Abstract
Aims/Introduction
Although mounting evidence has suggested an inverse association between the intake of whole grains and glycemic control, findings from randomized controlled trials are still conflicting. The current study was carried out to evaluate the effect of medium/long‐term whole grain intake on glycemic control in metabolic syndrome and healthy populations.
Materials and Methods
A literature search was carried out to identify qualified studies up to July 2021. The effects of whole grain consumption on glycemic control were calculated using a fixed effects model. Subgroup analysis was used to study whether grouping factors were important influencing factors of heterogeneity between research results.
Results
A total of 32 randomized controlled trials with 2,060 participants were included in the analyses. Whole grain consumption showed a significant inverse regulatory effect on fasting glucose concentration, but no significant effect was found for other glycemic measures, such as fasting insulin, homeostatic model assessment for insulin resistance, glycated hemoglobin and 2‐h glucose, in the pooled analysis. Through subgroup analyses, a significant decrease in fasting glucose concentration was observed for studies with a higher whole grain dose, with participants of normal glycemia, and with mixed types of whole grain.
Conclusions
Medium‐/long‐term whole grain intake reduced the fasting glucose concentration compared with similar refined foods. Appropriate intervention dose and accurate population selection might be the key links for whole grain consumption to exert its glycemic control effect.
Keywords: Glucose control, Insulin sensitivity, Meta‐analysis, Whole grain
Short abstract
Thirty‐two RCTs with 2060 participants were included in analyses. The whole grain consumption showed significant inverse regulation effect on fasting glucose concentration.
INTRODUCTION
Glycemic control, as a basic physiological function of the body, is important to maintain individual health 1 . However, for metabolic disorders, glycemic homeostasis is usually impaired and brings undesired hazards. Diabetes is considered to be a group of metabolic disorders as a result of the lack of insulin secretion, insulin activity, or both 2 . According to epidemiological research data, approximately 422 million people suffer from diabetes worldwide, and this figure has risen sharply 3 . The ensuing complications lead to numerous deaths and a heavy burden on medical systems. Recently, dietary intervention strategies have attracted increasing attention in diabetes treatment, because compared with hypoglycemic drugs, dietary intervention strategies have fewer side‐effects and are more cost‐effective 4 , 5 , 6 .
Among the existing dietary adjustment strategies, increasing intake of whole grains has been widely investigated for glycemic control 7 . A recent meta‐analysis including cohort studies suggested that increased whole grain food intake could reduce the risk of diabetes 8 . Evidence from another observational study also showed an inverse dose–response relationship between whole grain consumption and the incidence rate of type 2 diabetes mellitus 9 . It is worth noting that most studies have emphasized that the observed effect of whole grain intake is an increased sensitivity of insulin rather than a decrease in postprandial glycemia 10 . However, several recent studies suggest that whole grains can directly bring changes to postprandial blood glucose 11 , 12 . Thus, data on whether whole grains improve glucose control and the detailed mechanism of this effect remain conflicting. Although the results of a meta‐analysis have been reported, that study only included populations with specific health statuses and did not set the duration of the whole grain intervention. As a supplementary study with better evidence, the current study was carried out to evaluate the effect of medium‐/long‐term (≥2 weeks) whole grain intake on glycemic control in metabolic syndrome and healthy populations by including randomized controlled studies.
MATERIALS AND METHODS
Search strategy
PubMed (up to July 2021; http://www.ncbi.nlm.nih.gov/pubmed/), Embase (up to July 2021; http://www.embase.com/search/advanced/) and the Cochrane Library (up to July 2021; http://www.cochrane.org/) were searched in all fields with the following terms: wholegrain, whole grain, whole meal, wheat, whole wheat, rice, wild rice, brown rice, oat, maize, rye, barley, corn, millet, triticale, sorghum, amaranth, canary seed, quinoa and buckwheat, paired with the following words: glucose, glycemic control, glycemic, glucose control, insulin sensitivity and insulin. Two researchers independently screened each report, and any discrepancies were resolved by consensus and arbitration by a third investigator.
Study selection
We selected studies that met the following criteria: (i) medium‐/long‐term treatment duration ≥2 weeks; (ii) parallel or cross‐over designed randomized controlled studies with human participants; (iii) availability of end‐point values for blood glucose, fasting insulin, homeostatic model assessment for insulin resistance (HOMA‐IR), glycated hemoglobin (HbA1c) with standard error or standard deviation or 95% confidence intervals (CIs) for the control and intervention groups; (iv) participants received whole grain diet or product intervention; (v) inclusion of an isoenergetic control group with lower levels or no whole grain content; (vi) sole difference between observational groups of intervention of whole grains, and distinguishability of the effect value; (vii) examining lack of examination of the effects of individual grain components; and (viii) no special restrictions on the health status of the participants.
Risk of bias and quality of evidence
We used the Cochrane risk of bias tool to evaluate the quality of the literature. The assessment tool mainly divides bias into the following aspects: selection bias, implementation bias, measurement bias, follow‐up bias, reporting bias and other bias 13 . The evaluation was completed independently by two researchers. Any discrepancies were resolved with the participation of another coauthor. Trials were classified as being at a high risk of bias if one or more items were evaluated with a high bias risk, and at a low risk of bias if all of the items were evaluated with low bias risk, and all other trials were classified as being at unclear risk of bias.
