Abstract
Cytotoxicity assays provide an in vitro evaluation of the lytic activity of NK and T cells against tumors or transformed cells. However, none of these methods allow the recovery of cells or supernatants after the assay. We standardized a microcytotoxicity test using calcein-acetoxymethyl (calcein-AM) dye that requires very small quantities of cells while maintaining the same sensitivity as the traditional 51Cr assay. The assay is applicable to resting as well as activated human effector cells and uses different targets such as human cell lines that are adherent or growing in suspension and resistant or sensitive. The most important feature of the method is the possibility of recovering cells and supernatants for additional analyses such as phenotyping and evaluation of soluble factors.
Cytotoxicity assays provide an in vitro evaluation of the lytic activity of natural killer (NK) and T cells against tumors or transformed target cells (3, 16, 25). In in vitro experimental conditions, lytic activity is evaluated using isotopes or dyes either released from dead cells or retained by living ones. The 51chromium (51Cr) release assay is the most widely used method although it has several significant drawbacks. The major disadvantage results from the use of a radioactive compound, with the related problems of handling and disposal due to the short half-life of the isotope.
To overcome these problems, several nonradioactive methods have been developed, but none have yet found a broad acceptance, probably due to a lack of comparability of their results with the results obtained using the 51Cr release assay, which still remains the most popular. Some alternative methods consist of measuring endogenous or transfected reporter enzymes released in the supernatant by dead targets (1, 11, 19, 24). Other assays using nonradioactive compounds (dimethylthiazol-diphenyl bromide tetrazolium bromide, methylumbelliferyl heptanoate, Alamar blue) evaluate the variations in metabolic activity, which is directly proportional to the number of viable cells (6, 18). Alternative methods using fluorochromes (e.g., europium, D275, rhodamine-123, carboxyfluorescein diacetate, bis-carboxyethyl-carboxyfluorescein) (7, 9, 12, 14, 23, 26, 28) measure the amount of dye released from or remaining in prelabeled target cells (2, 8, 13). The major drawbacks for these methods appear to be the high spontaneous release of the fluorescent dye, the slow specific release of the fluorescein dye, and the low intensity of the fluorescence signal, all of which decrease the overall sensitivity of the test.
Among fluorescent dyes, the use of calcein-acetoxymethyl (calcein-AM) in cytotoxicity assays has already been described (13, 21, 22). This dye has good retention in targets and low pH sensitivity, and there is no stain transfer among cells (21). Acetoxymethyl ester of calcein is a lipid-soluble diester fluorogenic esterase substrate that passively crosses the cell membrane and that is frequently used to stain viable cells. Inside the cells it is converted by intracellular esterases into a polar lipid-insoluble fluorescent product (calcein) that is retained by cells with intact membranes but that is released by damaged ones (similarly to 51Cr), producing an intense green signal. Release of calcein in the supernatants recovered from cytotoxicity assays can be measured rapidly and with a high level of sensitivity by a fluorimeter. Alternatively, lytic activity could be determined by measuring the fluorescence retained in living cells after quenching the fluorescence released by dead targets (22, 27).
Frequently, cytotoxicity tests are limited by the number of available effector cells, as for poorly represented cellular subpopulations (such as NK subsets and sorted or cloned lymphocytes) or by small amounts of blood (e.g., from children and elderly people). In these situations, the ability to use a low number of effector cells and the ability to recover cells after the cytolytic activity test would greatly facilitate the performance of the assays.
Our objectives were (i) to test the possibility of recovering cells and supernatants after the calcein-AM cytotoxicity assay for additional analyses, such as membrane phenotyping tests and enzyme-linked immunosorbent assays (ELISA) in order to further optimize the use of the cell samples (this recovery is not possible when radioactive labeling is used), (ii) to standardize the calcein-AM cytotoxicity assay under the same conditions used in the 51Cr microassay previously described (16) and to demonstrate that the calcein-AM assay gives comparable results, and (iii) to evaluate the reliability of the method for the determination of NK lytic activity against adherent sensitive or resistant osteosarcoma tumor cell lines.
