Abstract
Background
Sexually transmitted infections (STIs) affect individuals of all ages, but adolescent girls and young women are disproportionately affected. We examined the prevalence and factors associated with self-reported STIs (SR-STIs) among adolescent girls and young women in sub-Saharan Africa (SSA).
Methods
Demographic and Health Survey data of 27 sub-Saharan African countries were used for the study. The sample size comprised 68944 adolescent girls and young women (15–24 y of age). The outcome variable was SR-STIs. Multilevel binary logistic regression analysis was performed to identify factors associated with SR-STIs.
Results
On average, the prevalence of SR-STIs among adolescent girls and young women in SSA was 6.92%. The likelihood of SR-STIs was higher among young women aged 20–24 y (adjusted odds ratio [aOR] 1.36 [confidence interval {CI} 1.27 to 1.46]), those not married (aOR 1.64 [CI 1.51 to 1.79]), those working (aOR 1.20 [CI 1.12 to 1.27]), those whose age at first sex was ≤19 y (aOR 1.99 [CI 1.80 to 2.20]), those with two or more sex partners (aOR 1.56 [CI 1.35 to 1.80]), those who listened to radio (aOR 1.26 [CI 1.17 to 1.35]), those in urban areas (aOR 1.42 [CI 1.30 to 1.51]) and those with a wealth index of rich (aOR 1.28 [CI 1.17 to 1.40]) compared with their counterparts. In contrast, those with a primary (aOR 0.86 [CI 0.78 to 0.94]) or secondary/higher level of education (aOR 0.83 [CI 0.75 to 0.92]) compared with those with no formal education and those who were exposed to television (aOR 0.90 [CI 0.84 to 0.98]) compared with those who were not exposed were less likely to report STIs.
Conclusions
Our findings demonstrate the need for countries in SSA to commit towards reducing the incidence of STIs. Community-based health educational programs are required to intensify the awareness of STIs and their prevention in various sub-Saharan African countries considering the factors that expose adolescent girls and young women to STIs.
Keywords: adolescent girls, prevalence, self-reported sexually transmitted infections, sub-Saharan Africa, young women
Introduction
Over the past 2 decades, sexually transmitted infections (STIs), including chlamydia, gonorrhoea, syphilis and human immunodeficiency virus (HIV), have been increasing and have become a public health concern for both developed and developing countries.1–5 STIs caused by sexual contact, including vaginal, oral and anal sex, have a great impact on the sexual and reproductive health of men and women age 15–49 y.6–10 STIs are a major global cause of infertility, acute illness, long-term disability and mortality, with severe medical and psychological consequences.1 Studies also show that STIs result in other negative health outcomes, including HIV/acquired immunodeficiency syndrome (AIDS), ectopic pregnancy, eye defects and pelvic inflammatory diseases.11,12
Globally, more than 1 million people contract STIs daily.13,14 In 2018, the World Health Organization reported 376 million new infections of four STIs—chlamydia, gonorrhoea, syphilis and trichomoniasis—among people ages 15–49 y in 2016.14 Prevalence rates of STIs vary from region to region, country to country and between urban and rural populations. The infections are more prevalent in Asia and sub-Saharan Africa (SSA), but SSA contributes a greater proportion of the cases of STIs every year, which is estimated at approximately 93 million.15
STIs affect individuals of all ages,16 but adolescent girls and young women are disproportionately impacted due to their high-risk sexual behaviours, including unprotected sex.17 Studies have shown that adolescent girls and young women are at a higher risk of acquiring STIs because of social circumstances and increased risk-taking.8,17,18 Self-reported STIs (SR-STIs) allows health facilities to provide specific prevention and control interventions to tackle the spread of infections.19,20
Yet, for SSA, studies that have focused on SR-STIs have failed to examine the factors associated with STIs among adolescent girls and young women.8,9,15,21 For example, Torrone et al.8 used combined data from 18 prospective HIV prevention studies in SSA to investigate the association between hormonal contraceptive use and contraction of HIV. Other studies on STIs in SSA have examined the prevalence and the associated factors of SR-STIs among men,15 men who have sex with men and transgender women.21,22 For Seidu et al.,15 sexually active men ages 25–34 y were more likely to report STIs compared with those age ≥45 y.
Given this gap in research knowledge, we utilized recent data from sub-Saharan African countries’ Demographic and Health Surveys (DHS) to examine the prevalence and associated factors of SR-STI among adolescent girls and young women in SSA. Findings from this study will strengthen our understanding of STIs and factors associated with SR-STIs among adolescent girls and young women in SSA. This study will also provide information on the country-level variations in the prevalence and factors associated with SR-STIs among adolescent girls and young women in this region.