Data extraction
The following study characteristics were extracted: (i) study characteristics, including publication year, author information, study design, treatment duration and sample size; (ii) population characteristics, including age, percentage of female subjects, body mass index (BMI) and participant health status; (iii) baseline and post‐treatment values in fasting glucose, plasma insulin, HOMA‐IR, HbA1c and 2‐h glucose; and (iv) dietary information/foods provided in the intervention group and control group. The study values were converted to unified measuring units for data standardization.
Statistical analysis
We carried out the present meta‐analysis using Stata version/SE 14.2 software (StataCorp, College Station, TX, USA). The postintervention values of anthropometric measures in the control and treatment groups were used to calculate the effect size. The outcomes of treatments were estimated using weighted mean differences (WMDs). The baseline and outcome values are shown in Table S1. The I 2 statistic and P‐value were used to determine the study heterogeneity. The random effects model was chosen if significant heterogeneity was detected, and otherwise, the fixed effects model was chosen.
In the meta‐analysis, we divided the research into subgroups according to the research characteristics of study design, treatment duration, type of whole grain, dose of whole grain, baseline mean BMI, glycemic condition, weight change and risk of bias. The purpose of subgroup analysis was to study whether the effect values were different in different populations or conditions. Funnel plots, Egger's regression test and sensitivity analyses were carried out. P‐values <0.05 were considered statistically significant.
RESULTS
Results of the literature search
The flow chart of the search strategy and study selection is shown in Figure 1. In total, 5,710 articles were initially identified. A total of 5,565 articles were excluded, because they were irrelevant to the present meta‐analysis or were duplicate publications. Of the 145 remaining articles, an additional 113 were excluded for the following reasons: the participants of 12 studies used treatment of individual grain components, 42 studies had an uncontrolled study design, 16 studies had incomplete data, 10 studies had a non‐randomized study design, 22 studies lasted <2 weeks and for 11 studies the data could not be extracted. Thus, 32 articles were finally included in the present meta‐analysis 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 .
Figure 1.
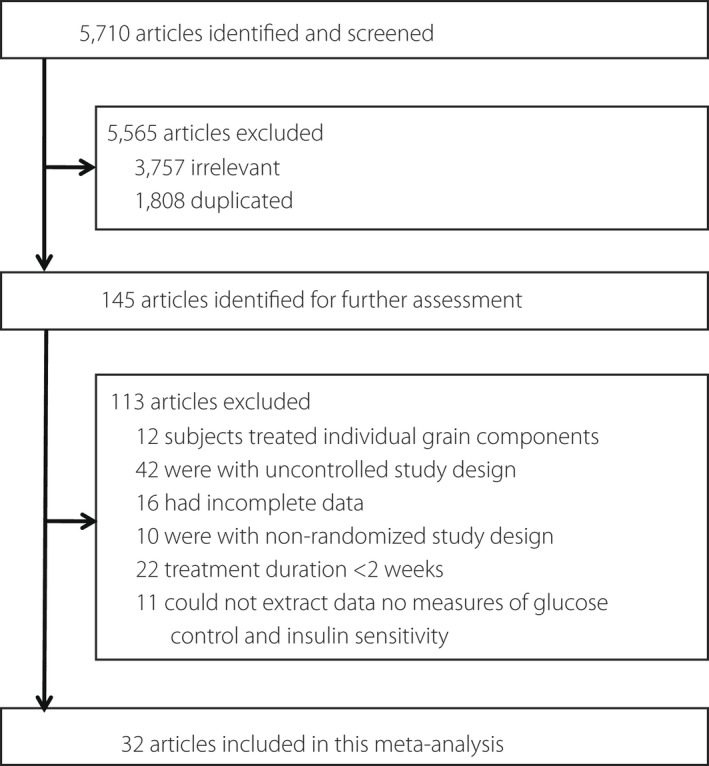
Flow diagram showing the number of citations retrieved in individual searches of articles included in the review.
Study characteristics
A summary of the article characteristics is presented in Table 1. In total, 2,060 participants were included in the 32 included studies (18 parallel design and 14 cross‐over design studies), and the number of participants in each study ranged from 11 to 206. A total of 13 randomized controlled studies were mixed whole grain interventions, and the other 19 trials examined the effects of individual whole grains. The intervention dose ranged from 45 to 301 mg/day (not reported in 8 studies), and the duration of treatment ranged from 3 to 16 weeks (median 8 weeks). According to the baseline mean BMI, 11 studies had obese populations (≥30 kg/m2), and the other 21 studies had non‐obese populations (<30 kg/m2). A total of 25 studies included participants with normal glycemia, whereas the other seven studies included participants with hyperglycemia.
Table 1.
