Abstract
Background:
Individuals with diabetes frequently have comorbid health conditions and suffer longer term complications. The control of blood glucose relies on diabetes management/self-care behaviors. Poor glycemic control, commonly encountered in underserved populations with type 2 diabetes (T2D) often results from inadequate diabetes self-care activities and/or perception. We aimed to assess the association between diabetes self-care activities/perception and glycemic control in adult Puerto Rican residents with T2D.
Design and methods:
We used a cross-sectional study design; our sample population was 260 individuals aged 40–65 years with T2D. We asked participants about their diabetes self-care over 8 weeks. High fasting blood glucose (≥130 mg/dL) and glycated hemoglobin (HbA1c; ≥7%) measures were defined. We estimated the strength of the following associations using logistic regression: each of three self-care activities and fasting glucose or HbA1c, adjusting for confounders.
Results:
Nearly 27% of the participants reported not checking their glucose levels, 7% did not take their medications as prescribed and 31% perceived their diabetes self-care as poor. Participants with less education perceived their diabetes self-care as poor more often than their counterparts (44% vs 25%; p = 0.003). Most participants had high glycemic levels (60%) or hbA1c levels (65%). Participants who perceived their diabetes self-care as poor had higher HbA1c levels than their counterparts (adj. odds ratio: 2.14, 95% CI (1.13, 4.08)).
Conclusion:
Poor diabetes self-care perception, possibly related to less education, likely explains poor glycemic control among adult Puerto Rican residents with T2D.
Keywords: Diabetes mellitus, self-care, perception, glycemic control, monitory health
Introduction
Diabetes is a chronic disease that currently affects 463 million people worldwide.1 In 2017, it was the seventh leading cause of death in the United States (US).2 An estimated 8.2% of the US population had a diagnosis of diabetes as of 2018.3 Hispanics in the US, a large minority group, have a 66% greater risk of developing Type 2 Diabetes (T2D).4 Among adults of Hispanic origin in the US, Puerto Ricans have one of the highest prevalence levels (12.4%).3 In Puerto Rico, the prevalence of diagnosed diabetes in adults is even higher at 16.8%,5 comprising the third leading cause of death in the Annual Health Report of 2016.6
About 80% of people living with diabetes are from low- and middle-income countries.1 Several researchers suggest that greater educational attainment, older age, BMI, and wealth are associated with treatment and better care performance.1,7 Based on the 2014 Behavioral Risk Factor Surveillance System (BRFSS) data, in Puerto Rico, income and education level showed an inverse relationship with diabetes prevalence; for example, the prevalence for those with income level ≥$50,000 was 8.8% (similar to the 8.7% prevalence for university graduates) yet for those with income <$15,000, it was 20.5% (similar to the 28% prevalence for people with less than a high school diploma). Less income and a lower education level tend to be conducive to difficulties in accessing medical care, producing an increased risk for diabetes.8
Individuals with diabetes are more likely to have high blood pressure, high cholesterol, as well obesity, and frequently suffer longer term complications such as blindness or kidney failure. Thus, diabetes management/self-care behaviors heavily rely on controlling blood glucose levels,9 blood pressure, and cholesterol levels.10,11 People with diabetes can achieve adequate glucose control through compliance with oral medications and insulin as well as through recommended physical activity and healthy eating. Poor glycemic control, commonly encountered in underserved populations with T2D, may be related to inadequate diabetes self-care. Knowledge of efficient diabetes self-care behaviors can improve HbA1c control among people with T2D, even those with a lower education level.12,13 Diabetes knowledge and perceived health status are important factors associated with glycemic control among underserved populations with a low level of income.3
Levels of self-care activities are frequently low despite the known significant benefits to be obtained by following and participating in the continuum of diabetes care.14 Adherence to recommendations to achieve glycemic goals is pursued by fewer than 50% of patients with diabetes15 in developed countries. Health literacy in diabetes includes the knowledge of the condition, the patient’s self-efficacy and self-care behaviors as well as their glycemic control.16 Low levels of health literacy are common among people with diabetes; for example, the level of literacy ranged from 15% to 40% in a sampled US population.16 Inadequate health literacy may be correlated with poor self-care behaviors and high hemoglobin A1c (HbA1c), which is an important indicator of possible long-term complications17 such as retinopathy.18
The diabetes intervention (traditional and technology assisted) conducted in one study achieved stability in HbA1c levels as well as improved nutrition, physical activity, and overall self-care.4 Factors which act as barriers to adequate diabetes management include a lack of resources, disease perception, patient age, the cost of items needed for adequate self-care, and the patient’s relationship with their healthcare provider, among others.15,19 Several reports in the literature have indicated an association between glycemic control and improved diabetes-related health outcomes through adherence to treatment.20 –22 However, cultural and socioeconomic factors may also influence long-term diabetes management and complications, thus should be taken into account.4 To our knowledge, no prior researchers have assessed the association between diabetes self-care perception and glycemic control in adults with T2D in Puerto Rico, so we therefore assessed this association using a convenience sample as a first step.
Design and methods
Study population
Our study population consisted of 260 non-institutionalized, predominantly Hispanic Puerto Ricans recruited conveniently, who were 40–65 years old and diagnosed with T2D, who had contributed to the “Lipid-Lowering agents use in Periodontitis and Diabetes Study” (LLIPDS). Participants resided or worked in Puerto Rico. Among these participants, 45% (N = 117) were from the general population, 50% (N = 130) were volunteers from the Puerto Rico Center for Diabetes (PRCD), and 5% (N = 13) were volunteers from COSSMA, a private island-wide decentralized health care organization; 7% of those from the general population were T2D participants from the San Juan Overweight Adults Longitudinal study (SOALS).
