Abstract
Medicinal plants have been employed as an alternative method to treat diabetes. One is Cissus sicyoides, a plant from the Amazon region (Northern Brazil), which is morphologically similar to Wedelia paludosa, a plant easily found in Southern Brazil. Thus, this study aimed to assess the potential toxicity of C. sicyoides and W. paludosa’s leaves water extracts. Through phytochemical screening, phenolic compounds and alkaloids were observed in both species and coumarins only W. paludosa’s aqueous extract. Phenolic compounds were quantified in both extracts and C. sicyoides presented 1.36 ± 0.04 mg/pyrogalic acid equivalent (PAE), whereas W. paludosa presented 3.27 ± 0.07 mg/PAE. Total antioxidant power was measured by the ferric reduction assay. Cissus sicyoides exhibited total antioxidant activity of 748.0 ± 104.5 μM and W. paludosa, 1971.5 ± 141.0 μM. Cissus sicyoides showed an inhibition rate for the alpha-glucosidases enzyme assay of 55.2 ± 1.7% and W. paludosa, 85.8 ± 9.7%. The formation of reactive oxygen species was evaluated by the DCFH-DA method, its formation being higher in W. paludosa’s water extracts than in C. sicyoides. Cell viability was evaluated by the Sulforhodamine B and MTT assays. Wedelia paludosa’s extracts’ exposure presented a cell viability close to positive control starting from 2 mg/mL to 30 mg/mL, whereas C. sicyoides demonstrated statistical significant low viability at the highest concentration when compared with the negative control. Moreover, cell death mechanism was investigated, having W. paludosa’s extract indicated death by necrosis. The results suggest low toxicity for C. sicyoides’ extract and high toxicity for W. paludosa’s extract.
Keywords: antioxidant, MTT, DCFH-DA, Wedelia paludosa, Cissus sicyoides
Introduction
Diabetes is a metabolic chronic disease1 classified mainly in type 1 diabetes, type 2 diabetes, gestational diabetes, and other types of specific causes. Diabetes is caused by flaws in insulin secretion, its action, or both. These flaws may be originated by autoimmune destruction of the pancreatic beta cells, inadequate secretion, or decrease of tissue response to insulin. The resulting hyperglycemia may cause damage, dysfunctions, and organ failure overtime.2
Diabetes is linked to oxidative stress, which is described as the unbalance between oxidant and antioxidant compounds and their overproduction or lack of elimination.3,4 For diabetes, glucose auto-oxidation and nonenzymatic glycation of proteins may increase the production of reactive oxygen species (ROS),5 in addition to decreasing insulin secretion by pancreatic beta-cells.6 Therefore, there is the antioxidant defense system, which may be enzymatic or nonenzymatic. Enzymes such as superoxide dismutase, catalase, and glutathione peroxidase are part of the first. Vitamins, minerals, and phenolic compounds are part of the latter.3 As hyperglycemia leads to a production of ROS, hence generating a state of oxidative stress there are several natural antioxidants that may eliminate these ROS and may prevent oxidative damage.5
Some of these antioxidant substances are in plants. Phenolic compounds, reducing agents that interrupt the oxidation chain by donating electrons to free radicals and making them stable. In this way, they may reduce the risk of diseases related to oxidative stress.7
Some plants have been traditionally used to treat diabetes. One of them is C. sicyoides L., known as ‘cipó-anil’ or ‘vegetal insulin’ in Brazil. It is usually found in the Amazon region in Northern Brazil and it is traditionally used to treat rheumatism, epilepsy, strokes, abscesses, arthritis, and diabetes.8 However, C. sicyoides is morphologically similar to Wedelia paludosa (also known as Acmela brasiliensis and Sphagneticola trilobata), a plant widely spread out in countries of tropical and temperate climate. Some of the metabolites found in W. paludosa have been previously linked to cytotoxic activities.9 Thus, constant research of the efficacy and safety of medicinal plants is extremely necessary. Wedelia paludosa may be mistaken for C. sicyoides, because the latter is not easily grown locally. Hence, raising concerns on their misuse and possible outcomes.
On that matter, assays were carried out to study the extent of the properties of the water extracts. Therefore, this study aimed to evaluate the potential toxicity, antioxidant activity, and identification of secondary metabolites present in the leaves water extracts of W. paludosa and C. sicyoides.
Materials and methods
Plant material
Plant material used to prepare the infusion of C. sicyoides was acquired as dry leaves in Igrejinha in South Brazil (−29.5766; −50.7907) in June 2020. Cissus sicyoides exsiccate is available in the academic collection of the Feevale University botany laboratory under the identification HEFE 462. As for W. paludosa, the plant was collected at Feevale University, Novo Hamburgo in South Brazil (−29.6790; −51.1128) in July 2020. Wedelia paludosa exsiccate is available in the academic collection of the Feevale University botany laboratory under the identification HEFE 461. Plant material for both species was cleaned and dried at room temperature (25°C). Samples were stored in paper bags until their use.
Water extract
Water extracts of both plants were prepared and concentration was chosen according to the popular use of C. sicyoides.10 Extracts were prepared using 1 g of the dry plant material and 150 mL of distilled water (6 g/mL). They were boiled up to 80°C and rested for 10 min before they were filtered. Extracts used for the assays were at room temperature.
Phytochemical screening
Colorimetric and chemicals reactions were used for the phytochemical screening of the extracts.11 The extracts were tested for anthraquinones, alkaloids, phenolic compounds, coumarins, flavonoids, saponins, and tannins.
Mayer, Draggendorf, and Bertrand reagents were used to identify alkaloids. Each water extract was divided in three parts and three drops of each reagent were added separately to each of them. Precipitate formed indicated positive result. For anthraquinones, six drops of acetic acid were added followed by 5 mL of toluene. After mixing, the supernatant was collected and 2 mL of potassium hydroxide 0.5 N was added. Red color indicated positive result. To identify coumarins, water extracts were boiled in an Erlenmeyer flask with a piece of filter paper impregnated with 5% potassium hydroxide covering the opening. Papers were observed under UV light at wavelengths 360 and 245 nm. For flavonoids, 1 mL of hydrochloric acid was added to the samples. After that, magnesium in powder was added. Red or orange color was formed if there was presence of flavonoids. Phenolic compounds were identified as one drop of ferric chloride was added to each extract and the formation of green color was observed if positive result. For saponins, extracts were shaken for 15 s. If the foam formed was kept after 15 min and the addition of 1 mL of hydrochloric acid 2 N the result was positive. Finally, for tannins, 1 mL of sodium chloride 2% was added to the water extracts’ samples. After that, three drops of 1% gelatin solution were added. Precipitate formed indicated positive result.
