Abstract
Duchenne muscular dystrophy (DMD) is an inherited X-linked disorder with an incidence of 1 in 3500 male births, and cardiomyopathy is becoming the leading cause of death. While Cardiac MRI (CMR) and late gadolinium enhancement (LGE) are important tools in recognizing myocardial involvement, myocardial strain imaging may demonstrate early changes and allow patients to avoid gadolinium contrast. We performed CMR feature tracking (FT) and echo-based speckle tracking (STE) strain measures on DMD patients and age/sex matched controls who had received a CMR with contrast and transthoracic echocardiogram. Data were collected for longitudinal strain in the apical four-chamber view and circumferential strain in the mid-papillary parasternal short axis. Segmental wall analysis was performed and compared with the presence of LGE. Data were analyzed using student’s t tests or one-way ANOVA adjusting for multiple comparisons. We measured 24 subjects with DMD and 8 controls. Thirteen of 24 DMD subjects were LGE positive only in the lateral segments in short-axis views. Average circumferential strain (CS) measured by FT was significantly decreased in DMD compared to controls (− 18.8 ± 6.1 vs. − 25.5 ± 3.2; p < 0.001) and showed significant differences in the anterolateral, inferolateral, and inferior segments. Average CS by STE trended towards significance (p = 0.06) but showed significance in only the inferior segment. FT showed significant differences in the inferolateral segment between LGE positive (− 15.5 ± 9.0) and LGE negative (− 18.2 ± 8.3) in DMD subjects compared to controls (− 28.6 ± 7.3; p ≤ 0.04). FT also showed significant differences between anteroseptal and inferolateral segments within LGE-positive (p < 0.003) and LGE-negative (p < 0.03) DMD subjects while STE did not. There were no significant differences in longitudinal strain measures. CMR-FT-derived myocardial strain was able to demonstrate differences between subjects with DMD and controls not detected by STE. FT was also able to demonstrate differences in LGE-positive and LGE-negative segments within patients with DMD. FT may be able to predict LGE-positive segments in DMD without the use of gadolinium contrast.
Keywords: Cardiac MR, Feature tracking, Duchenne muscular dystrophy cardiomyopathy, Speckle tracking echocardiography, Late gadolinium enhancement
Background
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder affecting approximately 1:3500 male births worldwide. Due to degeneration of skeletal, respiratory, and cardiac muscle cells, these patients suffer from restrictive lung disease and a dilated cardiomyopathy causing significant morbidity and mortality. As respiratory care has improved, cardiomyopathy is now becoming the leading cause of death usually during the second or third decade [1].
Cardiac care is now focused on early detection and early initiation of treatment with the goal of delaying cardiac remodeling and myocardial dysfunction [2]. Clinical detection can be difficult given the lack of classical symptoms due to wheelchair dependence and limitations in activity [3]. With a focus on early detection and treatment, standard measures of left ventricular function and remodeling have been inadequate as changes in ejection fraction and shortening fraction both occur late in the course of cardiomyopathy [4].
Advanced echocardiographic techniques have shown promise in diagnosing myocardial dysfunction prior to a decrease in ejection fraction. Speckle tracking echocardiography (STE) was developed as an assessment of global and segmental strain. Previous studies showed that STE has the ability to demonstrate abnormal strain values between healthy and DMD patients [4–6]. However, echocardiographic modalities are limited by image quality and difficulty in this specific patient population with body habitus and positioning.
Cardiovascular magnetic resonance (CMR) has gained popularity in the assessment of cardiomyopathy associated with DMD due to its ability to give more accurate volumetric measures and to show areas of edema and fibrosis with late gadolinium enhancement (LGE) [2, 3, 7]. However, LGE requires administration of gadolinium-based contrast. Gadolinium has a favorable safety profile but it requires an intravenous line, increases scan time, and is contraindicated in renal impairment. Recently, there are concerns with accumulation of some types of gadolinium contrast in the brain [8].