Characteristics of eligible studies
| Author, year, country | Study design | No. participants | Duration (weeks) | Dose (g/day) | Age, years (mean) | BMI | Female (%) | Population | Whole grain group | Control group |
|---|---|---|---|---|---|---|---|---|---|---|
| Ampatzoglou, 2014, UK 14 | R, C | 33 | 6 | >80 | 48.8 | 27.9 | 63.6 | Healthy | Mixed whole grain | Refined grain products |
| Andersson, 2007, Sweden 15 | R, C | 30 | 6 | 112 | 59 | 28.3 | 26.7 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Charlton, 2012, Australia 16 | R, P | 87 | 6 | 137 | 51 | 27.3 | 56.7 | Hypercholesterolemia without diabetes mellitus | Oats | Refined grain products |
| Connolly, 2016, UK 17 | R, C | 15 | 6 | 45.0 | 42 | 26.4 | 63.3 | Risk of developing cardiometabolic disease | Oats | Refined grain products |
| Giacco, 2013, Finland/Italy 18 | R, P | 123 | 12 | 185 | 40–65 | 31.6 | 52.8 | Metabolic syndrome | Mixed whole grain | Refined grain products |
| Giacco, 2014, Finland/Italy 19 | R, P | 54 | 12 | 136 | 57.2 | 31.7 | 57.4 | Metabolic syndrome | Mixed whole grain | Refined grain products |
| Giacco, 2009, Finland/Italy 20 | R, C | 15 | 3 | NR | 54.5 | 27.4 | 20 | Healthy | Wheat | Refined grain products |
| Jackson, 2014, USA 21 | R, P | 48 | 12 | 163–301 | 46.4 | 32.9 | 50 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Karl, 2017, USA 22 | R, P | 80 | 6 | 207 | 54.5 | 25.7 | 39.5 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Katcher, 2008, USA 23 | R, P | 50 | 12 | NR | 46 | 35.5 | 50 | Metabolic syndrome | Mixed whole grain | Refined grain products |
| Kazemzadeh, 2014, Iran 24 | R, C | 35 | 6 | 150 | 32.6 | 29.8 | 100 | Overweight and obesity | Brown rice | Refined grain products |
| Kikuchi, 2019, Japan 25 | R, P | 49 | 12 | 88.4 | 47.7 | 27.4 | 34.7 | Obesity | Wheat | Refined grain products |
| Kirwan, 2016, USA 26 | R, C | 33 | 8 | 93 | 39 | 33.1 | 51.5 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Kondo, 2016, Japan 27 | R, P | 28 | 8 | 150 | 66.7 | 24.6 | 55.6 | Type 2 diabetes | Brown rice | Refined grain products |
| Kristensen, 2012, Denmark 28 | R, P | 72 | 12 | 105 | 59.7 | 30.2 | 100 | Overweight | Wheat | Refined grain products |
| Liatis, 2009, Greece 29 | R, P | 41 | 3 | NR | 63 | 28.5 | 43.9 | Type 2 diabetes | Oats | Refined grain products |
| Liu, 2018, China 30 | R, P | 110 | 5 |
<69 >69 |
58 | 26.5 | 54.5 | Type 2 diabetes | Wheat | Refined grain products |
| Malik, 2019, USA 31 | R, C | 166 | 12 | 127.5 | 37.1 | 28.1 | 45 | Overweight | Brown rice | Refined grain products |
| Malin, 2019, USA 32 | R, C | 13 | 8 | 100 | 37.2 | 33.6 | 76.9 | Overweight and obesity | Mixed whole grain | Refined grain products |
| McIntosh, 2003, Australia 34 | R, C | 28 | 4 | NR | 40–65 | 30 | 0 | Overweight | Wheat Rye | Refined grain products |
| Pereira, 2002, USA 35 | R, C | 11 | 6 | NR | 41.6 | 30.2 | 54.5 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Pick, 1998, Canada 36 | R, C | 11 | 12 | NR | 51 | 27.4 | 0 | Type 2 diabetes | Barley | Refined grain products |
| Pins, 2002, USA 37 | R, P | 88 | 12 | 60 | 48.6 | 30.9 | 48.9 | Hypertension without diabetes mellitus | Oats | Refined grain products |
| Roager, 2017, Denmark 38 | R, C | 50 | 8 | 179 | 20–65 | 29 | 36.0 | Metabolic syndrome | Mixed whole grain | Refined grain products |
| Schutte, 2018, Netherlands 39 | R, P | 50 | 12 | 98 | 61 | 27.8 | 62 | Overweight with mildly elevated levels of plasma total cholesterol | Wheat | Refined grain products |
| Shimabukuro, 2013, Japan 40 | R, C | 27 | 8 | NR | NR | 26·7 | 0 | Obese and metabolic syndrome | Brown rice | Refined grain products |
| Steven, 2017, USA 33 | R, C | 14 | 8 | 90.5 | 38 | 34 | 78.6 | Overweight and obesity | Mixed whole grain | Refined grain products |
| Tighe, 2010, UK 41 | R, P | 206 | 12 | 100–120 | 51.8 | 28 | 50.4 | Overweight | Mixed whole grain wheat | Refined grain products |
| Vitaglione, 2015, Slovenia 42 | R, P | 68 | 8 | 70 | 38.6 | 29.8 | 66.2 | Overweight and obesity | Wheat | Refined grain products |
| Wang, 2013, USA 43 | R, P | 57 | 12 | NR | 52.5 | 25.8 | 66.7 | Prediabetes | Brown rice | Refined grain products |
| Zhang, 2011, China 44 | R, P | 202 | 16 | 100 | 49.7 | 25.7 | 46.5 | Diabetes or high risk for diabetes | Brown rice | Refined grain products |
| Zhang, 2012, China 45 | R, P | 166 | 6 | 100 | 53.2 | 25.5 | 38.6 | Hypercholesterolemia | Oats | Refined grain products |
BMI, body mass index; BP, blood pressure; C, cross‐over; CVD, cardiovascular disease; NR, not reported; P, parallel; R, random; WG, whole grain.
Risk of bias
The risk of bias of the studies included in the present meta‐analysis was rated as moderate or low. Overall, 11 studies had a low risk of bias for random sequence generation, and two had a high risk of bias for allocation concealment. Three studies had a high risk of bias on blinding, and two were at high risk of bias on blinding of outcome assessment. Most articles were at low risk of incomplete outcome data, selective reporting and other biases. Specific details of this part of the work are presented in Table S2.