Phenolics content
The phenolics content assay described by the Brazilian Pharmacopoeia12 was carried out in triplicate using the 6 mg/mL water extract (popular use). For that 2 mL of each extract was added to 2 mL of 0.2 M Folin–Ciocalteu reagent, followed by 4 mL of distilled water and completed to 10 mL with 20% sodium carbonate solution. Samples were vortexed and incubated for 30 min in the dark at room temperature (25°C). Absorbance was measured at 760 nm wavelength using an UV/Vis Spectrophotometer (Shimadzu 2600™, Japan). The phenolic content was expressed as mg of pyrogalic acid equivalent (PAE) from the calibration curve of pyrogalic acid standard solutions.
Ferric reducing ability of plasma
Total antioxidant power of the extracts was determined by the method recommended by Benzie and Strain.13 For measuring the total antioxidant power, 50 μL of each extract of C. sicyoides and W. paludosa was mixed with ferric reducing ability of plasma (FRAP) reagent (TPTZ—2,4,6-tripyridyl-s-triazin 10 mM in hydrochloric acid 40 mM, acetate buffer 300 mM pH 3.6; FeCl3 6H2O 20 mM). The ferric compound was reduced to ferrous and an intense blue color was formed. Samples were read in a 593-nm absorbance wavelength using an UV/Vis Spectrophotometer (Shimadzu 2600™, Japan). The assay was carried out in triplicate.
Alpha-glucosidases activity
The activity of the alpha-glucosidases enzyme was determined using an adapted method by Kwon et al.14 First, p-nitrophenyl-alpha-D-glucopyranoside 5 mmol/L (Sigma™) in citrate–phosphate buffer 0.1 mol/L, pH 7.0 was used as substrate. Then, 50 μL of the sample and 100 μL of the enzyme were incubated in warm bath at 37°C for 10 min after the substrate was added. Reaction was stopped by adding 1 mL of NaOH 0.05 mol/L. Samples were read in a 410-nm absorbance wavelength using an UV/Vis Spectrophotometer (Shimadzu 2600™, Japan) and the assay was carried out in triplicate. The percentage of inhibition was calculated according to the equation: % Inhibition = [(Δ control absorbance − Δ sample absorbance) / Δ control absorbance] × 100.
Cytotoxicity assay
Cell cultivation
Fibroblasts Balb/c 3 T3 cell lines were originally obtained from the Rio de Janeiro Cell Bank (BCRJ). Fibroblastic cells are naturally more sensible than epithelial cells, which turns this morphologic type more useful for toxicology screening of substances used as systemic effects. The 3 T3 cell was developed from BALB/c mouse embryos without transformation during the process of immortalization, representing a very sensible model to study toxicology with the advantage of to be a nontumoral cell. Besides, this cell line is indicated in several standardization guidelines from OECD (Organization for Economic Co-operation and Development), like Teste Guideline No. 432 and Series on the Safety of Manufactured Nanomaterials No. 85. In general, when a substance or mixture did not show expressive toxicity for to 3 T3 cells, it will not show for other cells.
Cell lines were seeded in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco™) with 10% v/v of fetal bovine serum and maintained at 37°C in a humid atmosphere at 5% of CO2. For cytotoxicity assays the cells were seeded at a rate of 2.5 × 104 cells/well in a 96-well polystyrene microplate with 150 μL of DMEM. They were maintained in the same conditions for 24 h until the assays were carried out.
Exposure medium
DMEM components (Sigma™) were weighted in their usual proportions and water extracts of both plant materials were used as solvent instead of water. After that, pH was confirmed as 7.4 and the extracts were filtered in a 0.22 μM cellulose acetate membrane and 1% of fetal bovine serum was added. Both extracts were diluted to 30, 15, 10, 6, 5, 4, 3, 2, 1, 0.5 and 0.25 mg/mL in order to compare to results obtained in a study conducted by Schmitz et al.15
ROS assay
The DCFH-DA (2′,7′-Dichlorofluorescein diacetate) method16 was employed in order to evaluate the formation of ROS.
DCFH-DA 4.0 μM was prepared in high glucose DMEM with 1% fetal bovine serum. Cells wells were washed with saline buffer and incubated with 100 μL of DCFH-DA for 1 h. Then, cells were washed again with saline buffer and exposed to the mediums of different concentrations previously mentioned. A group of wells was kept as negative control and was only exposed to DMEM. Positive control was exposed to 0.1% hydrogen peroxide (H2O2).
The fluorescence reading was held for 24 h. The measures were read at time zero and then at 0.5, 1, 2, 3, 4.5, 6.5, 16 and 24 h at 488 and 525 nm wavelengths with a microplate spectrophotometer Spectramax M3 (Molecular Devices™). The results were expressed in Absorbance versus time in hours.
Sulforhodamine B assay
For the Sulforhodamine B (SRB) assay17 cells were first exposed to the exposure mediums of different concentrations previously mentioned for 24 h. After 24 h they were washed with saline buffer and incubated with trichloroacetic acid (TCA) 10% for 1 h at 10°C. Then, the supernatant was discarded and the protein layer was washed five times with ultrapure water (MilliQ™). After that, the layer was incubated with SRB 0.4% for 30 min at room temperature. Then, the wells were washed four times with acetic acid 1% and the coloring was eluted with Tris buffer pH 10.5 on an orbital shaker for 5 min at 5 rotations per minute (rpm). The supernatant was transferred to a reading plate and absorbance was determined at 564 nm using a microplate spectrophotometer Spectramax M3 (Molecular Devices™). The results were calculated according to the equation: viability rate % = (sample absorbance/negative control absorbance) × 100.
MTT assay
For the MTT assay18 cells were exposed for 24 h to the exposure mediums of different concentrations previously mentioned. Then they were washed with saline buffer and phosphate buffer saline (PBS) containing 200 μg/mL of MTT was added to the wells. The plate was incubated for 2 h at 37°C. Then, the supernatant was discarded and the cell crystals were dissolved with dimethylsulphoxide. The absorbance reading was carried out at 570 nm using a microplate spectrophotometer Spectramax M3 (Molecular Devices™). The results were calculated according to the equation: viability rate % = (sample absorbance/ negative control absorbance) × 100.