Methods
Data source
Data for this study were obtained from the recent DHS of 27 sub-Saharan African countries. These surveys, which had information on SR-STIs, were conducted from 2010 to 2018. Given the focus of the present study, we used data from women's file which also contains information on adolescent girls and young women (ages 15–24 y) from the various countries. Generally, as a nationally representative survey, the DHS is globally undertaken in over 85 low- and middle-income countries. With regard to its sampling method and focus, the DHS adopts a two-stage stratified sampling protocol, focusing on essential maternal and child health markers and men's health, including SR-STIs.23 The dataset is freely accessible at https://dhsprogram.com/data/available-datasets.cfm. Details of the DHS methodology have been reported elsewhere.23,24 The present study used a sample of 68944 adolescent girls and young women in SSA who had ever had sex in the past 12 months and had complete information on all the variables of interest (Table 1). We followed the Strengthening the Reporting of Observational Studies in Epidemiology statement in conducting this research.
Table 1.
Description of study sample
| Country | Weighted frequency | Weighted percentage |
|---|---|---|
| Benin 2017/2018 | 3719 | 5.39 |
| Burkina Faso 2010 | 1709 | 2.48 |
| Burundi 2016/2017 | 2030 | 2.94 |
| Cameroon 2018 | 4280 | 6.21 |
| Chad 2014/2015 | 3696 | 5.36 |
| Comoros 2012 | 1669 | 2.42 |
| Congo 2011/2012 | 1039 | 1.51 |
| Cote D'Ivoire 2011/2012 | 683 | 0.99 |
| DR Congo 2013/2014 | 3292 | 4.78 |
| Ethiopia 2016 | 3045 | 4.42 |
| Gabon 2012 | 2507 | 3.64 |
| Gambia 2013 | 2376 | 3.45 |
| Ghana 2014 | 2324 | 3.37 |
| Guinea 2018 | 1600 | 2.32 |
| Kenya 2014 | 1646 | 2.39 |
| Lesotho 2014 | 516 | 0.75 |
| Liberia 2013 | 2738 | 3.97 |
| Malawi 2015/2016 | 6344 | 9.2 |
| Mali 2018 | 2634 | 3.82 |
| Mozambique 2011 | 2281 | 3.31 |
| Namibia 2013 | 2255 | 3.27 |
| Niger 2012 | 2054 | 2.98 |
| Senegal 2010/2011 | 2559 | 3.71 |
| Togo 2013/2014 | 1895 | 2.75 |
| Uganda 2016 | 4840 | 7.02 |
| Zambia 2018 | 3318 | 4.81 |
| Zimbabwe 2015 | 1893 | 2.75 |
| All countries | 68 944 | 100 |
Table 2.
Distribution of SR-STIs across the explanatory variables (weighted n=68 944)
| Had STI in the last 12 months, % | |||||
|---|---|---|---|---|---|
| Variables | Weighted frequency | Weighted percentage | No | Yes | p-Value |
| Age in 5-y groups | <0.001 | ||||
| 15–19 | 24 462 | 35.5 | 93.8 | 6.2 | |
| 20–24 | 44 482 | 64.5 | 92.7 | 7.3 | |
| Highest education level | <0.001 | ||||
| None | 15 663 | 22.7 | 94.5 | 5.5 | |
| Primary | 22 582 | 32.8 | 93.8 | 6.2 | |
| Secondary/higher | 30 699 | 44.5 | 91.8 | 8.2 | |
| Current marital status | <0.001 | ||||
| Not married | 36 043 | 52.3 | 91.0 | 9.0 | |
| Married | 32 901 | 47.7 | 95.3 | 4.7 | |
| Respondent's occupation | <0.001 | ||||
| Not working | 31 547 | 45.8 | 93.6 | 6.4 | |
| Working | 37 397 | 54.2 | 92.6 | 7.4 | |
| Age at first sex (years) | <0.001 | ||||
| ≤19 | 54 695 | 79.3 | 92.2 | 7.8 | |
| ≥20 | 14 249 | 20.7 | 96.4 | 3.6 | |
| Number of sex partners, excluding spouse, in last 12 months | <0.001 | ||||
| 0 | 43 629 | 63.3 | 94.2 | 5.8 | |
| 1 | 23 101 | 33.5 | 91.6 | 8.4 | |
| ≥2 | 2214 | 3.2 | 85.3 | 14.7 | |
| Condom use during last sex with most recent partner | 0.018 | ||||
| No | 56 854 | 82.5 | 92.9 | 7.1 | |
| Yes | 12 090 | 17.5 | 93.9 | 6.1 | |
| Comprehensive HIV knowledge | <0.001 | ||||
| No | 42 530 | 61.7 | 93.5 | 6.5 | |
| Yes | 26 414 | 38.3 | 92.5 | 7.5 | |
| Ever been tested for HIV | 0.68 | ||||
| No | 28 322 | 41.1 | 93.1 | 6.9 | |
| Yes | 40 622 | 58.9 | 93.1 | 6.9 | |
| Read newspaper or magazine | <0.001 | ||||
| No | 54 025 | 78.4 | 93.5 | 6.5 | |
| Yes | 14 919 | 21.6 | 91.7 | 8.3 | |
| Listen to radio | <0.001 | ||||
| No | 28 443 | 41.3 | 94.0 | 6.0 | |
| Yes | 40 501 | 58.7 | 92.5 | 7.5 | |
| Watch television | <0.001 | ||||
| No | 38 114 | 55.3 | 94.0 | 6.0 | |
| Yes | 30 830 | 44.7 | 92.0 | 8.0 | |
| Covered by health insurance | <0.001 | ||||
| No | 64 933 | 94.2 | 93.2 | 6.8 | |
| Yes | 4011 | 5.8 | 91.2 | 8.8 | |
| Place of residence | <0.001 | ||||
| Urban | 27 833 | 40.4 | 90.7 | 9.3 | |
| Rural | 41 111 | 59.6 | 94.7 | 5.3 | |
