Abstract
Background:
The treatment of chronic calcaneal osteomyelitis is a challenging and increasing problem because of the high prevalence of diabetes mellitus and operative fixation of heel fractures. In 1931, Gaenslen reported treatment of hematogenous calcaneal osteomyelitis by surgical excision through a midline, sagittal plantar incision. We have refined this approach to allow successful healing and early mobilization in a modern series of complex patients with hematogenous, diabetic, and postsurgical osteomyelitis.
Methods:
Twenty-eight patients (mean age 54.6 years, range 20-94) with Cierny-Mader stage IIIB chronic calcaneal osteomyelitis were treated with sagittal incision and calcaneal osteotomy, excision of infected bone, and wound closure. All patients received antibiotics for at least 6 weeks, and bone defects were filled with an antibiotic carrier in 20 patients. Patients were followed for a mean of 31 months (SD 25.4). Primary outcome measures were recurrence of calcaneal osteomyelitis and below-knee amputation. Secondary outcome measures included 30-day postoperative mortality and complications, duration of postoperative inpatient stay, footwear adaptions, mobility, and use of walking aids.
Results:
All 28 patients had failed previous medical and surgical treatment. Eighteen patients (64%) had significant comorbidities. The commonest causes of infection were diabetes ± ulceration (11 patients), fracture-related infection (4 patients), pressure ulceration, hematogenous spread, and penetrating soft tissue trauma. The overall recurrence rate of calcaneal osteomyelitis was 18% (5 patients) over the follow-up period, of which 2 patients (7%) required a below-knee amputation. Eighteen patients (64%) had a foot that comfortably fitted into a normal shoe with a custom insole. A further 6 patients (21%) required a custom-made shoe, and only 3 patients required a custom-made boot.
Conclusion:
Our results show that a repurposed Gaenslen calcanectomy is simple, safe, and effective in treating this difficult condition in a patient group with significant local and systemic comorbidities.
Level of Evidence:
Level III, case series.
Keywords: Osteomyelitis, calcaneus, calcaneal, Gaenslen, calcanectomy, split-heel, sinus, heel ulcer
Introduction
Chronic osteomyelitis of the calcaneus accounts for 3% to 8% of all patients with bone infection and represents an increasing problem with the high prevalence of diabetes mellitus, reportedly doubling in the last 15 years.16 Eradication of calcaneal osteomyelitis has historically proved challenging, particularly when trying to preserve normal hindfoot function, the specialized weight bearing surface of the heel, and the attachment of the Achilles tendon.1,26,29 Moreover, in patients with diabetes mellitus it is often associated with plantar skin ulceration, destruction of the specialized plantar fat pad, impaired peripheral perfusion, and protective sensation. Taken together, these local soft tissue factors greatly impair postoperative wound healing and can limit limb salvage options.
In 1931, Gaenslen reported treatment of hematogenous calcaneal osteomyelitis by surgical excision through a sagittal incision on the sole of the heel and a sagittal osteotomy of the calcaneus.13 Gaenslen described his technique to be performed with an open wound, regular dressings until granulation tissue created a secondary epithelialization around “stiff Vaseline” or a rubber insert, with no antibiotic use (systemic or local), and a prolonged period in hospital. This was before the discovery of antibiotics. We refined this technique to include modern principles around early wound healing, early mobilization, antibiotic use, and a more patient-friendly period in hospital. Our technique includes wound closure, often by skewing the two wound edges, and where possible using local antibiotic carriers to manage the dead space postdebridement.
The approach allows good access to the infected bone and has the advantages of preserving the Achilles tendon attachment, the weight bearing surface of the calcaneus, and the overlying soft tissue.
We present our experience in using a refined Gaenslen calcanectomy to treat 28 patients with chronic osteomyelitis of the calcaneus. We assessed the outcome of the technique, complications after surgery, and the need for modified footwear.
Materials and Methods
Theatre log books and paper and electronic patient records were accessed to identify 28 patients with chronic osteomyelitis of the calcaneus treated with a modified Gaenslen split heel calcanectomy at the Nuffield Orthopaedic Centre, Oxford, UK from 2000 to 2021. Retrospective data on patient demographics, diagnostic tests, surgical operative details, antimicrobial therapy, and postoperative progress were collected from electronic and paper patient records. Patients were diagnosed with calcaneal chronic osteomyelitis if they had clinical and magnetic resonance imaging (MRI) scan features of osteomyelitis, symptoms lasting at least 6 months, and at least 1 of the following: the presence of a sinus, an abscess or intraoperative pus, supportive histology, or 2 or more microbiological cultures of deep surgical samples (see below) with indistinguishable organisms.20,21
An MRI scan was performed on all patients and reported by a specialist musculoskeletal radiologist. This confirmed radiographic evidence of osteomyelitis and defined the extent of the infection based on the Cierny and Mader classification system.5 All cases were Cierny-Mader stage IIIB in that there was localized involvement of the medulla and the plantar cortex at the site of ulceration. Patients without evidence of deep infection and osteomyelitis were not included in this study.