LDH release assay and incorporation of acridine orange
After 24 h of being exposed to the exposure mediums, the supernatant was collected in polystyrene microtubes for the lactate dehydrogenase (LDH) assay19 (which activity in the supernatant indicates death by necrosis). The remaining cultures were used for the acridine orange assay20 (which suggests death by apoptosis). Wells incubated with only DMEM containing 1% of fetal bovine serum were used as negative control. Wells incubated with 0.1% hydrogen peroxide (H2O2) were used as positive control.
Acridine orange assay
After collecting the supernatant, cells were washed three times with PBS and 150 μL of TCA 10% was added to each well. After that, they were incubated for 30 min at 10°C. Then, supernatant was once again discarded and 100 μL of acridine orange (1 μg/mL) was added to the wells. The plate was incubated for 15 min in the dark at room temperature. Finally, wells were washed three times with ultrapure water and the supernatant was discarded. Total fluorescence was measured through well scan mode with an excitation wavelength of 490 nm and emission wavelength of 550 nm using a microplate spectrophotometer Spectramax M3 (Molecular Devices™). The experiment was carried out under light protection. The results were calculated according to the equation: % in relation to negative control = (sample absorbance/negative control absorbance) × 100.
LDH assay
Supernatant collected before the orange acridine assay was centrifuged for 10 min at 1200 rpm in a SL-703 refrigerated centrifuge (Solab™). Then, 75 μL of the supernatant was added to a UV–Vis plate followed by 125 μL of the reagent for the determination of LDH assay by NADH generation method. The LDH-LIQUID UV Kit (Human do Brasil™) was used for this assay. Absorbance was read at 340 nm at time zero and every 1 min for 3 min. The results were calculated according to the equation: {[(absorbance time 1 − absorbance time 0) + (absorbance time 2 − absorbance time 1) + (absorbance time 3 − absorbance time 2)] / 3} × 8095 and expressed in U/L.
Statistical analyses
Data were statistically analyzed by the SPSS™ 25.0 software. FRAP, alpha-glucosidases and total phenolic content results were analyzed by Student’s t-test. SRB, MTT, LDH assay, acridine orange assay and DCFH-DA results were analyzed by one-way analysis of variance followed by Tukey’s post-test. Statistically significant results were considered as P < 0.05.
Results
Results for the phytochemical screening of the water extracts (6 mg/mL) are shown in Table 1. Both species demonstrated the presence of phenolic compounds and alkaloids. However, only in W. paludosa extract was observed the presence of coumarins.
Table 1.
Phytochemical screening of C. sicyoides and W. paludosa.
| Phenolic compounds | Flavonoids | Coumarins | Tannnins | Anthraquinones | Saponins | Alkaloids | |
|---|---|---|---|---|---|---|---|
| Cissus sicyoides | + | − | − | − | − | − | + |
| Wedelia paludosa | + | − | + | − | − | − | + |
Positive result: +. Negative result: −.
Ferric reducing power, alpha-glucosidases assay, total phenolics content
Results are shown in Table 2. FRAP Assay is expressed in μM. The calibration curve used to calculate the results of the FRAP assay was y = 0.0007x + 0.085; R2 = 0.99. For the alpha-glucosidases assay a standard solution of acarbose (25 mg/mL) had an inhibition rate of 65.8%. Total phenolic content for both water extracts is expressed as mg of PAE per gram of dry material. The calibration curve used to calculate the results of the total phenolics content assay was y = 0.1682x + 0.0215; R2: 0.99. All results demonstrated statistical difference (P < 0.05). Wedelia paludosa’s extract demonstrated higher total antioxidant activity when compared with C. sicyoides’ (over 2.5-fold higher). In addition, W. paludosa also demonstrated to have more total phenolic compounds than C. sicyoides (amount of 2.4-fold higher). Both FRAP and total phenolic content assays showed P < 0.001. Furthermore, inhibition rate for W. paludosa’s extract was higher than C. sicyoides’ (P = 0.006).
Table 2.
Results of the analysis of FRAP expressed in μM, inhibition rate of the alpha- glucosidases enzyme of the water extracts of C. sicyoides (6 mg/mL) and W. paludosa (6 mg/mL) and phenolic content in PAE per gram of dry material.
| Variable | Cissus sicyoides | Wedelia paludosa | P (<0.05) |
|---|---|---|---|
| FRAP (μM/g) | 748.0 ± 104.5 | 1971.5 ± 141.0 | <0.001 |
| Alpha-glucosidases (% of inhibition) | 55.3 ± 1.7 | 85.8 ± 9.7 | 0.006 |
| Total phenolic content (mg PAE/g) | 1.36 ± 0.04 | 3.27 ± 0.07 | <0.001 |
PAE: pyrogalicacid equivalent, FRAP: Ferric Reducing Power. Results were expressed as mean ±standard deviation followed by the Student t test.
MTT and SRB assay
Results of the different concentrations were compared with the negative control. They are shown on Fig. 1.
Fig. 1.

Cell viability for C. sicyoides and W. paludosa’s water extracts and their standard deviation. Concentrations are expressed from left to right from 0.25 to 30 mg/mL. *Expresses significant results when compared with negative control (P < 0.05).
SRB
Results found were also compared with the positive control of 0.1% H2O2. Positive control demonstrated a viability rate of 6.3%. Wedelia paludosa’s water extracts demonstrated a high viability rate from 0.25 to 2 mg/mL. After that, viability rate is close if not lower than the positive control. It is important to notice that the lowest the cell viability, the higher is the toxicity presented by the water extract. Therefore, starting from 3 mg/mL W. paludosa presented toxicity rate over 80% On the other hand, C. sicyoides’ water extracts demonstrated a viability rate of over 80% from 0.25 to 15 mg/mL concentration. Viability rate only decreased to 68.75% (toxicity rate of 31.25%) on the 30 mg/mL concentration and was still high when compared with W. paludosa’s extracts.
MTT
Both W. paludosa’s and C. sicyoides’ water extracts showed similar cell viability up to 4 mg/mL where rates were even higher than the negative control results. After that, C. sicyoides kept its viability rate stable up to 15 mg/mL. Then, on 30 mg/mL, it decreased to 31.3%. On the other hand, W. paludosa’s extract from 5 mg/mL concentrations to 30 mg/mL showed decreasing cell viability. In total, 10, 15 and 30 mg/mL concentrations showed similar viability rate to the positive control, which was 23.2%.