CMR feature tracking (CMR-FT) is a tissue tracking technique similar to STE that allows an operator to derive strain measures on CMR cine of steady-state free processing (SSFP) images without the acquisition of additional sequences [9]. CMR-FT has been validated against traditional tagging methods of measuring strain including harmonic phase imaging and has demonstrated differences in myocardial deformation in other cardiac diseases including hypertrophic cardiomyopathy, cardiac amyloidosis, and myocarditis [10–13]. It has been used to identify subclinical changes in inflammatory myocardial diseases and in congenital heart diseases after repair [14, 15].
Both CMR-FT and STE may have clinical roles in the early identification and monitoring of DMD cardiomyopathy without the use of gadolinium. In the current study, we sought to evaluate CMR-FT and STE as measures of myocardial dysfunction in DMD patients and to identify whether differences in these strain measures would be present prior to the development of LGE. We hypothesize that the strain measured by CMR-FT would be more effective than STE in detecting regional differences in myocardial deformation and in finding myocardial abnormalities prior to the development of LGE.
Materials and Methods
A retrospective, observational, cross-sectional study was performed on DMD and control patients who had clinically indicated echocardiograms and CMR with gadolinium contrast. The study was approved by the Children’s National Health System Institutional Review Board (#4193). Control patients were selected from a database of patients who had undergone CMR with gadolinium for a separate indication with normal intracardiac anatomy, normal function, and no LGE (no history of cyanosis, cardiopulmonary bypass, and not evaluated for myocarditis or other inflammatory cardiomyopathies). Routine volumetry was performed by obtaining a standard SSFP cine image and calculation of cardiac output used a segmented, free-breathing method or using motion-corrected re-binning imagery as previously described, which allows patients who have difficulty with breath holding to free-breathe [16]. Late gadolinium enhancement imaging was performed as previously described by our CMR group [17].
Echocardiogram using a Phillips iE33 (Phillips Ultrasound, Amsterdam, The Netherlands) was obtained within 8 weeks of the CMR, and Simpson’s ejection fraction (EF) was derived from the apical four-chamber view. Both DMD and control patients underwent CMR with 0.15 mmol/kg of gadobutrol being administered prior to standard LGE imaging (same sequence parameters) on a Siemens Aera 1.5T MRI scanner (Siemens Healthcare, Erlangen, Germany). Data points collected included MR-derived EF and segmental LGE.
CMR-FT and STE measures were performed in post-processing after the indicated studies were completed using TomTec Arena software (TomTec Imaging Systems, Germany). Feature tracking was performed on SSFP cine or motion-corrected re-binning images of mid-papillary short-axis and four-chamber views. For STE, images were obtained from the standard apical four-chamber and mid-papillary parasternal short-axis views. Echo images were collected at an average of 68.8 frames/s in the parasternal short-axis view and 64.1 frames/s in the apical four-chamber view. Tracings were done in apical four-chamber and mid-papillary short-axis views for longitudinal and circumferential strain, respectively. The left ventricle was segmented according to the American Heart Association segmental model for chamber quantification [18]. Figure 1 demonstrates an echocardiographic image, a CMR SSFP still-frame, and a CMR still-frame with LGE on the same patient with DMD.
Fig. 1.
