Abstract
Cell surface protein antigen (PAc) and water-insoluble glucan-synthesizing enzyme (GTF-I) produced by cariogenic Streptococcus mutans are two major factors implicated in the colonization of the human oral cavity by this bacterium. We examined the effect of bovine milk, produced after immunization with a fusion protein of functional domains of these proteins, on the recolonization of S. mutans. To prepare immune milk, a pregnant Holstein cow was immunized with the fusion protein PAcA-GB, a fusion of the saliva-binding alanine-rich region (PAcA) of PAc and the glucan-binding (GB) domain of GTF-I. After eight adult subjects received cetylpyridinium chloride (CPC) treatment, one subgroup (n = 4) rinsed their mouths with immune milk and a control group (n = 4) rinsed with nonimmune milk. S. mutans levels in saliva and dental plaque decreased after CPC treatment in both groups. Mouth rinsing with immune milk significantly inhibited recolonization of S. mutans in saliva and plaque. On the other hand, the numbers of S. mutans cells in saliva and plaque in the control group increased immediately after the CPC treatment and surpassed the baseline level 42 and 28 days, respectively, after the CPC treatment. The ratios of S. mutans to total streptococci in saliva and plaque in the group that received immune milk were lower than those in the control group. These results suggest that milk produced from immunized cow may be useful for controlling S. mutans in the human oral cavity.
Dental caries is one of the most prevalent infectious diseases in humans, and Streptococcus mutans has been implicated as a causative organism in human dental caries (5, 12, 22). Colonization on tooth surfaces by this microorganism is considered to be the first step in the induction of dental caries. S. mutans adheres to tooth surfaces by sucrose-independent and sucrose-dependent mechanisms (8, 10). The former mechanism is due to the binding of a 190-kDa surface protein antigen (PAc) of S. mutans to human salivary components on tooth surfaces (9). The latter, sucrose-dependent binding, is due to the synthesis of water-insoluble glucan from sucrose catalyzed by glucosyltransferases (GTFs) (11). The important roles of PAc and water-insoluble-glucan-synthesizing GTF (GTF-I) in the cariogenicity of S. mutans make them rational targets for the development of an anticaries vaccine and an adhesion inhibitor (7). Simultaneous inhibition of these colonization factors may result in the protection of teeth from dental caries.
We previously constructed a fusion protein, PAcA-GB, that fuses the saliva-binding alanine-rich region (PAcA) of PAc with the glucan-binding (GB) domain of GTF-I (27) and have immunized Holstein cows with the fusion protein (19). Moreover, we have shown that antibodies purified from the milk of these immunized Holstein cows inhibit both the adhesion of S. mutans to saliva-coated hydroxyapatite beads and glucan synthesis by GTF-I (19). In this study, we investigated the effect of passive immunization with milk from an immunized cow on recolonization of the human oral cavity by S. mutans.
MATERIALS AND METHODS
Preparation of immune milk and control milk.
Immune milk and control milk were prepared as described previously (19). Immune milk was collected from a Holstein cow immunized with the fusion protein PAcA-GB (27), and control milk was collected from a Holstein cow that had not been immunized. After pasteurization at 65°C for 30 min, milk from each cow was processed and stored at −30°C until it was used.
rPAc and GTF-I.
Recombinant PAc (rPAc) and GTF-I were used as antigens for the enzyme-linked immunosorbent assay (ELISA). rPAc was purified from the culture supernatants of transformant S. mutans TK18 by ammonium sulfate precipitation, chromatography on DEAE-cellulose, and subsequent gel filtration on Sepharose CL-6B (Pharmacia, Uppsala, Sweden) (9). For preparation of GTF-I, transformant S. mutans UA130B+, which is defective in both the gtfC and gtfD gene products (27), was grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) at 37°C for 18 h. GFT-I was extracted from whole cells of the transformant by treatment with 8 M urea at 25°C for 1 h. The extract was centrifuged at 5,000 × g for 20 min, and the supernatant was dialyzed against 10 mM potassium phosphate buffer (pH 6.0). The supernatant was used as the GTF-I preparation (27).