| Wealth index | <0.001 | ||||
| Poor | 25 391 | 36.8 | 94.7 | 5.3 | |
| Middle | 13 620 | 19.8 | 94.0 | 6.0 | |
| Rich | 29 933 | 43.4 | 91.3 | 8.7 | |
| Geographical subregion | <0.001 | ||||
| Southern Africa | 2771 | 4.0 | 98.0 | 2.0 | |
| Western Africa | 24 291 | 35.2 | 93.4 | 6.6 | |
| Central Africa | 14 814 | 21.5 | 90.7 | 9.3 | |
| Eastern Africa | 27 068 | 39.3 | 93.6 | 6.4 | |
Study variables
Outcome variable
SR-STIs among adolescent girls and young women was the outcome variable in this research. Adolescent girls and young women were asked whether they had a disease they acquired through sexual contact in the past 12 months (yes/no). This implies that the variable has a dichotomous outcome.
Explanatory variables
Sixteen explanatory variables made up of 13 individual-level variables and 3 contextual-level variables were included in this analysis. The individual-level variables were age (15–19 y, 20–24 y), education level (no education, primary, secondary/higher), marital status (married, not married), occupation (not working, working), age at first sex (≤19, ≥20), number of sexual partners in the last 12 months excluding the spouse (0, 1, ≥2), condom use during last sex with most recent partner (yes, no), comprehensive HIV and AIDS knowledge (yes, no), HIV testing (yes, no), exposure to mass media (newspaper, radio, TV) (yes, no) and health insurance coverage (yes, no). The contextual-level variables were place of residence (rural, urban), wealth index (poor, middle, rich) and subregion (western Africa, eastern Africa, central Africa, southern Africa). These factors were chosen based on their theoretical and empirical relationship with SR-STIs in previous studies.23,24
Statistical analyses
Data analyses, which involved both descriptive and inferential analyses, were carried out using Stata version 14.0 (StataCorp, College Station, TX, USA). The descriptive statistics enabled us to characterize the adolescent girls and young women in the data. The data were weighted to account for sampling probability and non-response. Also, the data were adjusted using the SVY command in Stata to account for the complex survey design and robust standard errors. Pearson chi-sqaure analysis was conducted to select potential variables for the follow-up multilevel logistic regression analysis. At the bivariate analysis stage, due to multiple comparisons, we introduced the Bonferroni correction method.25 This was done by dividing the α rate (p=0.05) by the number of analyses performed (16 explanatory variables), that is, 0.05/16=0.003. Therefore, in the bivariate analysis, statistical significance was declared at p≤0.003. Variables with a p-value ≤0.003 in the bivariate analysis were included in the multilevel logistic regression model. Before fitting the final model, multicollinearity between the explanatory variables was checked (mean variance inflation factor [VIF] 1.35, minimum VIF 1.05, maximum VIF 2.01) and it was found to be satisfactory. The multilevel logistic regression analysis was performed to identify factors associated with SR-STIs. Four models were presented. Model 0 showed the variance in SR-STIs attributed to the clustering of the primary sampling units (PSUs) without the explanatory variables. Models 1 and 2 contained the individual- and contextual-level factors, respectively. The final model (model 3) had all the individual- and contextual-level factors. The Stata command melogit was used when fitting these models. The Akaike information criterion (AIC) test was used for model comparison. The descriptive results are presented as proportions while the regression results are presented as adjusted odds ratios (aORs) with 95% confidence intervals (CIs) and p-values.
Ethical consideration
Since this study used secondary data that is publicly available, the authors did not need further ethics approval. However, permission to use the dataset was sought from MEASURE DHS. Also, appropriate authorizations and procedures applicable in the respective countries were followed and these are reported in the various national reports. Further information on ethics for the DHS is available at https://dhsprogram.com/Methodology/Protecting-the-Privacy-of-DHS-Survey-Respondents.cfm.