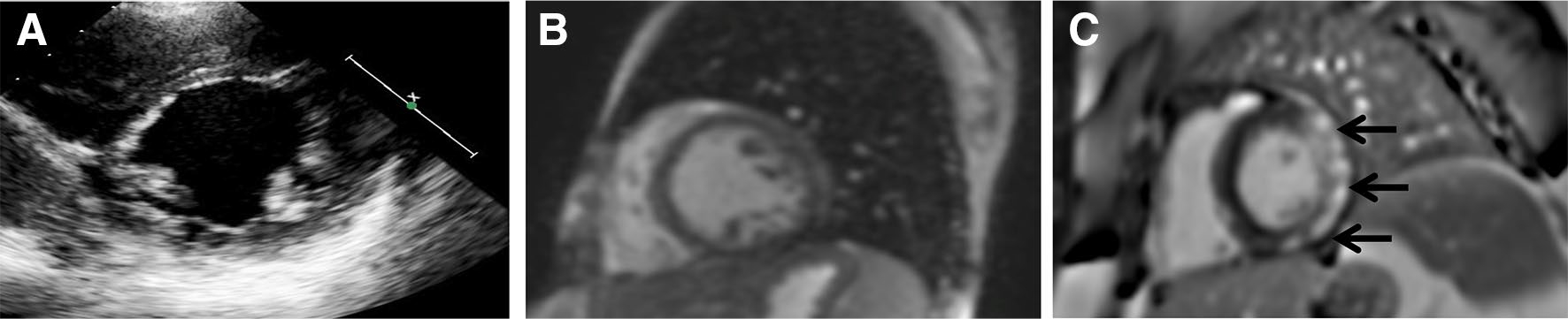
Three short-axis images of the same patient with Duchenne muscular dystrophy. a Echocardiographic image of mid-papillary short axis which is unable to show LGE but would be adequate for STE measures. b CMR short-axis steady-state free processing image without contrast which cannot show LGE but is able to be used for FT strain measures. c CMR short-axis imaging post-gadolinium contrast administration showing LGE (bright white) in the posterior and lateral segments (arrows)
Statistical Analysis
Data are presented as mean ± standard deviation. Normality of all strain outcomes was tested using a Shapiro–Wilk normality test. None of the outcomes showed deviations from normality; therefore, no data transformations were applied. We analyzed strain values measured in DMD patients and compared them to the values measured in controls. The DMD group was separated into two groups depending on the presence or absence of LGE. These groups were then compared to each other and to the control group. All comparisons were performed using either an independent student’s t test or a one-way analysis of variance model, appropriate for the number of groups being compared. For those outcomes tested using an ANOVA model and having an overall significant F test, post hoc pairwise comparisons were done and the resulting p value adjusted for multiple comparisons using the Sidak method.
Results
Patient Population
There were 24 patients with DMD and 8 controls. Age of the DMD cohort was 14.0 ± 4.2 years and the control cohort was age 16 ± 1.9 years. CMR-derived EF of the DMD cohort found 5 of 24 (29.2%) had an EF < 55% and 3 of 24 (12.5%) had an EF < 50%. None of the control cohort had an MRI determined EF < 55%. None of the controls had undergone cardiopulmonary bypass. The control patients initially underwent CMR and echocardiogram for arrhythmia or for initial concern of cardiomyopathy that was determined not to be present. LGE was present on 13 of 24 (54.2%) patients with DMD and none of the control patients.
Strain Measurements Between DMD and Control Patients
As shown in Table 1, strain measurements by CMR-FT differed in patients with DMD and controls in average circumferential strain (CS) (− 18.8 ± 6 vs. − 25.5 ± 3, p = 0.016). There were also differences in segmental CS using CMR-FT in the anterolateral, inferolateral, and inferior segments between DMD and controls (p < 0.03). Average CS by STE trended towards significance (p = 0.09) and statistical significance in segmental CS was only found in the inferior segment. Inter-observer reliability analysis was performed retrospectively on ten subjects for circumferential strain CMR-FT and STE measures and both had an interclass correlation coefficient (ICC) > 0.93. Intra-observer reliability analysis was also performed on ten subjects and had an ICC > 0.98 for both CMR-FT and STE. No significant differences were seen between groups when comparing longitudinal strain (data not shown).
Table 1.