Effects of whole grain consumption on glycemic control and insulin sensitivity
As shown in Figure 2 and Table S3, a significant reduction in fasting glucose concentration was observed in participants supplemented with whole grain (WMD −0.05 mmol/L, 95% CI −0.10, −0.01 mmol/L) when compared with control participants. In addition, there were 27 studies investigating fasting insulin concentration change after whole grain consumption, but no significant differences were observed (WMD −0.12 mIU/mL, 95% CI −0.52, −0.29 mIU/mL; Figure 3 and Table S3). Furthermore, no positive effect of whole grain on HOMA‐IR, HbA1c or 2‐h glucose was observed by the pooled analysis (Figure S1 and S2, and Table S3). No significant between‐study heterogeneity in the effects of whole grain intake on these measures was observed (Table S3).
Figure 2.
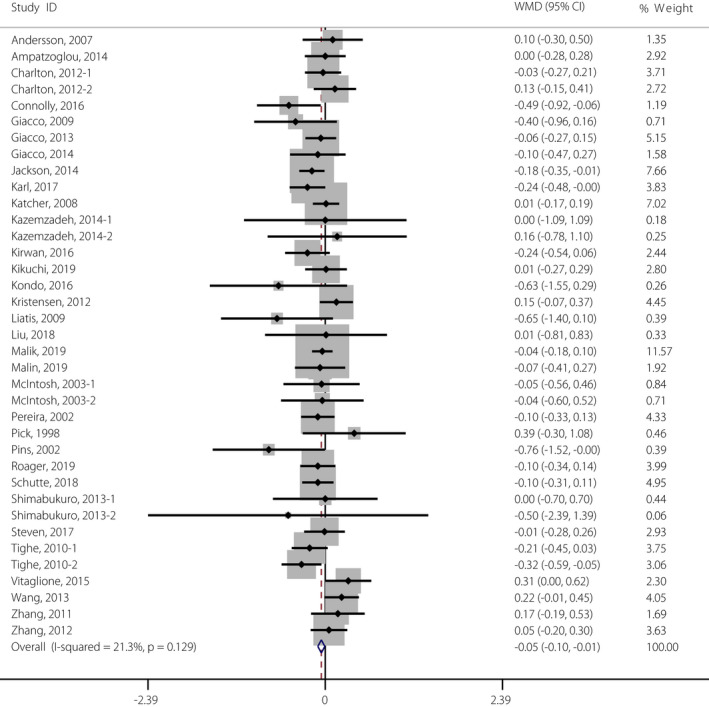
Meta‐analysis of the effects of whole grain on fasting glucose concentration. Weight was assigned with Stata (version 14.2; StataCorp, College Station, TX, USA) by using the number of participants and the standard deviation. Sizes of the data markers show the weight of each study in this analysis. The diamond represents the overall estimated effect and the result was obtained from a fixed effects model. WMD, weighted mean difference. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
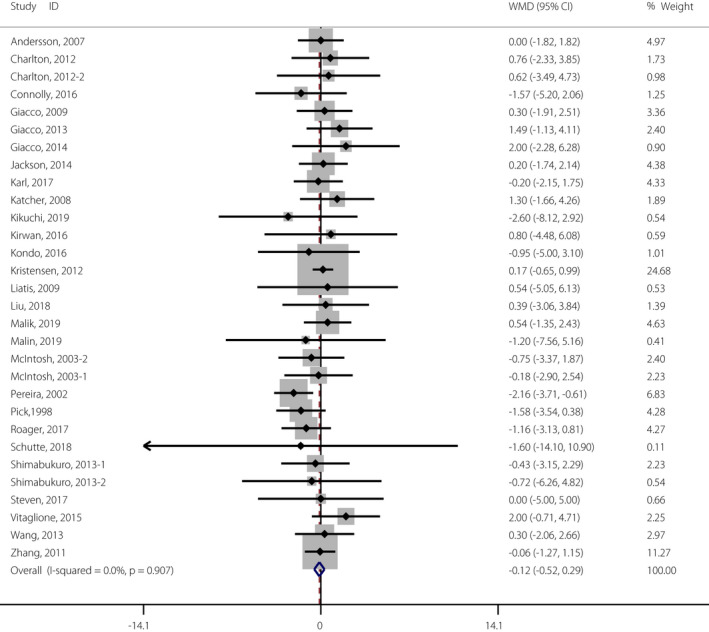
Meta‐analysis of the effects of whole grain on fasting insulin concentration. Weight was assigned with Stata (version 14.2; StataCorp, College Station, TX, USA) by using the number of participants and the standard deviation. Sizes of the data markers show the weight of each study in this analysis. The diamond represents the overall estimated effect, and the result was obtained from a fixed effects model. WMD, weighted mean difference. [Colour figure can be viewed at wileyonlinelibrary.com]
Subgroup analysis was carried out by categorizing treatment duration into shorter‐ (<8 weeks) and longer‐term subgroups (≥8 weeks). The dose of whole grain was divided into a higher dose (≥102.5 g/day) and a lower dose (<102.5 g/day). The baseline mean BMI was categorized as obese (≥30 kg/m2) or not obese (<30 kg/m2; Table 2). A significant reduction in fasting glucose concentration was observed for studies with higher whole grain doses (WMD −0.08 mmol/L, 95% CI −0.15, −0.02 mmol/L), and the subgroup analyses showed that whole grain consumption significantly lowered fasting glucose in mixed types of whole grain (WMD −0.10 mmol/L, 95% CI −0.17, −0.03 mmol/L; Table 2). Additionally, we found a significant reduction in insulin concentration for studies with only a cross‐over design (WMD −0.74 mIU/mL, 95% CI −1.39, −0.08 mIU/mL; Table 2). Sensitivity analysis showed that the comprehensive effect of whole grain consumption on fasting blood glucose and insulin concentration did not change after interpolation with a correlation coefficient of 0.5. Finally, the systematic deletion of each trial during sensitivity analysis did not significantly change the overall effect of whole grain consumption on fasting blood glucose or insulin concentration.
