Abstract
Background
In March 2020, Veterans Health Administration (VHA) enacted policies to expand treatment for Veterans with opioid use disorder (OUD) during COVID-19. In this study, we evaluate whether COVID-19 and subsequent OUD treatment policies impacted receipt of therapy/counseling and medication for OUD (MOUD).
Methods
Using VHA’s nationwide electronic health record data, we compared outcomes between a comparison cohort derived using data from prior to COVID-19 (October 2017-December 2019) and a pandemic-exposed cohort (January 2019-March 2021). Primary outcomes included receipt of therapy/counseling or any MOUD (any/none); secondary outcomes included the number of therapy/counseling sessions attended, and the average percentage of days covered (PDC) by, and months prescribed, each MOUD in a year.
Results
Veterans were less likely to receive therapy/counseling over time, especially post-pandemic onset, and despite substantial increases in teletherapy. The likelihood of receiving buprenorphine, methadone, and naltrexone was reduced post-pandemic onset. PDC on MOUD generally decreased over time, especially methadone PDC post-pandemic onset, whereas buprenorphine PDC was less impacted during COVID-19. The number of months prescribed methadone and buprenorphine represented relative improvements compared to prior years.
We observed important disparities across Veteran demographics.
Conclusion
Receipt of treatment was negatively impacted during the pandemic. However, there was some evidence that coverage on methadone and buprenorphine may have improved among some veterans who received them. These medication effects are consistent with expected COVID-19 treatment disruptions, while improvements regarding access to therapy/counseling via telehealth, as well as coverage on MOUD during the pandemic, are consistent with the aims of MOUD policy exemptions.
Keywords: Opioids, Opioid use disorder, Buprenorphine, Methadone, Naltrexone, Treatment, COVID-19, Veterans, Veterans Health Administration
1. Introduction
The COVID-19 pandemic occurred during an ongoing national opioid crisis (Scholl et al., 2018, Friedman et al., 2021). The prevalence of opioid use disorder (OUD) has been a significant and ongoing public health concern, with an estimated 2 million individuals in the U.S. meeting diagnostic criteria for OUD (SAMHSA, 2019) at the beginning of the pandemic. Military Veterans have been particularly at risk for OUD and rates of overdose death are nearly twice as high among Veterans compared to non-Veterans (Bohnert et al., 2011, Goldberg, 2017), and four times higher among Veterans with OUD who are not treated with buprenorphine (Vakkalanka et al., 2021). Veterans are also more likely than non-Veterans to experience disability and chronic pain (Center for Ethics and the Role of Law, 2017; Rhee and Rosenheck, 2019), psychiatric disorders (e.g., depression, posttraumatic stress disorder [PTSD]; Rhee and Rosenheck, 2019), and other psychosocial concerns (e.g., homelessness, financial insecurity; Midboe et al., 2019; Iheanacho et al., 2018) that can exacerbate risk and complicate effective treatment for OUD.
The gold-standard treatment for OUD combines FDA-approved medications for OUD (MOUD), primarily methadone, buprenorphine, and naltrexone, with therapy/counseling (Livingston et al., 2021, SAMHSA, 2020a, Connery, 2015). Buprenorphine and methadone are tightly regulated controlled substances (SAMHSA, 2015) and involve daily medication (i.e., oral formulations) or in-clinic administrations (e.g., extended-release buprenorphine injections), and close medication monitoring. Prior to the pandemic, MOUD typically required in person care (SAMHSA, 2020b, SAMHSA, 2022), and therapy/counseling tended to occur in person. In response to COVID-19 transmission concerns, in February and March 2020, there were complimentary efforts by the Centers for Disease Control and Prevention (CDC), U.S. Drug Enforcement Agency (DEA), Substance Abuse and Mental Health Services Administration (SAMHSA), and Centers for Medicare and Medicaid Services (CMS) to expand access to MOUD care (Centers for Disease, 2020; Lin et al., 2020). These changes included both guidelines to increase use of, and billing for, telehealth and telephone-only care, and exemptions to 42 CFR 8 permitting initial buprenorphine prescriptions without an in-person evaluation, as well as flexibility to dispense 14- and 28-day take-home methadone supplies for patients based on degree of stability on MOUD (SAMHSA, 2020a, SAMHSA, 2020b, SAMHSA, 2020c, SAMHSA, 2020d, SAMHSA, 2022). In the Veterans Health Administration (VHA), updated policy also expanded access to telehealth services, enabling providers and systems to provide more virtual care to patients (Jercich, 2022). In line with these shifts, the VHA officially adopted guidance from the CDC and SAMHSA to prevent OUD treatment disruptions (Gustavson et al., 2020).
