Graphical Abstract
Summary: A total of 389 microRNA (miRNA) were identified in small extracellular vesicles (sEV) isolated from bovine colostrum, with the top 50 miRNA contributing to more than 90% of total abundance and predicted to target 2,655 genes associated with cellular processes, environmental information processing, and organismal systems. The expression profiles of sEV-associated miRNA in bovine colostrum was independent of the concentrations of immunoglobulin G.
Highlights
-
•
The RNA concentration in sEV isolated from bovine colostrum was highest using the combination of the miRCURY Cell/Urine/CSF and miRNeasy Mini kits.
-
•
The top 50 miRNA were the same using miRDeep2 and sRNAbench, predominated by let-7b, let-7a-5p, miR-30a-5p, and miR-148a.
-
•
Predicted target genes of the top 50 miRNA regulate PI3K-Akt and MAPK signaling pathways, axon guidance, and focal adhesion.
-
•
The abundance of miR-27a-3p was higher in colostrum with high IgG concentrations.
Abstract
The consumption of bovine colostrum by newborn calves during the first days of life is essential to ensure the transfer of passive immunity. In addition to critical IgG, colostrum also contains non-IgG biomolecules, including microRNA (miRNA). The present study investigated the profiles of miRNA in small extracellular vesicles (sEV) isolated from bovine colostrum with high (256.5 ± 5.7 mg/mL, mean ± standard deviation, n = 4) and low (62.8 ± 3.6 mg/mL, n = 4) concentrations of IgG. Different combination of sEV extraction methods and bioinformatic pipelines (miRDeep2 and sRNAbench) for miRNA analysis were evaluated. Results showed that miRCURY exosome Cell/Urine/CSF and miRNeasy Mini kits yielded the highest RNA concentration. The miRNA-seq data analysis showed miRDeep2 yielded more comprehensive miRNAome compared with sRNAbench (527 versus 392 unique miRNA), whereas 389 shared miRNA were identified using both approaches. The profiles of top 50 miRNA were the same using both approaches, and their abundance contributed to 91.7% and 94.3% of total abundance of miRNA using miRDeep2 and sRNAbennch, respectively. These core miRNA were predicted to target 2,655 genes, which regulate 78 KEGG (Kyoto Encyclopedia of Genes and Genomes) level-3 pathways including PI3K-Akt and MAPK signaling pathway, axon guidance, and focal adhesion. The expression profiles of sEV-associated miRNA were similar between high- and low-IgG colostrum samples, despite the fact that the abundance of miR-27a-3p was higher in colostrum with high concentrations of IgG. In conclusion, a core miRNAome in bovine colostrum may play a role in regulating health and developmental stages in neonatal calves, independent of IgG concentration.
Colostrum provides the first source of nutrients and immunological protection for newborn calves and its consumption immediately or within a short period of time after birth is critical to calf health, survival (Chigerwe et al., 2015), long-term growth (Abuelo et al., 2021), and future milk production (Armengol and Fraile, 2020). Bovine colostrum contains high concentrations of IgG along with other bioactive compounds including growth factors, host defense peptides, and oligosaccharides (Arslan et al., 2021). It has been shown that bovine colostrum also contains abundant noncoding RNA such as microRNA (miRNA; Chen et al., 2010; Izumi et al., 2012; Zeng et al., 2019), which are small RNA molecules (i.e., 21–25 nucleotides; Lau et al., 2001) that regulate gene expression at a posttranscriptional level (Pritchard et al., 2012). Milk-derived miRNA are packaged in small extracellular vesicles (sEV), expecially exosomes (nanosized vesicles with 40–100 nm diameter) that make them resistant to acidic and RNase conditions (Izumi et al., 2012). These miRNA could be released to the extracellular environment through multivesicular bodies after budding with the plasma membrane (van Dommelen et al., 2012). For example, miR-148a, highly expressed in human and bovine milk exosomes (Izumi et al., 2015; Ma et al., 2019a), can be absorbed by intestinal cells and downregulate the expression of DNA methyltransferase 1, a gene involved in epigenetic regulation (Liao et al., 2017). Likewise, miR-223 is found in bovine milk exosome and decreases dual luciferase reporter gene expression in human HeLa cells (Benmoussa et al., 2020). Despite the knowledge of sEV-associated miRNA in bovine colostrum (Chen et al., 2010; Izumi et al., 2012) and their roles in neonatal homeostasis and immune development (Van Hese et al., 2020), their functions and associations with other immune effectors present in colostrum, including IgG, have been seldomly addressed. In this study, we aimed to determine the expression profiles and potential functions of sEV-associated miRNA in bovine colostrum containing different concentrations of IgG.