Table 2.
Subgroup analyses of fasting glucose and insulin concentrations stratified by previously defined study characteristics
| Variables | Fasting glucose | Fasting insulin | ||||||
|---|---|---|---|---|---|---|---|---|
| No. trials | Net change (95% CI) (mmol/L) | Test of heterogeneity † | No. trials | Net change (95% CI) (mIU/mL) | Test of heterogeneity † | |||
| P | I 2 (%) | P | I 2 (%) | |||||
| Study design | ||||||||
| Parallel | 18 | −0.07 (−0.10, 0.02) | 0.012 | 46.3 | 15 | 0.28 (−0.24, 0.80) | 0.985 | 0.0 |
| Cross‐over | 14 | −0.07 (−0.15, 0.00) | 0.874 | 0.0 | 12 | −0.74 (−1.39, −0.08) | 0.832 | 0.0 |
| Duration of intervention | ||||||||
| <8 weeks (lower than median) | 19 | −0.06 (−0.14, 0.01) | 0.443 | 1.3 | 16 | −0.45 (−1.08, 0.18) | 0.892 | 0.0 |
| ≥8 weeks (higher than median) | 11 | −0.05 (−0.11, 0.01) | 0.038 | 50.6 | 11 | 0.13 (−0.41, 0.66) | 0.762 | 0.0 |
| Type of whole grain | ||||||||
| Mixed | 13 | −0.10 (−0.17, −0.03) | 0.837 | 0.0 | 11 | −0.38 (−1.10, 0.35) | 0.370 | 7.8 |
| Others | 19 | −0.01 (−0.07, 0.06) | 0.057 | 33.6 | 19 | −0.01 (−0.49, 0.50) | 0.979 | 0 |
| Dose of whole grain | ||||||||
| Higher | 12 | −0.08 (−0.15, −0.02) | 0.293 | 14.3 | 10 | 0.15 (−0.40, 0.70) | 0.936 | 0.0 |
| Lower | 12 | −0.03 (−0.12, 0.06) | 0.117 | 34.1 | 9 | 0.04 (−0.90, 0.99) | 0.857 | 0.0 |
| Baseline mean of BMI | ||||||||
| Obese (≥30 kg/m2) | 11 | −0.07 (−0.14, 0.01) | 0.368 | 8.0 | 10 | −0.09 (−0.68, 0.50) | 0.364 | 8.4 |
| Non‐obese (<30 kg/m2) | 21 | −0.06 (−0.14, 0.02) | 0.055 | 33.3 | 17 | −0.14 (−0.70, 0.42) | 0.959 | 0.0 |
| Weight change | ||||||||
| Yes | 12 | −0.01 (−0.08, 0.07) | 0.254 | 17.8 | 11 | −0.16 (−0.40, 0.72) | 1.00 | 0.0 |
| No | 15 | −0.06 (−0.12, 0.01) | 0.307 | 13.1 | 12 | −0.27 (−0.95, 0.41) | 0.241 | 20.6 |
| Glucose | ||||||||
| Normal glycemia | 25 | −0.05 (−0.10, −0.003) | 0.165 | 20.4 | 21 | −0.03 (−0.49, 0.42) | 0.771 | 0.0 |
| Hyperglycemia | 7 | −0.07 (−0.30, 0.17) | 0.160 | 33.6 | 6 | −0.42 (−1.30, 0.46) | 0.907 | 0.0 |
| Risk of bias | ||||||||
| High | 8 | −0.07 (−0.11, 0.02) | 0.759 | 0.0 | 7 | −0.03 (−0.93, 0.99) | 0.800 | 0.0 |
| Others | 24 | −0.06 (−0.10, 0.11) | 0.049 | 21.3 | 20 | −0.15 (−0.60, 0.30) | 0.907 | 0.0 |
P for heterogeneity was assessed by using Cochran's test, and P < 0.1 was considered to show significant heterogeneity across studies. The I 2 statistic was calculated by using Cochran's test, and I 2 > 50% was considered to show significant heterogeneity across studies.
Publication bias
The funnel plots were symmetrical, and Egger's test showed no significant publication bias in the meta‐analysis of fasting insulin, fasting glucose, HOMA‐IR or HbA1c (Figures S3–S6; Egger's test: P = 0.39, 0.89, 0.53 and 0.06, respectively).
DISCUSSION
The present study suggested that whole grain consumption effectively reduced fasting glucose concentration in metabolic syndrome and healthy populations, but the inverse regulation effect was not observed on other glucose‐related measures, such as fasting insulin consumption, HOMA‐IR, HbA1c or 2‐h glucose level. Furthermore, subgroup analysis showed that fasting blood glucose concentration was significantly decreased in studies with higher whole grain doses, participants with normal glycemic baseline or mixed types of whole grains.