With its robust telehealth capabilities in place prior to the pandemic (Jercich, 2022; Gordon et a, 2020; Office of Public and Intergovernmental Affairs, 2018), VHA was well-positioned to deliver virtual care in line with OUD treatment recommendations during COVID-19. We believe it is critical to understand the impact of expanding access to MOUD through telehealth and the easing of MOUD restrictions, particularly in the context of COVID-19, which presented considerable barriers to initiating and maintaining treatment for OUD. The limited data published thus far suggests that VHA’s response to the pandemic resulted in continued access to buprenorphine treatment, largely attributable to VHA’s expansion of telehealth services during COVID-19 (Lin et al., 2022).
Even less is known regarding impacts on other front-line treatments, such as methadone, naltrexone, therapy/counseling, and research to date concerns non-Veterans (e.g., Zhang et al., 2022; Will et al., 2022; Morgan et al., 2022; Hoffman et al., 2022; Gomes et al., 2022; Joudrey et al., 2021). Each treatment was likely impacted differently during the pandemic, as the MOUD policy exemptions did not relate to naltrexone prescriptions and they differentially targeted access to methadone, buprenorphine, and therapy/counseling via telehealth. Given the critical role of each MOUD and therapy/counseling in combating OUD and overdose risk (Fairley et al., 2021), additional research is needed to discern COVID-19 versus MOUD policy impacts on buprenorphine, methadone, naltrexone, and therapy/counseling.
Our aim was to evaluate the impacts of COVID-19 and MOUD policy exemptions on MOUD and therapy/counseling receipt, using nationwide VHA electronic health record (EHR) data. To overcome limitations of single-group pre-post designs, and to control for some known confounds, we selected a quasi-experimental approach for this study to compare outcomes among Veterans with OUD during COVID-19 versus pre-pandemic onset. To accomplish this, we created two matched cohorts of Veterans with OUD—a comparison cohort derived using pre-pandemic data from October 2017 to December 2019 and a separate pandemic-exposed cohort using data from January 2019 to March 2021—to evaluate differences in receipt of therapy/counseling, buprenorphine, methadone, or naltrexone pre- and post-pandemic onset. Secondarily, we evaluated the number of months prescribed, as well as the percentage of days covered (PDC) on each MOUD, among Veterans who received them during the study period.
2. Material and methods
2.1. Data source and study population
This is a longitudinal retrospective cohort study of nationwide VHA EHR data, sourced from the VHA Corporate Data Warehouse (CDW). The current study procedures were determined exempt from IRB oversight and approved by the Research and Development Committee (R&DC) at VA Boston Healthcare System. We defined our cohorts using F11 (OUD) ICD-10 diagnosis codes (World Health Organization, 2004) attached to one or more VHA encounter(s) during the periods established for cohort creation (see Fig. 1). We excluded Veterans with only F11.21 and F11.11 codes (OUD in remission), unless they were prescribed one of the MOUD medications during the cohort building period, suggesting that the diagnosis was still being treated. Veterans who were identified as having current OUD between October 2016-September 2018 were included in the pre-pandemic comparison cohort and patients with a diagnosis between January 2018-December 2019 were included in the pandemic-exposed cohort (see Fig. 1). Duplicate patients across cohorts were randomly assigned to either the comparison or pandemic-exposed cohort, resulting in two mutually exclusive groups.
Fig. 1.
Diagram depicting the data ranges used for cohort creation and outcome observation between the camparison and pandemic-exposed.