This study was approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC13-0324, AC15-0150, AC16-0209, AC18-0204) and conducted in accordance with guidelines established by the Canadian Council on Animal Care.
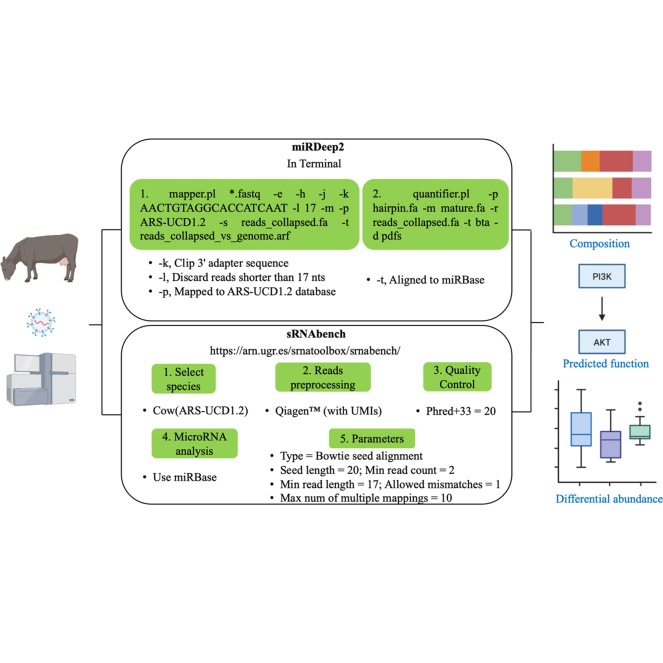
Colostrum samples were collected from 6 beef heifers (first parity) and 2 beef cows (third or higher parity) from 4 different privately owned cow-calf operations with either purebred Angus, Hereford, or Angus × Hereford crossbreds in southern Alberta, Canada. These samples were from 416 colostrum samples with extremely different IgG concentrations (Gamsjäger et al., 2020). Twenty milliliters of colostrum was collected manually, pooled from all teats into a clean plastic sampling cup, and stored at −80°C (Pearson et al., 2019a,b; Gamsjäger et al., 2020). The IgG concentration in these 8 colostrum samples were measured by radial immunodiffusion. Briefly, antiserum to bovine IgG was incorporated into agarose gels and serial dilutions of a reference serum containing known quantities of bovine IgG (Chelack et al., 1993). All colostrum samples were collected within 24 h postpartum. In addition, 3 colostrum samples collected from 3 dairy cows (Dairy Research and Technology Centre, University of Alberta, Edmonton, AB, Canada) in a similar way as those collected from beef cows were used as positive controls to evaluate 2 different methods for purification and RNA isolation from sEV. First, a Plasma/Serum Exosome Purification method (Norgen Biotek Corp.) was used, where 1 mL of colostrum was centrifuged once (10,000 × g, 15 min, room temperature) to remove fat, cells, large vesicles, and debris following the manufacturer's instructions. Norgen's proprietary resin was added into the supernatant to capture larger particles and exosomes were passed through the filter (sample 1; Table 1). Second, the miRCURY Cell/Urine/CSF method (Qiagen) was used where the supernatant was mixed with precipitation buffer and incubated at 4°C overnight to diminish the hydration of the subcellular particles and allow the precipitation of sEV with a low-speed centrifugation step. Both filtrates and precipitates were used to perform RNA extraction including miRNA using either Exosome RNA Isolation (sample 2; Norgen Biotek Corp.) or miRNeasy Mini kits (sample 3; Qiagen) following the manufacturer's instructions (Table 1). The RNA concentration of sEV isolated from the 3 samples was measured before miRNA isolation using a Qubit 3.0 Fluorometer (Invitrogen). The RNA yield was highest (45.46 ng/μL) using miRCURY Exosome Cell/Urine/CSF method for sEV purification followed by miRNeasy Mini kits (Table 1). The RNA concentrations in bovine colostrum exosome was reported to be ranging from 0 to 100 ng/μL using different kits as reported by Wijenayake et al. (2021). This combination was subsequently used for extracting sEV from 8 bovine colostrum samples with different IgG concentrations. It is noteworthy that both polymer-based precipitation methods (Exosome Purification, Norgen Biotek, and miRCURY Exosome Cell/Urine/CSF; García-Romero et al., 2019) do not require ultracentrifugation, thus reducing the likehood of protein aggregation, lipoprotein contamination (Li et al., 2017), and mechanical damage (Yang et al., 2020), and are less time consuming.
