Abstract
We evaluated the functional activities of antibodies, serum bactericidal activity (SBA), and immunoglobulin G (IgG) antibody avidity indices, using sodium thiocyanate (NaSCN) elution, elicited after vaccination with fractional doses of the Haemophilus influenzae type b conjugate (polyribosylribitol phosphate [PRP] conjugated to tetanus toxoid [PRP-T]) vaccine. A cohort of 600 infants from the Dominican Republic were randomized to receive one of three regimens of the PRP-T vaccine at ages 2, 4, and 6 months: full doses (10 μg of PRP antigen), one-half doses (5.0 μg), and one-third doses (3.3 μg) (J. Fernandez et al., Am. J. Trop. Med. Hyg. 62:485–490, 2000). Sixty serum samples, collected at age 7 months, with ≥2.0 μg of anti-PRP IgG per ml were randomly selected for avidity determinations. Geometric mean IgG concentrations were 13, 14, and 17 μg/ml for infants who received the full-dose (n = 19), one-half-dose (n = 19), and one-third-dose (n = 22) regimens, respectively. SBA geometric mean titers (1/dilution) were 85.0, 82.0, and 76.1 in sera from infants receiving the full-, one-half-, and one-third-dose regimens, respectively. Avidity indices (mean ± standard error weighted average of NaSCN molar concentration × serum dilution factor) were 71.9 ± 9.4, 123.6 ± 26.8, and 150.9 ± 24.9 for the full-, one-half-, and one-third-dose regimens, respectively. Upon comparison, the only significant difference (P = 0.024) found was a greater avidity index for sera from infants receiving the one-third-dose regimen than for sera from infants receiving the the full-dose regimen. We conclude that fractional doses elicit similar functional antibody activities in infants with ≥2 μg of anti-PRP IgG per ml, corresponding to 89, 90, and 97% of infants receiving three doses of either the full concentration or one-half or one-third of the labeled concentration, respectively. This approach offers an alternative strategy for the prevention of H. influenzae type b disease in countries with limited resources.
In the United States, there has been remarkable progress toward the elimination of Haemophilus influenzae type b (Hib) disease since the introduction of the Hib conjugate vaccines (2, 3). However, Hib remains one of the leading causes of bacterial pneumonia and meningitis worldwide (17). Hib disease accounts for up to 500,000 deaths around the world among children less than 5 years of age (12). Although an effective conjugate vaccine is available (10, 16), worldwide vaccine coverage is hampered by two major obstacles: local perceptions of disease burden and vaccine cost (7, 13, 18). One approach to reduce the cost of vaccination is the use of fractional doses of the existing vaccines, that is, to vaccinate more than one child with a single-dose vial.
Protection from Hib disease is correlated with the presence of antibodies to the capsular polysaccharide polyribosylribitol phosphate (PRP), and minimal levels of protection of 0.15 μg of anti-PRP antibody per ml for short-term protection and 1 μg/ml for long-term protection have been established (5, 8, 21). Previous studies have shown that the use of fractional doses can elicit long-term protective antibody concentrations in the majority of the study population (4, 11, 15). We reported that a one-half-dose or a one-third-dose regimen (given at 2, 4, and 6 months of age) elicits similar concentrations of immunoglobulin G (IgG) antibodies as a full-dose regimen of the Hib PRP conjugated to tetanus toxoid (PRP-T conjugate vaccine) in infants from the Dominican Republic (4). However, it remains unclear whether the functional abilities of the antibodies elicited by fractional-dose regimens would be equivalent to those elicited by full-dose regimens. Antibody avidity determinations have been used as indicators of the killing potential of sera and the induction of a memory response (1, 6). The present study evaluates the functional activities of antibodies, serum bactericidal activities (SBAs), and IgG antibody avidity indices, using sodium thiocyanate (NaSCN) elution, elicited by fractional doses of the Hib conjugate (PRP-T) vaccine. This fractional-dose approach offers alternative strategies for the prevention of Hib disease in countries with limited resources.
MATERIALS AND METHODS
Study design.