ROS assay
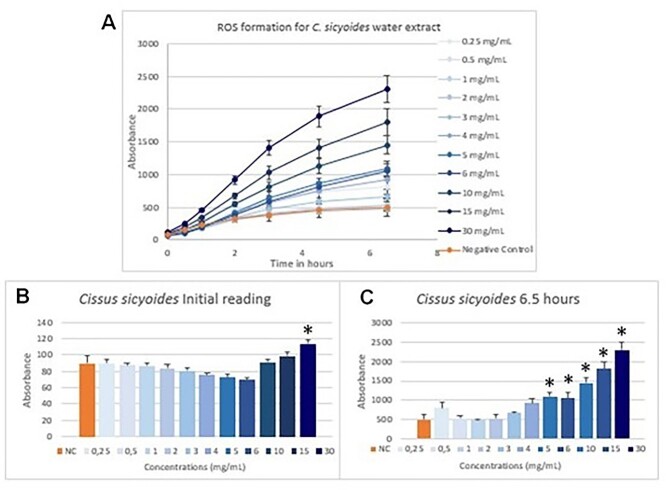
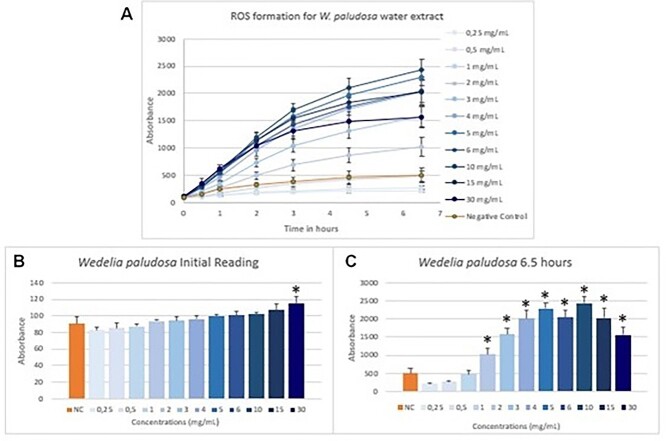
Formation of reactive species of oxygen was measured by the DCFH-DA method. Reading was held from 0 to 24 h. However, after 6.5 h of exposure the readings became stable. For this reason, only results from time 0 to 6.5 h are demonstrated in the following figures. Results for C. sicyoides’ extract are shown on Fig. 2 and W. paludosa’s on Fig. 3.
Fig. 2.
ROS formation for C. sicyoides’ water extract was measured at time 0, 1, 2, 3, 4.5, and 6.5 h (A) and detailed results for 0 h (B) and 6.5 h (C). Results are expressed as fluorescence vs. time in hours along with their standard deviation. Concentrations are expressed in mg/mL and are described in ascending order on general results. Negative control is expressed by orange column/line. On detailed results (B and C), starting from left to right side, are expressed results for negative control and concentrations from 0.25 to 30 mg/mL. *Expresses significant results when compared with negative control (P < 0.05).
Fig. 3.
ROS formation for W. paludosa’s water extract was measured at time 0, 1, 2, 3, 4.5 and 6.5 h (A) and detailed results for 0 h (B) and 6.5 h (C). Results are expressed as fluorescence vs. time in hours along with their standard deviation. Concentrations are expressed in mg/mL and are described in ascending order on general results. Negative control is expressed by orange column/line. On detailed results (B and C), starting from left to right side, are expressed results for negative control and concentrations from 0.25 to 30 mg/mL. *Expresses significant results when compared with negative control (P < 0.05).
Both water extracts showed similar formation of ROS at 0 h. Then, formation of ROS for C. sicyoides’ water extract remained close to negative control in several lower concentrations until 6.5 h. Higher concentrations seemed to have produced ROS in a dose-related way. On the other hand, W. paludosa’s extracts showed a production of ROS fourfold higher in some concentrations. Concentration 2 mg/mL already showed twice the amount of ROS produced when compared with negative control. ROS production increased significantly in higher concentrations. However, on 15 and 30 mg/mL concentrations, ROS production started to decrease in comparison to others since the third hour after exposure.
Acridine orange assay
Results for the acridine orange assay are shown in Fig. 4. Positive control (0.1% H2O2) demonstrated a result of 10.8% of acridine orange incorporation in cells.
Fig. 4.
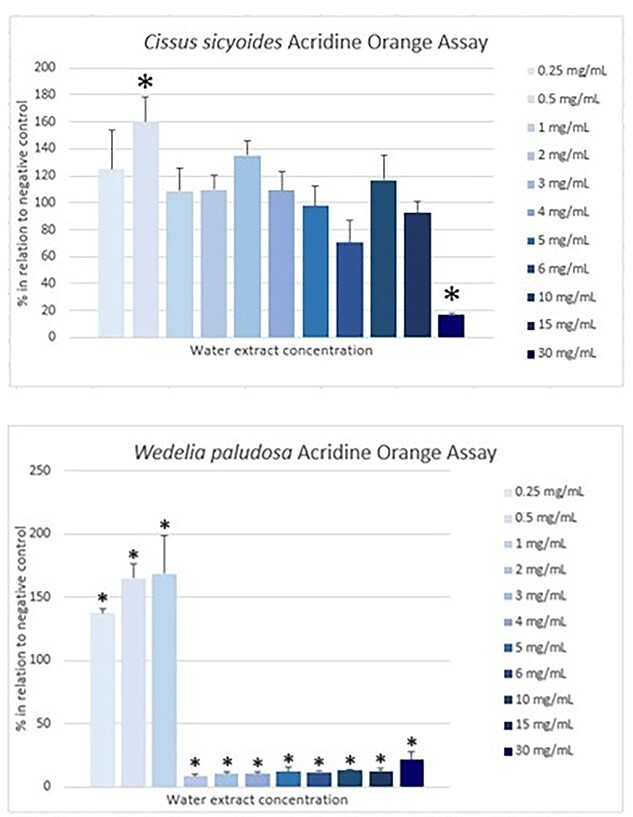
Cissus sicyoides’ and W. paludosa’s water extract acridine orange assay results expressed in % in relation to negative control and their standard deviation. Concentrations are expressed in mg/mL starting from 0.25 to 30 mg/mL. from left to right.*Expresses significant results when compared with negative control (P < 0.05).
For C. sicyoides, only 0.5 mg/mL concentration demonstrated to be significantly higher than negative control and 30 mg/mL, lower. Wedelia paludosa’s extract also demonstrated results higher than negative control for the first three concentrations (0.25, 0.5 and 1 mg/mL).
LDH assay
Results for the LDH assay are shown in Fig. 5. They are expressed in U/L. Negative and positive controls demonstrated a concentration of 60.9 and 4.5 U/L, respectively.
Fig. 5.