Consistent with the present results, several studies confirmed the regulatory effect of whole grain on blood glucose levels. In a meta‐analysis including 14 trials, Marventano et al. 11 explored the acute effects of whole grain intake on glucose control, and their results showed that whole grain intake significantly reduced the postprandial glucose values by 29.71 mmol min/L. In another 12‐week intervention study, the effects of different dietary carbohydrate sources on blood glucose concentrations were compared, and the blood glucose level in the unrefined carbohydrate group was improved significantly 12 . Of note, there was no significant association between the improvement of glucose‐related measures and whole grain food consumption, suggesting that an effect on insulin sensitivity might be slight or none. This finding is supported by Andersson's research results; in healthy and moderately overweight adults, replacing refined grain products with whole grains did not show a beneficial effect on the sensitivity of insulin or other inflammation markers 15 . The underlying mechanism of the observed effects of whole grain consumption might involve the following aspects: (i) whole grains are a better source of fiber and nutrients, and generally have a lower glycemic index/load than refined grains 46 ; (ii) multiple aspects of habitual consumption of whole grains seem to impact the effects of whole grain consumption, such as the low energy density of whole grains and the fermentation products of indigestible carbohydrates; (iii) in view of the aforementioned characteristics, whole grains directly affect the early postprandial blood glucose response, but do not cause significant changes in late blood glucose levels through hormonal (mainly insulin and glucagon) or metabolic (free fatty acid) mechanisms; and (iv) the intervention time of 6 weeks might be relatively short to have a significant effect on insulin sensitivity 15 .
For the present meta‐analysis, study design, duration of intervention, type of whole grain, dose of whole grain, baseline mean BMI, weight change and basal blood glucose status were potential factors that might influence the results. To evaluate these influences, we carried out detailed subgroup analysis and found only a few positive results, suggesting that the present study results are relatively stable. Subgroup analyses suggested that a higher dose of whole grain intake, but not a lower dose, was associated with improved glycemic control. This implies that the regulatory effect might be dose‐dependent. Chanson et al. 47 reported a significant inverse association between whole grain intake and the occurrence of type 2 diabetes mellitus, with a slope of −0.000293; an overall reduction of 0.3% in the incidence of type 2 diabetes mellitus for each additional 10 g of whole grain ingredients consumed per day was observed. Another study confirmed the non‐linear negative relationship between whole grains and the risk of type 2 diabetes mellitus. When the intake of whole grains increased to 60 g/day, most of the reductions were observed 48 . Although further original research is necessary to discover the underlying mechanisms of the observed non‐linear associations, the present study shows that a proper increase in daily whole grain intake might be beneficial for glucose control.
The present meta‐analysis showed that whole grain consumption significantly reduces fasting glucose in participants with normal basal fasting glucose levels. In contrast, whole grain intake did not significantly affect glucose control in the hyperglycemia subgroup. This phenomenon was supported by two recent meta‐analyses. A meta‐analysis found that the consumption of whole grain foods acutely improved postprandial glucose compared with controls in healthy participants 11 , but the inverse association disappeared when the included participants were prediabetes and type 2 diabetes mellitus patients from the research of Rahim's et al. 49 Furthermore, animal studies also showed that the intake of whole grain has no significant effect on glycemic measures in diabetic rodents. However, the underlying mechanisms remain unclear. A possible explanation is that non‐diabetic participants have normal blood glucose homeostasis, which is more sensitive to the regulatory effect of whole grains than the abnormal blood glucose homeostasis of prediabetes and type 2 diabetes mellitus patients. In contrast, for hyperglycemic patients with impaired function and decreased compensatory capacity, the protective effect from whole grains would not be observed. This potential effect needs to be evaluated through better designed animal experiments or high‐quality randomized controlled studies. The present results might be of great value in guiding the inclusion of populations that are suitable for whole grain intake.
This is the first meta‐analysis to evaluate the effect of medium‐/long‐term whole grain intake on glycemic control in metabolic syndrome and healthy populations. The results further enhance the understanding of the value of whole grain intake on glucose control. The present meta‐analysis also had several limitations. First, the lack of a clear definition of the concept of whole grain food, as well as different properties of dietary whole grain sources, might lead to some bias. A separate survey of designated types of whole grain foods produced by different crops might help to reduce heterogeneity, but due to the insufficient number of original studies, a meta‐analysis cannot be implemented. Second, our work increased the amount of evidence of the hypoglycemic effect from whole grain consumption, but we did not analyze the dose‐dependent relationship, and we were unable to further explain the hypoglycemic mechanisms. Third, the present study systematically focused on the medium‐term effect of whole grain consumption on blood glucose regulation, because most of the study durations were <1 year, and we could not investigate the protective effect of longer duration whole grain intake on health. Fourth, the studies included in the meta‐analysis mainly came from developed countries in Asia, North America and Europe. To better understand the effect of whole grains on human blood glucose control, further research in other regions is still required.
In conclusion, the present results of our meta‐analysis suggested that intake of whole grain foods could reduce the fasting glucose concentration compared with intake of similar refined foods. Appropriate intervention dose and accurate population selection might be the key links for whole grain intake to exert the effect of blood glucose regulation. To explain the underlying mechanism by which whole grain products have a positive impact on glucose regulation, it is necessary to carry out better designed studies on different groups of people using different forms of whole grain foods with different structures.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: This network meta‐analysis was registered at www.crd.york.ac.uk/PROSPERO as CRD42019131128.
Animal studies: N/A.
Supporting information
Table S1 | Baseline and outcome data used in the meta‐analysis.
Table S2 | Judgments of review authors for each risk of bias item according to the Cochrane risk of bias assessment tool.
Table S3 | Pooled effects of whole grain consumption on glucose control and insulin sensitivity.
Figure S1 | Meta‐analysis of the effects of whole grain on homeostatic model assessment for insulin resistance.
Figure S2 | Meta‐analysis of the effects of whole grain on glycated hemoglobin.