ELISA.
For ELISA, 96-well microtiter plates were coated with 100 μl of rPAc or GTF-I (5 μg/ml) in 50 mM carbonate-bicarbonate buffer (pH 9.6). After incubation at 37°C for 90 min, the plates were washed with phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween 20 (PBST) and blocked with PBST containing 1% (wt/vol) chicken egg albumin at 37°C for 90 min. After the plates were washed three times with PBST, twofold serial dilutions of pasteurized bovine milk were added (100 μl per well) and the plates were incubated at 37°C for 90 min. The bound antibodies were detected with alkaline phosphatase-conjugated rabbit anti-bovine immunoglobulin G (heavy and light chains) (Zymed Laboratories, South San Francisco, Calif.) followed by the addition of p-nitrophenylphosphate substrate solution (1 mg/ml). After 30 min of incubation at 37°C, the A405 was measured with a microplate reader (Bio-Rad Laboratories, Richmond, Calif.). The ELISA antibody titer was expressed as the reciprocal of the highest dilution giving an A405 of 0.1 above the conjugated control (no sample added) after 30 min of incubation with the substrate.
Subjects.
Eight healthy volunteers (ages 20 to 25) who had more than 5 × 105 CFU of S. mutans/ml in stimulated saliva were randomly divided into test (n = 4; 3 female, 1 male) and control (n = 4; 2 female, 2 male) groups. Written informed consent was obtained from all volunteers, and the protocol was approved by the ethics committee of the Faculty of Dental Science, Kyushu University, Fukuoka, Japan. None of the volunteers had any clinically detectable active carious lesions. For the baseline values of the mean S. mutans count, the ratio of S. mutans to total streptococci, and the mean dental caries experience (decayed, missing, filled teeth), there was no statistical difference between the groups.
Experimental design.
Three different samples of saliva and plaque were collected from each subject during a 2-week period. The baseline S. mutans level in each subject was determined from the mean value of the three samples. Before rinsing their mouths with milk, all of the subjects received cetylpyridinium chloride (CPC) treatment and professional mechanical tooth cleaning (PMTC) for 5 days to lower the level of S. mutans in the oral cavity. After PMTC with a rubber cup and an abrasive containing fluoride, 10 ml of 1.0% CPC solution was applied once a day for 5 min using a custom-made dentition tray. During this treatment period, the subjects also rinsed their mouths with 10 ml of 0.2% CPC solution for 1 min twice a day (morning and night) after brushing their teeth. On the day after the last CPC treatment, they started rinsing their mouths with milk. Mouth rinsing was performed with 10 ml of immune and control milk for 1 min twice a day (morning and night) after tooth brushing for a period of 14 days. The subjects were instructed not to change their oral hygiene habits during the study period, and they were asked to refrain from eating and drinking for 1 h after rinsing their mouths with CPC or milk.
Bacteriological analysis.
Stimulated whole saliva was collected by having the subjects chew paraffin and expectorate into a tube chilled on ice. Plaque samples collected using a sterilized periodontal probe from all the surfaces of the most posterior molars in each quadrant were immediately placed into 1 ml of PBS. Both saliva and plaque samples were homogenized by sonication for 10 s. Serial 10-fold dilutions of the suspensions were prepared in PBS. Aliquots of 100 μl of the appropriate dilutions were plated in duplicate on mitis salivarius (MS) agar and MS-bacitracin (MS-B) agar (2). The plates were incubated at 37°C under anaerobic conditions for 72 h. The number of S. mutans cells and the total number of streptococci per plate were determined by counting colonies on MS-B agar and MS agar plates, respectively. Identification of S. mutans was conducted by observation of colony morphology on MS-B agar plates under a microscope, and representative colonies of different morphotypes identified as S. mutans were confirmed by the PCR method as described previously (20).