Cissus sicyoides’ and W. paludosa’s water extract LDH assay results expressed in U/L and their standard deviation. Concentrations are expressed in mg/mL starting from negative control and 0.25 to 30 mg/mL from left to right. *Expresses significant results when compared with negative control (P < 0.05).
Results for the LDH assay show that C. sicyoides concentrations have a tendency to be similar if not lower than negative control; however, significant results were not reported. For W. paludosa’s extracts, the first three concentrations showed a number half the negative control. Then, 2–10 mg/mL concentrations showed the highest results among the samples—two- or threefold higher and were significantly different. However, 30 mg/mL concentration showed a result similar to the negative control.
Discussion
Phytochemical screening
Cissus genus is in general known for the presence of steroids, quinones, and phenolic compounds in its leaves and anthocyanidins in its fruit.21 However, the presence of steroids, tannins, and flavonoids has also been identified in a hydro alcoholic extract leaves of C. sicyoides. Other authors have indicated the presence of coumarins and anthocyanins as well.8 Furthermore, kaempferol 3-O-alpha-l-rhamnoside and quercetin-3-O-rhamnoside have been identified on C. sicyoides leaves.22 Moreover, Salazar et al.23 have identified terpenes besides phenolic compounds and flavonoids while using hexane (1:10 m/v) as solvent. Also, it was verified that the amount of phenolic compounds and flavonoids depended on the solvent type used for the extractions. The presence of phenolic compounds and alkaloids found in the current study is according to other studies mentioned previously. These reports are according to the present study where phenolic compounds and alkaloids were identified.
On the present study, phenolic compounds, alkaloids, and coumarins were identified in the water extract of W. paludosa. Other authors found the presence of diterpenes such as ent-kaur-16-em-19-oic and ent-kaur-9(11),16-dien-19-oic acid, besides other diterpenes, triterpenes, and lactones. Some of these metabolites were linked to the cytotoxic activity of its hydromethanolic and dichloromethane extracts, such effect being absent on the water extract.9
Furthermore, Leite et al.24 found flavonoids, anthracenes, and terpenes on its leaves hydroalcoholic extract (140 mg/mL). These authors also compared their findings to other studies that stated the presence of tannins, saponins, and essential oils. The study’s choice of solvent was ethanol, differently to the current study. Therefore, the different solvent used may have influenced the different metabolites found on each study. Since the popular use of the water extracts were investigated on this study, it is essential to compare results with authors’ papers with the same choice of solvent.
The metabolites found on this study are in general according to the authors mentioned previously. Although the presence or absence of some metabolites may be linked to the solvent of choice as some secondary metabolites are well extracted with some solvents than others due to their solubility, for instance. One example is the coumarins’ presence in W. paludosa’s water extract. Depending on the pH level, coumarins may have their lactone ring open and be more easily extracted with water. They are known for their immunosuppressive and hypolipidemic activities as well as being capable of inhibiting lipid peroxidation. On the other hand, some coumarins such as furanocoumarins have been linked to cytotoxic activity.25
However, it may also be linked to the climatic and environmental factors. These may alter the metabolic route of the plants and be reflected on the biosynthesis of secondary metabolites.26
Antioxidant activity and phenolic content
Cissus sicyoides’ water extract antioxidant activity has been discussed by many authors who employed distinct methods, for instance DPPH and ABTS. Salazar et al.27 found results that indicate the water extract is a potential source of natural antioxidants and may be used in treatment for prevention of conditions related to oxidative stress.
Sah et al.28 evaluated the water extract of another medicinal plant using alpha-tocopherol (<1 μg/mL) as standard. Alpha-tocopherol is a vitamin known for its antioxidant properties. The authors obtained an antioxidant activity of 1434.0 ± 109.4 μM for the standard. On this study, C. sicyoides’ water extract that showed 52% of antioxidant activity when compared with alpha-tocopherol vitamin. Therefore, it is suggested the water extract of C. sicyoides contains enough of antioxidant activity to be evaluated in further studies.
When it comes to W. paludosa’s water extract, Govindappa et al.29 found 630.7 μM for its leaves ethanolic extract (0.5 mg/mL). This amount was slightly lower when compared with ascorbic acid (vitamin C) standard, however, higher than butylated hydroxytoluene, which are other compounds known for their antioxidant properties. Govindappa et al.30 also evaluated W. paludosa leaves water extract (0.5 g/mL) where they obtained the amount of total antioxidant power of 1432.6 μM. This result corroborates to the findings of the current study. Thus, it may be suggested the water extract demonstrates higher antioxidant activity than the ethanolic extract.
Phenolic compounds and alkaloids are two secondary metabolites identified in both extracts that are known for their antioxidant properties. Due to phenolic compounds being part of the nonenzymatic defense system of the plant just like vitamins and minerals,3,4 their presence may be linked to the antioxidant activity found. Further, the amount of phenolic compounds quantified per gram of dry leaves (W. paludosa > C. sicyoides) may suggest why W. paludosa’s extract showed higher antioxidant activity when compared with C. sicyoides.
Alpha-glucosidases
Alpha-glucosidases is a key enzyme for carbohydrates metabolism. It is located on the mucosa of intestinal cells and inducts the hydrolysis of oligosaccharides to glucoses through the unbinding of an alpha-glucoside link. Thereby, these oligosaccharides are hydrolyzed to monomers like maltase, isomaltase, and lactase to be absorbed.31
Acabose, an inhibitor to alpha-glucosidases, is a pseudotetrasaccharide of microbial origin with <2% absorption. It works by competing with oligosaccharides for the enzyme link, hence interfering on the break of oligo to monosaccharides. Thus, the administration of an alpha-glucosidases inhibitor would decrease the amount of plasmatic glucose32 for it would slow the absorption of the carbohydrates.14
According to the results obtained, it is observed the C. sicyoides’ water extract inhibited 10.7% less when compared with the standard solution, whereas W. paludosa’s extract inhibited 22.9% more. Therefore, W. paludosa’s extract showed higher enzyme inhibition than standard solution. On the other hand, C. sicyoides indicated a significant result even though lower than standard.
