) |
| Triple negative (n = 211) | 4.6 (3.7–5.5) |
| Menopause | |
| No (n = 384) | 5.7 (5.2–6.6) |
| Yes (n = 263) | 5.4 (4.6–5.9) |
| Age (years) | |
| <65 (n = 957) | 5.5 (5.2–5.9) |
| ⩾65 (n = 56) | 6.3 (3.4–8.4) |
| Chemotherapy regimens | |
| LBP alone (n = 52) | 4.5 (3.0–5.8) |
| LBP + paclitaxel (n = 125) | 8.0 (5.7–9.7) |
| LBP + docetaxel (n = 240) | 7.0 (6.0–8.0) |
| LBP + gemcitabine (n = 201) | 5.1 (4.0–5.9) |
| LBP + vinorelbine (n = 342) | 4.6 (3.7–5.5) |
ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; LBP, lobaplatin; PFS, progression-free survival.
Discussion
This phase IV trial investigated the safety and efficacy of LBP-based chemotherapy in a large population with advanced breast cancer. Most of patients had recurrent/metastatic breast cancer (80.1%) and had previous use of anthracyclines and/or taxanes (87.4%). The results confirmed the manageable safety profile of LBP. The efficacy data supported the use of LBP-based chemotherapy in clinical practice.
Patients with recurrent/metastatic breast cancer have poor tolerance and bone marrow function after multiple courses of treatment. Platinum-based chemotherapy should be selected carefully considering its severe side effects.13 In our study, the incidence of liver toxicities was approximately 20% (grade 3–4: approximately 1%), and renal toxicities and peripheral neurotoxicities were infrequent. The incidence of nausea (30.6%; grade 3–4: 0.5%) and vomiting (15.8%; grade 3–4: 2.0%) was also low, which might be attributed to the high use rate of prophylactic antiemetic drugs (80.3%). Hematological toxicities were the main AEs of LBP-based chemotherapy, which were manageable by symptomatic treatments. No new safety signals were identified. Only a small proportion of patients reduced the dose (6.2%) or discontinued LBP (5.1%) due to AEs. A phase III trial in patients with locoregionally advanced nasopharyngeal carcinoma showed that LBP-based induction chemotherapy plus concurrent chemoradiotherapy resulted in significantly fewer grade 3–4 hematological (neutropenia, leukopenia, anemia) and gastrointestinal (nausea and vomiting) toxicity events than DDP-based therapy.14 Some other randomized controlled trials in small-cell lung cancer and ovarian cancer demonstrated better tolerance and quality of life with LBP-based chemotherapy than with DDP- or CBP-based chemotherapy.15,16 LBP could be a better option as platinum-based chemotherapy, and our results demonstrated the manageable safety profile of LBP-based chemotherapy in patients with advanced breast cancer.
No more than 10% of breast cancer are metastatic at diagnosis,17 and the development of anti-HER2 and endocrine therapies has greatly improved the survival of patients with early breast cancer. However, disease recurrence/metastasis and drug resistance still remain challenges in clinical practice, and chemotherapy was still the mainstay treatment for TNBC. The prognosis of metastatic breast cancer was poor, with a median OS of 4–5 years for HR-positive, HER2-negative subtype, 5 years for HER2-positive subtype, and only 10–13 months for TNBC.17 When most patients with advanced breast cancer had failed with anthracyclines and/or taxanes, DDP- or CBP-based chemotherapy would result in an ORR of 15.7–44.7% and median PFS of 4.3–8.6 months.18–20 LBP-based chemotherapy in our study showed an ORR of 36.8% and median PFS of 5.5 months, within the range of these previous results of platinum-based chemotherapy.18–20 Specifically, a 10-year retrospective study showed an ORR of 38.5% and median PFS of 8.6 months with CBP-based chemotherapy in 148 patients with HR-positive, HER2-negative metastatic breast cancer, and 49.2% and 9.65 months in 65 patients with HER2-positive metastatic breast cancer.19 However, another retrospective study showed a median PFS of 3.7 months with CBP-based chemotherapy in 48 patients with HR-positive, HER2-negative advanced breast cancer.20 In our study, LBP-based chemotherapy resulted in an ORR of 36.4% and median PFS of 5.7 months in patients with HR-positive, HER2-negative advanced breast cancer, and 37.0% and 5.4 months in patients with HER2-positive advanced breast cancer. Different patient characteristics and sample size should be considered when indirect comparisons were performed across studies. In addition, it should be noted that a huge proportion of patients in our study did not receive concomitant anti-HER2 therapy, possibly due to financial burden. This indicated that HER2-positive advanced breast cancer would achieve more clinical benefits if anti-HER2 therapy was used in combination with LBP-based chemotherapy. For TNBC, the most aggressive subtype, previous studies showed an ORR of 26.7–56.6% and median PFS of 4.3–8.4 months with platinum-based chemotherapy.19,21–24 The ORR (36.5%) and median PFS (4.6 months) with LBP-based chemotherapy in our study were within the range of these previous results of platinum-based chemotherapy in patients with TNBC.19,21–24 Taken the safety and efficacy results together, LBP could be a good option when platinum-based chemotherapy was selected for the treatment of advanced breast cancer.