Statistical analysis.
The ELISA titer, S. mutans count, and ratio of S. mutans to total streptococci were each expressed as the mean ± standard deviation. Statistical comparison between the test group and the control group was made using the Mann-Whitney U test with SPSS (version 6.1; SPSS Japan Inc., Tokyo, Japan).
RESULTS
ELISA titers in milk.
ELISA titers to rPAc and GTF-I of immune milk from a cow immunized with fusion protein PAcA-GB and control milk from a nonimmunized cow are shown in Table 1. Immune milk showed antibody titers to the two components of the fusion protein, rPAc and GTF-I, approximately 130- and 10-fold higher, respectively, than those of the control milk.
TABLE 1.
ELISA titers to rPAc and GTF-I of bovine milk
| Bovine milk | ELISA titer with coating antigena
|
|
|---|---|---|
| rPAc | GTF-I | |
| Immune | 4,667.7 | 2,791.8 |
| Control | 36.4 | 259.4 |
ELISA antibody titer expressed as the reciprocal of the highest dilution giving an A405 of 0.1 above the conjugated control (no sample added) after 30 min of incubation with the substrate.
S. mutans count.
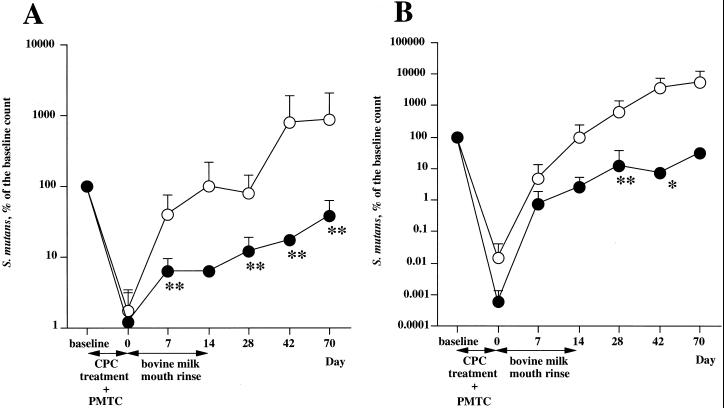
The numbers of S. mutans cells in saliva and dental plaque were expressed as the mean of the percentage of the baseline count (Fig. 1). S. mutans in saliva and plaque was suppressed by the CPC and PMTC treatments in both the test group and the control group. The numbers of S. mutans cells in saliva and plaque in the test group remained less than 1/10 of the baseline throughout the 4 weeks after mouth rinsing was begun. On the other hand, the numbers of S. mutans cells in saliva and plaque in the control group returned to near-baseline levels during the period of mouth rinsing and increased beyond the baseline after the end of the mouth rinsing.
FIG. 1.
Effects of milk from an immunized cow on oral recolonization by S. mutans in saliva (A) and dental plaque (B). The volunteers rinsed with milk (solid circles) from a Holstein cow immunized with fusion protein PAcA-GB or with control milk (open circles) (10 ml/rinse) twice a day (morning and night) after brushing for 14 days after an initial 5 days of CPC treatment. The values are the mean + standard deviation of S. mutans levels expressed as a percentage of the baseline S. mutans level. The statistical difference between the baseline S. mutans level and the S. mutans level at each sampling date was assessed using the Mann-Whitney U test. ∗, P < 0.1; ∗∗, P < 0.05.
Ratio of S. mutans to total streptococci.