Table 1.
Different small extracellular vesicle purification and isolation methods applied in bovine colostrum
| Sample1 | Purification kit | RNA isolation kit | RNA concentration2 (ng/μL) | miRNA library quality check3 |
|---|---|---|---|---|
| 1 | Plasma/Serum Exosome Purification Kit (Norgen Biotek Corp.) | Exosome RNA Isolation Kit (Norgen Biotek Corp.) | 18.05 | Passed |
| 2 | miRCURY Exosome Cell/Urine/CSF Kit (Qiagen) | Exosome RNA Isolation Kit (Norgen Biotek Corp.) | Failed | Failed |
| 3 | miRCURY Exosome Cell/Urine/CSF Kit (Qiagen) | miRNeasy Mini Kit (Qiagen) | 45.46 | Passed |
Three colostrum samples were collected from 3 dairy cows at the Dairy Research and Technology Centre (University of Alberta, Edmonton, Canada). Each colostrum sample was subjected to one combination of purification and RNA isolation kits.
The RNA concentration was measured using a Qubit 3.0 Fluorometer (Invitrogen) using 1 μL of RNA (1–1.5 mL of samples).
Quality check was done by analyzing 1 μL of the miRNA Sequencing Library on a 2200 TapeStation System (Agilent Technologies) using a High Sensitivity DNA chip according to the manufacturer's instructions.
The 8 sEV-associated miRNA libraries were then constructed using QIAseq miRNA Library Kit (Qiagen) adhering to the manufacturer's instructions. In brief, 100 ng of total RNA was ligated with adapters sequentially to the 3′ and 5′ ends of miRNA, cDNA synthesis, followed by amplification of 14 cycles. The size profile of the individual libraries (n = 8) was analyzed utilizing D1000 High Sensitivity Screen Tape on a 2200 TapeStation System (Agilent Technologies). The average size of the libraries was 170 ± 3 bp, and the average quality score expressed as the percentage of integrated area was 95% ± 3%. Libraries were quantified using a Qubit 3.0 Fluorometer (Invitrogen) and pooled libraries were sequenced using the platform of HiSeq 4000 SR to generate the sequences of 50 bp (Centre d'expertise et de services Génome Québec, McGill University, Montréal, QC, Canada). The profiles and expressions of miRNA were determined using 2 bioinformatic pipelines, miRdeep2 (Friedländer et al., 2012), and sRNAbench (Aparicio-Puerta et al., 2019), respectively. The predicted putative target genes of the core and differentially abundant (high vs. low IgG) miRNA were determined by TargetScan (http://www.targetscan.org/vert_80/). The cutoff of TargetScan PCT score was set to 0.8 as previously described (Lu and Clark, 2012). The putative target genes meeting this criterion were then submitted to Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov; Huang et al., 2009a,b) for functional annotation analysis.
The expression of miRNA in each library was normalized to counts per million reads (CPM) as follows: CPM = (miRNA read counts/total mapped read counts per library) × 1,000,000. The results of CPM was reported as mean ± standard deviation. Profiles of miRNA between high- and low-IgG groups were analyzed with permutational ANOVA (PERMANOVA) using ‘betadisper' function in ‘vegan' package and visualized by principal component analysis using ‘ggfortify' (Tang et al., 2016) package, whereas differential expression analysis was conducted using DESeq2 (Love et al., 2014) package in R studio (R Foundation for Statistical Computing). Significant differences were declared at Benjamini-Hochberg adjusted P < 0.05. For functional annotation analysis, a pathway was significantly enriched when it passed the count threshold of 3 genes per annotation term and P-values with Benjamini-Hochberg correction set to <0.05. The miRNA sequences were deposited in the National Center for Biotechnology Information Sequence Read Archive (SRR14121703-SRR14121710).