The original LLIPDS study included potential participants if they (a) were between 40 and 65 years old; (b) had at least four natural teeth (to conduct a valid evaluation of their periodontal status); and (c) had a T2D diagnosis confirmed by physicians or the medication(s) they used or the results from our fasting blood glucose analyses. The LLIPDS study excluded potential participants if they (a) had braces or orthodontic appliances or gross oral pathology that might interfere with periodontal probing with visual obstruction, and lead to invalid periodontal measures; (b) reported a regular use of steroids, anti-inflammatory drugs (except aspirin if the doses were ≤150 mg per day), immunosuppressants, thiazolidinediones, or glitazones; (c) reported systemic conditions, including hemophilia or other bleeding disorders, chronic inflammatory diseases (including autoimmune arthritis, Crohn’s disease, or multiple sclerosis), or active infectious diseases (e.g. hepatitis, HIV, or TB within the previous 6 months); (d) reported the use of antibiotic therapy prior to the clinical examination; or (e) reported the regular use of any medication known to influence periodontal status for 2 weeks or more within the month prior to the clinical examination. We also excluded individuals if they (f) had been diagnosed with congenital or chronic cardiovascular diseases, endocarditis, or rheumatic fever; (g) were undergoing active dialysis treatment; (h) were receiving anticoagulant therapy; (i) had undergone procedures related to CVD, including the implantation of a pacemaker or defibrillator, or CVD surgery; (j) had had any joint replacement surgery; or (k) had been diagnosed with cancer and were undergoing active radio/immunotherapy. These LLIPDS study’s inclusion/exclusion criteria derived from the previous SOALS study for consistency and comparable study outcomes.23 The data obtained from the LLIPDS study was carried out to be used in the present study. However, the present work did not set a particular criterion for the participants needed to be included in the study.
Recruitment and enrollment in the study
We used many strategies to recruit and screen potential participant candidates. We regularly distributed study brochures to individuals walking near the study center or patients from other physicians; we also provided the study brochures to pharmacists for inclusion in their advertisements to their patients at strategically important locations, including the University District Hospital, the University Internal Medicine Department, the Puerto Rico Diabetes Center (PRDC), and nearby Pharmacy Centers. We invited additional potential participants to be screened from the general population at nearby grocery stores, laundry facilities, shopping and employment centers, churches, schools, and primary care clinics. We also used word-of-mouth recruiting by some participants. We promoted the study on an annual basis through local news, magazines, a local TV channel, or a local radio station. We employed as additional sources of potential participants a list of former participants from SOALS who had a diagnosis of T2D, a list of volunteers from the Alliance Center’s (AC; formerly the Puerto Rico Clinical and Translational Research Consortium) data registry, and interactions with guests at local events such as “Expo Diabetes.” Due to our low rates of recruitment from the general population, we collaborated with other centers, such as PRDC and COSSMA, to obtain lists of their patients who might be interested in participating.
Participant screening
We administered a telephone script to screen potential participants. The script included a concise statement of our main objective and a small number of basic questions to determine eligibility, including their age, diabetic status, total number of natural teeth, their medication use, and the duration of such use. We obtained their phone numbers and physical mailing addresses. We scheduled a clinical examination visit for individuals who met our primary eligibility criteria and verbally consented to participate.
Ascertainment of diabetic status
All candidate participants were required to bring their physicians’ documentation of their diabetic status, laboratory results indicating that they were diabetic, or their diabetes-related medications labeled with their names to validate their status. Candidate participants from SOALS with a diagnosis of likely T2D who lacked paper evidence of their diabetic status were invited to participate if we were able to confirm their diabetic status from their study fasting blood glucose analyses.
Enrollment in the study
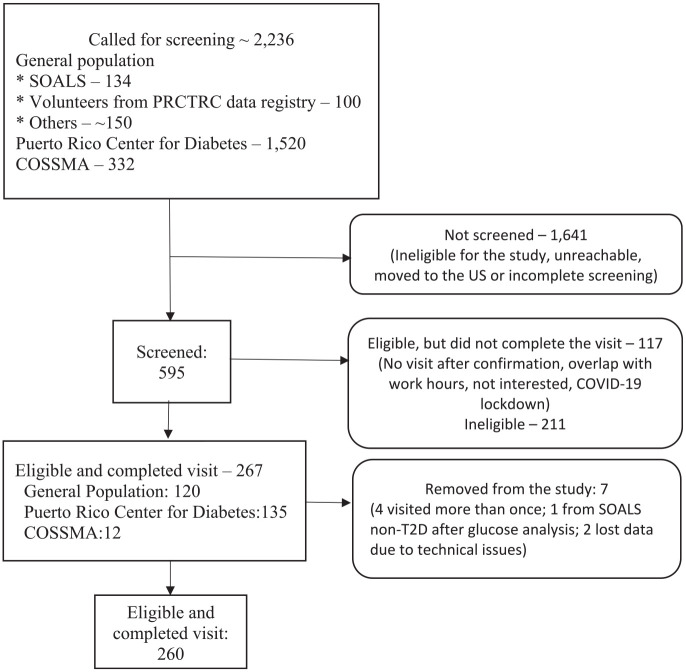
We began work on LLIPDS on April 26, 2017 and ended the study on March 9, 2020. We conducted study visits at the AC. Nearly 150 potential participants from the general population were interested in participating and contacted us (see Figure 1). We invited for screening the 134 candidates from SOALS and the 100 volunteers from the AC data registry. We also contacted almost 1520 potential participants from the PRCD, and 332 potential participants out of 494 from COSSMA.
Figure 1.
Diagram of LLIPDS’s participants recruitment: April 26, 2017–March 9, 2020 (N = 260).