The primary outcome measures were recurrence of calcaneal osteomyelitis (based on the same diagnostic criteria described above) and the rate of below-knee amputation for recurrent calcaneal osteomyelitis. Kaplan-Meier analysis was used to determine osteomyelitis recurrence-free survival. Secondary outcome measures included 30-day postoperative mortality, complications, duration of hospital stay after surgery, mobility, use of walking aids, and footwear adaptions.
All patients were seen regularly in outpatient clinics for the first 2 years after the operation, with reviews at 6 weeks, 3 months, 6 months, 1 year, and 2 years.
Patients were also contacted by telephone as part of a telemedicine follow-up consultation to assess if they had developed a recurrence of calcaneal osteomyelitis, to verify their mobility status and requisite walking aids, and to document their need for custom-made footwear on the operated side.
Inpatient Care Pathway
All patients were admitted to the Bone Infection Unit at our hospital under the combined care and responsibility of an orthopaedic surgeon (either specializing in foot ankle surgery or bone infection surgery), an infectious disease physician and, when required, a plastic surgeon. Unless the patient was septic, antibiotics were stopped for 2 weeks before the index procedure to maximize the chances of obtaining positive intraoperative samples for microbial culture.7,11
Based on regional microbiology guidance, intraoperative parenteral antibiotics were vancomycin and meropenem. These were only given after intraoperative sampling was complete. Standard operating procedure for bone infection cases included 5 deep microbiology and 2 histology samples taken per case.
Postoperative antibiotics began with empirical vancomycin and meropenem until appropriate bacterial culture and sensitivity results were available to determine the most appropriate antibiotic regimen. In general, patients completed a 6-week course that, more recently, has included conversion to oral antibiotics for the latter weeks based on the results of the OVIVA trial.17,19
Refined Gaenslen Approach and Surgical Procedure
Preparation and approach
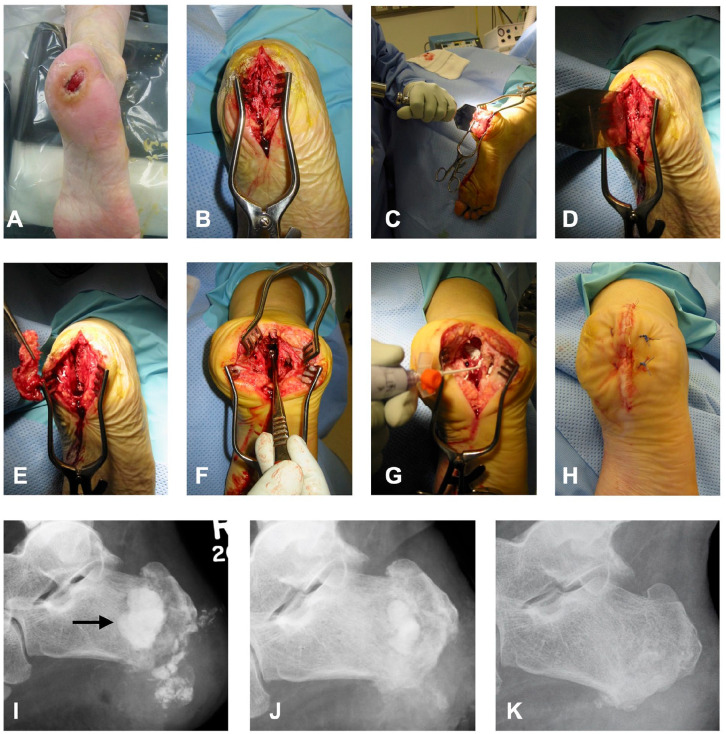
Where appropriate, the patient was safely placed in a floppy lateral position, with the affected side down, or prone, taking care to protect pressure areas (Figure 1). A thigh tourniquet was used. The incision was a plantar midline incision centered in the sagittal plane on the midline of the heel pad. It extended proximally from the top of the calcaneal tuberosity to the distal edge of the plantar heel pad. If there was a plantar ulcer or sinus, the incision was modified to incorporate the lesion in an ellipse. Sharp dissection straight down to the bone was performed with a fresh scalpel. Once appropriate soft tissue samples were obtained, a 7-cm osteotome was used to split the plantar cortex of the calcaneus in line with the incision. A 6-8mm-based wedge of calcaneus was removed. We found that in most cases, the apex of the osteotomy could stop short of the superior cortex and still provide good access to the infected calcaneal medulla for further sampling and excision. This is unlike Gaenslen’s original technique and was intended to help preserve the integrity of the subtalar joint. In those cases in which preoperative MRI suggested infection involving the subtalar joint, the sagittal osteotomy was extended into the superior cortex of the calcaneus to allow access to, and wash out, the subtalar joint.