Interestingly, our study found that LBP plus taxanes might be the optimal combination, with the best clinical benefits and lowest dose reduction or discontinuation rate, which might deserve further investigations.
There are some limitations in this study. Long-term OS was not analyzed. In addition, quality of life questionnaires were not collected in our study. The impact of toxicities with LBP-based chemotherapy on quality of life in a large population could not be analyzed. However, previous randomized controlled trials have demonstrated better quality of life with LBP-based chemotherapy than with other platinum-based chemotherapies.15,16
In conclusion, this is the largest study of LBP-based chemotherapy in patients with advanced breast cancer, which confirms its manageable safety profile. LBP-based chemotherapy shows favorable efficacy, similar to other platinum-based chemotherapy in patients with advanced breast cancer. LBP could be a good option as platinum-based chemotherapy in clinical practice.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221122715 for A prospective, open-label, multicenter phase IV clinical trial on the safety and efficacy of lobaplatin-based chemotherapy in advanced breast cancer by Min Yan, Peng Yuan, Quchang Ouyang, Ying Cheng, Guohui Han, Dewei Wang, Li Ran, Tao Sun, Da Zhao, Yuju Bai, Shun’e Yang, Xiaojia Wang, Rong Wu, Xiaohua Zeng, Herui Yao, Xuening Ji, Jun Jiang, Xiaohua Hu, Haifeng Lin, Liping Zheng, Zhitu Zhu, Wei Ge, Junlan Yang, Tongjian Cui, Xiaozhi Zhang, Fangyang Lu, Wenhui Li, Hongyan Xu, Mafei Kang, Ping Gong, Liqun Zou, Jiang Liu, Hongliang Zhang, Hao Yu and Binghe Xu in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Min Yan, Department of Breast Disease, Henan Breast Cancer Center, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China.
Peng Yuan, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Quchang Ouyang, Department of Breast Cancer Medical Oncology, Hunan Cancer Hospital, Changsha, China.
Ying Cheng, Department of Oncology, Cancer Hospital of Jilin Province, Changchun, China.
Guohui Han, Department of Breast Surgery, Shanxi Provincial Cancer Hospital, Shanxi Medical University, Taiyuan, China.
Dewei Wang, Department of Thoracic Surgery, Hainan General Hospital, Haikou, China.
Li Ran, Department of Oncology, The Affiliated Hospital of Guizhou Medical University/Guizhou Cancer Hospital, Guiyang, China.
Tao Sun, Department of Medical Oncology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, China.
Da Zhao, Department of Internal Medicine-Oncology, The First Hospital of Lanzhou University, Lanzhou, China.
Yuju Bai, Department of Oncology, The Affiliated Hospital of Zunyi Medical University, Zunyi, China.
Shun’e Yang, Department of Breast Cancer and Lymphoma, Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, China.
Xiaojia Wang, Department of Breast Medical Oncology, Cancer Hospital of the University of Chinese Academy of Sciences, Zhejiang Cancer Hospital and Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences, Hangzhou, China.
Rong Wu, Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, China.
Xiaohua Zeng, Breast Center, Chongqing Cancer Hospital, Chongqing University, Chongqing, China.
Herui Yao, Department of Oncology, Sun Yat-sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China.
Xuening Ji, Department of Oncology, Affiliated Zhongshan Hospital of Dalian University, Dalian, China.
Jun Jiang, Department of Surgical Oncology, General Hospital of Mining Industry Group Fuxin, Fuxin, China.
Xiaohua Hu, Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Haifeng Lin, Department of Medical Oncology, The Second Affiliated Hospital of Hainan Medical University, Haikou, China.
Liping Zheng, Department of Breast-Thoracic Tumor Surgery, Affiliated Hospital of Hainan Medical University, Haikou, China.
Zhitu Zhu, Cancer Center, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China.
Wei Ge, Center of Oncology, Renmin Hospital of Wuhan University, Wuhan, China.