We screened a total of 595 individuals. Screening was not conducted for certain individuals for the following reasons: individuals were ineligible; their phone was disconnected; participants declined to be screened (or to participate); they had moved to the US mainland; or they did not complete the screening process.
Of the 595 screened potential participants, 267 were eligible and completed their study visit, 117 were eligible but did not complete the visit, and 211 were excluded. We did attempt to contact the 117 individuals who failed to complete their study visit; those we succeeded in contacting told us they were no longer interested in participating.
Seven of the eligible participants who completed their study visit were subsequently found to be ineligible; of these, four participants came more than once, one SOALS participant was not diabetic, and two participants had their dental data lost due to technical issues, thus giving a final sample of 260 participants with complete data on the key variables for analysis. Our study was approved by the Institutional Board Review of the University of Puerto Rico (approved on 03/09/2016, IRB #B0930116). Eligible participants supplied signed informed consent forms prior to the study procedures.
Assessment of diabetes-selfcare activities as exposures
Interview-based questionnaire
Well trained and qualified interviewers administered an IRB approved questionnaire during an interview with the participants. The LLIPDS’ questionnaire derived from the previous SOALS’ questionnaire and other validated “Cholesterol Drugs and Adverse Events Study Questionnaire” from the Statin Study of the University of California, San Diego. Prior to the conduct of the study, we tested the questionnaire in 5–10 participants to confirm that participants understood each question and they could fill the questionnaire out easily and reliably within a time frame of a maximum 45 min. Unfortunately, the formal test of the answers from the questionnaire was not done, and the Cronbach alpha value was not computed.
The questionnaire data were directly recorded by the interviewers on the Redcap platform.24 The data obtained included information on socio-demographics, general health status, healthcare visits, medical treatments, lifestyle practices, and contact information. Basic socio-demographic data included the participant’s age, gender, and educational level (in years), along with lifestyle habits, such as smoking status (never, former, current) as well as alcohol consumption (abstainer, former, current).
Diabetes self-care activities
Participants were asked to complete each of the following statements describing diabetes self-care activities using this list of possible responses (1—applies to me very much, 2—applies to me to a considerable degree, 3—applies to me to some degree, or 4—does not apply to me): “Thinking about your self-care over the last 8 weeks, please specify the extent to which each statement applies to you” (1) I check my blood sugar levels with care and attention; (2) I take my diabetes medication (e.g. insulin, metformin tablets) as prescribed; and (3) My diabetes self-care is poor. We then created three exposure indicator variables from their responses: (1) glucose level check with care; (2) compliance with medications; and (3) perception of diabetes self-care.
Measurement of fasting glucose and glycated hemoglobin (HbA1c) as outcomes
All eligible participants were asked to fast for at least 9 h prior to their morning visit. A fasting serum glucose blood draw was performed at their morning visit; the analysis of the blood samples was performed at Clendo Reference Laboratories in Puerto Rico using commercially available methods. Glucose was assessed using a Vitros System 250 instrument with an intra-assay coefficient of variation of 1.21% and inter-assay coefficient of variation of 3.06%, using diffraction spectrometric technology. Uncontrolled fasting blood glucose was defined to be at a level ≥130 mg/dL. Glycosylated hemoglobin AIC (HbA1c) was measured by a latex immunoagglutination inhibition methodology with monoclonal antibody using a Siemens Kit for DCA 2000 and DCA Vantage Analyzer. Uncontrolled HbA1c measure was defined to be at a level ≥7%.
Other data collected
Anthropometric measurements
Height was measured three times to the nearest 0.1 cm using a regularly calibrated stadiometer and weight was measured three times to the nearest 0.5 kg using electronic scale (Analyzer-TBF-310A) according to the NHANES III anthropometric standard procedures.25 The average of the three measures was used as the individual’s height or weight measure. Body Mass Index (BMI) was calculated as the weight in kilograms divided by the square of height in meters (kg/m2). We securely stored all collected and calculated data in Redcap.
Statistical analysis
Data concerning socio-demographic characteristics (e.g. sex, age, education), general health status (BMI), lifestyle habits (smoking status, alcohol consumption), and diabetes self-care activities were summarized using means (standard deviations), medians (P25–P75), or frequencies (percentages), as appropriate (Table 1). Associations between selected socio-demographic variables and the exposures (i.e. diabetes self-care variables) were assessed using a Pearson Chi square test (Figure 2(a)–(f)). Associations between selected socio-demographic variables and the outcome variables (i.e. fasting glucose level or glycated HbA1c) are summarized in Table 2 (fasting blood glucose) and Table 3 (HbA1c). The crude odds ratios and the adjusted odds ratios (the latter from a logistic regression analysis) were used to evaluate the associations between the exposures (diabetes self-care activities or oral health care) and the outcomes (fasting glucose or HbA1c), while controlling for potential confounding factors, such as age, gender, educational level, smoking status, alcohol status, and BMI (Table 4). All hypothesis testing was two-sided, using a significance level of 0.05. All analyses were conducted using STATA SE version 16 (Stata Corp. Texas, USA).
Table 1.