MATERIALS AND METHODS
Target tumor cells.
Four different cell lines, all obtained from the American Type Culture Collection (Manassas, Va.), were used as targets: K562, Molt-4, HOS, and Saos-2. K562 is an NK-susceptible erythroleukemia cell line, and Molt-4 is a relatively NK-resistant T-lymphoblastoid cell line. They grow in suspension in complete medium: RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal calf serum (Biological Industries, Kibbutz Beit Hacmek, Israel), 4 mM glutamine (Sigma), and 200 μg of gentamicin (Biological Industries)/ml. Cells were kept at log growth phase before use in the experiments. HOS and Saos-2 are human osteoblastic-like tumor cell lines that grow as adherent cells in complete medium. Before use in the experiments, cells in the logarithmic growth phase were detached from monolayers with trypsin-EDTA (Sigma), washed twice, and used as targets.
2.2. Effector cells.
Total lymphocytes (peripheral blood mononuclear cells [PBMC]) were separated from the peripheral blood of 11 healthy human volunteers by density gradient centrifugation, resuspended at the optimal concentration (2.5 × 106/ml) in complete medium, and used as effector cells.
51Chromium release assay.
NK cell lytic activity was tested against K562 using a standard chromium release assay (16). The test was performed in V bottom 96-well microtiter plates (Nunc, Roskilde, Denmark). Cells at various effector-to-target (E:T) cell ratios (from 50:1 to 0.5:1) were seeded in triplicate together with 51Cr-labeled target cells, as previously described (15). After 4 h at 37°C in 5% CO2, 1/2 volume of each supernatant (75 μl) was harvested and counted in a Topcount microplate scintillation gamma counter (Packard, Meriden, Conn.). Data were acquired in counts per minute. Specific lysis was calculated according to the formula [(test release − spontaneous release)/(maximum release − spontaneous release)] × 100 (5, 20). Spontaneous release represents 51Cr release from target cells in medium alone, and maximum release is the 51Cr release from target cells lysed in medium plus 2% Triton X-100, each measured in at least six replicate wells.
Calcein-AM release assay (macro- and microassay).
Calcein-AM was purchased from Molecular Probes (Eugene, Oreg.) as a 1-mg/ml solution in dry dimethyl sulfoxide. Target cells were resuspended in complete medium at a final concentration of 106/ml and incubated with 15 μM calcein-AM for 30 min at 37°C with occasional shaking. After two washes in complete medium cells were adjusted to 105/ml (macroassay) or to 104/ml (microassay). The test was performed in the same way as the 51Cr assay in V bottom 96-well microtiter plates (Nunc), with E:T ratios ranging from 50:1 to 0.5:1, in triplicate, and with at least six replicate wells for spontaneous (only target cells in complete medium) and maximum release (only target cells in medium plus 2% Triton X-100). Various numbers of mononuclear effector cells and labeled tumor target cells were seeded as follows. For the macroassay (standard) each well contained from 2.5 × 105 to 2.5 × 103 lymphocytes in 100 μl of complete medium and 5 × 103 target cells/50 μl of complete medium; for the microassay a 10-fold-lower dilution of both effector and target cells was used. After incubation at 37°C in 5% CO2 for 4 h, 75 μl of each supernatant was harvested and transferred into new plates. Samples were measured using a Spectramax Gemini dual-scanning microplate spectrofluorimeter (Molecular Devices, Sunnyvale, Calif.) (excitation filter: 485 ± 9 nm; band-pass filter: 530 ± 9 nm). Data were expressed as arbitrary fluorescent units (AFU). Percent lysis was calculated with the same formula used for the 51Cr assay.
Cytokine treatment.
To evaluate the possibility of detecting an increase in the lytic activity using the calcein-AM assay, lymphocytes were previously incubated with 100 U of recombinant human interleukin-2 (rhIL-2; Glaxo, Geneva, Switzerland)/ml or 10 ng of rhIL-12 (R&D Systems Europe Ltd., Abingdon, United Kingdom)/ml for 18 h. Untreated lymphocytes were used as controls. After incubation, lymphocytes were washed in complete medium and used in the standard calcein-AM cytotoxicity assay.