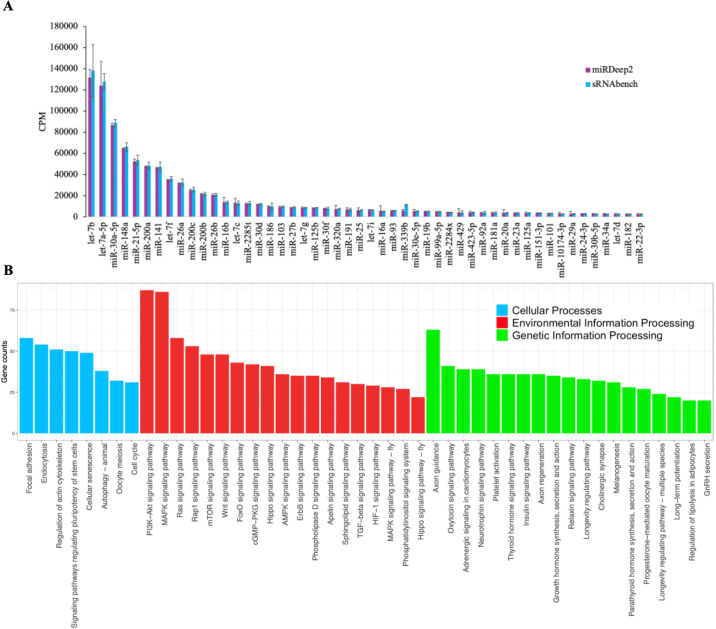
The sEV-associated miRNA profiles were assessed by miRDeep2 and sRNAbench using the same parameters when possible (e.g., minimum read count and minimum read length). These methods yielded similar number of differentially abundant miRNA in thioacetamide-treated rat liver samples with lesser variation compared with miRExpress and miRNAkey (Bisgin et al., 2018). In total, 527 and 392 miRNA (CPM >1) were identified in 8 samples using miRDeep2 and sRNAbench, respectively, with 389 miRNA identified by both methods. miR-29e, miR-2285bd, and miR-2368–3p were only identified using sRNAbench but in only 1 or 2 colostrum samples. Although more miRNA were uniquely identified using miRDeep2, these miRNA (n = 138) only accounted for 2% of the total abundance of 527 miRNA and were only identified in a few samples. The profiles of the top 50 miRNA were the same and were detetected in all 8 samples using miRDeep2 and sRNAbench, which accounted for 91.7% ± 0.44% and 94.3% ± 0.45% of total miRNA abundance, respectively, suggesting that both approaches identified a “core” miRNA in bovine colostrum. Both methods identified let-7b, let-7a-5p, miR-30a-5p, and miR-148a as the predominant miRNA (Figure 1A). In agreement, a sequencing-based study showed abundance of miR-148a (83.4%) among 290 miRNA (CPM >1) in milk from healthy cows (Ma et al., 2019a). In addition, miR-30a-5p was the second predominant exosomal miRNA in bovine colostrum collected 3 d postpartum (Yun et al., 2021). Moreover, miR-148a (15.6%), miR-21–5p (4.13%), and let-7a-5p (2.80%) were the most abundant milk miRNA in dairy cows from the last day of lactation to 21 d post dry-off (Putz et al., 2019). However, other microarray studies with a lower number of sampled cows (n = 3) showed a preponderance of miR-2478 in bovine milk whey exosomes (Izumi et al., 2015). These differences may be due to different miRNAome between colostrum and milk, lactating stages, breeds (dairy versus beef), as well as the utilized methods for sEV isolation and identification (microarray vs. RNA-seq).
Figure 1.
(A) Abundance of the top 50 small extracellular vesicle (sEV)-associated microRNA (miRNA) in the 8 bovine colostrum samples identified by 2 different bioinformatic pipelines: miRDeep2 (purple bar; Friedländer et al., 2012) and sRNAbench (blue bar; Aparicio-Puerta et al., 2019). (B) Main Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (generated using the Database for Annotation, Visualization, and Integrated Discovery; DAVID) regulated by the predicted target genes [generated using TargetScan (http://www.targetscan.org/vert_80/), probability of preferentially conserved targeting (PCT) score was set to 0.8] of the top 50 sEV-associated miRNA in the 8 bovine colostrum samples. CPM = counts per million reads.