Figure S3 | Funnel plots for the studies of the association of whole grain and fasting glucose concentrations.
Figure S4 | Funnel plots for the studies of the association of whole grain and fasting insulin concentrations.
Figure S5 | Funnel plots for the studies of the association of whole grain and homeostatic model assessment for insulin resistance.
Figure S6 | Funnel plots for the studies of the association of whole grain and glycated hemoglobin.
ACKNOWLEDGMENTS
This work was funded by the Research Project Foundation (CEP21J011). The funders had no role in the design and conduct of the study; in the collection, analysis and interpretation of the data; and in the preparation, review or approval of the manuscript.
Contributor Information
Suocheng Hui, Email: Suocheng.Hui@hotmail.com.
Kai Wang, Email: wangkai2005@126.com.
REFERENCES
- 1. Rodriguez‐Gutierrez R, Gonzalez‐Gonzalez JG, Zuniga‐Hernandez JA, et al. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 2019; 367: l5887. [DOI] [PubMed] [Google Scholar]
- 2. Zimmet P, Alberti KG, Magliano DJ, et al. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 2016; 12: 616–622. [DOI] [PubMed] [Google Scholar]
- 3. Ahmad R, Haque M. Oral health messiers: diabetes mellitus relevance. Diabetes Metab Syndr Obes 2021; 14: 3001–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2020; 16: 545–555. [DOI] [PubMed] [Google Scholar]
- 5. Brown TJ, Brainard J, Song F, et al. Omega‐3, omega‐6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta‐analysis of randomised controlled trials. BMJ 2019; 366: l4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med 2020; 383: 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu Y, Ding M, Sampson L, et al. Intake of whole grain foods and risk of type 2 diabetes: results from three prospective cohort studies. BMJ 2020; 370: m2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose‐response meta‐analysis of prospective studies. BMJ 2016; 353: i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker ED, Liu S, Van Horn L, et al. The association of whole grain consumption with incident type 2 diabetes: the Women's Health Initiative observational study. Ann Epidemiol 2013; 23: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seal CJ, Brownlee IA. Whole‐grain foods and chronic disease: evidence from epidemiological and intervention studies. Proc Nutr Soc 2015; 74: 313–319. [DOI] [PubMed] [Google Scholar]
- 11. Marventano S, Vetrani C, Vitale M, et al. Whole grain intake and glycaemic control in healthy subjects: a systematic review and meta‐analysis of randomized controlled trials. Nutrients 2017; 9: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang R, Kim M, Chae JS, et al. Consumption of whole grains and legumes modulates the genetic effect of the APOA5‐1131C variant on changes in triglyceride and apolipoprotein A‐V concentrations in patients with impaired fasting glucose or newly diagnosed type 2 diabetes. Trials 2014; 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu SS, Sun F, Zhan SY. Bias risk assessment: (3) revised Cochrane bias risk assessment tool for individual randomized, cross‐over trials (RoB2.0). Zhonghua Liu Xing Bing Xue Za Zhi 2017; 38: 1436–1440. [DOI] [PubMed] [Google Scholar]
- 14. Ampatzoglou A, Atwal KK, Maidens CM, et al. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low‐habitual whole grain consumers. J Nutr 2015; 145: 215–221. [DOI] [PubMed] [Google Scholar]
- 15. Andersson A, Tengblad S, Karlstrom B, et al. Whole‐grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 2007; 137: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 16. Charlton KE, Tapsell LC, Batterham MJ, et al. Effect of 6 weeks' consumption of beta‐glucan‐rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br J Nutr 2012; 107: 1037–1047. [DOI] [PubMed] [Google Scholar]
- 17. Connolly ML, Tzounis X, Tuohy KM, et al. Hypocholesterolemic and prebiotic effects of a whole‐grain oat‐based granola breakfast cereal in a cardio‐metabolic "at risk" population. Front Microbiol 2016; 7: 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giacco R, Clemente G, Cipriano D, et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr Metab Cardiovasc Dis 2010; 20: 186–194. [DOI] [PubMed] [Google Scholar]
- 19. Giacco R, Costabile G, Della Pepa G, et al. A whole‐grain cereal‐based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis 2014; 24: 837–844. [DOI] [PubMed] [Google Scholar]
- 20. Giacco R, Lappi J, Costabile G, et al. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two‐centre intervention study. Clin Nutr 2013; 32: 941–949. [DOI] [PubMed] [Google Scholar]
- 21. Harris Jackson K, West SG, Vanden Heuvel JP, et al. Effects of whole and refined grains in a weight‐loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled‐feeding trial. Am J Clin Nutr 2014; 100: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karl JP, Meydani M, Barnett JB, et al. Substituting whole grains for refined grains in a 6‐wk randomized trial favorably affects energy‐balance metrics in healthy men and postmenopausal women. Am J Clin Nutr 2017; 105: 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katcher HI, Legro RS, Kunselman AR, et al. The effects of a whole grain‐enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 2008; 87: 79–90. [DOI] [PubMed] [Google Scholar]
- 24. Kazemzadeh M, Safavi SM, Nematollahi S, et al. Effect of brown rice consumption on inflammatory marker and cardiovascular risk factors among overweight and obese non‐menopausal female adults. Int J Prev Med 2014; 5: 478–488. [PMC free article] [PubMed] [Google Scholar]
- 25. Kikuchi Y, Nozaki S, Makita M, et al. Effects of whole grain wheat bread on visceral fat obesity in Japanese subjects: a randomized double‐blind study. Plant Foods Hum Nutr 2018; 73: 161–165. [DOI] [PubMed] [Google Scholar]