Figure 1.
Refined Gaenslen calcanectomy technique. (A) The patient is safely placed in the prone or floppy lateral position. A thigh tourniquet is applied and inflated. (B) The midline plantar sagittal incision incorporates the ulcer in an ellipse. (C, D) A broad osteotome is used to split the calcaneum in the sagittal plane. (E) A plantar-based 10-mm wedge of bone is removed to allow access to the medullary bone. (F) The infected and dead bone is removed using curettes, burrs, osteotomes, and gouges. (G) Compared with the original Gaenslen approach, after thorough lavage, the bone defect is dried and the dead space is filled with an antibiotic carrier. (H) In addition, the wound is closed primarily in layers with interrupted sutures to the skin. (I) Lateral radiograph at day 1 postoperation with CERAMENT G filling defect (arrow). Radiograph of calcaneus at (J) 8 weeks and (K) 22 weeks postoperation showing absorption of antibiotic carrier and bone healing.
Sampling
In our center, we employ best practice techniques of microbiology and histology sampling. These have been well described previously.7,11 In essence, 5 microbiology samples and a minimum of 2 histology samples are obtained. Care is taken to avoid contamination; only deep samples are used for microbiology, a separate set of instruments is used for each sample, care is taken to avoid touching the wounds with anything but the sampling instrumentation, for example, suction tips and fingers. Intraoperative antibiotics are only given after the samples have been taken.
Debridement and excision
The wound edges of the ulcer were excised and sent as a histological sample. The subcutaneous and deeper soft tissues were debrided back to healthy bleeding tissue planes and any sinus tracts were excised as previously described.5,11 The amount of calcaneal osteomyelitis debridement and excision was based on preoperative MRI combined with intraoperative gross findings of infected and avascular calcaneal bone. It is standard practice in our Bone Infection Unit to continue systematic debridement until only healthy bleeding bone remains using a combination of curved curettes, gouges, and small osteotomes. Because this study only includes patients with Cierny-Mader stage IIIB osteomyelitis, there was sufficient healthy cortical (and medullary) bone left at the end of debridement to preserve skeletal stability.
Dead space management and wound closure
Two additional refinements of Gaenslen’s original approach were dead space management and primary wound closure. Dead space management was achieved using a variety of antibiotic impregnated carriers that dissolve over weeks to months. These included gentamicin beads, Herafill with gentamicin, Osteoset with tobramycin, and more recently, CERAMENT with gentamicin. The wound and bone defects were first washed with at least 3 L of either saline or aqueous chlorhexidine delivered systematically through 50-mL syringes. Bone defects were then dried with sterile gauze before the antibiotic carriers were packed (gentamicin beads, Herafill with gentamicin, Osteoset with tobramycin) or injected + pressurized into the defect (CERAMENT with gentamicin).
The wound was always closed, either direct closure in layers or with local full-thickness flaps (eg, keystone flap) or with a free flap and skin graft. The wound was never left open and vacuum-assisted dressings were not used postoperatively.
Results
Twenty-eight patients underwent a Gaenslen calcanectomy at the Nuffield Orthopaedic Centre, Oxford, UK since 2000 for Cierny-Mader stage IIIB osteomyelitis (Table 1). These were followed for a mean of 31 months (SD 25.4, range 4-94 months). One patient was lost to follow-up after 4 months. There were 21 male and 7 female patients with an average age of 54.6 years (SD 19.3, range 20-95 years). Eighteen patients had significant comorbidities, including diabetes mellitus (11 patients), peripheral neuropathy based on absent 10g monofilament foot sensory examination (10 patients),23 a history of treated peripheral vascular disease based on NICE guidelines, including Doppler-assessed ankle brachial pressure index22 (5 patients), and 6 patients had a history of ischemic heart disease (Table 1). Six patients continued to smoke tobacco products.
Table 1.
Patient Demographics.
| Sex, n (male/female) | 21:7 | Age, y, mean (SD); range | 54.6 y (19.3); 20-95 | ||
| Cause | Comorbidities (18 patients) | Previous treatment | |||
| Diabetic ulcer | 8 | Diabetes mellitus (IDDM) | 11 (10) | Previous antibiotics | 28 |
| Fracture-related infection | 4 (3 open) | Peripheral neuropathy | 10 | Of which >6 wk | 11 |
| Pressure ulcer | 4 | Tobacco smoker | 6 | Previous surgery | 12 |
| Haematogenous | 5 | Ischemic heart disease | 6 | ≥2 previous surgeries | 6 |
| Penetrating soft tissue trauma | 3 | Peripheral vascular disease | 5 | Previous local or free flap | 2 |
| Iatrogenic—post elective procedure | 2 | COPD/asthma | 6 | ||
| Cellulitis | 1 | Hypertension | 5 | Extent of disease | |
| Renal impairment | 3 | MRI osteomyelitis | 28 | ||
| Atrial fibrillation | 3 | Sinus tract | 15 | ||
| Depression | Ulcer | 14 | |||
| SLE | 1 | Subtalar joint extension | 1 | ||
| CES | 1 | ||||
| Heart failure | 1 | ||||
| DVT | 1 | ||||
Abbreviations: CES, cauda equina syndrome; COPD, chronic obstructive pulmonary disorder; DVT, deep vein thrombosis; IDDM, insulin-dependent diabetes mellitus; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus.