General Socio-demographic Characteristics of the study population, N = 260.
| Variables | N (%), mean ± SD, or median (P25–P75) | Missing values |
|---|---|---|
| Gender | ||
| Male | 116 (44.62) | |
| Female | 144 (55.38) | |
| Age | ||
| Mean ± SD | 54.48 ± 5.93 | |
| Median (P25–P75) | 55 (50–59) | |
| Education | ||
| ≤ High school diploma | 90 (34.62) | |
| > High school diploma | 170 (65.38) | |
| BMI | ||
| Under/normal | 17 (6.54) | |
| Overweight | 70 (26.92) | |
| Obese | 173 (66.54) | |
| Smoking status | ||
| Smoker | 92 (35.66) | 2 |
| Non-smoker | 166 (64.34) | |
| Alcohol consumption | 3 | |
| Yes | 192 (74.71) | |
| No | 65 (25.29) | |
| Revision of blood sugar levels with care | ||
| Yes | 189 (72.69) | |
| No | 71 (27.31) | |
| Use medication as prescribed | ||
| Yes | 242 (93.08) | |
| No | 18 (6.92) | |
| Perceived diabetes self-care | ||
| Yes | 178 (68.46) | |
| No | 82 (31.44) | |
| Fasting glucose | ||
| < than 130 | 103 (39.62) | |
| ≥ than 130 | 157 (60.38) | |
| A1C | ||
| Controlled (<7%) | 91 (35.00) | |
| Not controlled (≥7%) | 169 (65.00) | |
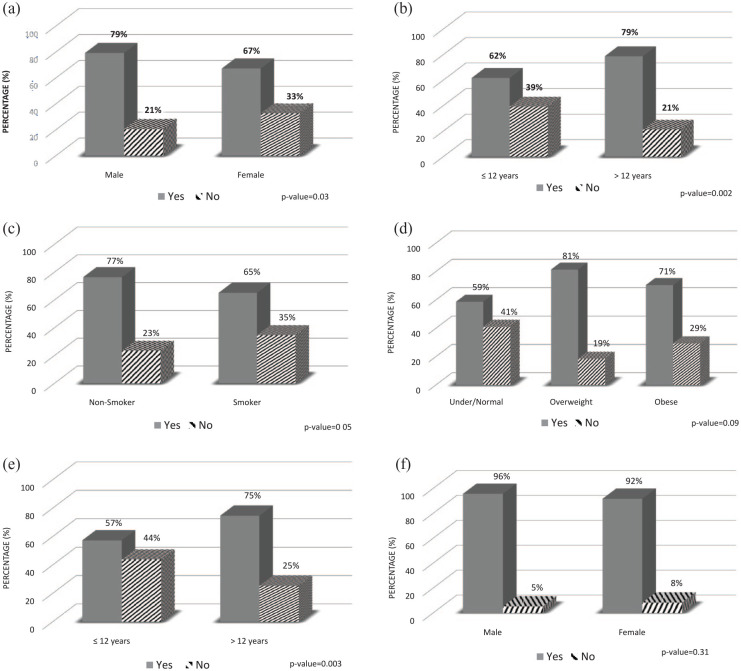
Figure 2.
Characteristics of the study population by diabetes self-care activities or perception (N = 260): (a) self-check of blood glucose by gender, (b) self-check of blood glucose by education, (c) self-check of blood glucose by smoking status, (d) self-check of blood glucose by BMI group, (e) diabetes self-care perception by education, and (f) medication compliance by gender.
Table 2.
Characteristics of the study population by fasting glucose level, N = 260.
| Variables | Fasting glucose | ||
|---|---|---|---|
| <than 130 mg | ≥ than 130 mg | p-Value* | |
| n = 103 (%) | n = 157 (%) | ||
| Gender | 0.62 | ||
| Male | 44 (42.72) | 72 (45.86) | |
| Female | 59 (57.27) | 85 (54.14) | |
| Age | 0.79 | ||
| Mean ± SD | 54.50 ± 5.74 | 54.47 ± 6.08 | |
| Median (P25–P75) | 55 (50–59) | 56 (50–59) | |
| Education | 0.13 | ||
| ≤ High school diploma | 30 (29.13) | 60 (38.22) | |
| > High school diploma | 73 (70.87) | 97 (61.78) | |
| BMI | <0.01 | ||
| Under/normal | 10 (9.71) | 7 (4.46) | |
| Overweight | 16 (15.53) | 54 (34.39) | |
| Obese | 77 (74.76) | 96 (61.15) | |
| Smoke° | 0.07 | ||
| Non-smoker | 73 (70.87) | 93 (60.00) | |
| Smoker | 30 (29.13) | 62 (40.00) | |
| Drink# | 0.13 | ||
| Yes | 71 (69.61) | 121 (78.06) | |
| No | 31 (30.39) | 34 (21.94) | |
| Revision of blood sugar levels with care | 0.37 | ||
| Yes | 78 (75.73) | 111 (70.70) | |
| No | 25 (24.27) | 46 (29.30) | |
| Use medication as prescribed | 0.95 | ||
| Yes | 96 (93.20) | 146 (92.99) | |
| No | 7 (6.80) | 11 (7.01) | |
| Diabetes self-care perception | 0.22 | ||
| Yes | 75 (72.82) | 103 (65.61) | |
| No | 28 (27.18) | 54 (34.39) | |
p-Values were obtained using the Pearson’s Chi Square Test.
°These associations have two missing values.
These associations have three missing values
Table 3.