The study group was selected from a cohort of 600 infants participating in an immunogenicity study of fractional doses of the Hib conjugate (PRP-T) vaccine (4). In this cohort, children were randomized to receive one of three regimens of PRP-T vaccine (Act Hib; produced by Pasteur Mérieux Connaught, Lyon, France) at ages 2, 4 and 6 months: full doses (10 μg of PRP antigen), one-half doses (5.5 μg), and one-third doses (3.3 μg). Blood specimens were obtained by venipuncture at ages 4, 6, and 7 months. Informed consent was obtained from all parents or guardians. For this analysis, serum specimens collected at age 7 months were analyzed for functional antibody activity. Sixty infants with anti-PRP IgG concentrations ≥2.0 μg/ml were randomly selected from among 241 infants receiving the PRP-T vaccine in separate arms from the whole-cell diphtheria-tetanus-pertussis toxoid vaccine (Pasteur Mérieux Connaught). This random selection resulted in the use of 19 of 85 serum specimens from infants receiving the full-dose regimen, 19 of 80 serum specimens from infants receiving the one-half-dose dose regimen, and 22 of 76 serum specimens from infants receiving the one-third-dose regimen. A concentration of at least 2.0 μg of anti-PRP IgG per ml was chosen since a full range of optical densities (4.0 to 0.03 at 420 nm) per serum sample was needed to accurately assess antibody avidity at the midpoint of the linear range. The percentage of infant sera with ≥2 μg of anti-PRP IgG per ml was 89% for the full-dose regimen, 90% for the one-half-dose regimen, and 97% for the one-third-dose regimen. Among the 500 infants completing the original study, 94% had anti-PRP IgG concentrations ≥1.0 μg/ml after a complete three-dose regimen. The distribution of IgG responses with concentrations ≥1.0 μg/ml was similar for all dose regimens when the Hib PRP-T vaccine was given in separate sites (94% for the full-dose regimen, 96% for the one-half-dose regimen, and 97% for the one-third-dose regimen) (4).
IgG antibody concentrations.
IgG antibody concentrations were determined by a previously described modification (4) of the enzyme-linked immunosorbent assay (ELISA) published by Madore et al. (14). The standard curve was generated by using reference serum lot 1983 (provided by Carl Frasch, Center for Biological Evaluation and Review, Food and Drug Administration, Bethesda, Md.) with a calculated IgG antibody concentration of 60.9 μg/ml. A quality control preparation (bacterial polysaccharide immune globulin [BPIG]) was used in each plate at a 1:20,000 dilution (24). The minimum level of detection of this assay was estimated to be 0.12 μg/ml at a 1:50 dilution of test serum and 0.06 μg/ml at a 1:25 dilution of test serum.
SBA.
Functional antibodies that can efficiently bind to the PRP capsule of Hib and fix complement onto the bacterial surface were measured by an assay for SBA. Infant sera were stored frozen at −70°C, and a 100-μl aliquot was heat inactivated at 56°C prior to performance of the assay for SBA. We followed the SBA method previously described by Schlesinger et al. (22). Human complement was not used, as a suitable donor could not be found among 20 donors screened. Healthy adults commonly have anti-PRP antibodies in circulation. Therefore, the selection of a suitable complement donor was quite difficult, especially when complement was added to the reaction mixture at high concentrations (25%). The alternative complement source used was sterile serum from 3- to 4-week-old baby rabbits (Pel-Freez, Brown Deer, Wis.), which has been shown to be an efficient source of complement for the measurement of SBA in human serum (23). SBA titers were defined as the reciprocal of the serum dilution that resulted in 50% killing of the initial inoculum compared to that achieved with the complement controls (22). Sandoglobulin (purified IgG; Sandoz Pharmaceuticals Co., East Hanover, N.J.) at a concentration of 6% was used as the quality control preparation in each microtiter plate. An SBA titer of 128 ± 1 dilution was consistently obtained with this preparation when bacterial target strain Hib DR 458 was used. This strain was selected from a panel of 10 strains isolated from the same study population that was part of an ongoing nasopharyngeal colonization study. The SBA titers obtained with these strains (see Table 1) for three reference quality control preparations (BPIG, Sandoglobulin, and the meningococcal pneumococcal reference [MPR] quality control serum [Centers for Disease Control and Prevention, Atlanta, Ga.]) were compared with the titers obtained with a reference strain (strain GB 3291) and a mutant unencapsulated strain (strain Eagan S-2).