Besides suggesting the antioxidant activity of the extracts is related to the presence and amount of phenolic compounds in their composition, it may also be suggested the enzyme inhibition is related to the same subject. Phenolic compounds may bind to digestive enzymes and stop their action. Hence, inhibit the alpha-glucosidases enzyme and slow down the carbohydrates break and their transformation to glucoses.33
ROS assay
This high ROS formation may be according to the increase of concentrations. This relation may be seen on the toxicity assays MTT and SRB. In both assays the higher concentration demonstrated the lowest cell viability after exposure. However, lowest concentration that did not alter much the ROS formation may be suggested to have a protective effect on the cells resulting in a higher cell viability. Some secondary metabolites such as phenolic compounds are related to the reduction of ROS formation thus reducing or slowing oxidative stress. Besides, they also show a protective effect against lipid peroxidation in cell membrane.26
On the other hand, W. paludosa’s extracts demonstrated a higher ROS production than negative control starting from concentration 2 mg/mL since the first hour. Initially, only 30 mg/mL concentration showed significant difference. However, after hour 3 the ROS production appeared to no longer be concentration like related. After that, in hour 6.5, this variation is even more apparent. Now, all concentrations from 2 to 30 mg/mL show significant difference when compared with negative control. It may be suggested that after that time the water extract induced such a high ROS production hence causing toxicity and cell death. This toxicity rate is shown on the cell viability assays where higher W. paludosa concentrations demonstrated cell viability close or even lower than positive control.
Some secondary metabolites that have been identified in W. paludosa—such as karenoic and grandiflorenic acids—have been liked to cytotoxic activities.9 Although the water extract presents a high potential antioxidant power, it also presents a high production of ROS. Thus, it may indicate the high toxicity of the water extract overlaps its antioxidant power through mechanisms not investigated on this study.
Cell viability
Normal cell viability after exposure to C. sicyoides’ water extract was expected. Only 30 mg/mL concentration showed decreased cell viability when compared with others, however still over 65% of viability. Sáenz et al.34 reported the water decoction of the plant (8.8%) demonstrated a LC50 (lethal concentration) of 254 ± 23.9 mg/mL. This concentration is way higher than the highest used for the present study and popular use. Other authors also indicated low toxicity like Vicentini et al.35 when analyzing Allium cepa L. cells. When compared with the local popular use (0.07 mg/mL) and a concentration tenfold higher (0.7 mg/mL), authors did not observe alteration in cell cycle. The author recommended more studies to confirm the nonmutagenic effects of the water extract but stated that there is no indication for such outcome.
The results for the present study are also according to Schmitz et al.15 When testing the water extracts from 1 to 30 mg/mL, low toxicity and high antioxidant power was observed, the latter may be why toxicity was low. This outcome is a good indication for the possible therapeutic use of the plant’s water extracts.
For W. paludosa, from 0.25 to 2 mg/mL concentration did not indicate decrease of cell viability. However, from 3 to 30 mg/mL cell viability substantially declined. Mardina et al.36 found an average LC50 of 58.1 μg/mL of the leaves extract using ethyl acetate as solvent. The author used a different extraction method that may lead to distinct metabolites being extracted. Significant antioxidant power was reported in both studies. Both samples were accompanied by high toxicity, though.
Schmitz et al.15 also reported possible toxicity for water extracts (concentration range from 0.5 to 6 mg/mL). Author suggested toxicity is related to low antioxidant power of the extract. However, total antioxidant activity found differs from the present study. Characteristics presented by the extract may vary due to the amount of secondary metabolites extracted. This amount depends on the place and time the plant material was collected.
Since W. paludosa’s water extract demonstrated high antioxidant power and toxicity, it is suggested this toxicity is no related to antioxidant power. Whereas the water extracted indicated the presence of alkaloids, their quantification may be recommended because these metabolites are known for their toxicity even though they present activities that may be considered beneficial, for instance antitumor activity and others.25 Therefore, the presence of alkaloids should be studied to verify the extent of their influence on its toxicity.
LDH and acridine orange assays
As mentioned previously, toxicity was low in cells exposed to C. sicyoides’ water extracts, except for 30 mg/mL concentration. This may also be observed in the acridine orange assay where almost every concentration showed results over 100% in relation to negative control. However, 6 and 30 mg/mL concentrations showed lower acridine intercalation which matches a higher LDH amount demonstrated in Fig. 5. This may indicate that cells exposed to these concentrations were more affected than others, suffering ruptures and releasing LDH. This result corroborates to the decrease in cell viability found in SRB and MTT assays for 30 mg/mL concentration.
Compounds such as stilbenes have been widely studied about their property of inducing apoptosis and have been identified in plants of the Cissus genus.37 Beyond that, C. sicyoides is known for containing a steroid called sitosterol and a polyphenol called resveratrol that are pointed out as capable of inducing apoptosis.38 Low results of the acridine assay for these two samples and higher LDH results may indicate that the higher concentrations were more harmful to cells hence causing them to rupture and release LDH.
On the other hand, W. paludosa demonstrated a different behavior. The acridine orange and LDH assays where 0.25, 0.5 and 1 mg/mL concentrations showed no significant difference from negative control in the first assay and a way lower LDH amount in the latter. Nevertheless, from 2 to 30 mg/mL concentrations showed a high amount of LDH release and a small percentage of cells in the acridine orange assay. This may indicate the water extracts caused necrosis to cells since concentrations as low as 2 mg/mL.
This was a different behavior from what was observed by Mardina et al.36 In their study, the ethyl acetate extract (1–200 μL/mL) showed mainly an apoptotic effect. The solvent used, concentration, and origin of the plant material were different from the current study. However, according to Amgalan et al.,39 it is suggested that cells that receive apoptotic stimulation later on might have a cell death pathway transition and generated inflammation, hence causing secondary necrosis. Since W. paludosa had rising results for the acridine orange assay on the first concentrations (0.25–1 mg/mL), it is possible to suggest that, according to the increase of concentration, apoptotic stimulation rose as well and for some, it caused necrosis. However, studies to investigate these mechanisms further were not carried out.
One important thing to notice is the amount of phenolic compounds observed in W. paludosa’s water extract. While C. sicyoides’ extract showed approximately half the amount antioxidant power however lower than W. paludosa, this could mean it was the right amount for redox equilibrium. Antioxidant power has been extensively linked to phenolic compounds. Although their activity is influenced by the number and position of the hydroxyl group,40 it is a relevant outcome that should be more deeply studied.
On the other hand, other substances such as pro-oxidants should also be discussed. These are substances capable to oxidase other molecules. This way resulting in the formation of ROS. According to their amount, certain substances may show different effects on cells (both antioxidant and pro-oxidant).40Wedelia paludosa showed an extremely high formation of ROS when compared with negative control and C. sicyoides. This could indicate that just as antioxidant compounds are present in its extract, pro-oxidants are as well. As the water extracted indicated the presence of alkaloids, metabolites known for their toxicity,36 these compounds should be more extensively studied. Other metabolites known for their possible toxic effect are coumarins, metabolites that were identified in W. paludosa’s water extract but not in C. sicyoide’s water extract. While alkaloids in general have not had their mechanism of action fully elucidated, it is only possible to suggest they are somehow responsible for this pro-antioxidant action—along with phenolic compounds and coumarins—until more studies are carried out.