The commonest causes of calcaneal osteomyelitis were diabetic ulceration (8 patients), hematogenous spread (5 patients), fracture-related infection (4 patients), and penetrating soft tissue trauma (3 patients).
All patients had received previous antibiotics and 11 had received 1 or more 6-week courses of antibiotics. Twelve patients (43%) had already undergone a previous operation elsewhere, and 21% had undergone 2 or more prior operations (Table 2). Intraoperative sampling revealed that the commonest causative organisms were Staphylococcus aureus, coagulase-negative staphylococcus and Streptococcus spp. Polymicrobial infection based on intraoperative sampling was found in 46% of patients (Table 2).
Table 2.
Perioperative Findings and Management.
| Pathogen Isolated Intraoperatively | Adjuvant Antibiotic Treatment | ||
|---|---|---|---|
| Polymicrobial infection | 13 | Perioperative antibiotics | |
| Staphylococcus aureus | 10 | Intravenous broad spectrum after sampling | 24 |
| Coagulase-negative staphylococcus | 6 | Oral broad spectrum | 4 |
| Streptococcus spp | 6 | Postoperative antibiotics | |
| Proteus mirabilis | 4 | 6 wk; Intravenous then oral based on sensitivities | 18 |
| Enterococcus faecalis | 4 | 3 months; intravenous then oral based on sensitivities | 3 |
| Escherichia coli | 3 | 6 wk intravenous alone | 3 |
| Klebsiella spp | 2 | 6 wk oral alone | 3 |
| Enterobacter spp | 2 | 6 mo oral (anti-TB) | 1 |
| Corynebacterium striatum | 2 | Dead space management | |
| Streptococcus anginosus | 1 | CERAMENT + gentamicin |
13 |
| Diphtheroid spp. | 1 | OsteoSet + tobramycin | 2 |
| Pseudomonas aeruginosa | 1 | Collagen fleece | 2 |
| Candida sp | 1 | Gentamicin beads | 2 |
| Mycobacterium tuberculosis | 1 | Herafill beads + gentamicin | 1 |
| Morganella morganii | 1 | ||
| Soft tissue closure | |||
| Direct closure | 22 | ||
| Free flap + split skin graft | 3 | ||
| Keystone flap | 2 | ||
| Lateral calcaneal flap | 1 | ||
None of the patients died as a result of the infection or the procedure (Table 3). One patient died of unrelated causes, 16 months after the operation. The overall recurrence rate of calcaneal osteomyelitis was 18% (5 patients) over the follow-up period. All of these occurred within the first 24 months of the index Gaenslen procedure (Figure 2). Two patients (7%) required a below-knee amputation for recurrent infection at 12 and 19 months, respectively. The remaining 3 patients each underwent a further surgical procedure to debride and excise infected tissue. They had no further recurrence at final review.
Table 3.
Outcomes Post Gaenslen Calcanectomy.a
| Outcome | |
| All recurrence of bone infection | 18% |
| Subsequent BKA | 7% |
| Intraoperative complications | 0% |
| 30-d mortality rate | 0% |
| 30-d postoperative complications (11/28 patients) | 39% |
| Breakdown of 30-d postoperative complications | |
| Wound leak | 6 |
| Ulcer slow to heal | 4 |
| Flap revision/reexplored | 2 |
| Foot drop | 1 |
| Chest pain | 1 |
| Pulmonary embolism | 1 |
| Recurrent heel ulceration (<1 y), n (%) | 1 (3%) |
| Postoperative stay, d, mean (SD); range | 19.7 (18.1); 6-78 |
| Period of NWB, wk, mean (SD) | 5 (1.3) |
| Mobility, n (%) | |
| Unaided | 18 (64%) |
| Crutches | 4 (14%) |
| Frame | 6 (21%) |
| Footwear, n (%) | |
| Normal shoe and custom insole | 18 (64%) |
| Orthotic custom shoe | 6 (21%) |
| CROW/Rocker boot | 3 (11%) |
| BKA prosthesis | 2 (7%) |
Abbreviations: BKA, below-knee amputation; CROW, Charcot restraint orthotic walker; NWB, non weight bearing.
Follow-up period: 31 months (SD 25.4, range 4-94).
Figure 2.
