Abstract
Background
Gamma-glutamyl transpeptidase to platelet ratio (GPR) and gamma-glutamyl transpeptidase to lymphocyte ratio (GLR) are assumed to be prognostic factors in liver fibrosis, cirrhosis and hepatocellular carcinoma. However, the reference values of GPR and GLR were not known.
Objectives
The study aimed to investigate the reference ranges of GPR and GLR in Chinese Han population in Chaoshan region in South China.
Methods
A retrospective study was conducted in the First Affiliated Hospital of Shantou University Medical College in South China. 2400 healthy adults aged 20~79 years were included. GPR and GLR were determined.
Results
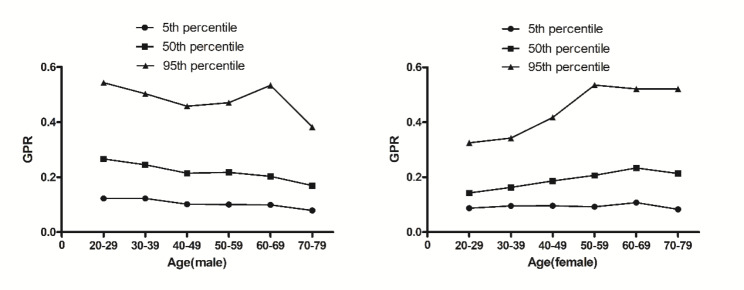
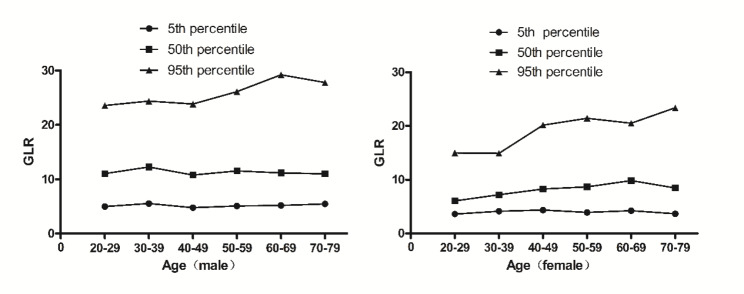
Of 2400 healthy adults, 1200 men and 1200 women were included. The median GPR and GLR for men were 0.22 and 11.28, for women were 0.18 and 7.86, respectively. The 95% reference range of GPR in normal male and female are 0.09~0.54 and 0.08~0.55, GLR are 4.55~29.64 and 3.52~23.08, respectively. The male had a higher GPR at age 20~49 than the female while the GPR at age 60~79 was higher in the female than in the male. The GPR was affected by age, decreased with aging in male and increased in female. The GLR was higher in the male than in the female and varied with aging in the female but not in the male.
Conclusion
The study provides reference data on GPR and GLR from different age and sex groups in South China. GPR and GLR varied with age and sex.
Keywords: Gamma-glutamyl transpeptidase to platelet ratio, Gamma-glutamyl transpeptidase to lymphocyte ratio, Reference range
Introduction
Chronic hepatitis B virus (HBV) infection is a major public health problem, especially in countries of the African region and Western Pacific region, with HBsAg seroprevalence 8.83% and 5.26%, respectively [1]. It was estimated that HBsAg prevalence was 3.61% worldwide and about 248 million people were HBsAg positive in 2010 [1]. Hepatitis B is a common cause of liver fibrosis, which may lead to cirrhosis, liver failure and hepatocellular carcinoma if untreated. Therefore, it is important to identify liver fibrosis for decreasing the burden of disease attributable to HBV infection.
Recently Lemoine M et al. reported that the gamma-glutamyl transpeptidase (GGT) to platelet ratio (GPR) can predict significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa [2], which can be used in other populations and hepatitis B virus-related liver cancer [3–7]. More recently, gamma-glutamyl transpeptidase to lymphocyte ratio (GLR) were reported to be predictors of microvascular invasion in hepatocellular carcinoma [8] and intrahepatic cholangiocarcinoma patients following hepatectomy [9]. These tests supplied simple, cheap and convenient methods to assess the hepatic fibrosis level of patients with chronic hepatitis B and prognosis of patients with hepatocellular carcinoma.