CMR-FT demonstrates significant difference in average circumferential strain and in more segments than STE between DMD patients and controls
| Left ventricular segment | CMR-FT |
STE |
||||
|---|---|---|---|---|---|---|
| Control | DMD | p value | Control | DMD | p value | |
|
| ||||||
| Anterior | −20.23 ± 5.34 | −19.82 ± 8.58 | 0.90 | −23.36 ± 7.94 | −19.14 ± 7.71 | 0.19 |
| Anterolateral | −20.95 ± 8.44 | −14.59 ± 6.08 | 0.027 | −22.56 ± 8.02 | −18.58 ± 6.88 | 0.18 |
| Inferolateral | −28.64 ± 7.33 | −16.74 ± 8.61 | 0.002 | −21.73 ± 4.91 | −20.18 ± 6.82 | 0.56 |
| Inferior | −27.24 ± 6.18 | −17.45 ± 8.15 | 0.004 | −25.80 ± 4.28 | −19.07 ± 7.02 | 0.017 |
| Inferoseptal | −22.05 ± 4.70 | −20.10 ± 7.60 | 0.50 | −24.56 ± 6.71 | −19.40 ± 7.12 | 0.08 |
| Anteroseptal | −28.07 ± 5.25 | −24.00 ± 9.19 | 0.25 | −21.60 ± 9.45 | −21.70 ± 6.74 | 0.97 |
| Average | −25.53 ± 3.20 | −18.78 ± 6.05 | 0.016 | −23.26 ± 4.76 | −19.68 ± 5.15 | 0.09 |
Bold values indicate statistically significant
Data reported as mean ± SD
Relationship of Strain Measures to Development of Late Gadolinium Enhancement
Focusing on the left ventricular (LV) lateral wall segments that initially develop LGE in DMD patients, we compared strain measures in the LV anterolateral and inferolateral segments in LGE-positive and LGE-negative DMD groups compared to control patients. Significant differences were noted in the antero- and inferolateral segments using CMR-FT strain between the LGE-positive group and the control group. Table 2 also shows a statistically significant difference between both the control group and LGE-negative group in the inferolateral segment. For STE strain measures, there was no statistically significant difference between control and LGE-positive or LGE-negative groups with reference to circumferential strain in the antero- or inferolateral segments. This shows that with CMR-FT strain measures in the inferolateral segment, significant changes were seen in the DMD population in the absence of LGE.
Table 2.
Segmental comparison of strain values between control patients, DMD patients without LGE, and DMD patients with LGE using both STE and CMR-FT shows significant differences in CMR-FT measures
| Segment | Control (N = 8) | LGE− (N = 11) | LGE+ (N = 13) | Statistically different groups |
|---|---|---|---|---|
|
| ||||
| STE | ||||
| Anterolateral | −22.56 ± 8.02 | −21.14 ± 5.15 | −16.41 ± 7.58 | None |
| Inferolateral | −21.73 ± 4.91 | −21.96 ± 4.94 | −18.68 ± 7.96 | None |
| CMR-FT | ||||
| Anterolateral | −20.95 ± 8.44 | −16.47 ± 7.07 | −13.00 ± 4.83 | Control versus LGE+ (p = 0.037) |
| Inferolateral | −28.64 ± 7.33 | −18.23 ± 8.31 | −15.49 ± 9.00 | Control versus LGE− (p = 0.036) |
| Control versus LGE+ (p = 0.005) | ||||
Bold values indicate statistically significant
Data reported as mean ± SD
To further evaluate whether changes in myocardial strain can measure early myocardial disease, we compared septal and lateral segments within LGE-positive and LGE-negative DMD groups. Table 3 shows the differences in myocardial strain between the anterolateral and inferolateral segments compared to both the inferoseptal and anteroseptal segments in LGE-positive and LGE-negative patients. The LGE-negative patients trended towards significance comparing septal segments to the anterolateral wall. In the anteroseptal segment compared to the inferolateral segment, both LGE-positive and LGE-negative DMD groups showed significant differences in CMR-FT strain. Using STE strain measures, only the anterolateral to anteroseptal comparison segments showed differences in the LGE-positive patients.
Table 3.