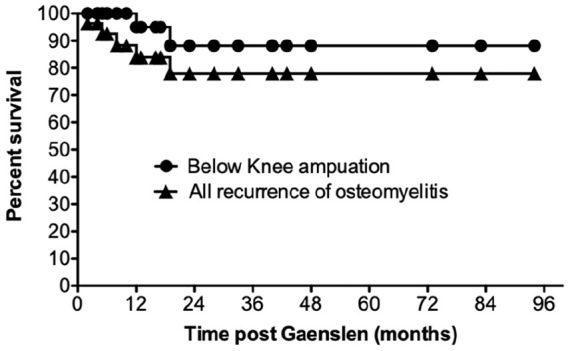
Infection-free survival post modified Gaenslen calcanectomy. Overall, 5 patients (18%) had recurrence of calcaneal osteomyelitis (triangles), of which 2 patients (7%) required a below-knee amputation (circles). The remaining 3 patients were successfully treated with a further surgical debridement.
Postoperative complications occurred in 11 patients (39%) and these included wound leak in 5 patients (often related to the calcium sulphate-based antibiotic carriers used to manage the dead space), an ulcer that was slow to heal in 5 patients (one of which developed deep soft tissue infection 40 days postoperation), revision or reexploration of the soft tissue flap (2 patients) and a case of postoperative foot drop that resolved (Table 3). One patient developed a recurrent superficial ulcer within 12 months of the operation that healed with nonoperative treatment and was not associated with recurrent osteomyelitis.
Most patients (64%) were able to walk unaided, and 64% has a foot that would comfortably fit into a normal shoe with a molded insole (Table 3). A further 5 patients (21%) required a custom-made shoe, and only 3 patients required a custom-made boot, for example, Charcot restraint orthotic walker (CROW).
Discussion
Calcaneal osteomyelitis remains a difficult condition to treat. The weight bearing surface of the heel is prone to fracture-related infections, penetrating injury, and pressure ulceration, particularly in the context of peripheral neuropathy.15,16 The fundamental steps in treating chronic osteomyelitis include physiological optimization of the patient, a thorough debridement of poorly vascularized and infected bone, dead space management, ensuring skeletal stability, appropriate antibiotics based on careful intraoperative sampling, and adequate soft tissue coverage.11 Accomplishing these objectives can be difficult when there is extensive osteomyelitis of the calcaneus. Excision of devascularized infected bone risks destroying the weight bearing plantar cortex, detaching the Achilles tendon and disrupting the hindfoot complex. Moreover, in cases of exogenous osteomyelitis, the overlying plantar fat pad and skin are often compromised and limit soft tissue closure. For these reasons, a below-knee amputation understandably remains a curative surgical option for established osteomyelitis of the calcaneus, particularly in patients with systemic and local comorbidities that compromise wound healing.
Limb salvaging surgery attempts to achieve a curative resection that avoids the impact on mobility and the increased mortality rates following a below-knee amputation. Historically this has involved either partial or total calcanectomy. These reports began in 1959 when Wiltse et al described excision of the posterior calcaneus in 7 patients with osteomyelitis with re-attachment of the Achilles tendon to the plantar fascia.28 Subsequently, Horwitz14 and Martini et al18 reported their results in the 1970s of partial calcanectomy to treat 4 and 13 patients, respectively, with calcaneal bone infection. All 13 of the latter cohort were reported to be able to walk “almost normally” with heel inserts in normal shoes, thereby being spared the specialized footwear requisite in the other 7 who underwent a total calcanectomy.18
In the intervening 50 years, multiple further cohort series of partial, subtotal, and total calcanectomies have been published. Two systematic reviews have summarized these studies. The first in 2012 included 100 patients from 16 studies.25 The majority underwent partial calcanectomy, with only 28 undergoing a total calcanectomy. A subsequent below- or above-knee amputation was required in 10% for recalcitrant osteomyelitis. They found that 85% of patients receiving a partial calcanectomy maintained their pre-infection mobility levels. In 2021, Yammine et al30 collated data on 300 calcanectomies on 295 patients from 20 studies. Again, the majority were partial or subtotal calcanectomies (90%). In this review, a subsequent total calcanectomy (5%) was used as an additional outcome measure post partial calcanectomy. All patients had diabetes mellitus and associated heal ulcers. This may at least partially explain the higher rates of recurrent infection (20%), subsequent below-knee amputation (17%), and mortality (13%) in this complex patient group.