Results
Prevalence of SR-STIs among adolescent girls and young women in SSA
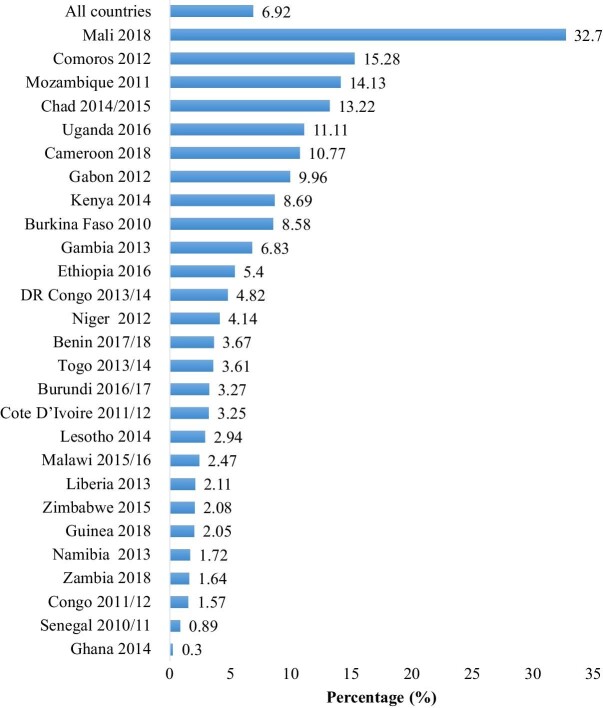
Figure 1 shows the prevalence of SR-STIs among adolescent girls and young women across 27 countries in SSA. On average, the prevalence of SR-STIs among adolescent girls and young women in SSA was 6.92%. In terms of cross-country variations, the prevalence ranged from 32.7% in Mali to 0.3% in Ghana.
Figure 1.
Prevalence of SR-STIs by country.
Sociodemographic characteristics and SR-STIs among adolescent girls and young women in SSA
At p≤0.003, all the explanatory variables showed statistically significant associations with SR-STIs among adolescent girls and young women in SSA, except condom use during last sex with the most recent partner and ever been tested for HIV. Specifically, a higher prevalence of STIs was recorded among those ages 20–24 y (7.3%); those with secondary or higher education (8.2%); those not married (9.0%); those who were working (7.4%); those ≤19 y of age at first sex (7.8%); those with two or more partners (14.7%); those with comprehensive knowledge of HIV (7.5%); those who read newspapers (8.3%), listened to radio (7.5%) or watched television (8.0%); those covered by health insurance (8.8%); those residing in urban areas (9.3%); those with a wealth index of rich (8.7%) and adolescent girls and young women in central Africa (9.3%) compared with their counterparts in the other variable categories.
Multilevel logistic regression results on the factors associated with SR-STIs among adolescent girls and young women in SSA
Table 3 shows the results from the multilevel logistic regression analysis of the factors associated with SR-STIs among adolescent girls and young women in SSA. The final model, which contains all the explanatory variables, revealed that in terms of individual-level factors, the likelihood of SR-STIs was higher among adolescent girls and young women ages 20–24 y (aOR 1.36 [CI 1.27 to 1.46]) compared with those ages 15–19 y. Again, those not married (aOR 1.64 [95% CI 1.51 to 1.79]), those working (aOR 1.20 [95% CI 1.12 to 1.27]) and those whose age at first sex was ≤19 y (aOR 1.99 [95% CI 1.80 to 2.20]) were more likely to report an STI as compared with those who were married, those not working and those with an age at first sex of ≥20 y, respectively. Also, those with two or more sex partners (aOR 1.56 [95% CI 1.35 to 1.80]) compared with those with no sex partner and those who listened to radio (aOR 1.26 [95% CI 1.17 to 1.35]) compared with those who did not listen to radio were more likely to self-report STI. On the other hand, those with a primary (aOR 0.86 [95% CI 0.78 to 0.94) or secondary/higher level of education (aOR 0.83 [95% CI 0.75 to 0.92]) compared with those with no formal education and those who watched television (aOR 0.90 [95% CI 0.84 to 0.98]) compared with those who did not watch television were less likely to report an STI. For the contextual factors, those who lived in urban areas (aOR 1.42 [95% CI 1.30 to 1.51]) compared with rural residents, those a wealth index of rich (aOR 1.28 [95% CI 1.17 to 1.40]) compared with those with a wealth index of poor and those in all subregions compared with those in southern Africa were more likely to report an STI.
Table 3.