TABLE 1.
Hib strains used in this study and their corresponding SBA titers obtained with quality control preparations
| Hib strain designation | SBA titer (50% killing) by antibody source
|
||
|---|---|---|---|
| Sandoglobulin | MPR | BPIG | |
| GB 3291a | 128 | 1,024 | 16,384 |
| DR 247 | 128 | 2,048 | 32,768 |
| DR 458b | 128 | 2,048 | 32,768 |
| DR 519 | 512 | 16,384 | 65,536 |
| DR 671 | 128 | 4,096 | 32,768 |
| DR 715 | 256 | 4,096 | 32,768 |
| DR 729 | 128 | 4,096 | 32,768 |
| DR 761 | 128 | 512 | 16,384 |
| DR 810 | 128 | 2,048 | 32,768 |
| Eagan S-2 mutantc | No titer | No titer | No titer |
Reference Hib strain (Centers for Disease Control and Prevention) used in the generation of Hib antiserum.
Hib strain from the Dominican Republic used in this study for the determination of SBA titers.
Unencapsulated mutant strain.
IgG antibody avidity indices.
Functional antibodies capable of binding to the PRP substrate even after elution treatment with NaSCN were measured by an ELISA, as described by Goldblatt et al. (6), with minor modifications. Briefly, the ELISA IgG antibody titer curves for each serum specimen were analyzed by a four-parameter logistic curve-fitting technique (19), and a serum dilution that yielded an optical density of 2.0 at 420 nm was calculated for the avidity assays. Diluted sera were allowed to incubate with the PRP antigen (coating concentration, 1 μg/ml) for 1 h. After the unbound antibodies were washed, an elution treatment was performed with NaSCN solutions at various concentrations (0 to 4 M) for 15 min. After removing both the unbound antibodies and the chaotropic agent by washing, detection of the remaining bound IgG antibodies was done in a manner similar to that for the ELISA described above. The bacterial polysaccharide immune globulin was also used as a quality control preparation at a dilution of 1:20,000. Avidity indices were calculated as the mean ± standard error weighted average of the NaSCN molar concentration × serum dilution factor.
Statistical analysis.
IgG antibody concentrations and SBA titers were log2 transformed, and the geometric mean concentrations (GMCs) and the geometric mean titers (GMTs) were calculated. Since serum dilutions for SBA determinations were made in a twofold scheme, we applied logarithmic transformations in base 2 to decrease the effect caused by data at the extremes of the distribution curve. Weighted averages of NaSCN concentrations were calculated as described previously (20). Pearson's product moment correlation coefficient was used for comparisons among groups. Significant differences among study groups were assessed by using Student's t test for data normally distributed or the Mann-Whitney rank sum test for data not normally distributed. Comparisons between paired data were done by Fisher's two-tailed exact test. Levels of significance were set at a P value of <0.05.
RESULTS
Bacterial strains.
The Hib strains used in this study and the corresponding SBA titers obtained with quality control preparations are given in Table 1. For most strains tested, the SBA titers were the same or differed by 1 dilution for the three control preparations used. SBA titers were the same or 1 dilution apart from those obtained with reference Hib strain GB 3291. Strain DR 519 was the only strain for which there was more than a 1 dilution difference in titer. In addition, the rate of killing by complement alone was 30 to 40% for this strain. SBA titers could not be determined with the unencapsulated mutant strain, as no PRP is present on this mutant and the bacterial strain was susceptible to killing (100%) by complement. Among those strains with low levels of variability in titer, strain DR 458 was randomly selected to be the target strain for the study after reproducible titers were obtained for at least five consecutive assays.
IgG antibody concentrations.