Though there have been extensive investigations on GPR and GLR, the normal ranges of GPR and GLR were not investigated. It is important to investigate the ranges of GPR and GLR. The aim of this study is to explore the reference values of GPR and GLR among the Han population in Chaoshan District of Guangdong Province in South China.
Methods
The study was conducted retrospectively in the First Affiliated Hospital of Shantou University Medical College in South China. Hepatic function and Complete blood count (CBC) tests between January 2019 and December 2019 were reviewed from healthy persons aged 20~79 years without diagnosed diseases including acute or chronic infection, heart failure, renal failure, autoimmune or hematopoietic diseases, acute or chronic hepatitis, hepatic fibrosis, cirrhosis and hepatocellular carcinoma. The healthy adults had no history of smoking, alcohol abuse and drug usage for diseases as noted above and were divided into groups according to gender and age. A total of 2400 healthy adults were included. Blood samples were obtained from fasting subjects. Platelet and lymphocyte cell counts were determined by the Coulter method with the Beckman Coulter LH780 analyzer. The near month-coefficient of variation of platelet and lymphocyte cell was 3.53% and 2.14%, respectively (The set value of coefficient of platelet and lymphocyte cell variation was 4.49% and 3.49%). GGT was determined by the rate method with Beckman Coulter AU5800. The near month-coefficient of GGT variation was 1.19% (The set value of coefficient of variation was 3.0%).
GPR and GLR were calculated as GGT/ULN of GGT/platelet count (109/L)×100 [2] and as the ratio of GGT to lymphocyte cell count [8], respectively. The samples were excluded with White blood cell (WBC) less than 3.5 × 109/L or more than 9.5 × 109/L, lymphocyte less than 1.1 × 109/L or more than 3.2 × 109/L and platelet less than 125 × 109/L or more than 350 × 109/L and hepatic insufficiency with GGT more than 100 U/L. The study was approved by the ethics committee of Shantou University Medical College.
Data are presented as mean ± SD or median and interquartile range. Differences between group means were assessed by an unpaired Student’s t-test or Mann-Whitney test for single comparisons or by Kruskal-Wallis test for multiple comparisons using SPSS 24.0. P value < 0.05 was considered significant.
Results
As shown in the Table 1, there are 2400 individuals in present study, which included 1200 men and 1200 women. The differences of age between the male and female were not significant. The male had a higher GGT, lymphocyte cell counts, GPR and GLR than the female while the female had a higher platelet counts. The median GPR and GLR for men and women were 0.22, 11.28, 0.18 and 7.86, respectively. The 95% reference range of GPR in normal male and female are 0.09~0.54 and 0.08~0.55, GLR are 4.55~29.64 and 3.52~23.08, respectively. GPR and GLR were analyzed based on sex and age (shown in Figs. 1 and 2; Tables 2 and 3,). The GPR was affected by age, decreased with aging in male and increased in female. There are significant differences of GPR among at age 20~39, 40~69 and 70~79 in the male and among at age 20~29, 30~39, 40~49 and 50~79 in the female. The GPR did not vary from age 20 to 39, 50 to 69, respectively in the male and from age 50 to 79 in the female. The male had a higher GPR at age 20~49 than the female while the GPR at age 60~79 was higher in female than in male. At age 50~59 group there are no difference of the GPR between the male and the female. The differences of GLR between the male and female were significant different from age 20 to 79. The effect of aging on GLR varied with sex. There was significant effect of aging on GLR in the female while not in the male.
Table 1.