Since these two species of plants are similar and they have been mistakenly used by the population, this study was essential to understand more about their effects when drunk as an infusion. More studies on their in vivo cytotoxicity and their mechanism of action are needed, although the results found on this study already suggest W. paludosa’s toxic and C. sicyoides’ atoxic effects.
Conclusions
Cissus sicyoides did not demonstrate toxicity in most of the concentrations tested, including traditional use. It showed significant total antioxidant power and inhibition rate of the alpha-glucosidases enzyme. Its capacity of generating ROS was similar to negative control, except for higher concentrations, which might mean its feature is dose related. Its water extract did not seem to harm cell culture when usually infusions doses were tested; on other hand, it seemed to allow cells to keep replicating after exposure leading to the conclusion it does not indicate in vitro toxicity.
Instead W. paludosa demonstrated high decrease of cell viability (suggested to be by necrosis) in almost all concentrations tested. Even though it showed higher total antioxidant power and alpha-glucosidases enzyme inhibition when compared with C. sicyoides, its capacity of generating ROS was at least twofold higher than negative control starting from 2 to 30 mg/mL concentrations tested. It indicates possible in vitro toxicity and further studies should be carried out to investigate the extent of cell deleterious caused by its extract and the mechanisms involved.
Funding
This work was supported by Feevale University and PPSUS 03/2017: CNPq and FAPERGS.
Conflict of interest statement. None declared.
Author contribution
ASP, ESS, ALZ, and MSP conceived of the present idea. ASP collected and processed the plant material. ASP and DRV performed the laboratory analyzes. ASP wrote the manuscript whit support from ESS, ALZ, and MSP critically revised the work. All authors discussed the results and contributed to the final manuscript.
Contributor Information
Amanda Schu Ponath, Master degree in Toxicology and Toxicological Analysis, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil.
Débora Rech Volz, Cytotoxicity Laboratory, Institute of Helth Sciences, Feevale University, Novo Hamburgo, Brazil.
Edna Sayuri Suyenaga, Master degree in Toxicology and Toxicological Analysis, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil; Department of Pharmacy, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil.
Ana Luíza Ziulkoski, Master degree in Toxicology and Toxicological Analysis, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil; Cytotoxicity Laboratory, Institute of Helth Sciences, Feevale University, Novo Hamburgo, Brazil; Department of Pharmacy, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil.
Magda Susana Perassolo, Master degree in Toxicology and Toxicological Analysis, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil; Department of Pharmacy, Institute of Health Sciences, Feevale University, Novo Hamburgo, Brazil.
References
- 1. Meiners MMMA, Tavares NUL, Guimarães LSP, Bertoldi AD, dal Pizzol TS, Luiza VL, Mengue SS, Merchan-HamannI E. Acesso e adesão a medicamentos entre pessoas com diabetes no Brasil: evidências da PNAUM. Rev Bras Epidemiol. 2017:20:445–459. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Classification and Diagnosis of Diabetes: standards of medical care in diabetes. Diabetes Care. 2019:42:13–28. [Google Scholar]
- 3. Barbosa KBF, Costa NMB, Alfenas RCG, Paula SP, Minim VPR, Bressan J. Estresse oxidativo: conceito, implicações e fatores modulatórios. Rev Nutr. 2010:23:629–643. [Google Scholar]
- 4. Li J, Wuliji O, Li W, Jiang JG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013:14:24438–24475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alkholy UM, Abdalmonem N, Zaki A, Elkoumi MA, Hashim MIA, Basset MAA, Salah HE. The antioxidant status of coenzyme Q10 and vitamin E in children with type 1 diabetes. J Pediatr. 2018:95:224–230. [DOI] [PubMed] [Google Scholar]
- 6. Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. Glycation-dependent, reactive oxygen species – mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Investig. 1997:99:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonçalves EM, Carvalho ER. Antioxidant compounds in pixirica fruits. https//www.cea-unesp.org.br/holos/issue/view/1060 (accessed September 8, 2019).
- 8. Dias GT, Lima CMBL, Lira AB, Lira AB, Ramalho JA, Oliveira KM, Diniz MFFM. Toxicidade do extrato hidroalcoólico das folhas de Cissus sicyoides. Acta Brasiliensis. 2017:1:8–12. [Google Scholar]
- 9. Batista R, Brandão GC, Braga FC, Oliveira AB. Cytotoxicty of Wedelia paludosa D.C. extracts and constituents. Rev Bras Farm. 2009:19:36–40. [Google Scholar]
- 10. Santos HB, Modesto-Filho J, Diniz MFFM, Vasconcelos THC, Pereira FSB, Ramalho JÁ, Dantas JG, Sants EB. Avaliação do efeito hipoglicemiante de Cissus sicyoides em estudos clínicos fase II. Rev Bras Farm. 2008:18:70–76. [Google Scholar]
- 11. Debiyi OO, Sofowora FA. Pytochemical screening of medical plants. Iloyidia. 1978:3:234–246. [Google Scholar]
- 12. Agência Nacional de Vigilância Sanitária (ANVISA) . Farmacopeia Brasileira. 6th ed. Brasil: Agência Nacional de Vigilância Sanitária (ANVISA); 2019 [Google Scholar]
- 13. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996:239:70–76. [DOI] [PubMed] [Google Scholar]
- 14. Kwon YI, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J Food Biochem. 2006:32:15–31. [Google Scholar]
- 15. Schmitz AP, Weimer P, Weschenfelder AM, Hansen AW, Maluf RW, Rossi RC, Basso LS, Ziulkoski AL, Perassolo MS, Suyenaga ES. In vitro cytotoxic and genotoxic effects of Cissus verticillata and Sphagneticola trilobata used for treatment of diabetes mellitus in Brazilian folk medicine. Acta Sci Biol Sci. 2021:43:e56549. [Google Scholar]
- 16. Moysés FS, Bertoldi K, Elsner VR, Cechinel LR, Basso C, Stulp S, Rodrigues MAS, Siqueira IR. Effect of tannery effluent on oxidative status of brain structures and liver of rodents. Environ Sci Pollut Res. 2017:24:15689–15699. [DOI] [PubMed] [Google Scholar]
- 17. Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990:82:1107–1112. [DOI] [PubMed] [Google Scholar]
- 18. Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983:65:55–63. [DOI] [PubMed] [Google Scholar]
- 19. Human Do Brasil. LDH Liqui UV. In: Sce Mod. Human do Brasil. 2011:Vol. 5. https://quibasa.bioclin.com.br/anexos/INSTRUCOES_DESIDROGENASE_LATICA_LDH_UV.pdf (accessed September 22, 2022). [Google Scholar]
- 20. Laboratório De Sinalização e Palsticidade Celular – LABSINAL . Autophagy: acridine orange staining and quantification by cytometry. 2020. http://www.ufrgs.br/labsinal/autophagy.html (accessed September February 10, 2021).