Next, we exact-matched patients in our comparison and pandemic-exposed cohorts on age (i.e., 18–29, 30–39, 40–49, 50–65, 65 +), sex assigned in their medical record, and state, followed by 1:1 propensity score matching on race/ethnicity and whether Veterans resided in rural versus urban areas based on available location information in the medical record at baseline. The standardized mean differences (SMD) between groups were below 1, suggesting a balanced match (see Table 1). After omitting two Veterans with invalid ages, we ended up with 53,803 Veterans in each cohort, and 107,606 Veterans overall (see Table 1 for Veteran demographics).
Table 1.
Characteristics of Analytic Sample by Cohort.
| Demographics | Total |
Comparison Cohort |
Pandemic-Exposed Cohort |
SMD |
|---|---|---|---|---|
| N = 107,606 | N = 53,803 | N = 53,803 | ||
| Sex assigned at birtha | < .001 | |||
| Female | 7210(6.70%) | 3605(6.70%) | 3605(6.70%) | |
| Male | 100,396(93.30%) | 50,198(93.30%) | 50,198(93.30%) | |
| Age groupa | < .001 | |||
| 18–29 | 4432(4.12%) | 2216(4.12%) | 2216(4.12%) | |
| 30–39 | 19,514(18.13%) | 9757(18.13%) | 9757(18.13%) | |
| 40–49 | 12,552(11.66%) | 6276(11.66%) | 6276(11.66%) | |
| 50–64 | 42,512(39.51%) | 21,256(39.51%) | 21,256(39.51%) | |
| 65 + | 28,596(26.57%) | 14,298(26.57%) | 14,298(26.57%) | |
| Race/Ethnicitya | .063 | |||
| White | 74,517(69.25%) | 37,931(70.50%) | 36,586(68.00%) | |
| Black | 21,979(20.43%) | 10,317(19.20%) | 11,662(21.70%) | |
| Hispanic or Latino | 5386(5.00%) | 2701(5.00%) | 2685(5.00%) | |
| Asian | 220(0.20%) | 110(0.20%) | 110(0.20%) | |
| American Indian | 616(0.60%) | 310(0.60%) | 306(0.60%) | |
| Pacific Islander | 422(0.40%) | 199(0.40%) | 223(0.40%) | |
| Unknown | 3752(3.50%) | 1876(3.50%) | 1876(3.50%) | |
| Rural vs. Urbana | .002 | |||
| Rural | 29,364(27.29%) | 14,664(27.25%) | 14,700(27.32%) | |
| Urban | 78,242(72.71%) | 39,139(72.75%) | 39,103(72.68%) |
Note. VHA = Veterans Health Administration, SMD = standard mean difference
aMatching variable; patients also matched on state of residence (not shown); SMD < 0.001
We used different observation periods for cohort creation versus outcome monitoring. Our outcome observation period for the pre-pandemic comparison cohort spanned October 2017-December 2019, and January 2019-March 2021 for the pandemic-exposed cohort. As noted above, our cohort creation dates predate the start dates of our observation periods of interest by one year. Since OUD diagnoses are active for a minimum of 12 months (American Psychiatric Association, 2013), OUD diagnoses identified up to 12 months prior to the start of our observation periods could still be considered valid and eligible for treatment during patients’ respective observation period.
2.2. Predictor variables and covariates
We used a quasi-experimental difference-in-difference design to examine whether there were changes from year one to two of each cohort’s respective observation period, and whether these changes differed by cohort. We expected that healthcare utilization would decrease from the first to second year generally, but that this effect would be more pronounced among the pandemic-exposed cohort, for whom year two corresponds with COVID-19 onset, and that this effect would be detectable through examination of a cohort by year (one versus two) interaction terms. We chose a 27-month observation period for both cohorts. This decision was based largely on the pandemic-exposed cohort, as we wanted to include a full calendar year of data from before the start of COVID-19 (World Health Organization declared a Public Health Emergency on March 11, 2020), and a full 12 months of data post-pandemic onset (through March 2021). We then matched the 27-month duration for our pre-pandemic comparison cohort, for whom we also wanted to ensure a calendar year of overlap with our pandemic-exposed cohort (January-December 2019). As depicted in Fig. 1, for the comparison cohort this coding scheme corresponded with dates October 2017-November 2018 for year one and December 2018-December 2019 for year two. For the pandemic-exposed cohort, we used data from January 2019-February 2020 for year one and March 2020-March 2021 for year two, with year two coinciding with the start of the COVID-19 public health emergency and MOUD policy exemptions. Covariates included age, race/ethnicity, sex assigned in the medical record, whether patients lived in rural versus urban settings, medical comorbidity at baseline (via the Charlson Comorbidity Index; CCI; Charlson et al., 1987), and number of baseline psychiatric comorbidities (via Psychiatric Diagnostic Groups score; PDG; Ashcroft et al., 1989).