Analysis of cell pellets and supernatants recovered after the cytotoxicity assay.
PBMC used for immunophenotyping were either fresh or recovered after the cytotoxicity assay. Samples were washed three times with phosphate-buffered saline–2% fetal calf serum–0.1% sodium azide, adjusted to approximately 105 cells in 100 μl of the same buffer, and labeled with phycoerythrin (PE)-conjugated monoclonal antibody (MAb) CD-8 or CD-56 (Becton Dickinson) and Cy5-conjugated MAb CD-16 (Caltag Laboratories, Burlingame, Calif.). Incubations with MAbs were performed for 30 min at 4°C. Negative-control cells were incubated with an immunoglobulin isotype control. Analysis was performed by flow cytometry (4, 16).
Cell-free supernatants from 10 samples were collected before and after the calcein-AM cytotoxicity test (E:T ratio, 50:1). Four of these samples were also tested after 18 h of incubation with 100 U of rhIL-2/ml. RANTES levels were measured by a sandwich quantitative immunoassay with anti-human RANTES matched antibody pairs (Pharmingen). The standard curve range was from 15.6 to 2,000 pg/ml.
Statistical analysis.
Experimental data are expressed as means ± standard errors of the means (SEM). Statistical significance was evaluated using Student's t test for paired data, and regression analysis was performed to compare results. The Statistics for Windows package was used to carry out statistical analyses.
RESULTS
Comparison of cytotoxicity evaluated by 51Cr and calcein-AM release assays.
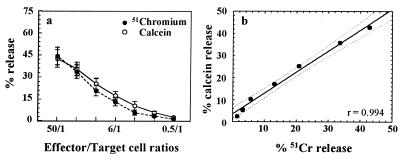
To evaluate the efficacy of the calcein-AM cytotoxicity assay, tests were performed in parallel to the 51Cr standard procedure using the same target (K562) and effector cells, the only difference being the labeling of the targets. Maximum- and spontaneous-release values corresponded, respectively, to 17,769 ± 3,991 and 1,506 ± 318 cpm for the 51Cr assay and 5,935 ± 405 and 2,186 ± 171 AFU for the calcein-AM assay. The mean spontaneous releases from K562 cells were about 10 and about 34% of the maximum for 51Cr and calcein-AM assays, respectively . Target cell viability was not affected by calcein-AM staining, as assessed by the eosin exclusion test (data not shown). To further confirm that the higher spontaneous release with calcein-AM than with 51Cr was not due to a poor dye-dependent target cell viability, a double labeling of K562 cells, first with calcein-AM and subsequently with 51Cr, was performed. Spontaneous release of 51Cr in these cells was similar to spontaneous release in the same cells labeled only with 51Cr (4.5 versus 6% of 51Cr release; mean of three experiments).
As shown in Fig. 1a, the mean curves from all 11 analyzed samples obtained with both methods overlapped. Regression analysis performed by plotting mean percentages of 51Cr release against the corresponding mean percentages of calcein-AM release (Fig. 1b) indicated a close positive correlation between the two methods (P < 0.001).
FIG. 1.
Comparison of the 51Cr and the calcein-AM cytotoxicity release assays of K562 target cells. (a) Results are expressed as mean percentages of release ± SEM for 11 experiments run in parallel. (b) Regression analysis. Each point represents the mean values of paired observations for all the E:T ratios tested. The fitted line is y = 3.6322 + 0.94794x (correlation coefficient, r = 0.994; P < 0.001). Dotted lines, 95% confidence interval.
Comparison between calcein-AM macro- and microassay.