- 21. Guerrero AMQ, Gimenéz MDG, Rodríguz MTS. Plantas utilizadas em procesos inflamatorios y cancEROos em el área del Caribe. Rev Fitoter. 2006:6:59–63. [Google Scholar]
- 22. Khalil N, Pepato MT, Brunetti IL. Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: Bauhinia forficate and Cissus sicyoides. Biol Res. 2008:41:165–171. [PubMed] [Google Scholar]
- 23. Salazar MAR, Costa JV, Urbina GRO, Cunha VMB, Silva MP, Bezerra PN, Pinheiro WBS, Gomes-Leal W, Lopes AS, Junior RNC. Chemical composition, antioxidant activity, neuroprotective and inflammatory effects of cipó-pucá (Cissus sicyoides L.) extracts obtained from supercritical extraction. J Supercritic Fluids. 2018:138:36–45. [Google Scholar]
- 24. Leite AGB, Farias ETN, Oliveira AP, Abreu REF, Costa MM, Almeida JRGS, Estevão LRM, Evêncio-Neto J. Triagem fitoquímica e atividade antimicrobiana do extrato hidroalcoólico bruto das folhas de Sphagneticola trilobata (Asteraceae). Ciência Rural. 2019:49:1–6. [Google Scholar]
- 25. Simões CMO, et al. Farmacognosia: do produto natural ao medicamento. Porto Alegre, RS: Artmed, 2017:p. 486.
- 26. Oliveira RS, Lucas CP, Antonucci G, Silva FC. Compostos Bioativos Naturais: Agentes Promissores na Redução do Estresse Oxidativo e Processos Inflamatórios. South Am J Basic Educ Tech Technol. 2018:5:258–273. [Google Scholar]
- 27. Salazar MAR, Urbina GRO, Bezerra PN, Cunha VMB, Silva MP, Pires FCS, Souza e Silva AP, Sousa SHB, Carvalho RN Jr.. Antioxidant and biological activity of cissus sicyoides and Rosmarinus officinalis extracts. https://www.intechopen.com/books/antioxidants/antioxidantand-biological-activity-of-em-cissus-sicyoides-em-and-em-rosmarinus-officinalis-em-extra (accessed December 23, 2020).
- 28. Sah SY, Sia CM, Chang SK, Ang YK, Yim HS. Antioxidant capacity and total phenolic contente of lemon grass (Cymbopogon citratus) leaves. Ann Food Sci Technol. 2012:13:150–155. [Google Scholar]
- 29. Govindappa M, Sravya SN, Poojashri MN, Sadananda TS, Chandrappa CP. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. J P P. 2011:3:43–51. [Google Scholar]
- 30. Govindappa M, Sravya SN, Poojashri MN, Sadananda TS, Chandrappa CP. Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata (L.) Hitchc. J Med Plant Res. 2011:5:5718–5729. [Google Scholar]
- 31. Silva HCP.. Triagem virtual de compostos provenientes de plantas da biodiversidade brasileira, com potencial atividade inibitória das enzimas alfa-amilase, alfa-glicosidase humanas. 2017. 33 f. Trabalho de Conclusão de Curso (Graduação em Biotecnologia) – Universidade Federal de Uberlândia, Uberlândia, 2017. https://repositorio.ufu.br/handle/123456789/20582 (accessed September 22, 2022). [Google Scholar]
- 32. Lebovitz HE. Alpha-glucosidase inhibitors. Endocrinol Metab Clin N Am. 1997:26:539–551. [DOI] [PubMed] [Google Scholar]
- 33. Camargo TM.. Morango (Fragaria x ananassa), amora-preta (Rubus spp.) e mirtilo (Vaccinium ashei Reade): caracterização química, atividade antioxidante e ação sobre as enzimas digestivas alfa-glicosidase e alfa-amilase em dois ciclos produtivos das frutíferas.2019. 94 f. Dissertação (Mestrado em Ciência e Tecnologia de Alimentos) – Programa de Pós-Graduação em Ciência e Tecnologia de Alimentos, Faculdade de Agronomia Eliseu Maciel, Universidade Federal de Pelotas, Pelotas, 2019. http://guaiaca.ufpel.edu.br:8080/handle/prefix/4322 (accessed September 22, 2022). [Google Scholar]
- 34. Sáenz MT, Garcia MD, Quílez A, Ahumada MC. Cytotorix activity of Agave intermixta L. (Agavaceae) and Cissus sicyoides L. (Vitaceae). Phytother Res. 2000:14:552–554. [DOI] [PubMed] [Google Scholar]
- 35. Vicentini VEP, Camparoto ML, Teixeira RO, Mantovani MS. Averrhoa carambola L., Syzygium cumini (L.) Skeels and Cissus sicyoides L.: medicinal herbal tea effects on vegetal and animal test systems. Acta Sci. 2001:23:593–598. [Google Scholar]
- 36. Mardina V, Ilyas S, Harmawan T, Halimatussakdiah H, Tanjung M. Antioxidant and cytotoxic activities of the ethyl acetate extract of Sphagneticola trilobata (L.) J. F. Proski on MCF-7 breast cancer cell. J Adv Pharm Technol Res. 2020:11:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandes G, Banu J. Medicinal properties of plants from the genus Cissus: a review. J Med Plant Res. 2012:6:3080–3086. [Google Scholar]
- 38. Lucena FRS, Almeida ER, Aguiar JS, Silva TG, Souza VMO, Nascimento SC. Cytotoxic, antitumor and leukocyte migration activities of resveratrol and sitosterol present in the hydroalcoholic extract of Cissus sicyoides L., Vitaceae, leaves. Rev Bras Farm. 2010:20:729–733. [Google Scholar]
- 39. Amgalan D, Pekson R, Kitsis RN. Troponin release following brief myocardial ischemia. JACC Basic Transl Sci. 2017:2:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerqueira FM, Medeiros MHG, Augusto O. Antioxidantes dietéticos: controvérsias e perspectivas. Química Nova. 2007:30:441–449. [Google Scholar]