The ELISA GMCs of IgG were 13, 14, and 17 μg/ml for infants receiving the full-, one-half-, and one-third-dose regimens at age 7 months, respectively (Table 2). Although there was a slight increase in the IgG antibody GMCs as the dose concentration was reduced, there were no significant differences (P ≥ 0.21) in the IgG antibody concentrations between regimens. In addition, these concentrations were very similar to the concentrations obtained for the entire cohort (4).
TABLE 2.
Functional antibody activity and IgG antibody concentrations in infant sera following administration of full or fractional-dose regimens of the Hib PRP-T conjugate vaccine
| Regimen | SBA GMTa | Avidity index (mean ± SE)b | ELISA IgG GMC (μg/ml) |
|---|---|---|---|
| Full dose (n = 19)c | 85.0 | 71.9 ± 9.4 | 13.0 |
| One-half dose (n = 19) | 82.0 | 123.6 ± 26.8 | 13.7 |
| One-third dose (n = 22) | 76.2 | 150.9 ± 24.9d | 17.0 |
Titers were defined as the reciprocal of the dilution with ≥50% killing of the initial inoculum.
Avidity index of the IgG anti-PRP antibodies in the presence of 0 to 4 M NaSCN. The avidity index was calculated as the weighted average of the NaSCN molar concentration times the dilution factor.
The SBA GMTs for the full-dose and one-half dose regimens were calculated with 18 instead of 19 serum samples. One serum sample in each of these groups exhibited >25% killing in the growth controls; SBA titers could not be determined.
A significant difference (P = 0.024) compared to the full-dose regimen. The Mann-Whitney rank sum test was used for comparison of groups of data. No other significant differences were found.
SBA.
SBA GMTs were 85.0, 82.0, and 76.2 (1/dilution) in sera from infants receiving the full-, one-half-, and one-third-dose regimens, respectively (Table 2). No significant differences (P ≥ 0.20) in SBA titers were found between regimens. SBA titers were not correlated to ELISA IgG antibody concentrations, as SBA does not distinguish between IgG and IgM antibodies and antibodies of the IgM class are very potent activators of complement.
IgG antibody indices.
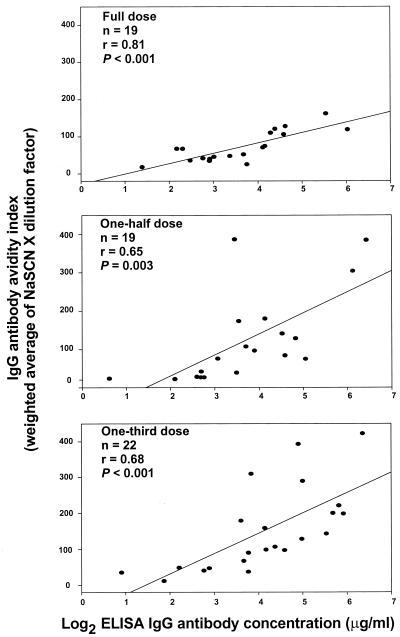
IgG antibody avidity indices were 71.9 ± 9.4, 123.6 ± 26.8, and 150.9 ± 24.9 for the full-, one-half-, and one-third-dose regimens, respectively (Table 2). There was a tendency for the IgG antibody indices to be higher as the regimen dose concentration was reduced. In fact, the IgG avidity indices for sera from infants receiving the one-third-dose regimen were significantly greater than those for sera from infants receiving the full-dose regimen (P = 0.024). However, this difference was not observed when the other two regimens were compared. IgG antibody concentrations and antibody avidity indices were highly correlated in all study groups, with the highest level of correlation achieved for the full-dose regimen (r = 0.81, P < 0.001), as shown in Fig. 1.
FIG. 1.
Correlation between log2 ELISA IgG antibody concentration and the corresponding IgG antibody avidity index measured as the weighted average of NaSCN concentration times the dilution factor for all three dose regimens. Pearson's product moment correlation coefficient was used to calculate the r and P values. The lines designate the linear correlations between assays.