The top 50 miRNA identified in this study were predicted to target 2,655 unique genes. Among them, 2,504 genes were successfully converted in DAVID, of which 1,065 were involved in KEGG pathways. Functional analyses showed these genes regulate 78 KEGG level-3 pathways, with most genes regulating phosphatidylinositol 3-kinase/protein kinase B (PI3K-Akt) and mitogen-activated protein kinase (MAPK) signaling pathways, axon guidance, and focal adhesion (Figure 1B). The PI3K/AKT pathway consists of several kinases, phosphatases, and transcription factors that are key for cell migration, metabolism, and cycle progression (Plotnikov et al., 2011; Ashry et al., 2018) and activate glycolysis and inhibit gluconeogenesis in humans (Puigserver et al., 2003). We speculate that miRNA regulating the genes invovled in the PI3K/Akt signaling pathway could be key in affecting physiology in the neonatal calf. The miR-30a, abundant in our colostrum samples, regulates insulin sensitivity (McLean et al., 2015) and islet functions (Jiang et al., 2017) by signaling PI3K/Akt. Moreover, miR-30a-5p could regulate glucose metabolism through PI3K/Akt signaling in neonatal calves, mainly in young calves that often experience glucose dysbalances (e.g., postprandial hyperglycemia, hyperinsulinemia, and insulin resistance) (Bach et al., 2013; Pantophlet et al., 2016). The MAPK pathway regulates mammary epithelial cell proliferation, differentiation, and mammary gland branching morphogenesis (Huebner et al., 2016). Indeed, differentially expressed miRNA including miR-21–5p in mammary glands from dairy cattle under normal and heat stress were reported to be regulated by the MAPK pathway (Li et al., 2018). Axon guidance is necessary for growing neural axons to reach their target locations and let-7 downregulated the axon guidance genes during peripheral nerve regeneration in rats (Wang et al., 2019). Another key role of colostrum in calf's homeostasis (Fischer et al., 2018; Ma et al., 2019b) is the establishment of the microbiome in the small intestine (Malmuthuge et al., 2015), including the colonization of beneficial bacteria such as Bifidobacterium (Song et al., 2019) and Lactobacillus (Ma et al., 2019b). The exosome miRNA in colostrum identified in our study could directly or indirectly modulate these gut-microbial interactions. Indeed, the expression of miR-15/16, miR-29, and miR-196 in bovine colostrum correlated with the copy numbers of Bifidobacterium and Lactobacillus species in the gut of neonatal calves (Liang et al., 2014). Taken together, core sEV-associated miRNA in bovine colostrum seem to be associated with the metabolism and health of neonatal calves and dams.
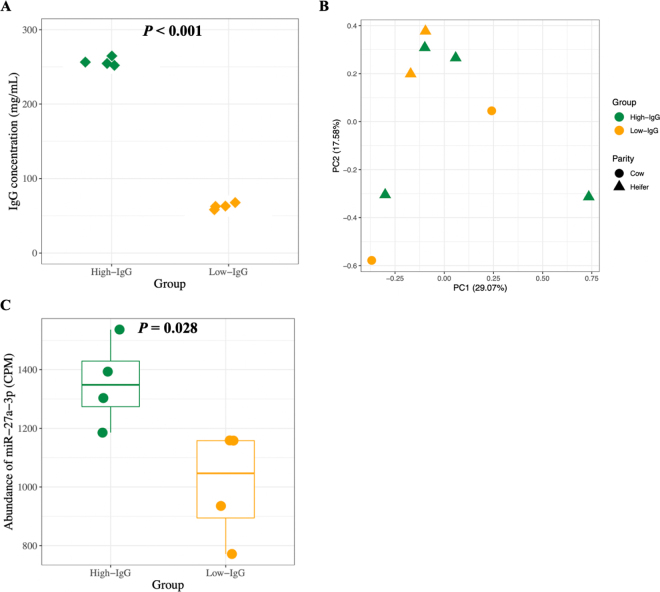
We categorized our bovine colostrum samples based on IgG concentrations as high (256.5 ± 5.7 mg/mL, mean ± SD, n = 4) and low (62.8 ± 3.6 mg/mL, n = 4; P < 0.001; Figure 2A). However, no miRNA was uniquely identified in all samples from one group except for miR-758, which was only expressed in all low-IgG samples using miRDeep2 (average CPM = 19). We showed similar miRNA profiles in colostrum with high- and low-IgG concentrations using both approaches. For example, principal component analysis showed no separation of miRNA with different IgG concentrations or parity (Figure 2B). The PERMANOVA analysis showed no difference in profiles of miRNA between 2 groups of samples (P = 0.565). No differentially abundant miRNA was identified based on differential expression analysis except for miR-27a-3p, which was more abundant in high- compared with low-IgG samples (P = 0.028; Figure 2C). This miR-27a-3p showed to promote osteogenic differentiation in pre-osteoblast (MC3T3-E1) cells (Ren et al., 2021), suggesting a role in the osteogenesis of neonatal calves.
Figure 2.