(a) Segmental analysis of CMR-FT comparing LV segments that do not get LGE (inferoseptal and anteroseptal) with segments shown to have LGE (inferolateral and anterolateral) in DMD subjects; (b) segmental analysis of STE comparing LV segments that do not get LGE (inferoseptal and anteroseptal) with segments shown to have LGE (inferolateral and anterolateral) in DMD subjects
| (a) | |||||||
| CMR-FT | Group | Inferoseptal | Anterolateral | p value | Anteroseptal | Anterolateral | p value |
| Segmental circumferential strain | LGE− (N = 11) LGE+ (N = 13) |
−21.13 ± 8.82 −19.23 ± 1.84 |
−16.47 ± 7.07 −13.00 ± 1.34 |
0.08 0.004 |
−23.34 ± 10.08 −24.55 ± 8.73 |
−16.47 ± 7.07 −13.00 ± 4.84 |
0.06 0.002 |
| Group | Inferoseptal | Inferolateral | p value | Anteroseptal | Inferolateral | p value | |
| Segmental circumferential strain | LGE− (N = 11) LGE+ (N = 13) |
−21.13 ± 8.82 −19.23 ± 6.65 |
−18.23 ± 8.32 −15.49 ± 8.99 |
0.28 0.10 |
−23.34 ± 10.08 −24.55 ± 8.73 |
−18.23 ± 8.32 −15.49 ± 8.99 |
0.03
0.003 |
| (b) | |||||||
| STE | Group | Inferoseptal | Anterolateral | p value | Anteroseptal | Anterolateral | p value |
| Segmental circumferential strain | LGE− (N = 11) LGE+ (N = 13) |
−21.32 ± 6.27 −17.78 ± 7.62 |
−21.14 ± 5.15 −16.41 ± 7.58 |
0.95 0.55 |
−22.30 ± 6.78 −21.20 ± 6.94 |
−21.14 ± 5.15 −16.41 ± 7.58 |
0.63 0.023 |
| Group | Inferoseptal | Inferolateral | p value | Anteroseptal | Inferolateral | p value | |
| Segmental circumferential strain | LGE− (N = 11) LGE+ (N = 13) |
−21.32 ± 6.27 −17.78 ± 7.62 |
−21.96 ± 4.94 −18.68 ± 7.96 |
0.81 0.71 |
−22.30 ± 6.78 −21.20 ± 6.94 |
−21.96 ± 4.94 −18.68 ± 7.96 |
0.90 0.10 |
Data reported as mean ± SD
Discussion
This study showed that CMR-FT strain was able to demonstrate differences between DMD patients and control patients that were not detected by STE strain. Importantly, CMR-FT also demonstrated changes in strain measurements in LGE-negative segments of myocardium in DMD patients compared to controls. This signifies that CMR-FT may have utility as a biomarker in identifying early myocardial changes in the DMD patient population. This is the first study to demonstrate differences in CMR-FT strain in DMD patients and to show that CMR-FT changes occur prior to the development of LGE.
Historically, echocardiography is the gold standard for assessment of cardiac function in DMD and is often more accessible than CMR. However, echo can be limited in this population due to body habitus, chest wall deformities, lung interference, and difficulties with positioning that limit the acoustic windows, especially in older patients [4, 7]. Newer echo techniques including tissue Doppler imaging and STE are able to provide earlier assessments of myocardial function in DMD [4, 6]. However, the imaging limitations of DMD patients also affect these techniques. CMR is not affected by these limitations and offers improved imaging quality in DMD, especially in the teenage years. CMR can also assess myocardial strain using harmonic phase (HARP) imaging and feature tracking (CMR-FT) software. Previous studies using CMR HARP imaging showed that myocardial strain is an early biomarker of myocardial dysfunction in DMD patients [19]. However, HARP imaging requires additional tagged images that limit its clinical practicality while CMR-FT imaging can be performed on clinically acquired SSFP images. Good correlation between HARP circumferential strain and CMR-FT strain measures was demonstrated in DMD patients [10]. Therefore, using CMR-FT strain imaging can make strain measurements more clinically applicable. Also, while echo-based STE and CMR-FT were comparable in evaluating left ventricular function, they were not compared in the DMD patient population [20]. In our study, CMR-FT demonstrated changes in myocardial deformation in the DMD population that were not detected by STE.