Predictors of SR-STIs among adolescent girls and young women in SSA
| Variables | Model 0 | Model 1, aOR (95% CI) | Model 2, aOR (95% CI) | Model 3, aOR (95% CI) |
|---|---|---|---|---|
| Age in 5-y groups | ||||
| 15–19 | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| 20–24 | 1.40*** (1.31 to 1.50) | 1.36*** (1.27 to 1.46) | ||
| Highest education level | ||||
| None | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Primary | 0.87** (0.79 to 0.96) | 0.86** (0.78 to 0.94) | ||
| Secondary/higher | 0.98 (0.89 to 1.07) | 0.83*** (0.75 to 0.92) | ||
| Current marital status | ||||
| Not married | 1.75*** (1.60 to 1.90) | 1.64*** (1.51 to 1.79) | ||
| Married | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Respondents occupation | ||||
| Not working | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Working | 1.19*** (1.11 to 1.26) | 1.20*** (1.12 to 1.27) | ||
| Age at first sex (years) | ||||
| ≤19 | 2.01*** (1.82 to 2.22) | 1.99*** (1.80 to 2.20) | ||
| ≥20 | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Number of sex partners, excluding spouse, in the last 12 months | ||||
| 0 | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| 1 | 0.97 (0.89 to 1.05) | 0.91* (0.84 to 0.99) | ||
| ≥2 | 1.71*** (1.48 to 1.96) | 1.56*** (1.35 to 1.80) | ||
| Comprehensive HIV knowledge | ||||
| No | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Yes | 1.07* (1.00 to 1.14) | 1.06 (0.99 to 1.13) | ||
| Read newspaper or magazine | ||||
| No | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Yes | 0.97 (0.90 to 1.05) | 0.99 (0.92 to 1.07) | ||
| Listen to radio | ||||
| No | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Yes | 1.18*** (1.10 to 1.26) | 1.26*** (1.17 to 1.35) | ||
| Watch television | ||||
| No | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Yes | 1.12*** (1.05 to 1.20) | 0.90** (0.84 to 0.98) | ||
| Covered by health insurance | ||||
| No | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Yes | 1.05 (0.94 to 1.19) | 1.10 (0.97 to 1.23) | ||
| Residence | ||||
| Urban | 1.54*** (1.42 to 1.66) | 1.42*** (1.30 to 1.54) | ||
| Rural | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Wealth index | ||||
| Poor | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Middle | 1.02 (0.92 to 1.11) | 1.00 (0.91 to 1.10) | ||
| Rich | 1.27*** (1.17 to 1.39) | 1.28*** (1.17 to 1.40) | ||
| Geographical subregion | ||||
| Southern Africa | 1 (1.00 to 1.00) | 1 (1.00 to 1.00) | ||
| Western Africa | 3.07*** (2.32 to 4.05) | 2.37*** (1.79 to 3.14) | ||
| Central Africa | 4.74*** (3.59 to 6.26) | 3.48*** (2.62 to 4.61) | ||
| Eastern Africa | 3.51*** (2.66 to 4.62) | 2.41*** (1.82 to 3.20) | ||
| Random effects model | ||||
| PSU variance (95% CI) | 0.27 (0.23 to 0.33) | 0.26 (0.21 to 0.31) | 0.27 (0.22 to 0.32) | 0.26(0.21 to 0.31) |
| ICC | 0.0765 | 0.0727 | 0.0751 | 0.0723 |
| Wald χ2 | Ref | 861.60*** | 581.52*** | 1168.69*** |
| Model fitness | ||||
| log-likelihood | −17 047.5 | −16 948.16 | −16 731.03 | −16 402.99 |
| AIC | 34 098.98 | 33 197.06 | 33 478.06 | 32 847.97 |
| BIC | 34 117.25 | 33 334.07 | 33 551.13 | 33 039.78 |
| Number of clusters | 1479 | 1479 | 1479 | 1479 |
Exponentiated coefficients.
*p<0.05, **p<0.01, ***p<0.001.
Ref: reference category; PSU: primary sampling unit; ICC: intraclass correlation; BIC: Bayesian information criterion.
Discussion
This study examined the prevalence and factors associated with SR-STIs among adolescent girls and young women in SSA. We found that overall there was a relatively low prevalence of SR-STIs (6.92%) among adolescent girls and young women from the 27 sub-Saharan African countries included in the study. Nevertheless, this prevalence is higher than that found among men (3.8%) in a related study by Seidu et al.15 Women are known to have better health-seeking behaviours compared with men, which may explain the high prevalence of SR-STIs among adolescent girls and young women in this study.26 The SR-STI prevalence in this study implies that there is much to be done in SSA to attain the global health strategy for STIs, which envisions a world where everybody has free and easy access to STI prevention and treatment services.27
Even though the overall prevalence across the countries in this study was low, we found intercountry variations in the prevalence of SR-STIs, with adolescent girls and young women from Mali reporting the highest prevalence while those from Ghana reported the lowest prevalence of SR-STIs. The results are substantiated by recent findings by Smolak,28 who also reported a higher prevalence of STIs and HIV among Malian women. This could possibly be explained by the pervasiveness of some sociocultural practices, such as female genital mutilation in Mali, which has been found to be linked with an increased likelihood of engaging in multiple sexual partnerships, a factor that increases the risk of contracting STIs.29 As reported in Ghana, good access to sexual and reproductive health services could account for the low prevalence of SR-STIs among Ghanaian women.30 Other possible reasons for the wide difference in the prevalence of SR-STIs in Ghana and Mali could be country-level variations in the distribution of factors associated with SR-STIs, such as age and socio-economic status, which were found to have significant associations with SR-STIs in our study.
We recorded a statistically significant association between age and SR-STIs among adolescent girls and young women in SSA. Specifically, women ages 20–24 years were more likely to report STIs compared with girls of younger ages. This is in agreement with previous studies that found women ages 20–24 y have a higher risk of STIs.31 A plausible explanation for this result may be found in the study by Sathiyasusuman,31 which revealed that women ages 20–24 y are more likely to engage in multiple sexual partnerships, predisposing them to a higher likelihood of contracting an STI. Multiple sexual partnerships, whether serial or concurrent, have been found to be significantly associated with the risk of STIs among women.32 It is therefore not surprising that women with two or more sexual partners had a higher risk of reporting STIs.