Main characteristics of the study based on sex
| Male | Female | P Value | |
|---|---|---|---|
| Number | 1200 | 1200 | |
| Age (years, mean ± SD) | 49.40 ± 16.96 | 49.29 ± 16.79 | 0.869 |
| Median GGT (U/L) (IQR) | 26.00 (19.00–36.00) | 18.00 (13.00–25.00) | 0.000 |
| Mean PLT (×109/L) | 205.58 ± 41.55 | 219.78 ± 42.15 | 0.000 |
| Mean LY (×109/L) | 2.36 ± 0.45 | 2.31 ± 0.47 | 0.008 |
| Median GPR (IQR) | 0.22 (0.15–0.31) | 0.18 (0.14–0.26) | 0.000 |
| 95% reference range | 0.09 ~ 0.54 | 0.08 ~ 0.55 | |
| Median GLR (IQR) | 11.28 (8.03–16.06) | 7.86 (5.83–11.15) | 0.000 |
| 95% reference range | 4.55 ~ 29.64 | 3.52 ~ 23.08 |
GGT, gamma-glutamyl transpeptidase; PLT, platelet; LY, lymphocyte; GPR, GGT to platelet ratio; GLR, GGT to lymphocyte ratio
Fig. 1.
The percentile nomogram for GPR in the male and the female
Fig. 2.
The percentile nomogram for GLR in the male and the female
Table 2.
Gamma-glutamyltranspeptidase-to-platelet ratio at different groups
| Subgroup (age) | Gamma-glutamyltranspeptidase-to-platelet ratio (male) |
Gamma-glutamyltranspeptidase-to-platelet ratio (female) |
P value |
|---|---|---|---|
| 20 ~ 29 | 0.27 (0.19–0.35) | 0.14 (0.11–0.18) ∆∆ | 0.000 |
| 30 ~ 39 | 0.24 (0.18–0.32) | 0.16 (0.13–0.21)* | 0.000 |
| 40 ~ 49 | 0.21 (0.15–0.29) ∆ | 0.19 (0.15–0.25) ## | 0.033 |
| 50 ~ 59 | 0.22 (0.16–0.32) ∆ | 0.21 (0.15–0.29) | 0.204 |
| 60 ~ 69 | 0.20 (0.14–0.28) ∆ | 0.23 (0.17–0.31) | 0.002 |
| 70 ~ 79 | 0.17 (0.12–0.25) ∆ # | 0.21 (0.14–0.31) | 0.001 |
∆compared with group age 20 ~ 39 (P < 0.05) #compared with group age 20 ~ 69(P < 0.01)
∆∆compared with group age 30 ~ 79(P < 0.05)*compared with group age 40 ~ 79 (P < 0.05)
## compared with group age 50 ~ 79(P < 0.05)
Table 3.
Gamma-glutamyltranspeptidase-to-lymphocyte ratio at different groups
| Subgroup (age) | Gamma-glutamyltranspeptidase-to-lymphocyte ratio (male) |
Gamma-glutamyltranspeptidase-to-lymphocyte ratio (female) |
P value |
|---|---|---|---|
| 20 ~ 29 | 11.03 (8.31–14.81) | 6.06 (5.08–7.41)# | 0.000 |
| 30 ~ 39 | 12.26 (8.03–16.58) | 7.21 (5.63–9.56)∆ | 0.000 |
| 40 ~ 49 | 10.80 (7.55–15.60) | 8.27 (6.18–11.57) | 0.000 |
| 50 ~ 59 | 11.53 (8.25–17.46) | 8.67 (6.51–12.13) | 0.000 |
| 60 ~ 69 | 11.16 (8.22–16.83) | 9.83 (6.74–13.34) | 0.000 |
| 70~79 | 11.02 (7.98–15.95) | 8.45 (5.93–12.58) | 0.000 |
#compared with group age 30 ~ 79 (P < 0.05); ∆compared with group age 40 ~ 79 (P < 0.01)
Discussion
Liver fibrosis is a wound-healing response to liver injury caused by various factors such as viral hepatitis, alcohol abuse, and non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD). Though liver fibrosis is a reversible process, advanced liver fibrosis can result in cirrhosis, liver failure and hepatocellular carcinoma (HCC). It is important to assess fibrosis stage. Liver biopsy has been considered the gold standard for diagnosing liver fibrosis, however it is invasive and can cause serious complications [10, 11]. Transient elastography is a noninvasive tool for staging liver fibrosis, but the Fibroscan device is expensive. Furthermore the diagnostic accuracy of the method was poorer for significant fibrosis [12]. Recently GPR and GLR were reported to be predictors of liver fibrosis, cirrhosis and hepatocellular carcinoma [2–9]. However, it has remained unknown about the reference ranges of GPR and GLR in healthy adults.