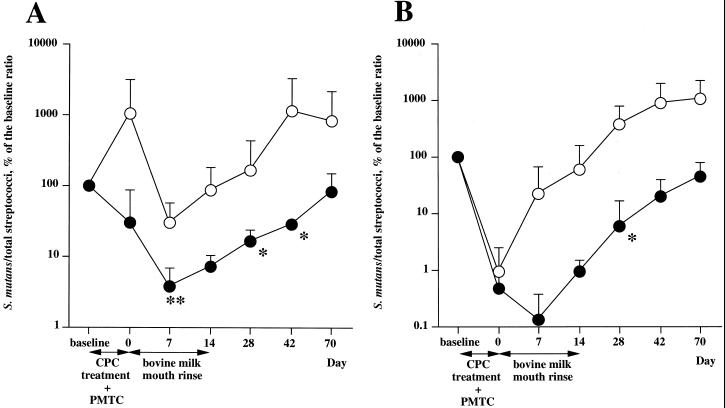
In the test group, the ratios of S. mutans to total streptococci in saliva and plaque decreased after the CPC treatment and PMTC and further decreased during the first week of mouth rinsing with the immune milk (Fig. 2). From the second week of mouth rinsing, the ratios increased gradually. On the other hand, the ratio of S. mutans to total streptococci in plaque in the control group increased immediately after the CPC treatment and PMTC and continued to increase beyond the baseline level. The ratios of S. mutans to total streptococci in the test group were lower than in the control group throughout the study period.
FIG. 2.
Effects of milk from an immunized cow on the ratio of S. mutans to total streptococci in saliva (A) and dental plaque (B). The volunteers rinsed with milk (solid circles) from a Holstein cow immunized with fusion protein PAcA-GB or with control milk (open circles) (10 ml/rinse) twice a day (morning and night) after brushing for 14 days after an initial 5 days of CPC treatment. The values are the mean + standard deviation of the ratio of S. mutans to total streptococci expressed as a percentage of the baseline level. The statistical difference between the baseline level and the ratio of S. mutans to total streptococci at each sampling date was assessed using the Mann-Whitney U test. ∗, P < 0.1; ∗∗, P < 0.05.
DISCUSSION
Passive immunization for prevention of dental caries has recently received much attention (3, 4, 23), and monoclonal antibodies, chicken egg yolk antibodies, and bovine milk antibodies have been used (1, 6, 13, 14). Of these, bovine milk antibodies are, in theory, most easily obtained on a large scale. In practice, colostrum or concentrated milk has been used in previous studies (1, 13, 17) because of the difficulty of obtaining normal milk containing high titers of antibodies. However, the use of colostrum as a daily food for humans is prohibited by the Ministry of Health, Labour and Welfare in Japan, because colostrum contains a large amount of proteins, including blood cells, readily forms a coagulum on warming, and is colored. Moreover, the concentration of normal milk containing low titers of antibodies is a time-consuming job. We have recently succeeded in preparing large amounts of normal milk in which the antibody titers to PAc and GTF-I are very high (19). In this study, we examined the effect of passive immunization with normal milk containing antibodies against the fusion protein PAcA-GB on the recolonization of S. mutans in the human oral cavity. The immune milk significantly inhibited recolonization of S. mutans in saliva and dental plaque. Neither the smell nor the taste of our immune milk is different from that of control milk. In this study, none of the volunteers complained of an unpleasant taste or of being in bad health after rinsing with the immune milk. Passive immunization with normal immune milk may be useful for control of S. mutans in humans.
Significant reduction of the resting infection level of S. mutans in the oral cavity is considered to be an effective starting point at which to assess the subsequent effect of passive immunization, in which antibodies are administered to test subjects. Chlorhexidine (CHX) is often used to decrease the S. mutans level (24, 25). The application of CHX to the mucosal region, however, is known to be capable of inducing anaphylactic shock and drug-mediated eruption (18, 21). Therefore, in our initial intervention, we used CPC, anticipating an antibacterial effect equivalent to that of CHX (26). It is very difficult to lower the S. mutans level using only an antibacterial agent, however, because oral bacteria form biofilms on tooth surfaces (26). In this study, we combined mechanical cleaning of the tooth surfaces with the CPC treatment. The combined use of the CPC treatment and PMTC lowered the S. mutans level effectively.