In our patient cohort, 11 patients had diabetes mellitus, 8 of whom had an associated ulcer. There was only 1 bone infection recurrence (9%) in this subgroup of patients with diabetes. It occurred after 5 months and required another debridement, which was successful. One patient refused to non weight bear immediately postprocedure, developed delayed wound healing, and a subsequent soft tissue infection after 90 days, spreading proximally and posteriorly. He underwent urgent excision and skin grafting and remains bone infection free. Postoperative compliance with non weight bearing can be challenging for our patients, particularly those who have lost protective sensation. Increasingly we ask them to remain in hospital, non weight bearing and elevating the operated limb for at least 2 weeks or until the wound has healed. This is reflected in our average postoperative hospital stay of 19.7 days (SD 18.1). However, this is very similar to the national average for inpatient treatment of osteomyelitis in all bones.9
A concern about any partial or total calcanectomy is the affect it has on a patient’s ability to mobilize. In one study, 14 patients completed a questionnaire on their subjective function post partial calcanectomy.26 Eight required a custom-made orthotic shoe and 3 needed a heel raise. Ten reported being pain free, 3 had mild weight bearing pain, and 1 reported “continuous moderate pain.” All were reportedly satisfied with the cosmetic result of their foot and 12 of 14 would undergo the same operation again. This supports the view that preservation of some calcaneal bone is a reasonable goal in management.
Our Gaenslen calcanectomy allowed access to the calcaneal medullary bone and, when required, the subtalar joint by extending the osteotomy to the dorsal cortex—a true split calcanectomy. Overall, the disruption to the weight bearing surface of the calcaneus is minimized, at worse the heel print is slightly widened. Two-thirds of our patients treated with this refined Gaenslen procedure were able to mobilize without additional aid and in normal footwear. Six patients (21%) required a custom-made orthotic shoe and 3 (11%) required a CROW or rocker boot.
Since Gaenslen’s original publication in 1931, 3 papers have described the results of a split-heel calcanectomy. Two were mini-series of 3 pediatric patients each,3,27 and 1 included 3 adults.2 Our study therefore represents the largest patient cohort since his original description of 10 much younger patients, ranging from 1.5 to 39 years old with an average age of 15 years.13 They were followed for an average of 31 months (range 12-72 months) and 3 were able to walk without a limp and without any additional orthosis. The remaining 7 patients walked with a limp, 2 of whom developed a 10-15-degree equinovarus as a result of the operation, and 5 patients required padded shoes. In an age before antibiotics, trauma with associated soft tissue infection led to osteomyelitis in 4 patients and hematogenous bacterial spread accounted for a further 6 patients, of which 2 were tuberculous. The causative organisms in Gaenslen’s series included Streptococcus pyogenes in 4 patients, Staphylococcus aureus in 3 patients, tuberculosis in 2 patients, and hemolytic Streptococcus in 1 patient. In comparison, the most common cause in our series was chronic diabetic ulceration and the commonest causative organisms were Staphylococcus aureus, coagulase-negative staphylococcus and Streptococcus spp. This is a clinically complex group of patients. Eighteen of our cohort had major comorbidities, nearly half had polymicrobial infection, all had previous failed antibiotic treatment, and 12 had failed surgical treatment. In spite of these poor local and systemic prognostic factors, our Gaenslen approach in combination with local antibiotic carrier and targeted systemic antibiotics (based on intraoperative samples) resulted in a cure rate of 82%.
A major advance over the last decade in surgical management of osteomyelitis has been the development and use of local antibiotic carriers. These have the dual benefit of reducing the dead space following bone excision and eluting very high doses of broad-spectrum antibiotic locally and without systemic side effects.21 Thirteen patients received CERAMENT, a composite carrier of calcium sulphate and calcium hydroxyapatite that can be pre-mixed with gentamicin and/or vancomycin. Although the calcium sulphate typically dissolves over a few weeks, the calcium hydroxyapatite has been shown to encourage bone mineralization and is ultimately replaced by normal bone.10
Wound leak is an issue when using calcium sulphate–based antibiotic carriers and our experience is it rarely lasts more than 2-4 weeks. In order to improve wound healing, our protocol includes a “watertight” wound closure, strict elevation, and non weight bearing for the first 2-3 weeks to encourage the wounds to seal. This may also explain why the healing rate was shorter in our cohort, limiting non weight bearing to a mean of 5 weeks compared to 4 months in the original Gaenslen cohort, in which the wound was left to granulate and heal by secondary intention.
Alternative limb-preserving surgical treatments of calcaneal osteomyelitis include using muscle flaps to fill in the bone defect post debridement12,24 and modifications of the partial calcanectomy to improve wound closure. In particular, the vertical contour calcanectomy4,6,8 uses a Gaenslen skin incision, and complete detachment of the Achilles tendon and plantar fascia from the calcaneus. Three sequential osteotomies are then performed to (1) remove the weight bearing surface of the calcaneus, (2) remove the tubercle posteriorly, and (3) one at 45 degrees to chamfer the posteroinferior corner. This technique has the advantage of treating patients with larger plantar heel ulcers and those with posterior heel ulcers. Cook et al6 found only 31% of 51 patients were healed without recurrence at 12 months and a major lower limb amputation was required in 31%. Many of these patients had very large ulcers, peripheral vascular ischemia (56%) and significant systemic comorbidities, for example, diabetes mellitus (86%).6 This may explain these poorer outcomes in comparison to our series which did not include patients with very large plantar heel ulcers.