Profiles of small extracellular vesicle (sEV)-associated microRNA (miRNA) in bovine colostrum with different IgG concentrations identified by miRDeep2 (Friedländer et al., 2012). (A) Difference in concentrations of IgG between 2 groups of samples. (B) The principal component (PC) analysis plot of the miRNA profiles in high- and low-IgG colostrum. (C) Differential abundance of miR-27a-3p in high- and low-IgG colostrum samples (P = 0.028). The differential expression analysis was conducted using DESeq2 (Love et al., 2014). Significant differences were declared at Benjamini-Hochberg adjusted P < 0.05. CPM = counts per million reads.
This proof-of-concept study, although with a limited number of samples, summarizes that miRCURY Exosome Cell/Urine/CSF and miRNeasy Mini methods are suitable for sEV-associated miRNA examination in bovine colostrum. Both miRDeep2 and sRNAbench identified a similar core miRNA, which may play a role in regulating health and immune development functions in newborn calves beyond the known passive immunity associated with IgG. Although the overall miRNA profiles are similar, it is noticeable that expression of certain miRNA was only detected in one or a few individual animals (data not shown). Due to the small sample size, it is not possible to confirm the individualized expression of certain miRNA. Future study using a large amount of samples are needed to validate our findings and to reveal the effect of breed, farm, or parity on the profiles and expressions of sEV-associated miRNA in colostrum samples collected from dairy and beef cows.
Notes
This work was supported by an Natural Sciences and Engineering Research Council (NSERC) Discovery Grant, Alberta Agriculture and Forestry (RDAR 2019F041R; Edmonton, AB, Canada), Major Innovation Fund Program for the AMR–One Health Consortium (Alberta Ministry of Jobs, Economy, and Innovation; Edmonton, AB, Canada), and Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2017-FRI-04; Beijing, China), and Central Public-Interest Scientific Institution Basal Research Fund (Y2022QC10; Beijing, China).
We thank the Dairy Research and Technology Centre (University of Alberta, Edmonton, Canada) staff for their assistance with colostrum sample collection, students and staff (particularly Jennifer Pearson) at the University of Calgary (Calgary, AB, Canada) for their involvement in collection of beef cow colostrum, and Saskatoon Colostrum Company Ltd. (Saskatoon, SK, Canada) for the IgG measurments.
The authors have not stated any conflicts of interest.
References
- Abuelo A., Cullens F., Hanes A., Brester J.L. Impact of 2 versus 1 colostrum meals on failure of transfer of passive immunity, pre-weaning morbidity and mortality, and performance of dairy calves in a large dairy herd. Animals (Basel) 2021;11:782. doi: 10.3390/ani11030782. 33799858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio-Puerta E., Lebrón R., Rueda A., Gómez-Martín C., Giannoukakos S., Jaspez D., Medina J.M., Zubkovic A., Jurak I., Fromm B., Marchal J.A., Oliver J., Hackenberg M. sRNAbench and sRNAtoolbox 2019: Intuitive fast small RNA profiling and differential expression. Nucleic Acids Res. 2019;47(W1):W530–W535. doi: 10.1093/nar/gkz415. 31114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengol R., Fraile L. Feeding calves with pasteurized colostrum and milk has a positive long-term effect on their productive performance. Animals (Basel) 2020;10 doi: 10.3390/ani10091494. 32847051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan A., Kaplan M., Duman H., Bayraktar B., Ertürk M., Henrick B.M., Frese S.A., Karav S. Bovine colostrum and its potential for human health and nutrition. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.651721. 34235166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashry M., Rajput S.K., Folger J.K., Knott J.G., Hemeida N.A., Kandil O.M., Ragab R.S., Smith G.W. Functional role of AKT signaling in bovine early embryonic development: Potential link to embryotrophic actions of follistatin. Reprod. Biol. Endocrinol. 2018;16:1. doi: 10.1186/s12958-017-0318-6. 29310676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach A., Domingo L., Montoro C., Terre M. Short communication: Insulin responsiveness is affected by the level of milk replacer offered to young calves. J. Dairy Sci. 2013;96:4634–4637. doi: 10.3168/jds.2012-6196. 23660138. [DOI] [PubMed] [Google Scholar]
- Benmoussa A., Laugier J., Beauparlant C.J., Lambert M., Droit A., Provost P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 2020;103:16–29. doi: 10.3168/jds.2019-16880. 31677838. [DOI] [PubMed] [Google Scholar]