2.3. Outcome variables
Code lists used in this study included OUD-relevant CPT codes (patient-provider encounters) identified from an exhaustive list of services offered in VHA, and MOUD prescription fills (e.g., oral buprenorphine) and procedure codes (e.g., injectable buprenorphine), developed and finalized through consensus among key study team members and expert MOUD providers (see Supplemental Attachment). Presence of outcomes in Veterans’ medical record were aggregated within Veterans’ year one versus two and included receipt of buprenorphine, methadone, or naltrexone, or in-person or telehealth therapy/counseling (yes/no) within a given year. We also created separate outcomes for therapy/counseling delivered in-person vs. telehealth. MOUD data was indexed by NDC codes or drug name, documented prescription fills, or procedure codes to capture any instance of medication dispensing (e.g., buprenorphine prescription) or administration (e.g., buprenorphine injection) in VHA, regardless of formulation.
Secondary outcomes included the (1) average annual percentage of days covered (PDC) for Veterans who received an MOUD; (2) the number of months MOUD was provided in the year; and (3) the number of therapy/counseling appointments attended each year. These analyses focused solely on Veterans who received the respective intervention.
2.4. Data management and analytic strategy
Data were analyzed using a difference-in-differences framework, with observations aggregated within years one or two and between cohorts. We estimated models for our primary outcomes separately for buprenorphine, methadone, naltrexone, and therapy/counseling (all yes/no within year), using binary logistic mixed models. Secondary analyses, all estimated separately using linear mixed models, included PDC on buprenorphine, methadone, and naltrexone; the number of months prescribed each MOUD in a given year; and the number of in-person or telehealth therapy/counseling visits attended. Each model was estimated using fixed effects for predictors and a random intercept for patient. Primary predictors included time (i.e., year two versus year one, with year two for the pandemic-exposed cohort coinciding with the start of the COVID-19 public health emergency and MOUD policy exemptions), cohort grouping (comparison cohort = 0, pandemic-exposed cohort = 1), and a cohort grouping by year interaction. Additional covariates included sex assigned in the medical record, age, race/ethnicity, rural versus urban, and CCI and PDG scores. Age, CCI, and PDG variables were centered and standardized to facilitate model convergence. Data were analyzed in R using lme4 (Bates et al., 2015) and plots were created using Excel.
3. Results
3.1. Primary analyses
Primary analyses include all matched Veterans to evaluate the probability of receiving any of the following eligible treatments, yes/no, among both cohorts.
3.1.1. MOUD prescriptions or administrations
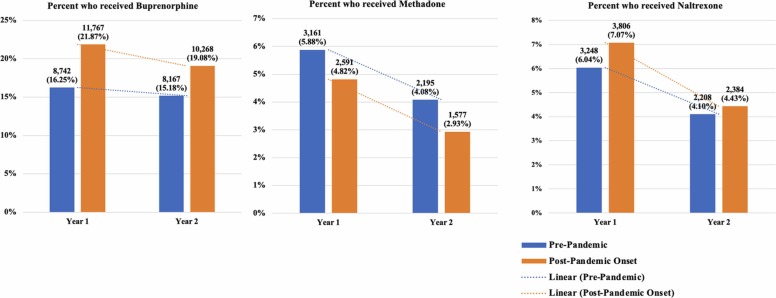
As expected, we observed statistically significant time by cohort interactions suggesting that Veterans in the pandemic-exposed cohort were significantly less likely than the pre-pandemic cohort to receive MOUD in year two, post-pandemic onset, relative to year two in the pre-pandemic cohort (see Table 2, Fig. 2). Covariate effects suggest that Veterans of color were less likely to receive buprenorphine and more likely to receive naltrexone. Older Veterans were more likely to receive methadone and less likely to receive buprenorphine and naltrexone. Rural Veterans were less likely to receive any MOUD. Lastly, Veterans with more medical comorbidities (higher CCI index) were less likely to receive buprenorphine and naltrexone; and Veterans with more psychiatric comorbidities (PDG) were more likely to receive naltrexone and less likely to receive methadone.