Junlan Yang, Department of Medical Oncology, People’s Liberation Army General Hospital, Beijing, China.
Tongjian Cui, Department of Oncology, Fujian Provincial Hospital, Fuzhou, China.
Xiaozhi Zhang, Department of Radiotherapy and Oncology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China.
Fangyang Lu, Department of Oncology, The Second Affiliated Hospital of Guiyang Medical University, Guiyang, China.
Wenhui Li, Department of Radiotherapy, Tumor Hospital of Yunnan Province, The Third Affiliated Hospital of Kunming Medical College, Kunming, China.
Hongyan Xu, Department of Oncology, Jilin Second People’s Hospital, Jilin, China.
Mafei Kang, Department of Medical Oncology, The Affiliated Hospital of Guilin Medical University, Guilin, China.
Ping Gong, Department of Oncology, The First Affiliated Hospital, Shihezi University School of Medicine, Shihezi, China.
Liqun Zou, Department of Oncology, West China Hospital, Sichuan University, Chengdu, China.
Jiang Liu, Department of Oncology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China.
Hongliang Zhang, Department of Oncology, Xinjiang Uygur Autonomous Region Chinese Medicine Hospital, Urumqi, China.
Hao Yu, School of Public Health, Nanjing Medical University, Nanjing, China.
Binghe Xu, Department of Medical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences, No.17 Panjiayuannanli, Chaoyang District, Beijing 100021, China.
Declarations
Ethics approval and consent to participate: This study was conducted in accordance with the Declarations of Helsinki and Good Clinical Practice, and was approved by the institutional review boards of the participating centers. All patients provided written informed consent.
Consent for publication: Not applicable.
Author contribution(s): Min Yan: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Peng Yuan: Conceptualization; Data curation.
Quchang Ouyang: Conceptualization; Data curation.
Ying Cheng: Conceptualization; Data curation.
Guohui Han: Conceptualization; Data curation.
Dewei Wang: Conceptualization; Data curation.
Li Ran: Conceptualization; Data curation.
Tao Sun: Conceptualization; Data curation.
Da Zhao: Conceptualization; Data curation.
Yuju Bai: Conceptualization; Data curation.
Shun’e Yang: Conceptualization; Data curation.
Xiaojia Wang: Conceptualization; Data curation.
Rong Wu: Conceptualization; Data curation.
Xiaohua Zeng: Conceptualization; Data curation.
Herui Yao: Conceptualization; Data curation.
Xuening Ji: Conceptualization; Data curation.
Jun Jiang: Conceptualization; Data curation.
Xiaohua Hu: Conceptualization; Data curation.
Haifeng Lin: Conceptualization; Data curation.
Liping Zheng: Conceptualization; Data curation.
Zhitu Zhu: Conceptualization; Data curation.
Wei Ge: Conceptualization; Data curation.
Junlan Yang: Conceptualization; Data curation.
Tongjian Cui: Conceptualization; Data curation.
Xiaozhi Zhang: Conceptualization; Data curation.
Fangyang Lu: Conceptualization; Data curation.
Wenhui Li: Conceptualization; Data curation.
Hongyan Xu: Conceptualization; Data curation.
Mafei Kang: Conceptualization; Data curation.
Ping Gong: Conceptualization; Data curation.
Liqun Zou: Conceptualization; Data curation.
Jiang Liu: Conceptualization; Data curation.
Hongliang Zhang: Conceptualization; Data curation.
Hao Yu: Conceptualization; Data curation; Formal analysis.
Binghe Xu: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the ‘Twelfth Five-Year Plan’ National Major New Drug Development Major Special Project (grant number: 2013ZX09104001).
Role of the funding source: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. A steering committee of study investigators was involved in study design, data collection, data interpretation, writing of the report, and the decision to submit for publication. The corresponding author had full access to all the data in the study and had ultimate responsibility for submitting it for publication.