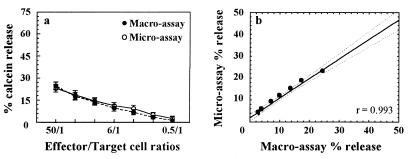
The correlation between the calcein-AM release macro- and microassays was evaluated in comparative experiments. For the microassays we utilized a 10-fold-lower concentration of targets and effectors than in the macroassays. The curves obtained using 5,000 or 500 K562 target cells overlapped (Fig. 2a). Mean maximum release obtained from 500 K562 cells was 4,994 ± 568 AFU, with a spontaneous release that never exceeded 40% of the maximum. Regression analysis performed by plotting mean percentages of calcein release obtained with macro- and microassays indicated a very close positive correlation (P < 0.001) (Fig. 2b). A significant positive correlation was also observed by plotting mean percentages of calcein and chromium released with a microassay (r = 0.997, P < 0.005) (not shown).
FIG. 2.
Comparison of cell-mediated cytotoxicity detected using the calcein-AM macro- or microassay of K562 target cells. (a) Results are expressed as mean percentages of release ± SEM for 11 experiments run in parallel. (b) Regression analysis. The fitted line is y = 1.9009 + 0.89479x (r = 0.993; P < 0.001). Dotted lines, 95% confidence interval.
Evaluation of NK stimulation by calcein-AM assay.
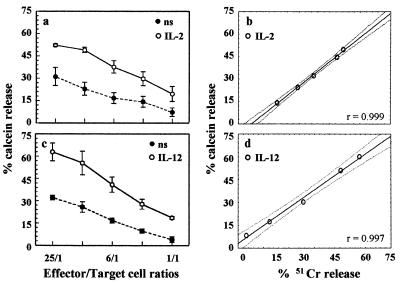
We also tested the ability of the calcein-AM method to detect the increase of lytic activity induced by interleukin-2 (IL-2) or IL-12 treatment of PBMC against K562 cells. An evident increase in calcein release occurs after 18 h of IL-2 incubation for all E:T ratios tested (P < 0.05) (Fig. 3a). Incubation of PBMC with IL-12 similarly enhanced lytic activity (Fig. 3c) (P < 0.05) for all the E:T ratios except at 3:1. Regression analysis performed by plotting mean percentages of calcein release versus chromium release obtained following cytokine incubation showed a very close positive correlation for both IL-2 (Fig. 3b) and IL-12 (Fig. 3d) stimulations (P < 0.001, at least).
FIG. 3.
Increase in lytic activity after cytokine treatment, detected by calcein-AM release assay. Shown is the lytic activity of four samples of PBMC, which were either resting (ns) or treated for 18 h with 100 U of IL-2/ml (a) or with 10 ng of IL-12/ml (c), against K562 cells. Results are mean percentages of release ± SEM (P < 0.05 for all the E:T ratios, except for the 3:1 ratio (c). (b and d) Regression analysis. Each point represents the mean value of paired observations for all the E:T ratios tested. The fitted line for IL-2-stimulated lymphocytes (b) is y = −6.758 + 1.0778x (correlation coefficient, r = 0.999; P < 0.001). The fitted line for IL-12-stimulated lymphocytes (d) is y = −14.53 + 1.1770x (correlation coefficient, r = 0.997; P < 0.005). Dotted lines, 95% confidence interval.
Calcein-AM assay using sensitive and resistant cell lines.
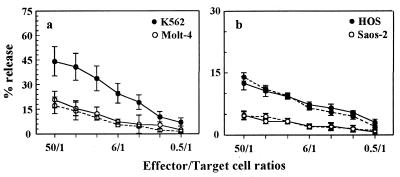
The calcein-AM method is also efficient using cell lines relatively resistant to NK lysis, such as Molt-4, as targets. Results indicated a significant difference in percent lysis at all E:T ratios compared to K562 targets, in agreement with the traditional 51Cr release assay (Fig. 4a).
FIG. 4.
Cytolytic activity against sensitive and resistant human cell lines measured by calcein-AM assay. (a) Human cell lines growing in suspension. Shown are means ± SEM of four experiments with PBMC tested against K562 and Molt-4 cells. Dashed line, results obtained by 51Cr assay using Molt-4 cells. (b) Adherent human osteosarcoma cell lines. Shown are means ± SEM of four experiments with PBMC tested against HOS and Saos-2 cells. Dashed lines, results obtained by 51Cr assay using HOS (closed symbols) and Saos-2 (open symbols).