We argue that one of the advantages of the Gaenslen approach is that it is technically relatively simple to perform, requires no high-tech equipment, and can therefore be used in diverse health care settings throughout the world. Only 3 patients in our study had a free flap (2 gracilis and 1 serratus anterior muscle) and split skin graft to close the wound. Two of these patients had suffered open calcaneal fractures in the past and subsequently developed osteomyelitis, and the third was part of revision surgery for recurrent bone infection with a large lateral sinus. In addition, 1 patient with heel necrosis following prolonged inotropes in the intensive care unit underwent a lateral calcaneal artery–based local flap, and 2 patients required a local keystone flap to close the skin. In general, we have found our approach works best in patients in which any skin and soft tissue defect can be closed primarily. We incorporate the heel pad ulcer within the midline elliptical incision and by deliberately skewing the 2 wound edges it is often possible to close the central defect primarily, addressing the small resultant “dog-ears” at the extremes of the S-shaped wound.
Conclusion
Osteomyelitis of the calcaneus is a difficult condition to treat. In our cohort, we used a refined Gaenslen’s technique with the addition of modern elements (limitation of the osteotomy, local antibiotics, wound closure) to achieve low recurrence rates of infection, low rates of below-knee or hindfoot amputation, and a reduced stay in hospital. This allowed the majority of patients to mobilize without walking aids and in a normal shoe.
Footnotes
Ethical Approval: Ethical approval for this study was waived by UKRI Medical Reseach Council Review as no NHS REC reviews are required for sites in England for this study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Adrian Kendal, MA, BMBCh, DPhil, FRCS,  https://orcid.org/0000-0002-2208-0246
https://orcid.org/0000-0002-2208-0246
Martin McNally, MBBCh, MD, FRCSEd, FRCS,  https://orcid.org/0000-0003-2003-9044
https://orcid.org/0000-0003-2003-9044
References
- 1. Beals TC, MacWilliams BA, Webster J, Nickisch F. Gait and functional implications of bilateral, partial calcanectomy: case report. Foot Ankle Int. 2010;31(5):448-451. doi: 10.3113/fai.2010.0448. [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharyya A, Das R. Gaenslen’s split heel approach for the treatment of chronic osteomyelitis of the calcaneus: a series of three cases. Foot Ankle Online J. 2010;3(11):3. [Google Scholar]
- 3. Broudy AS, Scott RD, Watts HG. The split-heel technique in the management of calcaneal osteomyelitis in children. Report of three cases. Clin Orthop Relat Res. 1976(119):202-205. [PubMed] [Google Scholar]
- 4. Cates NK, Wang KH, Stowers JM, Attinger CE, Kim PJ, Steinberg JS. The vertical contour calcanectomy, an alternative approach to surgical heel ulcers: a case series. J Foot Ankle Surg. 2019;58(6):1067-1071. doi: 10.1053/j.jfas.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 5. Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003(414):7-24. doi: 10.1097/01.blo.0000088564.81746.62 [DOI] [PubMed] [Google Scholar]
- 6. Cook H, Kennedy C, Delijani K, et al. Early clinical, functional, and mortality outcomes for heel ulcers treated with a vertical contour calcanectomy. J Foot Ankle Surg. 2022;61(1):117-122. doi: 10.1053/j.jfas.2021.06.015 [DOI] [PubMed] [Google Scholar]
- 7. Dudareva M, Barrett LK, Morgenstern M, Atkins BL, Brent AJ, McNally MA. Providing an evidence base for tissue sampling and culture interpretation in suspected fracture-related infection. J Bone Joint Surg Am. 2021;103(11):977-983. doi: 10.2106/JBJS.20.00409 [DOI] [PubMed] [Google Scholar]
- 8. Elmarsafi T, Pierre AJ, Wang K, et al. The vertical contour calcanectomy: an alternative surgical technique to the conventional partial calcanectomy. J Foot Ankle Surg. 2019;58(2):381-386. doi: 10.1053/j.jfas.2018.08.040 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson J, Alexander M, Bruce S, O'Connell M, Beecroft S, McNally M. A retrospective cohort study comparing clinical outcomes and healthcare resource utilisation in patients undergoing surgery for osteomyelitis in England: a case for reorganising orthopaedic infection services. J Bone Jt Infect. 2021;6(5):151-163. doi: 10.5194/jbji-6-151-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferguson J, Athanasou N, Diefenbeck M, McNally M. Radiographic and histological analysis of a synthetic bone graft substitute eluting gentamicin in the treatment of chronic osteomyelitis. J Bone Jt Infect. 2019;4(2):76-84. doi: 10.7150/jbji.31592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kendal AR, Ferguson J, Wong THN, Atkins BL, McNally M. Osteomyelitis - symptoms, diagnosis and treatment. BMJ Best Practice Update. BMJ Best Pract. April 7, 2021. [Google Scholar]