Characteristics of the study population by glycated hemoglobin (HBA1c) control, N = 260.
| Variables | HbA1C | ||
|---|---|---|---|
| Not controlled | Controlled | p-Value* | |
| n = 169 (%) | n = 91 (%) | ||
| Gender | 0.23 | ||
| Male | 80 (47.34) | 36 (39.56) | |
| Female | 89 (52.66) | 55 (60.44) | |
| Age | 0.06 | ||
| Mean ± SD | 53.90 ± 5.96 | 55.55 ± 5.77 | |
| Median (P25–P75) | 55 (49–58) | 56 (51–61) | |
| Education | 0.68 | ||
| ≤ High school diploma | 60 (35.50) | 30 (32.97) | |
| > High school diploma | 109 (64.50) | 61 (67.03) | |
| BMI | 0.01 | ||
| Under/normal | 8 (4.73) | 9 (9.89) | |
| Overweight | 56 (33.14) | 14 (15.38) | |
| Obese | 105 (62.13) | 68 (74.73) | |
| Smoke° | <0.01 | ||
| Non-smoker | 96 (57.49) | 70 (76.92) | |
| Smoker | 71 (42.51) | 21 (23.08) | |
| Drink# | 0.02 | ||
| Yes | 132 (79.52) | 60 (65.93) | |
| No | 34 (20.48) | 31 (34.07) | |
| Revision of blood sugar levels with care | 0.36 | ||
| Yes | 126 (74.56) | 63 (69.23) | |
| No | 43 (25.44) | 28 (30.77) | |
| Use medication as prescribed | 0.38 | ||
| Yes | 159 (94.08) | 83 (91.21) | |
| No | 10 (5.92) | 8 (8.79) | |
| Diabetes self-care | 0.03 | ||
| Yes | 108 (63.91) | 70 (76.92) | |
| No | 61 (36.09) | 21 (23.08) | |
p-Values were obtained using the Pearson’s Chi Square Test.
°These associations have two missing values.
These associations have three missing values.
Table 4.
Odds Ratio (OR, 95% CI) of the associations between diabetes selfcare activities and fasting glucose level or glycosylated hemoglobin (HbA1c) N = 260.
| Independent variables | Fasting glucose | HbA1C | ||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR° (95% CI) | Crude OR (95% CI) | Adjusted OR° (95% CI) | |
| Revision of blood sugar levels with care# | ||||
| Yes | Reference | Reference | Reference | Reference |
| No | 1.29 (0.73–2.28) | 1.35 (0.72–2.50) | 0.77 (0.44–1.35) | 0.75 (0.40–1.40) |
| Use medication as prescribed§ | ||||
| Yes | Reference | Reference | Reference | Reference |
| No | 1.03 (0.39–2.76) | 1.16 (0.39–3.50) | 0.65 (0.25–1.72) | 0.71 (0.23–2.15) |
| Diabetes self care# | ||||
| Yes | Reference | Reference | Reference | Reference |
| No | 1.40 (0.81–2.42) | 1.53 (0.84–2.77) | 1.88 (1.05–3.36) | 2.14 (1.13–4.08) |
°Odds Ratio were adjusted by sex, age, education level, BMI, smoking status, and alcohol usage.
These adjusted associations have four missing values.
These adjusted associations have five missing values.
Results
Approximately 55% of the participants were women (Table 1). The average age was 55 years (median 55 years). Nearly 65% of the participants had an educational level of a high school diploma or more (i.e. ≥12 years of education). More than 66% of the participants were obese, 36% were current or former smokers, and 75% were current or former alcohol drinkers. Approximately 27% of participants reported not checking their blood sugar level with care, 7% did not take their medications as prescribed, and approximately 31% perceived their diabetes self-care as poor. Nearly 60% of the participants had a fasting blood glucose ≥130 mg/dL; around 65% had a HbA1c ≥ 7%.
The profiles of the participants’ exposures (i.e. diabetes self-care activities or perception) varied with the activity. Females checked their glucose levels less often than males (67% vs 79%; p = 0.03, Figure 2(a)), participants with less education (≤12 years) also monitored their blood sugar less than their counterparts (62% vs 79%; p = 0.002, Figure 2(b)), and current or former smokers performed blood sugar checks less than non-smokers (65% vs 77%; p = 0.05, Figure 2(c)). There was a borderline association across BMI groups and checking the sugar level: underweight (BMI < 18.5 kg/m2) or normal weight (18.5–24.99 kg/m2) participants tended to check their sugar levels (59%) less than their counterparts who were overweight (BMI: 25–29.99 kg/m2, 81%) or obese (BMI ≥ 30 kg/m2, 71%) counterparts (three-way comparison; p = 0.09, Figure 2(d)). Similarly, more participants with a lower educational level perceived their diabetes self-care as poor compared to those with higher education (44% vs 25%; p = 0.003, Figure 2(e)). No significant association between gender and adherence to medication (92% females vs 96% males, p = 0.31, Figure 2(f)) or participants’ general characteristics (including income status) (data not shown, p > 0.05) was observed.
The characteristics of the outcomes (i.e. fasting blood glucose, HbA1c) varied slightly less (Tables 2 and 3). BMI was significantly associated with fasting glucose levels (p ≤ 0.01); and smoking habits showed a borderline association (p = 0.07) (Table 2). Similarly, factors such as older age (p = 0.06), overall BMI (p = 0.01), current or former smokers (p < 0.01), alcohol non-drinkers (p = 0.02), and good diabetes self-care perception (p = 0.03), varied in the strength of their association with HbA1c levels (Table 3).
Neither the crude odds ratios nor the adjusted odds ratios obtained from logistic regression models (adjusted for age, gender, education level, BMI, smoking, and alcohol status) showed any statistically significant associations between diabetes self-care activities or participants’ perception of their self-care and high fasting glucose (Table 4). However, we did find a significant association between poor diabetes self-care perception and high HbA1c (Table 4). In other words, after adjusting for age, gender, smoking status, alcohol consumption, education, and BMI, participants with poor diabetes self-care perception had about twice the odds of having higher HbA1c levels than those with good diabetes self-care perception (OR: 2.14; 95% CI: 1.13, 4.08). We did not see any association between the other diabetes self-care activities and HbA1c levels.