- Bisgin H., Gong B., Wang Y., Tong W. Evaluation of bioinformatics approaches for next-generation sequencing analysis of microRNAs with a toxicogenomics study design. Front. Genet. 2018;9:22. doi: 10.3389/fgene.2018.00022. 29467792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelack B.J., Morley P.S., Haines D.M. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can. Vet. J. 1993;34:407–412. 17424250. [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gao C., Li H., Huang L., Sun Q., Dong Y., Tian C., Gao S., Dong H., Guan D., Hu X., Zhao S., Li L., Zhu L., Yan Q., Zhang J., Zen K., Zhang C.Y. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010;20:1128–1137. doi: 10.1038/cr.2010.80. 20548333. [DOI] [PubMed] [Google Scholar]
- Chigerwe M., Hagey J.V., Aly S.S. Determination of neonatal serum immunoglobulin G concentrations associated with mortality during the first 4 months of life in dairy heifer calves. J. Dairy Res. 2015;82:400–406. doi: 10.1017/S0022029915000503. 26383079. [DOI] [PubMed] [Google Scholar]
- Fischer A.J., Song Y., He Z., Haines D.M., Guan L.L., Steele M.A. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J. Dairy Sci. 2018;101:3099–3109. doi: 10.3168/jds.2017-13397. 29397179. [DOI] [PubMed] [Google Scholar]
- Friedländer M.R., Mackowiak S.D., Li N., Chen W., Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. 21911355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsjäger L., Elsohaby I., Pearson J.M., Levy M., Pajor E.A., Haines D.M., Windeyer M.C. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J. Vet. Intern. Med. 2020;34:1662–1673. doi: 10.1111/jvim.15805. 32463548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Romero N., Madurga R., Rackov G., Palacín-Aliana I., Núñez-Torres R., Asensi-Puig A., Carrión-Navarro J., Esteban-Rubio S., Peinado H., González-Neira A., González-Rumayor V., Belda-Iniesta C., Ayuso-Sacido A. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J. Transl. Med. 2019;17:75. doi: 10.1186/s12967-019-1825-3. 30871557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. 19131956. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. 19033363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R.J., Neumann N.M., Ewald A.J. Mammary epithelial tubes elongate through MAPK-dependent coordination of cell migration. Development. 2016;143:983–993. doi: 10.1242/dev.127944. 26839364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H., Kosaka N., Shimizu T., Sekine K., Ochiya T., Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012;95:4831–4841. doi: 10.3168/jds.2012-5489. 22916887. [DOI] [PubMed] [Google Scholar]
- Izumi H., Tsuda M., Sato Y., Kosaka N., Ochiya T., Iwamoto H., Namba K., Takeda Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015;98:2920–2933. doi: 10.3168/jds.2014-9076. 25726110. [DOI] [PubMed] [Google Scholar]
- Jiang X., Xu C., Lei F., Liao M., Wang W., Xu N., Zhang Y., Xie W. MiR-30a targets IL-1α and regulates islet functions as an inflammation buffer and response factor. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-05560-1. 28706254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. 11679671. [DOI] [PubMed] [Google Scholar]
- Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. 28255367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yang C., Du J., Zhang B., He Y., Hu Q., Li M., Zhang Y., Wang C., Zhong J. Characterization of miRNA profiles in the mammary tissue of dairy cattle in response to heat stress. BMC Genomics. 2018;19:975. doi: 10.1186/s12864-018-5298-1. 30593264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., Malmuthuge N., McFadden T.B., Bao H., Griebel P.J., Stothard P., Guan L.L. Potential regulatory role of micrornas in the development of bovine gastrointestinal tract during early life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092592. 24682221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Du X., Li J., Lönnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700082. 28688106. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. 25516281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Clark A.G. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22:1243–1254. doi: 10.1101/gr.132514.111. 22456605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Tong C., Ibeagha-Awemu E.M., Zhao X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genomics. 2019;20:934. doi: 10.1186/s12864-019-6338-1. 31805863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., O'Hara E., Song Y., Fischer A.J., He Z., Steele M.A., Guan L.L. Altered mucosa-associated microbiota in the ileum and colon of neonatal calves in response to delayed first colostrum feeding. J. Dairy Sci. 2019;102:7073–7086. doi: 10.3168/jds.2018-16130. 31202657. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Chen Y., Liang G., Goonewardene L.A., Guan L.L. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. J. Dairy Sci. 2015;98:8044–8053. doi: 10.3168/jds.2015-9607. 26342981. [DOI] [PubMed] [Google Scholar]