- 12. Fish DN, Goulet JA, Stevenson T. Chronic osteomyelitis of the calcaneus - treatment with a vascularized muscle flap. Orthopedics. 1993;16(1):81-85. [DOI] [PubMed] [Google Scholar]
- 13. Gaenslen F. Split-heel approach in osteomyelitis of os calcis. J Bone Joint Surg. 1931;13:759-772. [Google Scholar]
- 14. Horwitz T. Partial resection of os calcis and primary closure in treatment of resistant large ulcers of heel, with or without osteomyelitis of os calcis. Clin Orthop Relat Res. 1972;84:149-153. [DOI] [PubMed] [Google Scholar]
- 15. Huang K, Guo QF, Zhu YS. The epidemiology and clinical features of calcaneus osteomyelitis following calcaneus fracture: a retrospective study of 127 cases. Ann Palliat Med. 2021;10(3):3154-3161. doi: 10.21037/apm-21-208 [DOI] [PubMed] [Google Scholar]
- 16. Iacobucci G. One in 10 UK adults could have diabetes by 2030, warns charity. BMJ. 2021;375:n2453. [DOI] [PubMed] [Google Scholar]
- 17. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi: 10.1056/NEJMoa1710926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martini M, Martinib Y, Bekhechi T, Daoud A. Treatment of chronic osteomyelitis of calcaneus by resection of calcaneus - report of 20 cases. J Bone Joint Surg Am. 1974;56(3):542-548. doi: 10.2106/00004623-197456030-00011 [DOI] [PubMed] [Google Scholar]
- 19. McMeekin N, Geue C, Briggs A, et al. Cost-effectiveness of oral versus intravenous antibiotics (OVIVA) in patients with bone and joint infection: evidence from a non-inferiority trial. Wellcome Open Res. 2019;4:108. doi: 10.12688/wellcomeopenres.15314.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNally M, Govaert G, Dudareva M, Morgenstern M, Metsemakers WJ. Definition and diagnosis of fracture-related infection. EFORT Open Rev. 2020;5(10):614-619. doi: 10.1302/2058-5241.5.190072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNally MA, Ferguson JY, Lau AC, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016;98-B(9):1289-1296. doi: 10.1302/0301-620X.98B9.38057 [DOI] [PubMed] [Google Scholar]
- 22. NICE Guidelines. Peripheral arterial disease: diagnosis and management. Clinical guideline [CG147]. National Institute for Health and Care Excellence (NICE). Published August 8, 2012. Last updated December 11, 2020. https://www.nice.org.uk/guidance/cg147
- 23. NICE Guidelines. Diabetic foot problems: prevention and management. NICE guideline [NG19]. National Institute for Health and Care Excellence (NICE). Published August 26, 2015. Last updated October 11, 2019. [Google Scholar]
- 24. Peng P, Dong ZG, Liu LH, Wei JW, Luo ZB, Cao S. An effective technique for managing the calcaneus osteomyelitis combined with soft-tissue defect. Int J Low Extrem Wounds. Published online May 3, 2021. doi: 10.1177/15347346211016696 [DOI] [PubMed] [Google Scholar]
- 25. Schade VL. Partial or total calcanectomy as an alternative to below-the-knee amputation for limb salvage: a systematic review. J Am Podiatr Med Assoc. 2012;102(5):396-405. doi: 10.7547/1020396. [DOI] [PubMed] [Google Scholar]
- 26. Van Riet A, Harake R, Stuyck J. Partial calcanectomy: a procedure to cherish or to reject? Foot Ankle Surg. 2012;18(1):25-29. doi: 10.1016/j.fas.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 27. Wang EB, Zhao Q, Zhang LJ, Ji SJ, Li JJ. The split-heel technique in the management of chronic calcaneal osteomyelitis in children. J Pediatr Orthop B. 2009;18(1):23-27. doi: 10.1097/BPB.0b013e3283195440 [DOI] [PubMed] [Google Scholar]
- 28. Wiltse L, Batemen JG, Kase S. Resection of major portion of the calcaneus. Clin Orthop Relat Res. 1959;13:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woll TS, Beals RK. Partial calcanectomy for the treatment of osteomyelitis of the calcaneus. Foot Ankle. 1991;12(1):31-34. doi: 10.1177/107110079101200106 [DOI] [PubMed] [Google Scholar]
- 30. Yammine K, El-Alam A, Assi C. Outcomes of partial and total calcanectomies for the treatment of diabetic heel ulcers complicated with osteomyelitis. A systematic review and meta-analysis. Foot and Ankle Surgery 2021;27(6):598-605. doi: 10.1016/j.fas.2020.07.014. [DOI] [PubMed] [Google Scholar]