Available evidence suggests that being married is a protective factor against STIs among adolescent girls and young women.33–35 Our findings confirm this. We found that women who are married are less likely to report STIs compared with those who are not married. Perhaps this could be attributed to the premium that Africans place on marriage. A married person is supposed to live up to societal and cultural expectations,36 and in the case of women, they are expected to be humble and not engage in acts of infidelity. Therefore it is not expected that married adolescent girls and young women would engage in multiple sexual partnerships. Another plausible explanation for the lower likelihood of SR-STIs among married adolescent girls and young women could be that in many countries in SSA, like Nigeria37 and Ghana,38 the phenomenon of compulsory premarital STI testing, including HIV/AIDS testing, has become common. Thus married adolescent girls and young women know their status before marriage. Therefore it is expected that the odds of SR-STIs would be low among married adolescent girls and young women. However, our findings are not in agreement with previous findings that married individuals have higher likelihoods of reporting STIs since they are more likely to report non-use of condoms.39
Our findings also suggest that adolescent girls and young women who reside in rural areas are less likely to report STIs. Living in an urban area comes with a host of concomitant risks. The anonymity of being a migrant increases one's likelihood of engaging in risky sexual behaviours, including commercial sex work and multiple sexual partners.40 For instance, reports from Ghana indicate that in rural areas, women are less exposed to risky sexual behaviours that contribute to STIs; however, when they migrate to urban areas, they work as head porters, known as kayayei in Ghana, sleeping in the open and becoming targets of rapists who have unprotected sex with them.41 Also, adolescent girls and young women living in rural areas are marginalized and disempowered and face geographical barriers in terms of accessing STI knowledge and services, including testing,42,43 thus explaining the low odds of SR-STIs among rural adolescent girls and young women in this study.
Access to the media (i.e. radio) was found to be significantly associated with the likelihood of reporting STIs among adolescent girls and young women in SSA. This study provided evidence showing that having greater access to the media increased the odds of reporting STIs. The result is in line with evidence from Nigeria44 showing that the odds of reporting an STI is positively associated with the frequency of access to the media. This could be explained from the perspective that often these media platforms show health promotional messages that encourage people to go for testing and by so doing many adolescent girls and young women become aware of their STI status.45 Another reason for this finding could be that some newspapers carry sexually explicit content that could entice adolescent girls and young women to engage in risky sexual behaviours, which increases their chances of reporting STIs.15,44 This supports the finding in the present study that adolescent girls and young women in rich households had a higher risk of reporting STIs. A wealth index of rich implies that they can afford access to mass media that has become a conduit through which STI screening and testing are promoted.
Strengths and limitations
We used data from the recent DHS, which employed robust methodologies in its data collection and has been validated by several studies. Also, the use of nationally representative data ensures that our findings are generalizable to adolescent girls and young women in SSA. Nonetheless, the study had some limitations. The use of secondary data limited our analysis to variables that were present in the datasets. Therefore important variables such as the effect of culture and patriarchal norms that may be associated with the risk of reporting STIs among women could not be assessed. Also, due to the cross-sectional design employed by the DHS, causal inferences cannot be made from the findings. Moreover, the outcome variable was based on self-reports, which are prone to recall bias. Therefore readers are cautioned to interpret the findings taking these limitations into account. Finally, the large sample size may affect conclusions on associations since any small association could be found to be statistically significant. However, this was addressed to some extent by using a multilevel analysis.
Conclusions
Our study found a relatively low prevalence of SR-STIs among adolescent girls and young women in SSA. Despite the low prevalence, much effort will be needed to increase the ability of SSA countries to attain the global health strategy for STIs prevention and treatment. Our findings demonstrate the urgent need for countries within SSA to commit to further reductions in the prevalence of STIs. In particular, countries like Mali, which recorded a high prevalence of STIs, should make conscious efforts to target priority populations that are at higher risk of STIs (i.e. younger women, those in the urban areas, those with a wealth index of rich and those engaged in multiple sexual partnerships). From our findings, we conclude that the media plays a critical role in promoting SR-STIs. Therefore STI education through the media should be strengthened in order for adolescent girls and young women to seek STI testing. Public education on the effects of multiple sexual partnership should also be increased across the region. Future studies should explore the effects of age and socio-economic status on the awareness of STI symptoms.
Contributor Information
Louis Kobina Dadzie, Department of Population and Health, University of Cape Coast, Cape Coast, Ghana.
Ebenezer Agbaglo, Department of English, University of Cape Coast, Cape Coast, Ghana.
Joshua Okyere, Department of Population and Health, University of Cape Coast, Cape Coast, Ghana.
Richard Gyan Aboagye, School of Public Health, University of Health and Allied Sciences, Ho, Ghana.