Discussion
Diabetes requires consistent care and medication adherence to avoid long term complications. We evaluated the diabetes self-care activities and perception of individuals with T2D in Puerto Rico to understand the risk involved in not engaging in attentive diabetes care. Around one-third of our participants perceived their diabetes self-care as poor. Researchers have demonstrated that such a perception is more likely in people with less education,1,26 which we confirmed (44% participants with less education perceived poor diabetes self-care vs 25% of their more-educated counterparts; p = 0.003). We also observed an objective corroboration of such perception with actual poor glucose control, especially via HbA1c in individuals with T2D. Findings by D’Souza et al.,27 which indicated poor HbA1c control to be more likely associated with poor self-efficacy and less engagement in self-care behaviors among adults with T2D, supported our results.
The Annual Health Report of Puerto Rico from 2016 and the 2014 BRFSS data suggested that the prevalence of T2D is three times higher in patients with a low educational level or a low socioeconomic status than in those with either a higher educational level or a high socioeconomic status, but neither of these reports documented self-care behaviors among those with a higher risk of having T2D.
We described the profiles or characteristics of participants who were less engaged in diabetes self-care activities and perception, which in turn might affect poor glycemic control in T2D. We did not find an association between educational status and fasting glucose or HbA1c levels. However, our findings suggest both associations between lower educational level and poorer diabetes self-care perception and between the latter and less adequate glycemic control might be related to lack of knowledge of the diabetic condition. These associations could be the result of a more complex interaction between socio-economic status, education level, specific disease knowledge, and the ability to act on that knowledge. It is possible that participants with less education may simply have less knowledge about diabetes, in addition to less effective self-management behaviors thereby leading to poorer glycemic control. Previous researchers have suggested the effect of empowerment of patients through education to improve their diabetes knowledge, which in turn improves their glycemic control.28 Indeed, individuals with greater diabetes knowledge should also have better self-management behaviors.29,30
We found poor perception of diabetes self-care to be associated with high HbA1c but not fasting blood glucose. Fasting glucose level reflects the short term daily glycemic level, while HbA1c measures the average longer-term blood glucose level (e.g. over the past 2–3 months); the latter may be impacted more by an individual’s tendency to poorly conduct diabetes self-care behaviors.
Apart from our findings on poor perceived diabetes self-care to be associated with poor glycemic control, we did not observe any statistically significant associations between revision of blood sugar levels with care or better adherence to medication and reduced fasting blood glucose or HbA1C. Previous reports, such as those of Shao et al.31 or Saad et al.,32 however, have indicated that better self-care management behaviors, including adherence to medication or self-monitoring of blood glucose (SMBG) to be associated with improved HbA1c. On the other hand, Brown et al.33 suggested that SMBG was the most predictive factor for fasting blood glucose. Basically, current clinical recommendations on the frequency of self-testing blood glucose for T2D patients depend upon individual factors such as the type of treatment (oral medications, insulin, and/or lifestyle changes), A1C level, risk of hypoglycemia, and treatment goals.34
Approximately one in four individuals with T2D in our study population did not check their blood glucose levels during the previous 8 weeks. Specifically, we found that being female, having an education level at or less than a high school diploma, or being a current/former smoker were associated with a higher probability of not carefully checking blood glucose levels. Despite the potential beneficial use of SMBG to improve glycemic control, reports in the literature have indicated that only a very low percentage of patients with T2D adhere to this diabetes self-care management technique, and that the profile characteristics of those who usually self-monitor their blood glucose are complex and vary across countries and regions.35 –37 Similar to our findings, other results suggest that higher educational levels may increase the frequency of blood glucose monitoring, including the SMBG.35,37 On the other hand, apart from the individual factors dictating the recommended frequency of use of SMBG in patients with T2D cited above, the cost, pain of constant finger-pricking, and the stress of having to do blood glucose measurements38,39 might constitute significant barriers to SBGM among women versus men in our study population, although evidence is needed to prove this conjecture. We did not observe any significant differences in specific characteristics between men and women which might have produced the difference in the diabetes self-care management we observed.
A greater adherence to medication has been known to improve glycemic control.20,21 Different factors have been known to be associated with poor medication adherence, and losing this benefit, including the following: patients’ poor diabetes self-care behaviors including lifestyle habits, age, gender (male or female), countries of origins, or absence of other chronic health conditions (e.g. hypertension or hyperlipidemia comorbidities), especially among patients with a new diagnosis of diabetes.21,22,26 Many of our participants reported using their medication as prescribed (93.1%), yet nearly 31% of them still perceived their diabetes self-care behavior as poor. There could be many factors which may play a role in this apparent paradox. Participants might have believed that medication compliance alone would be enough to control their diabetes condition, thus they otherwise engaged in poor diabetes self-care behaviors. Other conditions, including low socio-economic status, are also known to affect diabetes management.
Our findings might also have been affected by social desirability bias, in which the participants, who were asked to fast prior to the blood draw, might have answered that they had fasted either to make them appear to be compliant for the interviewers or to satisfy a part of our inclusion criteria thereby to be rewarded through participation. For instance, 66.5% of participants in this study were obese, 35.7% reported that they currently smoked or were former smokers, and 74.7% consumed or previously consumed alcohol.
There are few reports of assessments of self-care behaviors or self-management of T1D or T2D among Puerto Ricans.40,41 To the best of our knowledge ours is a novel approach in the evaluation of the association between diabetes self-care activities or self-care perception and either fasting glucose or HbA1c in Puerto Rican adults with T2D. We performed rigorous diabetes self-care activity assessments along with high-quality blood measurements. Moreover, our study dataset contains a very large number of factors possibly related to other health outcomes, which we plan to employ for further hypothesis generation, and which may provide insights into general public health issues in Hispanics with T2D which need to be addressed.