The calcein-AM assay was also able to measure cytotoxicity against two adherent osteosarcoma cell lines, HOS and Saos-2. After detachment, a macroassay was performed. Maximum- and spontaneous-release values were, respectively, 5,854 ± 1,/0 and 1,849 ± 277 AFU for HOS cells and 5,292 ± 345 and 1,485 ± 68 AFU for Saos-2. Results indicated that HOS cells are more sensitive to NK lysis than Saos-2 cells (Fig. 4b), with a statistically significant difference between release values from 50:1 to 0.5:1 ratios (t test for paired data; P < 0.05), similar to results for the 51Cr release assay.
Recovery of cell pellets and supernatants after the calcein-AM assay.
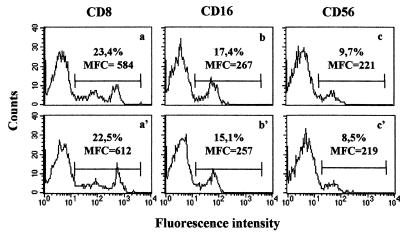
The use of a nonradioactive compound makes it possible to recover cells after the cytotoxicity assay for additional analyses. Lymphocytes recovered from cytotoxicity plates together with target cells were directly labeled with MAbs that recognize surface molecules on cytolytic cells: PE-conjugated anti-CD8 and anti-CD16 and Cy5-conjugated anti-CD56. The percentages of positive lymphocytes recovered after cytotoxicity assays were similar to those obtained with the same cells before performing cytotoxicity assays, as were the fluorescence intensities (Fig. 5). K562 cells were electronically excluded from the analysis on the basis of their different size.
FIG. 5.
Fluorescence histograms obtained by fluorescence-activated cell sorter analysis of membrane phenotype after a calcein-AM cytotoxicity assay. PBMC effectors that were fresh (a to c) or recovered after the assay (a′ to c′) were labeled with CD8-PE and CD16-Cy5 or CD56-PE. Percentages of positive lymphocytes and mean fluorescence channels (MFC) are indicated. One representative observation of 10 performed is shown.
Cell-free supernatants recovered after the cytotoxicity assay could also be used in an ELISA. Supernatants before and after the cytotoxicity assay (E:T ratio, 50:1) were collected from 10 samples and used to evaluate the production of the chemokine RANTES, which seems to be unmodulated during cytotoxicity assays (personal preliminary observations). RANTES levels in unstimulated samples before and after calcein-AM test were, respectively, 607 ± 81 and 645 ± 73 pg/ml. After treatment of four of these samples with IL-2 for 18 h, the RANTES level in supernatants recovered after the cytotoxicity assay was significantly increased (850.3 ± 183 pg/ml) with respect to that for the corresponding unstimulated samples (625 ± 169 pg/ml; P < 0.05), but it was similar to the amount of chemokine found in supernatants of IL-2-stimulated lymphocytes for which the cytotoxicity assay was not performed (927 ± 145 pg/ml).
DISCUSSION
NK cells play an important role in the cellular recognition and killing of virus-infected and tumor cells. The evaluation of lytic activity is therefore of great importance in monitoring the functional capability of these cells. Traditionally, cytotoxicity is measured by the 51Cr release assay (3), which has several disadvantages, including the use of a radioactive label and the time required to process samples. Several alternative approaches using fluorescent dyes have been proposed (2, 7–9, 12–14, 21–23, 26–28).