Francis Arthur-Holmes, Department of Sociology and Social Policy, Lingnan University, 8 Castle Peak Road, Tuen Mun, Hong Kong.
Abdul-Aziz Seidu, Department of Estate Management, Takoradi Technical University, Takoradi, Ghana; Centre for Gender and Advocacy, Takoradi Technical University, Takoradi, Ghana; College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, QLD, Australia.
Bright Opoku Ahinkorah, School of Public Health, Faculty of Health, University of Technology Sydney, Sydney, NSW, Australia.
Authors’ contributions
LKD, BOA and AS conceived and designed the study. LKD, RGA and BOA analysed and interpreted the data. LKD, EA, JO, RGA, FA, AS and BOA implemented the study, contributed to draft writing and read and approved the final version.
Acknowledgements
None.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Data availability
References
- 1. World Health Organization . Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organization; 2001. [Google Scholar]
- 2. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson DA, Chen MY. Emerging and reemerging sexually transmitted infections. N Engl J Med. 2020;382(21):2023–32. [DOI] [PubMed] [Google Scholar]
- 4. Scott-Sheldon LA, Chan PA.. Increasing sexually transmitted infections in the US: a call for action for research, clinical, and public health practice. Arch Sex Behav. 2020;49(1):13–7. [DOI] [PubMed] [Google Scholar]
- 5. Grant JS, Stafylis C, Celum Cet al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis. 2020;70(6):1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Sexually transmitted infections. Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)#:∼:text=Key%20facts,trichomoniasis%20(1%2C%202) [accessed 19 March 2021]. [Google Scholar]
- 7. Wiyeh AB, Mome RK, Mahasha PWet al. Effectiveness of the female condom in preventing HIV and sexually transmitted infections: a systematic review and meta-analysis. BMC Public Health. 2020;20:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torrone EA, Morrison CS, Chen PLet al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 2018;15(2):e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalichman SC, Simbayi LC.. Sexual assault history and risks for sexually transmitted infections among women in an African township in Cape Town, South Africa. AIDS Care. 2004;16(6):681–9. [DOI] [PubMed] [Google Scholar]
- 10. Unemo M, Bradshaw CS, Hocking JSet al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235–79. [DOI] [PubMed] [Google Scholar]
- 11. Dagnew GW, Asresie MB, Fekadu GA.. Factors associated with sexually transmitted infections among sexually active men in Ethiopia. Further analysis of 2016 Ethiopian demographic and health survey data. PLoS One. 2020;15(5):e0232793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chesson HW, Mayaud P, Aral SO.. Sexually transmitted infections: impact and cost-effectiveness of prevention. In: Holmes KK, Bertozzi S, Bloom BRet al. (eds.). Disease control priorities. Washington, DC: World Bank Publications; 2017:203–32. [PubMed] [Google Scholar]
- 13. Rowley J, Vander Hoorn S, Korenromp Eet al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Org. 2019;97(8):548–62P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Report on global sexually transmitted infection surveillance. Available from: https://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691-eng.pdf [accessed 19 March 2021]. [Google Scholar]
- 15. Seidu AA, Ahinkorah BO, Dadzie LKet al. A multi-country cross-sectional study of self-reported sexually transmitted infections among sexually active men in sub-Saharan Africa. BMC Public Health. 2020;20:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman DR, Rahman MM, Brantley Aet al. Rates of new human immunodeficiency virus (HIV) diagnoses after reported sexually transmitted infection in women in Louisiana, 2000–2015: implications for HIV prevention. Clin Infect Dis. 2020;70(6):1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karim QA, Baxter C, Birx D.. Prevention of HIV in adolescent girls and young women: key to an AIDS-free generation. J Acquir Immune Defic Syndr. 2017;75(Suppl 1):S17–26. [DOI] [PubMed] [Google Scholar]
- 18. Dehne KL, Riedner G.. Sexually transmitted infections among adolescents: the need for adequate health services. Reprod Health Matters. 2001;9(17):170–83. [DOI] [PubMed] [Google Scholar]
- 19. Aggarwal P, Bhattar S, Sahani SKet al. Sexually transmitted infections and HIV in self reporting men who have sex with men: a two-year study from India. J Infect Public Health. 2016;9(5):564–70. [DOI] [PubMed] [Google Scholar]
- 20. Abdul R, Gerritsen AA, Mwangome Met al. Prevalence of self-reported symptoms of sexually transmitted infections, knowledge and sexual behaviour among youth in semi-rural Tanzania in the period of adolescent friendly health services strategy implementation. BMC Infect Dis. 2018;18:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones J, Sanchez TH, Dominguez Ket al. Sexually transmitted infection screening, prevalence and incidence among South African men and transgender women who have sex with men enrolled in a combination HIV prevention cohort study: the Sibanye Methods for Prevention Packages Programme (MP3) project. J Int AIDS Soc. 2020;23:e25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mmbaga EJ, Moen K, Makyao Net al. HIV and STIs among men who have sex with men in Dodoma municipality, Tanzania: a cross-sectional study. Sex Transm Infect. 2017;93(5):314–9. [DOI] [PubMed] [Google Scholar]