- McLean C.S., Mielke C., Cordova J.M., Langlais P.R., Bowen B., Miranda D., Coletta D.K., Mandarino L.J. Gene and microRNA expression responses to exercise; Relationship with insulin sensitivity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127089. 25984722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet A.J., Gilbert M.S., van den Borne J.J.G.C., Gerrits W.J.J., Priebe M.G., Vonk R.J. Insulin sensitivity in calves decreases substantially during the first 3 months of life and is unaffected by weaning or fructo-oligosaccharide supplementation. J. Dairy Sci. 2016;99:7602–7611. doi: 10.3168/jds.2016-11084. 27289153. [DOI] [PubMed] [Google Scholar]
- Pearson J.M., Pajor E.A., Campbell J.R., Caulkett N.A., Levy M., Dorin C., Windeyer M.C. Clinical impacts of administering a nonsteroidal anti-inflammatory drug to beef calves after assisted calving on pain and inflammation, passive immunity, health, and growth. J. Anim. Sci. 2019;97:1996–2008. doi: 10.1093/jas/skz094. 30896739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.M., Pajor E., Campbell J., Levy M., Caulkett N., Windeyer M.C. A randomised controlled trial investigating the effects of administering a non-steroidal anti- inflammatory drug to beef calves assisted at birth and risk factors associated with passive immunity, health, and growth. Vet. Rec. Open. 2019;6 doi: 10.1136/vetreco-2019-000364. 31673377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta Mol. Cell Res. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. 21167873. [DOI] [PubMed] [Google Scholar]
- Pritchard C.C., Cheng H.H., Tewari M. MicroRNA profiling: approaches and considerations. Nat. Rev. Genet. 2012;13:358–369. doi: 10.1038/nrg3198. 22510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P., Rhee J., Donovan J., Walkey C.J., Yoon J.C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B.M. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. 12754525. [DOI] [PubMed] [Google Scholar]
- Putz E.J., Putz A.M., Jeon H., Lippolis J.D., Ma H., Reinhardt T.A., Casas E. MicroRNA profiles of dry secretions through the first three weeks of the dry period from Holstein cows. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56193-5. 31873189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.-R., Yao R.-B., Wang S.-Y., Gong X.-D., Xu J.-T., Yang K.-S. MiR-27a-3p promotes the osteogenic differentiation by activating CRY2/ERK1/2 axis. Mol. Med. 2021;27:43. doi: 10.1186/s10020-021-00303-5. 33902432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Malmuthuge N., Li F., Guan L.L. Colostrum feeding shapes the hindgut microbiota of dairy calves during the first 12 h of life. FEMS Microbiol. Ecol. 2019;95 doi: 10.1093/femsec/fiy203. 30307547. [DOI] [PubMed] [Google Scholar]
- Tang Y., Horikoshi M., Li W. ggfortify: Unified interface to visualize statistical results of popular r packages. R J. 2016;8:474–485. doi: 10.32614/RJ-2016-060. [DOI] [Google Scholar]
- van Dommelen S.M., Vader P., Lakhal S., Kooijmans S.A., van Solinge W.W., Wood M.J., Schiffelers R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release. 2012;161:635–644. doi: 10.1016/j.jconrel.2011.11.021. 22138068. [DOI] [PubMed] [Google Scholar]
- Van Hese I., Goossens K., Vandaele L., Opsomer G. Invited review: MicroRNAs in bovine colostrum—Focus on their origin and potential health benefits for the calf. J. Dairy Sci. 2020;103:1–15. doi: 10.3168/jds.2019-16959. 31677833. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen Q., Yi S., Liu Q., Zhang R., Wang P., Qian T., Li S. The microRNAs let-7 and miR-9 down-regulate the axon-guidance genes Ntn1 and Dcc during peripheral nerve regeneration. J. Biol. Chem. 2019;294:3489–3500. doi: 10.1074/jbc.RA119.007389. 30626732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijenayake S., Eisha S., Tawhidi Z., Pitino M.A., Steele M.A., Fleming A.S., McGowan P.O. Comparison of methods for pre-processing, exosome isolation, and RNA extraction in unpasteurized bovine and human milk. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257633. 34591894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Zhang W., Zhang H., Zhang F., Chen L., Ma L., Larcher L.M., Chen S., Liu N., Zhao Q., Tran P.H.L., Chen C., Veedu R.N., Wang T. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–3707. doi: 10.7150/thno.41580. 32206116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun B., Kim Y., Park D.J., Oh S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J. Anim. Sci. Technol. 2021;63:593–602. doi: 10.5187/jast.2021.e39. 34189507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B., Chen T., Xie M.-Y., Luo J.-Y., He J.-J., Xi Q.-Y., Sun J.-J., Zhang Y.-L. Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 2019;102:6726–6737. doi: 10.3168/jds.2019-16257. 31155266. [DOI] [PubMed] [Google Scholar]