Comparative analysis of functional antibodies.
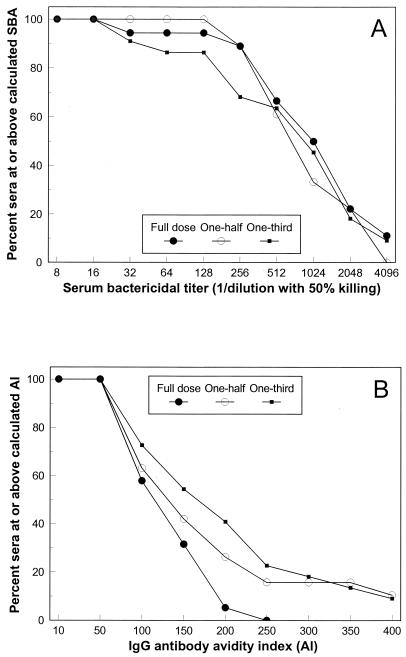
The reverse cumulative distributions of infant sera above given SBA titers or IgG avidity indices following administration of each of the regimens are given in Fig. 2. All infant sera tested had SBA titers ≥16 after administration of the complete full-dose, one-half-dose, or one-third-dose regimen. Although the distribution of SBA titers did not differ among the sera from infants receiving the different regimens, the antibody avidity indices tended to be higher as the regimen dose was decreased. Sera from 16 and 23% of the infants receiving the one-half-dose and the one-third dose regimens attained IgG avidity indices ≥200, respectively, whereas none of the sera from infants receiving the full-dose regimen had avidity indices ≥200. The ≥200 difference in antibody avidity distribution between the full-dose regimen and the one-third-dose regimen was significant (P < 0.05) by Fisher's exact two-tailed test. A limited number of serum samples (n = 7) with concentrations <2 μg/ml were randomly selected from among the serum samples for all dose regimens and were analyzed for antibody avidity and SBA. The GMC of IgG was 0.61 μg/ml, the avidity index was 20.4, and the SBA titer was 6.5. All except one of the serum samples tested had SBA titers below the level of detection of our assay (a titer of 4); the exception was one serum sample that had a titer of 128 and an antibody concentration of 1.4 μg/ml. Additional studies are needed to determine if differences in avidity indices have any clinical significance.
FIG. 2.
Reverse cumulative distribution of infant sera at or above a corresponding SBA titer (A) or at or above a corresponding IgG antibody avidity index (AI) (B).
DISCUSSION
The study of the immunogenicities of fractional doses in the Dominican Republic provided valuable information on the serum IgG concentrations that can be attained by vaccination with fractions of the full dose (4). Although the antibodies elicited reached levels that are considered protective for invasive Hib disease, we questioned whether they lacked the capacity to kill Hib or had the avidity characteristics of those elicited by the full-dose regimen. In the present study, we demonstrated that fractional doses of the Hib PRP-T conjugate vaccine elicit similar functional antibody activities as well as similar IgG antibody titers in infants receiving a three-dose regimen of either full, one-half, or one-third of the labeled concentration. No significant differences in SBA titers or anti-PRP IgG antibody concentrations were found between the regimens. The only significant difference found was a higher IgG avidity index in sera from infants receiving the one-third-dose regimen compared with that in infants receiving the full-dose regimen. In addition to eliciting high IgG concentrations, PRP-T can elicit functional antibodies that are capable of killing strains endogenous to the target population even when used at one-third of the labeled concentration. It is possible that higher avidities were observed for the one-third-dose regimen because of the higher IgG concentrations obtained in this group. The avidity index takes into account the dilution factor of the serum, and therefore, it measures the strength of diluted antibodies. Goldblatt et al. have found that antibody avidities were lower in infants with anti-PRP antibody concentrations <1.0 μg/ml after receipt of a booster dose, indicating the absence of priming when antibody concentrations are low (6).