- 23. Wandera SO, Golaz V, Kwagala Bet al. Factors associated with self reported ill health among older Ugandans: a cross sectional study. Arch Gerontol Geriatr. 2015;61(2):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corsi DJ, Neuman M, Finlay JEet al. Demographic and health surveys: a profile. Int J Epidemiol. 2012;41(6):1602–13. [DOI] [PubMed] [Google Scholar]
- 25. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–8. [DOI] [PubMed] [Google Scholar]
- 26. Thompson AE, Anisimowicz Y, Miedema Bet al. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization . Global health sector strategy on HIV 2016–2021. Towards ending AIDS. WHO/HIV/2016.05. Geneva: World Health Organization; 2017. [Google Scholar]
- 28. Smolak A. Multilevel factors associated with uptake of biomedical HIV prevention strategies in the Muslim world: a study of Central Asia, India, and Mali. PhD dissertation, Columbia University, 2013. [Google Scholar]
- 29. Ahinkorah BO, Hagan JE, Seidu AAet al. Empirical linkages between female genital mutilation and multiple sexual partnership: evidence from the 2018 Mali and 2013 Sierra Leone Demographic and Health Surveys. J Biosoc Sci. 2021;doi: 10.1017/S0021932021000109. [DOI] [PubMed] [Google Scholar]
- 30. Dela H, Attram N, Behene Eet al. Risk factors associated with gonorrhea and chlamydia transmission in selected health facilities in Ghana. BMC Infect Dis. 2019;19:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sathiyasusuman A. Associated risk factors of STIs and multiple sexual relationships among youths in Malawi. PLoS One. 2015;10(8):e0134286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dimbuene ZT, Emina JB, Sankoh O.. UNAIDS ‘multiple sexual partners’ core indicator: promoting sexual networks to reduce potential biases. Global Health Action. 2014;7(1):23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Francis SC, Ao TT, Vanobberghen FMet al. Epidemiology of curable sexually transmitted infections among women at increased risk for HIV in northwestern Tanzania: inadequacy of syndromic management. PLoS One. 2014;9(7):e101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ginindza TG, Stefan CD, Tsoka-Gwegweni JMet al. Prevalence and risk factors associated with sexually transmitted infections (STIs) among women of reproductive age in Swaziland. Infect Agent Cancer. 2017;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nyarko C, Unson C, Koduah Met al. Risk factors of sexually-transmitted infections (STIs) among men and women in a mining community in western Ghana: a study of lifetime occurrence. Int J Sci Technol Res. 2014;3(12):361–70. [Google Scholar]
- 36. Chuick CD. Gender and infidelity: a study of the relationship between conformity to masculine norms and extrarelational involvement. PhD dissertation, University of Iowa, 2009. [Google Scholar]
- 37. Arulogun OS, Adefioye OA.. Attitude towards mandatory pre-marital HIV testing among unmarried youths in Ibadan northwest local government area, Nigeria. Afr J Reprod Health. 2010;14(1):83–94. [PubMed] [Google Scholar]
- 38. Darteh EK, Amo-Adjei J, Awusabo-Asare K.. Correlates of HIV testing among young people in Ghana. J HIV AIDS Soc Serv. 2014;13(3):219–33. [Google Scholar]
- 39. Pinchoff J, Boyer CB, Mutombo Net al. Why don't urban youth in Zambia use condoms? The influence of gender and marriage on non-use of male condoms among young adults. PLoS One. 2017;12(3):e0172062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tiruneh K, Wasie B, Gonzalez H.. Sexual behavior and vulnerability to HIV infection among seasonal migrant laborers in Metema district, northwest Ethiopia: a cross-sectional study. BMC Public Health. 2015;15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wumbee YIF. Empowering returnee migrants: the case of “kayayei” in the Kubori community of the Mamprugu Moaduri district of Ghana. PhD dissertation, University of Development Studies, Tamale, 2018. [Google Scholar]
- 42. Bandali S. HIV risk assessment and risk reduction strategies in the context of prevailing gender norms in rural areas of Cabo Delgado, Mozambique. J Int Assoc Provid AIDS Care 2013;12(1):50–4. [DOI] [PubMed] [Google Scholar]
- 43. Madiba S, Ngwenya N.. Cultural practices, gender inequality and inconsistent condom use increase vulnerability to HIV infection: narratives from married and cohabiting women in rural communities in Mpumalanga province, South Africa. Global Health Action. 2017;10(Suppl 2):1341597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asekun-Olarinmoye OS, Asekun-Olarinmoye EO, Adebimpe WOet al. Effect of mass media and internet on sexual behavior of undergraduates in Osogbo metropolis, southwestern Nigeria. Adolesc Health Med Ther. 2014;5:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Onsomu EO, Moore D, Abuya BAet al. Importance of the media in scaling-up HIV testing in Kenya. SAGE Open. 2013;3(3):2158244013497721. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.