Our study results had certain limitations. The data were obtained from a non-probability convenience sample thus findings may not be generalizable to the island’s diabetic population. Nonetheless, our study population included 45% of its participants from the general population, 50% from the PRCD, and 5% from COSSMA. In fact, most individuals with T2D residing around San Juan (the most populated municipality area in PR) attend the PRCD for regular health checkups. Hence, the non-random sampling method we used might not have affected the external validity or generalizability of our findings. Other than the social desirability bias that might have occurred, recall bias is inherent to our cross-sectional study design. However, the recall of participants’ routine diabetes self-care activities is unlikely to be impaired within the relatively short period of 8 weeks.
Conclusion
Poor diabetes self-care perception, possibly related to low educational level, was associated with high HbA1C among Puerto Rican residents with T2D. A comprehensive health educational campaign to improve self-care practices by learning about glycemic goals and the importance of reaching these goals should be promoted among Puerto Ricans with T2D to avoid or at least delay long-term complications.
Acknowledgments
The authors acknowledge the sponsors, the National Institute of Dental and from the Craniofacial Research (NIDCR, K23 DE025313) and the National Institute of General Medical Sciences (NIGMS, U54GM133807) National Institute of Health, which funds the Hispanic Alliance for Clinical and Translational Research. The authors also acknowledge the LLIPDS team (Dr. Francisco Jiménez, Mr. Francisco Muñoz-Torres, Mr. Abdiel Castillo, Ms. Claudia Díaz, Mr. Alexis Acevedo, Ms. Patricia Serrano, and all who participated in the study), the Alliance (formerly PRCTRC) personnel, who contributed to the conduct/oversight/planning of data collection of the study (administrative and regulatory affairs: Ms. Antonia Ortiz, Ms. Ivette Molina, and Ms. Adelma Rivera; nurses: Ms. Bárbara Guzmán, Ms. Sheyla Garced, Ms. Ladimila De Lima, and Mr. Robert Pinder; laboratory work: Mrs. Nilda González and Ms. Carola López-Cepero). The authors also acknowledge the Puerto Rico Diabetes Center, COSSMA, and all participants, who contributed to and participated in the study. They would also like to thank Stephen Campbell, who has edited their work.
Footnotes
Author contributions: ALF contributed to the study conception, data acquisition and interpretation, drafted and critically revised the manuscript. NP contributed to the data analysis and interpretation, and critically revised the manuscript. DMCM contributed to the data interpretation, drafted and critically revised the manuscript. MRV contributed to the design of the work, data acquisition and interpretation, and critically revised the manuscript. OMA contributed to the study conception, design of the work, data acquisition, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was fully supported by Award K23 DE025313-05 from the National Institute of Dental and Craniofacial Research (NIDCR), partially supported by the National Institute of General Medical Sciences (NIGMS) grant U54MD007587, which funded the former Puerto Rico Clinical and Translational Research Consortium (PRCTRC now is called ALLIANCE). Non-financial support was provided for the statistical analysis by The Hispanic Alliance for Clinical and Translational Research (ALLIANCE) (Award Number U54GM133807). The content of the research study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethics approval and consent to participate: The study was approved by the Institutional Board Review of the University of Puerto Rico (approved on 03/09/2016, IRB #B0930116). Eligible participants willing to participate in the study provided signed informed consent forms prior to the study procedures.
Patient consent for publication: Eligible participants provided signed informed consent forms for publication prior to the study procedures.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Significance for public health: Individuals with diabetes frequently have comorbid health conditions and suffer longer term complications. Glycemic control relies on diabetes management/self-care behaviors. Poor glycemic control, commonly encountered in underserved populations with type 2 diabetes, results from a lack of diabetes self-care activities, such as glucose monitoring, adherence to prescribed medications, or poor diabetes self-care perception. Knowledge of efficient diabetes self-care behaviors can improve glycemic control among people with type 2 diabetes even with less education, and diabetes knowledge and perceived health status are important factors associated with glycemic control among underserved populations with less income. To our knowledge, ours is the first study to assess the potential impact of diabetes self-care activities and perception on glycemic control among Hispanic majority adults with type 2 diabetes residing in Puerto Rico. This work is needed to provide insights into public health issues in need of resolution.
ORCID iD: Alejandro Llera-Fábregas  https://orcid.org/0000-0001-5984-9808
https://orcid.org/0000-0001-5984-9808
Availability of data and materials: The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1. Flood D, Seiglie JA, Dunn M, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Healthy Longev 2021; 2(6): e340–e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. America Diabetes Association. Statistics about diabetes, https://www.diabetes.org/resources/statistics/statistics-about-diabetes (2020, accessed 2 November 2020).
- 3. Center for Disease Control and Prevention. National Diabetes Statistics Report 2020: estimates of diabetes and its burden in the United States, 2020. [Google Scholar]
- 4. Fortmann AL, Savin KL, Clark TL, et al. Innovative diabetes interventions in the U.S. Hispanic population. Diabetes Spectr 2019; 32(4): 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Diabetes Federation. IDF SACA region, https://idf.org/our-network/regions-members/south-and-central-america/members/90-puerto-rico.html (accessed 29 October 2020).
- 6. Report AsHRA. America’s Health Rankings Annual Report 2016. [Google Scholar]
- 7. Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med 2019; 16(3): e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seiglie JA, Marcus M-E, Ebert C, et al. Diabetes prevalence and its relationship with education, wealth, and BMI in 29 low- and middle-income countries. Diabetes Care 2020; 43(4): 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Protheroe J, Rowlands G, Bartlam B, et al. Health literacy, diabetes prevention, and self-management. J Diabetes Res 2017; 2017: 1298315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Diabetes, https://www.who.int/news-room/fact-sheets/detail/diabetes (2021, accessed 11 May 2021).