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Murphy CG, Seidman AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer 2009; 9: S58–S65. [DOI] [PubMed] [Google Scholar]
- 3. Park IH, Im S-A, Jung KH, et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01). Cancer Res Treat 2019; 51: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alsaloumi L, Shawagfeh S, Abdi A, et al. Efficacy and safety of capecitabine alone or in combination in advanced metastatic breast cancer patients previously treated with anthracycline and taxane: a systematic review and meta-analysis. Oncol Res Treat 2020; 43: 694–702. [DOI] [PubMed] [Google Scholar]
- 5. Decatris MP, Sundar S, O’Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev 2004; 30: 53–81. [DOI] [PubMed] [Google Scholar]
- 6. Sikov WM. Assessing the role of platinum agents in aggressive breast cancers. Curr Oncol Rep 2015; 17: 3. [DOI] [PubMed] [Google Scholar]
- 7. Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol 2015; 33: 1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chew HK, Doroshow JH, Frankel P, et al. Phase II studies of gemcitabine and cisplatin in heavily and minimally pretreated metastatic breast cancer. J Clin Oncol 2009; 27: 2163–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol 2016; 102: 37–46. [DOI] [PubMed] [Google Scholar]
- 10. Wang Z, Xu L, Wang H, et al. Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment. Saudi J Biol Sci 2018; 25: 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Xu X-Y, Yan F, et al. Retrospective study of the efficacy and toxicity of lobaplatin in combined chemotherapy for metastatic breast cancer. Onco Targets Ther 2019; 12: 4849–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breast Cancer Group, Branch of Oncologist and Chinese Medical Doctor Association. Expert consensus on the clinical application of platinums in advanced breast cancer (2020 version). Zhonghua Zhong Liu Za Zhi 2021; 43: 167–175. [DOI] [PubMed] [Google Scholar]
- 13. Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol 2016; 77: 1103–1124. [DOI] [PubMed] [Google Scholar]
- 14. Lv X, Cao X, Xia W-X, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol 2021; 22: 716–726. [DOI] [PubMed] [Google Scholar]
- 15. Cheng Y, Fan Y, Liu X, et al. Randomized controlled trial of lobaplatin plus etoposide vs. cisplatin plus etoposide as first-line therapy in patients with extensive-stage small cell lung cancer. Oncol Lett 2019; 17: 4701–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Liu M, Ding B. Comparison of the short-term efficacy and serum markers between lobaplatin/paclitaxel- and carboplatin/paclitaxel-based adjuvant chemotherapy in patient with ovarian cancer. J Clin Pharm Ther 2021; 46: 166–172. [DOI] [PubMed] [Google Scholar]
- 17. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019; 321: 288–300. [DOI] [PubMed] [Google Scholar]
- 18. Xu B, Jiang Z, Kim S-B, et al. Biweekly gemcitabine-paclitaxel, gemcitabine-carboplatin, or gemcitabine-cisplatin as first-line treatment in metastatic breast cancer after anthracycline failure: a phase II randomized selection trial. Breast Cancer 2011; 18: 203–212. [DOI] [PubMed] [Google Scholar]
- 19. Vernieri C, Milano M, Mennitto A, et al. Antitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective study. Breast Cancer Res Treat 2017; 165: 365–373. [DOI] [PubMed] [Google Scholar]
- 20. Vernieri C, Prisciandaro M, Milano M, et al. Single-agent gemcitabine vs. carboplatin-gemcitabine in advanced breast cancer: a retrospective comparison of efficacy and safety profiles. Clin Breast Cancer 2019; 19: e306–e318. [DOI] [PubMed] [Google Scholar]
- 21. Staudacher L, Cottu PH, Diéras V, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol 2011; 22: 848–856. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Wang L, Wang Z, et al. A phase II trial of biweekly vinorelbine and oxaliplatin in second- or third-line metastatic triple-negative breast cancer. Cancer Biol Ther 2015; 16: 225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, Fan Y, Li Q, et al. Vinorelbine plus platinum in patients with metastatic triple negative breast cancer and prior anthracycline and taxane treatment. Medicine (Baltimore) 2015; 94: e1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Guan Y, Wang J, et al. Platinum-based chemotherapy in advanced triple-negative breast cancer: a multicenter real-world study in China. Int J Cancer 2020; 147: 3490–3499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221122715 for A prospective, open-label, multicenter phase IV clinical trial on the safety and efficacy of lobaplatin-based chemotherapy in advanced breast cancer by Min Yan, Peng Yuan, Quchang Ouyang, Ying Cheng, Guohui Han, Dewei Wang, Li Ran, Tao Sun, Da Zhao, Yuju Bai, Shun’e Yang, Xiaojia Wang, Rong Wu, Xiaohua Zeng, Herui Yao, Xuening Ji, Jun Jiang, Xiaohua Hu, Haifeng Lin, Liping Zheng, Zhitu Zhu, Wei Ge, Junlan Yang, Tongjian Cui, Xiaozhi Zhang, Fangyang Lu, Wenhui Li, Hongyan Xu, Mafei Kang, Ping Gong, Liqun Zou, Jiang Liu, Hongliang Zhang, Hao Yu and Binghe Xu in Therapeutic Advances in Medical Oncology