DMD cardiomyopathy is present almost universally in DMD patients as they age into adulthood [21]. It is characterized by inflammation, cell death, and fibrotic replacement of muscular tissue due to instability in the myocardial cell membrane [22]. This fibrosis is detected using CMR-based contrast administration. LGE occurs in a predictable pattern in DMD cardiomyopathy, with primary areas affected including the LV inferolateral, anterolateral, inferior, and other free wall segments [23]. LGE in septal segments occurs only in patients with advanced disease [24]. The segmental analyses of CMR-FT presented in this study correlate well with the known development of LGE in the inferolateral, inferior, and anterolateral segments. These segments showed a significantly decreased circumferential strain compared to controls, while only the inferior segment showed that difference using STE. In LGE-negative DMD patients in particular, our results showed that CMR-FT strain detected changes in the segments known to develop LGE. This suggests that FT may be able to detect changes in damaged myocardium prior to the development of LGE. Comparisons between the segments known to develop LGE (inferolateral and anterolateral) and those known to not develop LGE (inferoseptal and anteroseptal) were made to further evaluate these measures. Differences in LGE-negative DMD patients between the inferolateral segment and the anteroseptal segment also provided evidence that CMR-FT may be able to detect changes prior to the development of LGE. This may allow for earlier diagnosis of myocardial damage and allow for initiation of earlier treatment and outcome monitoring.
Given recent concerns about the usage of gadolinium contrast, non-contrast imaging techniques such as STE and CMR-FT may be of increased utility in evaluating DMD patients [8]. Our study demonstrates that CMR-FT is superior to STE and still avoids the use of gadolinium. This is especially important for the longitudinal assessment of myocardial strain in these patients. Future research directions should focus on measuring changes in circumferential strain prospectively in DMD patients using CMR-FT to better correlate with the development of LGE with an ultimate goal of establishing guidelines for imaging that reduces overall gadolinium exposure. Limitations include that this is a single-center, retrospective study on a rare disease, thus limiting the study to a small number of patients. One reader also performed strain analysis on STE and one reader on CMR-FT but inter-observer reliability has previously been shown to be high in STE strain measures (ICC > 0.95) and our own analysis of inter-observer reliability was strong as well [4].
Conclusions
CMR-FT detects changes between DMD patients and controls not seen with STE. CMR-FT is able to detect changes in DMD myocardium in the absence of LGE. CMR-FT is a novel imaging biomarker for identifying early myocardial changes in DMD cardiomyopathy without the use of gadolinium and could be used as an outcome measure for early therapeutic trials and for clinical monitoring.
Funding
This study is funded through NHLBI Intramural Grant Number NHLBI-CSB-(HL)-2013-013-JML. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest Bryan Siegel declares that he has no conflict of interest. Laura Olivieri declares that she has no conflict of interest. Heather Gordish-Dressman declares that she has no conflict of interest. Christopher Spurney declares that he has no conflict of interest.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Research Involving Animal and Human Rights This article does not contain any studies with animals performed by any of the authors.
References
- 1.Eagle M, Baudouin SV, Chandler C et al. (2002) Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 12:926–929. 10.1016/S0960-8966(02)00140-2 [DOI] [PubMed] [Google Scholar]
- 2.McNally EM, Kaltman JR, Woodrow Benson D et al. (2015) Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 131:1590–1598. 10.1161/CIRCULATIONAHA.114.015151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spurney C, Shimizu R, Morgenroth LP et al. (2014) Cooperative international neuromuscular research group duchenne natural history study demonstrates insufficient diagnosis and treatment of cardiomyopathy in duchenne muscular dystrophy. Muscle Nerve 50:250–256. 10.1002/mus.24163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spurney CF, McCaffrey FM, Cnaan A et al. (2015) Feasibility and reproducibility of echocardiographic measures in children with muscular dystrophies. J Am Soc Echocardiogr 28:999–1008. 10.1016/j.echo.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taqatqa A, Bokowski J, Al-Kubaisi M et al. (2016) The use of speckle tracking echocardiography for early detection of myocardial dysfunction in patients with duchenne muscular dystrophy. Pediatr Cardiol 37:1422–1428. 10.1007/s00246-016-1451-2 [DOI] [PubMed] [Google Scholar]