In our study, the passive immunization period during which the volunteers were exposed to immune milk was 2 weeks. The S. mutans level in the test group remained lower than that in the control group during the entire 8-week experimental period but increased gradually and approached the baseline by the eighth week after the beginning of mouth rinsing. It may be necessary to extend the period of passive immunization to enhance the inhibitory effect of immune milk on the recolonization of S. mutans.
In the control group, the S. mutans level increased beyond the baseline level after the CPC treatment. The volunteers rinsed with nonimmune milk twice a day in the morning and at night after tooth brushing and, as with the test group, were not to eat or drink for 1 h after rinsing their mouths with milk. Normal milk may contain various components that enhance the growth of S. mutans or colonization on teeth by the organism. Long-time stagnation of such milk components in the oral cavity might increase the number of S. mutans cells there. Rapid elimination of other oral bacteria by an antibacterial agent may be responsible for the incremental increase of S. mutans. A similar rebound phenomenon was observed in other studies using CHX (15, 16). Since the concentration of CPC used in this study was higher than that in commercially available toothpaste and mouth rinse, most of the subjects complained of taste disorder or a burning sensation in the oral cavity during the CPC treatment. Such side effects, however, were temporary, and the symptoms subsided within a few days after the CPC treatment. Similar side effects have been reported during clinical research studies using high concentrations of CHX (25).
In conclusion, bovine milk containing antibody against the fusion protein PAcA-GB was effective in controlling the recolonization of the oral cavity by S. mutans. Milk from immunized cattle may be useful not only for controlling S. mutans in humans who are already infected, but also for preventing initial infection with S. mutans in infancy.
ACKNOWLEDGMENTS
We thank Takusaburo Ebina of the Division of Immunology, Research Institute Miyagi Cancer Center, Miyagi, Japan, for helpful suggestions concerning the immunization method for cows.
This work was supported in part by Grants-in-Aid for Developmental Scientific Research (A) 12357013 (T.K.) and (C) 11672051 (T.O.) from the Ministry of Education, Science, Sports and Culture of Japan and by the Kyushu University Interdisciplinary Programs in Education and Projects in Research Development (T.K.).
REFERENCES
- 1.Filler S J, Gregory R L, Michalek S M, Katz J, McGhee J R. Effect of immune bovine milk on Streptococcus mutans in human dental plaque. Arch Oral Biol. 1991;36:41–47. doi: 10.1016/0003-9969(91)90052-v. [DOI] [PubMed] [Google Scholar]
- 2.Gold O G, Jordan H V, Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G, Michalek S M. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1999;14:1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamada S, Kodama Y. Passive immunity for protection against mucosal infections and vaccination for dental caries. In: Kiyono H, Ogura P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 187–197. [Google Scholar]
- 5.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, Hirasawa M, Katz J, Childers N K, Michalek S M. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31:268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- 7.Irvin R T, Bautista D L. Hope for the post-antibiotic era? Nat Biotechnol. 1999;17:20. doi: 10.1038/5189. [DOI] [PubMed] [Google Scholar]
- 8.Koga T, Asakawa H, Okahashi N, Hamada S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J Gen Microbiol. 1986;132:2873–2883. doi: 10.1099/00221287-132-10-2873. [DOI] [PubMed] [Google Scholar]