In present study we measured the GPR and GLR in 2400 Chinese healthy adults. We found that the 95% reference range of GPR in normal male and female are 0.09~0.54 and 0.08~0.55, GLR are 4.55~29.64 and 3.52~23.08, respectively. The GPR and GLR were affected by sex and age.
GGT, a cell-membrane-bound protease, has long been regarded as a marker of liver disease [13]. Recent evidences have shown the association between GGT and cancer [14, 15], cardiovascular diseases, lung inflammation and neurological diseases [16]. Moreover it has been found that GPR and GLR are associated with significant liver fibrosis, cirrhosis and liver cancer [2–9]. There was significant positive correlation between GPR and fibrosis stage. The optimal cut-off value of GPR for significant fibrosis and cirrhosis was 0.32 and 0.56, respectively [2]. High GPR (> 0.23) was an independent risk factor for hepatocellular carcinoma development in chronic hepatitis patients [17]. High GLR was also an independent prognostic factor of hepatocellular carcinoma and intrahepatic cholangiocarcinoma [8, 9].
It has been demonstrated that there are significant male-female differences in the reference range for serum or plasma GGT [18], platelet and lymphocyte cell counts [19]. Furthermore, the geographic and ethnic difference of platelet counts was significant [20–22]. These studies showed that GPR and GLR varied significantly among sex, geographic region and race.
Though GPR and GLR were used widely in many diseases, the cut-off points for risk stratification varied in these studies, which were affected by the disease category, age, and race of patients. In the studies from West Africa a lower cut-off value of GPR for predicting significant liver fibrosis was suggested than that in China (0.32 vs. 0.448). The optimal cut-off value of GLR was 33.7 for predicting prognosis of intrahepatic cholangiocarcinoma while 56 for hepatocellular carcinoma [8, 9]. In present study, we found that GPR and GLR varied with age and sex, which suggested that factors affecting GPR and GLR should be considered when the cut-off values for risk stratification were determined.
There are a few limitations in present study. First, the study is a retrospective study and routine blood analyses were collected from healthy population in the checkup center of hospital, the effects of high alcohol consumption and use of enzyme-inducing drugs on GGT can not be excluded [18]. Secondly, owing to the geographic and ethnic difference of platelet counts [20–22], the reference range of GPR in healthy population from Han population in Chaoshan region may be different from other races in local region or other regions in China.
In summary, we found that the reference ranges of GPR and GLR in male were different from in female from Chaoshan region in South China. The GPR and GLR varied with age and sex.
Acknowledgements
We have no one to acknowledge to.
List of abbreviations
- HBV
Hepatitis B virus
- GGT
Gamma-glutamyl transpeptidase
- GPR
Gamma-glutamyl transpeptidase to platelet ratio
- GLR
Gamma-glutamyl transpeptidase to lymphocyte ratio
- CBC
Complete blood count
- WBC
White blood cell
Authors’ contributions
WJC drafted the manuscript. DMH, ZLC, JC and XQD were involved in data collection and analysis. MY and LPL edited the manuscript and conceived of the study. All authors read and approved the final manuscript.
Funding
None.