- 6.Ryan TD, Taylor MD, Mazur W et al. (2013) Abnormal circumferential strain is present in young duchenne muscular dystrophy patients. Pediatr Cardiol 34:1159–1165. 10.1007/s00246-012-0622-z [DOI] [PubMed] [Google Scholar]
- 7.Soslow JH, Xu M, Slaughter JC et al. (2016) Evaluation of echocardiographic measures of left ventricular function in patients with duchenne muscular dystrophy: assessment of reproducibility and comparison to cardiac magnetic resonance imaging. J Am Soc Echocardiogr 37232:1–9. 10.1016/j.echo.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malayeri AA, Brooks KM, Bryant LH et al. (2016) National institutes of health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13:237–241. 10.1016/j.jacr.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 10.1186/s12968-016-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hor KN, Gottliebson WM, Carson C et al. (2010) Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Bogarapu S, Puchalski MD, Everitt MD et al. (2016) Novel cardiac magnetic resonance feature tracking (CMR-FT) analysis for detection of myocardial fibrosis in pediatric hypertrophic cardiomyopathy. Pediatr Cardiol 37:663–673. 10.1007/s00246-015-1329-8 [DOI] [PubMed] [Google Scholar]
- 12.Baessler B, Schaarschmidt F, Dick A et al. (2016) Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol 85:218–227. 10.1016/j.ejrad.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 13.Bhatti S, Vallurupalli S, Ambach S et al. (2016) Myocardial strain pattern in patients with cardiac amyloidosis secondary to multiple myeloma: a cardiac MRI feature tracking study. Int J Cardiovasc Imaging [DOI] [PubMed] [Google Scholar]
- 14.Bratis K, Hachmann P, Child N et al. (2017) Cardiac magnetic resonance feature tracking in Kawasaki disease convalescence. Ann Pediatr Cardiol 10:18–25. 10.4103/0974-2069.197046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berganza FM, de Alba CG, Özcelik N, Adebo D (2017) Cardiac magnetic resonance feature tracking biventricular two-dimensional and three-dimensional strains to evaluate ventricular function in children after repaired tetralogy of fallot as compared with healthy children. Pediatr Cardiol 38:566–574. 10.1007/s00246-016-1549-6 [DOI] [PubMed] [Google Scholar]
- 16.Cross R, Olivieri L, Brien KO et al. (2016) Improved workflow for quantification of left ventricular volumes and mass using free-breathing motion corrected cine imaging. J Cardiovasc Magn Reson. 10.1186/s12968-016-0231-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivieri L, Cross R, Brien KJO et al. (2016) Free-breathing motion-corrected late-gadolinium-enhancement imaging improves image quality in children. Pediatr Radiol. 10.1007/s00247-016-3553-7 [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V et al. (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J Cardiovasc Magn Reson 4:203–210. 10.1081/JCMR-120003946 [DOI] [PubMed] [Google Scholar]
- 19.Hor KN, Kissoon N, Mazur W et al. (2014) Regional circumferential strain is a biomarker for disease severity in duchenne muscular dystrophy heart disease: a cross-sectional study. Pediatr Cardiol 36:111–119. 10.1007/s00246-014-0972-9 [DOI] [PubMed] [Google Scholar]
- 20.Aurich M, Keller M, Greiner S et al. (2016) Left ventricular mechanics assessed by two-dimensional echocardiography and cardiac magnetic resonance imaging: comparison of high-resolution speckle tracking and feature tracking. Eur Hear J Cardiovasc Imaging 17:1370. 10.1093/ehjci/jew042 [DOI] [PubMed] [Google Scholar]
- 21.Nigro G, Comi LI, Politano L, Bain RJI (1990) The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 26:271–277. 10.1016/0167-5273(90)90082-G [DOI] [PubMed] [Google Scholar]
- 22.Kamdar F, Garry DJ (2016) Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol 67:2533–2546. 10.1016/j.jacc.2016.02.081 [DOI] [PubMed] [Google Scholar]
- 23.Bilchick KC, Salerno M, Plitt D et al. (2011) Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy. J Cardiovasc Magn Reson 13:20. 10.1186/1532-429X-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hor KN, Taylor MD, Al-khalidi HR et al. (2013) Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 10.1186/1532-429X-15-107 [DOI] [PMC free article] [PubMed] [Google Scholar]