Recent studies on the use of fractional doses of the Hib vaccine (4, 11) investigated only the antibody concentrations elicited but did not address the functionalities of such antibodies. In the present study we investigated this question, and the results obtained should encourage investigators to conduct immunogenicity studies in the future to determine the minimum quantity of antigen that can elicit protective antibody concentrations and functional antibody activity. It is very likely that vaccination with the conjugate vaccine, which can induce a memory response to the PRP polysaccharide, requires very minimal amounts of antigen to induce an appropriate immune response (9). Recently, Goldblatt et al. proposed the use of antibody avidity determination as an indicator of immune memory following vaccination with the Hib vaccine (6). Other investigators have also found the avidities of the antibodies elicited to be a crucial factor for SBA in immune sera (1). The results of this study suggest that lowering of the concentration of the regimen dose induced higher IgG antibody avidities, with a tendency to attain also higher antibody concentrations. Antibody avidity assays provide an ELISA-based laboratory tool for the assessment of functional antibody activity, which highly correlates with ELISA IgG antibody concentrations.
The findings of this study should be encouraging to countries with limited resources and where Hib vaccine use is often limited because of the high cost of the vaccine. Although this implies the use of off-label concentrations, which have already been approved for use in the infant population, this approach offers alternative strategies for the prevention of Hib disease in countries that normally could not afford the vaccine.
ACKNOWLEDGMENTS
We thank Leslie LaClaire at the Centers for Disease Control and Prevention for providing the isolates of strains used in this study. Our special thanks go to the vaccine research team in Clínica Infantil Robert Reid Cabral, Santo Domingo, Dominican Republic, for work in recruiting and monitoring the participants.
This work was supported by financial assistance from the Children's Vaccine Program, USAID.
REFERENCES
- 1.Amir J, Liang X, Granoff D M. Variability in the functional activity of vaccines induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990;27:358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Carlone G M, Wenger J D, Perkins B A, Maslanka S E, Popovic T. Haemophilus influenzae type B, Neisseria meningitidis, Streptococcus pneumoniae, and Corynebacterium diphtheriae vaccines. In: Rose N R, de Macario E C, Folds J D, Lane H C, Nakamura R M, editors. Manual of clinical laboratory immunology. 5th ed. Washington, D.C.: American Society for Microbiology; 1997. pp. 458–469. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type B disease among infants and children—United States, 1987–1995. Morb Mortal Wkly Rep. 1996;45:901–906. [PubMed] [Google Scholar]
- 4.Fernandez J, Balter S, Feris J, Gomez E, Garib Z, Castellanos P L, Sánchez J, Romero-Steiner S, Levine O S. Randomized trial of the immunogenicity of fractional-dose regimens of PRP-T Haemophilus influenzae type b conjugate vaccine. Am J Trop Med Hyg. 2000;62:485–490. doi: 10.4269/ajtmh.2000.62.485. [DOI] [PubMed] [Google Scholar]
- 5.Fothergill L, Wright J. Influenzal meningitis: relation of age incidence to the bactericidal power of blood against the causal organism. J Immunol. 1933;24:273–284. [Google Scholar]
- 6.Goldblatt D, Pinto Vaz A R J P M, Miller E. Antibody avidity as a surrogate marker of successful priming to Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 7.Gomez E, Moore A, Sánchez J, Kool J, Castellanos P L, Feris J, Kolczak M, Levine O S. The epidemiology of Haemophilus influenzae type b carriage among infants and young children in Santo Domingo, Dominican Republic. Pediatr Infect Dis. 1998;17:782–786. doi: 10.1097/00006454-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Käyhty H, Peltola H, Kankako V, Mäkelä P H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 9.Käyhty H. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals. 1994;22:397–402. doi: 10.1006/biol.1994.1062. [DOI] [PubMed] [Google Scholar]