In the present study, we describe a modification of an easy and fast method for measuring lytic activity using a fluorescent nonradioactive compound (calcein-AM). This dye has good retention in living cells, bright fluorescence, and reduced spontaneous leakage compared to other fluorescent molecules such as, bis-carboxyethyl-carboxyfluorescein and carboxyfluorescein diacetate (13, 27). Labeling target cells with calcein-AM has been shown to have no effect on the formation of effector-target conjugates (4), and the intense green fluorescent stain is stable for at least 4 h (13). Calcein-AM cytotoxicity assays based on the evaluation of dye released in the supernatant used a relatively high number of target cells (15 × 103) (13); the number of targets used for the evaluation of dye retained by living cells (3 × 103) was also relatively high (22), except in a study in which the test was performed using Terasaki trays and 5 × 102 targets in 5 μl of medium (27). The method that we standardized requires very small quantities of cells to evaluate the dye released in the supernatant (500) and maintains the same experimental procedures and working volumes as in the 51Cr assay. We found a strict correlation between results, with no significant differences in the experimental release values, making it possible to compare data obtained using the two different techniques. Furthermore, by reducing in the number of cytolytic effector and target cells 10-fold, without reducing working volumes, we obtained results very similar to those of the standard calcein-AM test. The microassay is therefore as reliable as the standard one, and this is of great importance when small amounts of blood are available, as in studies on elderly people or children. Fluorescent calcein released from lysed cells can be easily and readily measured in the recovered supernatants using a standard automated plate-reading fluorimeter. The entire procedure is analogous to the chromium release assay but requires less time to perform. In fact, while the standard 51Cr assay requires 1 h to label target cells, calcein-AM labeling is performed in 30 min. In addition, the former method has slow processing times when large numbers of samples have to be counted (1 min per well), while with the calcein-AM assay and the Spectramax fluorimeter the detection time can be standardized to less than 1 s per well. We found that spontaneous release of calcein is significantly higher than that of 51Cr, particularly in the microassay, in accordance with previous findings (13). This higher spontaneous release does not depend on poor target cell viability caused by calcein-AM, as demonstrated by performing a double labeling of K562 cells with calcein-AM and subsequently with 51Cr. The technique shows sensitivities similar to those for 51Cr labeling while maintaining specificity. In effect, the difference between results never reached statistical significance, both assays revealing the same functional correlation. The slightly higher sensitivity of the calcein-AM assay could allow a reduction of the incubation time to less than 4 h, probably even reducing the spontaneous release rate. However, spontaneous release did not seem to be influenced by the dye concentration (13).
IL-2- and IL-12-activated total PBMC lyse the NK-susceptible K562 cells with more efficiency (10, 15), and this effect may also have been revealed by the calcein-AM assay, which is therefore applicable to studies of cytotoxicity with resting as well as activated human effector cells.
We also tested the calcein-AM assay in cytotoxicity tests against the relatively resistant Molt-4 cells, obtaining results comparable to those of the standard chromium technique. To our knowledge, there were no available data regarding the use of the calcein-AM assay to test lytic activity against adherent human osteosarcoma cell lines. Using cell lines HOS and Saos-2 as targets we observed that both lines retained calcein, maintained viability after staining, and displayed a spontaneous release similar to that of targets not treated with detaching enzymes (K562 and Molt-4). HOS cells were found to be more susceptible to lysis than Saos-2, in accordance with previous observations obtained with the 51Cr assay (17). The amount of lysis and the degree of the susceptibility of the two cell lines obtained with the calcein-AM assay were equivalent to those obtained with 51Cr.
A very important feature of the method is that cells can be recovered and the supernatants can be used again for additional biological analyses. This may provide numerous advantages in terms of quantities of material required and also may enable the characteristics of the cells or their soluble products to be studied after their lytic potential has been determined. We demonstrated that recovered cells could be labeled with MAbs. Labeling with PE or Cy5 MAbs that recognize molecules of the lymphocyte cell surface gave very similar results before and after determination of cytotoxicity with calcein. Fluorescein isothiocyanate (FITC)-conjugated antibodies were not used to avoid possible interference with the green fluorescence of calcein. In effect, even if K562 calcein-labeled targets could be electronically excluded from the analysis because of their different size, there is a weak interference of calcein present in the medium with effector cells, giving rise to a little shifting of the lymphocyte green fluorescence or a randomly distributed low autofluorescence signal (<10%) (data not shown). FITC-conjugated antibodies could therefore be used only to label surface molecules with high density or expressed in well-represented subpopulations.