- 11. UCSF Health. Diabetes mellitus treatments, https://www.ucsfhealth.org/conditions/diabetes-mellitus/treatment (2021, accessed 27 July 2021).
- 12. Maleki Chollou K, Gaffari-fam S, Babazadeh T, et al. The Association of Health Literacy level with self-care behaviors and glycemic control in a low education population with type 2 diabetes mellitus: a cross-sectional study in Iran. Diabetes Metab Syndr Obes 2020; 13: 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bains SS, Egede LE. Associations between health literacy, diabetes knowledge, self-care behaviors, and glycemic control in a low income population with type 2 diabetes. Diabetes Technol Ther 2011; 13(3): 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013; 12(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Pérez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013; 4(2): 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavanaugh KL. Health literacy in diabetes care: explanation, evidence and equipment. Diabetes Manag 2011; 1(2): 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayberry LS, Bergner EM, Chakkalakal RJ, et al. Self-care disparities among adults with type 2 diabetes in the USA. Curr Diab Rep 2016; 16(11): 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA 2002; 288(4): 475–482. [DOI] [PubMed] [Google Scholar]
- 19. Hu J, Amirehsani K, Wallace DC, et al. Perceptions of barriers in managing diabetes: perspectives of Hispanic immigrant patients and family members. Diabetes Educ 2013; 39(4): 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011; 33(1): 74–109. [DOI] [PubMed] [Google Scholar]
- 21. Lin LK, Sun Y, Heng BH, et al. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diabetes Res Care 2017; 5(1): e000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olickal JJ, Chinnakali P, Suryanarayana BS, et al. Medication adherence and glycemic control status among people with diabetes seeking care from a tertiary care teaching hospital, south India. Clin Epidemiol Glob Health 2021; 11: 100742. [Google Scholar]
- 23. Joshipura KJ, Muñoz-Torres FJ, Dye BA, et al. Longitudinal association between periodontitis and development of diabetes. Diabetes Res Clin Pract 2018; 141: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. CDC. BRFSS summary data quality report. U.S. Department of Health and Human Services, 2008. [Google Scholar]
- 26. Kassahun T, Gesesew H, Mwanri L, et al. Diabetes related knowledge, self-care behaviours and adherence to medications among diabetic patients in southwest Ethiopia: a cross-sectional survey. BMC Endocr Disord 2016; 16(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Souza MS, Karkada SN, Parahoo K, et al. Self-efficacy and self-care behaviours among adults with type 2 diabetes. Appl Nurs Res 2017; 36: 25–32. [DOI] [PubMed] [Google Scholar]
- 28. Panja S, Starr B, Colleran KM. Patient knowledge improves glycemic control: is it time to go back to the classroom? J Investig Med 2005; 53(5): 264–266. [DOI] [PubMed] [Google Scholar]
- 29. Ji M, Ren D, Dunbar-Jacob J, et al. Self-management behaviors, glycemic control, and metabolic syndrome in type 2 diabetes. Nurs Res 2020; 69(2): E9–E17. [DOI] [PubMed] [Google Scholar]
- 30. Kugbey N, Oppong Asante K, Adulai K. Illness perception, diabetes knowledge and self-care practices among type-2 diabetes patients: a cross-sectional study. BMC Res Notes 2017; 10(1): 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shao Y, Liang L, Shi L, et al. The effect of social support on glycemic control in patients with type 2 diabetes mellitus: the mediating roles of self-efficacy and adherence. J Diabetes Res 2017; 2017: 2804178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saad AMJ, Younes ZMH, Ahmed H, et al. Self-efficacy, self-care and glycemic control in Saudi Arabian patients with type 2 diabetes mellitus: a cross-sectional survey. Diabetes Res Clin Pract 2018; 137: 28–36. [DOI] [PubMed] [Google Scholar]
- 33. Brown SA, García AA, Brown A, et al. Biobehavioral determinants of glycemic control in type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns 2016; 99(10): 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruth W. Patient education: blood glucose monitoring in diabetes (Beyond the Basics), https://www.uptodate.com/contents/blood-glucose-monitoring-in-diabetes-beyond-the-basics (2021, accessed 11 November 2021).
- 35. Yao J, Wang H, Yan J, et al. Understanding the profiles of blood glucose monitoring among patients with type 2 diabetes mellitus: a cross-sectional study in Shandong, China. Patient Prefer Adherence 2021; 15: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Platt KD, Thompson AN, Lin P, et al. Assessment of self-monitoring of blood glucose in individuals with type 2 diabetes not using insulin. JAMA Intern Med 2019; 179(2): 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng Y, Wu J, Han Y, et al. Educational disparities in the associations between self-monitoring of blood glucose and glycemic control in type 2 diabetes patients in Xiamen, China. J Diabetes 2018; 10(9): 715–723. [DOI] [PubMed] [Google Scholar]
- 38. Sue Cradock JH. Pain, distress and blood glucose monitoring. J Diabetes Nurs 2002; 6(6): 188–191. [Google Scholar]
- 39. Tanaka N, Yabe D, Murotani K, et al. Mental distress and health-related quality of life among type 1 and type 2 diabetes patients using self-monitoring of blood glucose: a cross-sectional questionnaire study in Japan. J Diabetes Invest 2018; 9(5): 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Streisand R, Respess D, Overstreet S, et al. Brief report: self-care behaviors of children with type 1 diabetes living in Puerto Rico. J Pediatr Psychol 2002; 27(8): 759–764. [DOI] [PubMed] [Google Scholar]
- 41. Bernal H. Self-management of diabetes in a Puerto Rican population. Public Health Nurs 1986; 3(1): 38–47. [DOI] [PubMed] [Google Scholar]