- 10.Lagos R, Horowitz I, Toro J, San Martin O, Abrego P, Bustamante C, Wasserman S, Levine O S, Levine M M. Large-scale, post licensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: practicality and effectiveness in preventing invasive of Haemophilus influenzae type b infections. Pediatr Infect Dis J. 1996;15:216–222. doi: 10.1097/00006454-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lagos R, Valenzuela M T, Levine O S, Losonsky G A, Erazo A, Wasserman S S, Levine M M. Economizing vaccination against Haemophilus influenzae type b: a randomized trial of the immunogenicity of fractional- and two-dose regimens. Lancet. 1998;351:1472–1476. doi: 10.1016/S0140-6736(97)07456-4. [DOI] [PubMed] [Google Scholar]
- 12.Levine O, Wenger J, Perkins Y B, Rosenstein N, Schuchat A. Haemophilus influenzae type B infection. Bull W H O. 1998;76(Suppl. 2):131–132. [PMC free article] [PubMed] [Google Scholar]
- 13.Levine O S, Lagos R, Muñoz A, Villaroel J, Alvarez A M, Abrego P, Levine M M. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Madore D, Anderson P, Baxter B D, Carlone C M, Edwards K M, Hamilton R G, Holder P, Käyhty H, Phipps D C, Peeters C C A, Schneerson R, Siber G R, Ward J I, Frasch C E. Interlaboratory study evaluating quatitation of antibodies to Haemophilus influenzae type b polysaccharide by enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1996;3:84–88. doi: 10.1128/cdli.3.1.84-88.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelman P M, Feeley L, Bird S, Staub T, Overturf G, Lee A, Ellis J, Staub S, Szymanski S, Donnelly J, Hennessey J P, Kniskern P. Immunogenicity and safety of Haemophilus influenzae type b polysaccharide-Neisseria meningitidis conjugate vaccines in 7.5 micrograms liquid formulation: a comparison of three lots with the 15.0 micrograms lyophilized formulation. Vaccine. 1997;15:775–781. doi: 10.1016/s0264-410x(96)00129-6. [DOI] [PubMed] [Google Scholar]
- 16.Mulholland K, Hilton S, Adegbola R, Usen S, Oparaugo A, Omosigho C, Weber M, Palmer A, Schneider G, Jobe K, Lahai G, Jaffar S, Secka O, Lin K, Ethevenaux C, Freenwood B. Randomized trial of Haemophilus influenzae type b tetanus protein conjugate for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland K, Levine O, Nohynek H, Greenwood B M. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21:43–55. doi: 10.1093/oxfordjournals.epirev.a017987. [DOI] [PubMed] [Google Scholar]
- 18.Peltola H. Spectrum and burden of severe Haemophilus influenzae type b diseases in Asia. Bull W H O. 1999;77:878–879. [PMC free article] [PubMed] [Google Scholar]
- 19.Plikaytis B, Holder P F, Pais L B, Maslanka S E, Gheesling L L, Carlone G M. Determination of parallelism and nonparallelism in bioassay dilution curves. J Clin Microbiol. 1994;32:2441–2447. doi: 10.1128/jcm.32.10.2441-2447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Steiner S, Musher D M, Cetron M S, Pais L B, Groover J E, Fiore A E, Plikaytis B D, Carlone G M. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 21.Santhosham M, Reid R, Ambrosino D, Wolff M C, Ameido-Hill J, Priehs C, Aspery K M, Garrett S, Croll L, Foster S, Burge G, Page P, Zacher B, Moxon R, Siber G R. Prevention of Haemophilus influenzae type b infections in high-risk infants treated with bacterial polysaccharide immune globulin. N Engl J Med. 1987;317:923–929. doi: 10.1056/NEJM198710083171503. [DOI] [PubMed] [Google Scholar]
- 22.Schlesinger Y, Granoff D M the Vaccine Study Group. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 23.Schneerson R, Robbins J B. Induction of serum Haemophilus influenzae type b capsular antibodies in adult volunteers fed cross-reacting Escherichia coli O75:K100:H5. N Engl J Med. 1975;292:1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- 24.Siber G R, Ambrosino D, McIver J, Ervin T J, Schiffman G, Sallan S, Grady G F. Preparation of human hyperimmune globulin to Haemophilus influenzae b, Streptococcus pneumoniae and Neisseria meningitidis. Infect Immun. 1984;45:248–254. doi: 10.1128/iai.45.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]