We also demonstrated the possibility of using supernatants to evaluate soluble factors produced during the cytotoxicity assay. We observed that the presence of calcein was compatible with the development of an ELISA either with fresh cells or with interleukin-activated lymphocytes, for example, for the detection of the RANTES chemokine, chosen because it is not modulated during effector-target cell interactions.
In conclusion, the calcein-AM-based method seems to be a very good candidate for a substitute for the standard 51Cr assay because it is sensitive, easy, safe, and useful for different types of targets and because cells and supernatants can be recovered to perform additional analyses, especially when limited amounts of blood are available.
ACKNOWLEDGMENTS
This work was partially supported by grants from MURST (60% fund), Ricerca Corrente IOR, FP Health Ministry from Italy and ImAginE framework from the EU (QLK6-CT-1999-02031).
We thank Keith Smith for revising the English manuscript.
REFERENCES
- 1.Bachy M, Bonnin-Rivalland A, Tilliet V, Trannoy E. Beta galactosidase release as an alternative to chromium release in cytotoxic T-cell assays. J Immunol Methods. 1999;230:37–46. doi: 10.1016/s0022-1759(99)00118-0. [DOI] [PubMed] [Google Scholar]
- 2.Blomberg K, Ulfstedt A C. Fluorescent europium chelates as target cell markers in the assessment of natural killer cell cytotoxicity. J Immunol Methods. 1993;160:27–34. doi: 10.1016/0022-1759(93)90005-r. [DOI] [PubMed] [Google Scholar]
- 3.Brunner K T, Mauel J, Cerottini J C, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and drugs. Immunology. 1968;14:181–196. [PMC free article] [PubMed] [Google Scholar]
- 4.Callewaert D M, Radcliff G, Waite R, LeFevre J, Poulik M D. Characterization of effector-target conjugates for cloned human natural killer and human lymphokine activated killer cells by flow cytometry. Cytometry. 1991;12:666–676. doi: 10.1002/cyto.990120711. [DOI] [PubMed] [Google Scholar]
- 5.Facchini A, Mariani E, Mariani A R, Papa S, Vitale M, Manzoli F A. Increased number of circulating Leu 11+ (CD16) large granular lymphocytes and decreased NK activity during human ageing. Clin Exp Immunol. 1987;68:340–347. [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain R F, Nouri A M E, Oliver R T D. A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods. 1993;160:89–96. doi: 10.1016/0022-1759(93)90012-v. [DOI] [PubMed] [Google Scholar]
- 7.Iwao M, Ochi C, Kurane I, Muraoka S. A non-radioisotopic human natural killer cell assay using rhodamine-123 fluorescent dye as labelling probe. Immunol Investig. 1998;27:31–45. doi: 10.3109/08820139809070888. [DOI] [PubMed] [Google Scholar]
- 8.Johann S, Blumel G, Lipp M, Forster R. A versatile flow cytometry-based assay for the determination of short- and long-term natural killer cell activity. J Immunol Methods. 1995;185:209–216. doi: 10.1016/0022-1759(95)00116-r. [DOI] [PubMed] [Google Scholar]
- 9.Kolber M A, Quinones R R, Gress R E, Henkart P A. Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF) J Immunol Methods. 1988;108:255–264. doi: 10.1016/0022-1759(88)90427-9. [DOI] [PubMed] [Google Scholar]
- 10.Konjevic G, Schlesinger B, Cheng L, Olsen K J, Podack E R, Spuzic I. Analysis of perforin expression in human peripheral blood lymphocytes, CD56+ natural killer cell subsets and its induction by interleukin-2. Immunol Investig. 1995;24:499–507. doi: 10.3109/08820139509066846. [DOI] [PubMed] [Google Scholar]