Data availability
Raw data supporting the obtained results are available at the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Shantou University Medical College. The need for consent was waived by the ethics committee of Shantou University Medical College because of the retrospective data. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–76. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, et al. The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Med (Baltim) 2016;95(16):e3372. doi: 10.1097/MD.0000000000003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park YE, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 2017;32(6):1221–9. doi: 10.1111/jgh.13653. [DOI] [PubMed] [Google Scholar]
- 5.Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, et al. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Med (Baltim) 2016;95(27):e4087. doi: 10.1097/MD.0000000000004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang Q, Bi JB, Wang ZX, Xu XS, Qu K, Miao RC, et al. Simple models based on gamma-glutamyl transpeptidase and platelets ratiofor predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther. 2016;9:2099–109. doi: 10.2147/OTT.S101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd A, Bottero J, Lacombe K. The γ-glutamyl transpeptidase-to-platelet as a predictor of liver fibrosis in patients co-infected with HBV and HIV. Gut. 2016;65(4):718–20. doi: 10.1136/gutjnl-2015-310607. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhou Y, Li Y, Qin W, Zi Y, Liu Y, et al. Predictive value of gamma-glutamyl transpeptidase to lymphocyte count ratio in hepatocellular carcinoma patients with microvascular invasion. BMC Cancer. 2020;20(1):132. doi: 10.1186/s12885-020-6628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JJ, Li H, Li JX, Xu L, Wu H, Zeng Y. Preoperative gamma-glutamyltransferase to lymphocyte ratio predicts long-term outcomes in intrahepatic cholangiocarcinoma patients following hepatic resection. World J Gastroenterol. 2020;26(13):1501–12. doi: 10.3748/wjg.v26.i13.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JF, Hsieh MY, Dai CY, Hou NJ, Lee LP, Lin ZY, et al. The incidence and risks of liver biopsy in non-cirrhotic patients: An evaluation of 3806 biopsies. Gut. 2007;56(5):736–7. doi: 10.1136/gut.2006.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705–12. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 12.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. FIBROSTIC study group. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53(6):1013–21. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Zein M, Discombe G. Serum gamma-glutamyl transpeptidase as a diagnostic aid. Lancet. 1970;2(7676):748–50. doi: 10.1016/S0140-6736(70)90222-9. [DOI] [PubMed] [Google Scholar]
- 14.Luo M, Sun W, Wu C, Zhang L, Liu D, Li W, et al. High pretreatment serum gamma-glutamyl transpeptidase predicts an inferior outcome in nasopharyngeal carcinoma. Oncotarget. 2017;8(40):67651–62. doi: 10.18632/oncotarget.18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YJ, Lee DH, Han KD, Yoon H, Shin CM, Park YS, et al. Elevated serum gamma-glutamyltransferase is associated with an increased risk of oesophageal carcinoma in a cohort of 8,388,256 Korean subjects. PLoS ONE. 2017;12(5):e0177053. doi: 10.1371/journal.pone.0177053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corti A, Belcastro E, Dominici S, Maellaro E, Pompella A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radic Biol Med. 2020;160:807–19. doi: 10.1016/j.freeradbiomed.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhu YF, Tan YF, Xu X, Zheng JL, Zhang BH, Tang HR, et al. Gamma-glutamyl transpeptidase-to-platelet ratio and the fibrosis-4 index in predicting hepatitis B virus-related hepatocellular carcinoma development in elderly chronic hepatitis B patients in China: A single-center retrospective study. Med (Baltim) 2019;98(50):e18319. doi: 10.1097/MD.0000000000018319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Zou S, Wang C, Tan X, Yu M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc Disord. 2019;19(1):125. doi: 10.1186/s12872-019-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Zhao M, Pan B, Zhang J, Peng M, Wang L, et al. Complete blood count reference intervals for healthy Han Chinese adults. PLoS ONE. 2015;10(3):e0119669. doi: 10.1371/journal.pone.0119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong J, Min Z, Bai-shen P, Jie Z, Ming-ting P, Xian-zhang H, et al. Investigation on reference intervals and regional differences of platelet indices in healthy Chinese Han adults. J Clin Lab Anal. 2015;29(1):21–7. doi: 10.1002/jcla.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu Q, Zhang R, Zhao M, Zeng S, Huang X, Jiang H, et al. Differences in platelet indices between healthy Han population and Tibetans in China. PLoS ONE. 2013;8(6):e67203. doi: 10.1371/journal.pone.0067203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data supporting the obtained results are available at the corresponding author.