- 9.Koga T, Okahashi N, Takahashi I, Kanamoto T, Asakawa H, Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58:289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga T, Yamashita Y, Nakano Y, Kawasaki M, Oho T, Yu H, Nakai M, Okahashi N. Surface proteins of Streptococcus mutans. Dev Biol Stand. 1995;85:363–369. [PubMed] [Google Scholar]
- 11.Kuramitsu H K, Smorawinska M, Nakano Y J, Shimamura A, Lis M. Analysis of glucan synthesis by Streptococcus mutans. Dev Biol Stand. 1995;85:303–307. [PubMed] [Google Scholar]
- 12.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loimaranta V, Tenovuo J, Virtanen S, Marnila P, Syvaoja E L, Tupasela T, Korhonen H. Generation of bovine immune colostrum against Streptococcus mutans and Streptococcus sobrinus and its effect on glucose uptake and extracellular polysaccharide formation by mutans streptococci. Vaccine. 1997;15:1261–1268. doi: 10.1016/s0264-410x(97)00027-3. [DOI] [PubMed] [Google Scholar]
- 14.Ma J K, Hikmat B Y, Wycoff K, Vine N D, Chargelegue D, Yu L, Hein M B, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- 15.Ma J K, Hunjan M, Smith R, Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol. 1989;77:331–337. [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J K, Lehner T. Prevention of colonization of Streptococcus mutans by topical application of monoclonal antibodies in human subjects. Arch Oral Biol. 1990;35:115S–122S. doi: 10.1016/0003-9969(90)90140-6. [DOI] [PubMed] [Google Scholar]
- 17.Michalek S M, Gregory R L, Harmon C C, Katz J, Richardson G J, Hilton T, Filler S J, McGhee J R. Protection of gnotobiotic rats against dental caries by passive immunization with bovine milk antibodies to Streptococcus mutans. Infect Immun. 1987;55:2341–2347. doi: 10.1128/iai.55.10.2341-2347.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghadam B K, Drisko C L, Gier R E. Chlorhexidine mouthwash-induced fixed drug eruption. Case report and review of the literature. Oral Surg Oral Med Oral Pathol. 1991;71:431–434. doi: 10.1016/0030-4220(91)90424-b. [DOI] [PubMed] [Google Scholar]
- 19.Oho T, Shimazaki Y, Mitoma M, Yoshimura M, Yamashita Y, Okano K, Nakano Y, Kawagoe H, Fukuyama M, Fujihara N, Koga T. Bovine milk antibodies against cell surface protein antigen PAc-glucosyltransferase fusion protein suppress cell adhesion and alter glucan synthesis of Streptococcus mutans. J Nutr. 1999;129:1836–1841. doi: 10.1093/jn/129.10.1836. [DOI] [PubMed] [Google Scholar]
- 20.Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol. 2000;15:258–262. doi: 10.1034/j.1399-302x.2000.150408.x. [DOI] [PubMed] [Google Scholar]
- 21.Okano M, Nomura M, Hata S, Okada N, Sato K, Kitano Y, Tashiro M, Yoshimoto Y, Hama R, Aoki T. Anaphylactic symptoms due to chlorhexidine gluconate. Arch Dermatol. 1989;125:50–52. [PubMed] [Google Scholar]
- 22.Russell R R. The application of molecular genetics to the microbiology of dental caries. Caries Res. 1994;28:69–82. doi: 10.1159/000261625. [DOI] [PubMed] [Google Scholar]
- 23.Smith D J, Taubman M A. Vaccines against dental caries infection. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 913–930. [Google Scholar]
- 24.Twetman S, Petersson L G. Comparison of the efficacy of three different chlorhexidine preparations in decreasing the levels of mutans streptococci in saliva and interdental plaque. Caries Res. 1998;32:113–118. doi: 10.1159/000016440. [DOI] [PubMed] [Google Scholar]
- 25.Wallman C, Krasse B, Birkhed D, Diacono S. The effect of monitored chlorhexidine gel treatment on mutans streptococci in margins of restorations. J Dent. 1998;26:25–30. doi: 10.1016/s0300-5712(96)00075-9. [DOI] [PubMed] [Google Scholar]
- 26.Wilson M, Patel H, Fletcher J. Susceptibility of biofilms of Streptococcus sanguis to chlorhexidine gluconate and cetylpyridinium chloride. Oral Microbiol Immunol. 1996;11:188–192. doi: 10.1111/j.1399-302x.1996.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Nakano Y, Yamashita Y, Oho T, Koga T. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect Immun. 1997;65:2292–2298. doi: 10.1128/iai.65.6.2292-2298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]