- 26. Kirwan JP, Malin SK, Scelsi AR, et al. A whole‐grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr 2016; 146: 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kondo K, Morino K, Nishio Y, et al. Fiber‐rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: a randomized controlled trial. PLoS One 2017; 12: e0179869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kristensen M, Toubro S, Jensen MG, et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12‐week, energy‐restricted dietary intervention in postmenopausal women. J Nutr 2012; 142: 710–716. [DOI] [PubMed] [Google Scholar]
- 29. Liatis S, Tsapogas P, Chala E, et al. The consumption of bread enriched with betaglucan reduces LDL‐cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab 2009; 35: 115–120. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Qiu J, Yue Y, et al. Dietary black‐grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: a randomized controlled trial. Ther Clin Risk Manag 2018; 14: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik VS, Sudha V, Wedick NM, et al. Substituting brown rice for white rice on diabetes risk factors in India: a randomised controlled trial. Br J Nutr 2019; 121: 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malin SK, Kullman EL, Scelsi AR, et al. A whole‐grain diet increases glucose‐stimulated insulin secretion independent of gut hormones in adults at risk for type 2 diabetes. Mol Nutr Food Res 2019; 63: e1800967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malin SK, Kullman EL, Scelsi AR, et al. A whole‐grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: a randomized‐controlled trial. Metabolism 2018; 82: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McIntosh GH, Noakes M, Royle PJ, et al. Whole‐grain rye and wheat foods and markers of bowel health in overweight middle‐aged men. Am J Clin Nutr 2003; 77: 967–974. [DOI] [PubMed] [Google Scholar]
- 35. Pereira MA, Jacobs DR Jr, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002; 75: 848–855. [DOI] [PubMed] [Google Scholar]
- 36. Pick ME, Hawrysh ZJ, Gee MI, et al. Barley bread products improve glycemic control of type 2 subjects. Int J Food Sci Nutr 2009; 49: 71–78. [Google Scholar]
- 37. Pins JJ, Geleva D, Keenan JM, et al. Do whole‐grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J Fam Pract 2002; 51: 353–359. [PubMed] [Google Scholar]
- 38. Roager HM, Vogt JK, Kristensen M, et al. Whole grain‐rich diet reduces body weight and systemic low‐grade inflammation without inducing major changes of the gut microbiome: a randomised cross‐over trial. Gut 2019; 68: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schutte S, Esser D, Hoevenaars FPM, et al. A 12‐wk whole‐grain wheat intervention protects against hepatic fat: the Graandioos study, a randomized trial in overweight subjects. Am J Clin Nutr 2018; 108: 1264–1274. [DOI] [PubMed] [Google Scholar]
- 40. Shimabukuro M, Higa M, Kinjo R, et al. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr 2014; 111: 310–320. [DOI] [PubMed] [Google Scholar]
- 41. Tighe P, Duthie G, Vaughan N, et al. Effect of increased consumption of whole‐grain foods on blood pressure and other cardiovascular risk markers in healthy middle‐aged persons: a randomized controlled trial. Am J Clin Nutr 2010; 92: 733–740. [DOI] [PubMed] [Google Scholar]
- 42. Vitaglione P, Mennella I, Ferracane R, et al. Whole‐grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr 2015; 101: 251–261. [DOI] [PubMed] [Google Scholar]
- 43. Wang B, Medapalli R, Xu J, et al. Effects of a whole rice diet on metabolic parameters and inflammatory markers in prediabetes. e‐SPEN Journal 2013; 8: e15–e20. [Google Scholar]
- 44. Zhang G, Pan A, Zong G, et al. Substituting white rice with brown rice for 16 weeks does not substantially affect metabolic risk factors in middle‐aged Chinese men and women with diabetes or a high risk for diabetes. J Nutr 2011; 141: 1685–1690. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Li L, Song P, et al. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutr J 2012; 11: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol 2007; 18: 3–8. [DOI] [PubMed] [Google Scholar]
- 47. Chanson‐Rolle A, Meynier A, Aubin F, et al. Systematic review and meta‐analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PLoS One 2015; 10: e0131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aune D, Norat T, Romundstad P, et al. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis of cohort studies. Eur J Epidemiol 2013; 28: 845–858. [DOI] [PubMed] [Google Scholar]
- 49. Abdul Rahim AF, Norhayati MN, Zainudin AM. The effect of a brown‐rice diets on glycemic control and metabolic parameters in prediabetes and type 2 diabetes mellitus: a meta‐analysis of randomized controlled trials and controlled clinical trials. PeerJ 2021; 9: e11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Baseline and outcome data used in the meta‐analysis.
Table S2 | Judgments of review authors for each risk of bias item according to the Cochrane risk of bias assessment tool.
Table S3 | Pooled effects of whole grain consumption on glucose control and insulin sensitivity.
Figure S1 | Meta‐analysis of the effects of whole grain on homeostatic model assessment for insulin resistance.
Figure S2 | Meta‐analysis of the effects of whole grain on glycated hemoglobin.
Figure S3 | Funnel plots for the studies of the association of whole grain and fasting glucose concentrations.
Figure S4 | Funnel plots for the studies of the association of whole grain and fasting insulin concentrations.
Figure S5 | Funnel plots for the studies of the association of whole grain and homeostatic model assessment for insulin resistance.
Figure S6 | Funnel plots for the studies of the association of whole grain and glycated hemoglobin.