- 11.Korzeniewski C, Callewaert D M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 12.Kroesen B J, Mesander G, ter Haar J G, The T H, de Leij L. Direct visualisation and quantification of cellular cytotoxicity using two colour fluorescence. J Immunol Methods. 1992;156:47–54. doi: 10.1016/0022-1759(92)90009-i. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenfels R, Biddison W E, Schulz H, Vogt A B, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 14.Lövgren J, Blomberg K. Simultaneous measurement of NK cell cytotoxicity against two target cell lines labelled with fluorescent lanthanide chelates. J Immunol Methods. 1994;173:119–125. doi: 10.1016/0022-1759(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 15.Mariani E, Meneghetti A, Tarozzi A, Cattini L, Facchini A. IL-12 induces efficient lysis of NK sensitive and NK resistant human osteosarcoma cells. The synergistic effect of IL-2. Scand J Immunol. 2000;51:618–625. doi: 10.1046/j.1365-3083.2000.00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Mariani E, Monaco M C G, Sgobbi S, de Zwart J F, Mariani A R, Facchini A. Standardization of a micro-cytotoxicity assay for human natural killer cell lytic activity. J Immunol Methods. 1994;172:173–178. doi: 10.1016/0022-1759(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 17.Mariani E, Tarozzi A, Meneghetti A, Cattini L, Facchini A. Human osteosarcoma cell susceptibility to natural killer cell lysis depends on CD54 and increases after TNFα incubation. FEBS Lett. 1997;406:83–88. doi: 10.1016/s0014-5793(97)00247-0. [DOI] [PubMed] [Google Scholar]
- 18.Nociari M M, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods. 1998;213:157–167. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 19.Ohmori H, Ikeda H, Tanigawa T, Hikida M. Enzyme release assay of human NK cell activity using beta-galactosidase-expressing K562 target cell line. J Immunol Methods. 1993;164:131–135. doi: 10.1016/0022-1759(93)90283-d. [DOI] [PubMed] [Google Scholar]
- 20.Ortaldo J R, Bonnard G D, Herbermann R B. Cytotoxic reactivity of human lymphocytes cultured in vitro. J Immunol. 1977;119:1351–1357. [PubMed] [Google Scholar]
- 21.Papadopoulos N G, Dedoussis G V Z, Spanakos G, Gritzapis A D, Baxevanis C N, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177:101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 22.Roden M M, Lee K-H, Panelli M C, Marincola F M. A novel cytolysis assay using fluorescent labelling and quantitative fluorescent scanning technology. J Immunol Methods. 1999;226:29–41. doi: 10.1016/s0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Yoshikawa K, Yokochi T. A new sensitive and rapid automated fluorometric assay for detection of natural killer activity using carboxyfluorescein diacetate. J Immunoassay. 1991;12:145–157. doi: 10.1080/01971529108055062. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres J, Pacsa A S, Pejtsik B. Measurement of lymphocyte cytotoxicity by assessing endogenous alkaline phosphatase activity of the target cells. J Immunol Methods. 1981;40:151–154. doi: 10.1016/0022-1759(81)90061-2. [DOI] [PubMed] [Google Scholar]
- 25.Virag L, Kerekgyarto C, Fachet J. A simple, rapid and sensitive fluorimetric assay for the measurement of cell-mediated cytotoxicity. J Immunol Methods. 1995;185:199–208. doi: 10.1016/0022-1759(95)00115-q. [DOI] [PubMed] [Google Scholar]
- 26.Von Zons P, Crowley-Nowick P, Friberg D, Bell M, Koldovsky U, Whiteside T L. Comparison of europium and chromium release assays: cytotoxicity in healthy individuals and patients with cervical carcinoma. Clin Diagn Lab Immunol. 1997;4:202–207. doi: 10.1128/cdli.4.2.202-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X M, Terasaki P I, Rankin G W, Jr, Chia D, Zhong H P, Hardy S. A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol. 1993;37:264–270. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 28.Wierda W G, Mehr D S, Kim Y B. Comparison of fluorochrome-labeled and 51Cr-labeled targets for natural killer cytotoxicity assay. J Immunol Methods. 1989;122:15–24. doi: 10.1016/0022-1759(89)90329-3. [DOI] [PubMed] [Google Scholar]