Table 2.
Fixed and random parameter estimates for the logistic mixed model predicting the likelihood of MOUD and therapy/counseling receipt between cohorts and over time.
| Therapy/Counseling |
Buprenorphine |
Methadone |
Naltrexone |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In person |
Telehealth |
Total |
||||||||||
| Fixed Effects | Est. (SE) | adjOR (95% CI) | Est. (SE) | adjOR (95% CI) | Est. (SE) | adjOR (95% CI) | Est. (SE) | adjOR (95% CI) | Est. (SE) | adjOR (95% CI) | Est. (SE) | adjOR (95% CI) |
| Intercept | 1.96(0.02)* ** | 7.07 (6.80, 7.36) |
-3.07(0.03)* ** | 0.05 (.04,0.05) |
2.07(0.02)* ** | 7.96 (7.64, 8.31) |
-9.24(0.06) * ** | 0.00 (.00,0.00) |
-11.26(0.12)* ** | 0.00 (.00,0.00) |
-9.38(0.08)* ** | 0.00 (.00,0.00) |
| Agea | -.18(0.01)* ** | 0.83 (.82,0.85) |
-0.18(0.01)* ** | 0.83 (.81,0.84) |
-0.20(0.01)* ** | 0.82 (.80,0.83) |
-0.48(0.03)* ** | 0.62 (.59,0.66) |
0.15(0.07)* | 1.16 (1.02, 1.32) |
-0.41(0.04)* ** | 0.67 (.62,0.72) |
| Gender (male ref.) |
0.30(0.03)* ** | 1.34 (1.26, 1.44) |
0.32(0.03)* ** | 1.38 (1.30, 1.47) |
0.37(0.036)* ** | 1.45 (1.35, 1.55) |
-0.13(0.10) | 0.87 (.71, 1.07) |
-0.35(0.29) | 0.70 (.40, 1.25) |
-0.21(0.14) | 0.81 (.62, 1.06) |
| Race/Ethnicity (White ref.) |
0.25(0.02)* ** | 1.28 (1.24, 1.33) |
0.08(0.02)* ** | 1.09 (1.05, 1.13) |
0.25(0.02)* ** | 1.28 (1.24, 1.33) |
-0.47(0.06)* ** | 0.62 (.55,0.71) |
0.02(0.13) | 1.02 (.79, 1.31) |
0.19(0.08)* | 1.21 (1.04, 1.42) |
| Rural vs. Urban (urban ref.) |
-0.36(0.02)* ** | 0.70 (.67,0.72) |
0.04(0.02)* | 1.05 (1.01, 1.08) |
-0.36(0.02)* ** | 0.70 (.67,0.73) |
-0.08(0.06)* ** | 0.92 (.82, 1.04) |
-0.48(0.15)* * | 0.62 (.46,0.83) |
-0.26(0.09)* * | 0.77 (.65,0.91) |
| CCIa | -.15(0.01)* ** | 0.86 (.85,0.88) |
-0.10(0.01)* ** | 0.90 (.89,0.92) |
-0.19(0.01)* ** | 0.83 (.82,0.85) |
-0.10(0.03)* * | 0.90 (.85,0.97) |
0.09(0.06) | 1.09 (.97, 1.22) |
-0.17(0.05)* ** | 0.84 (.77,0.92) |
| PDGa | .46(0.01)* ** | 1.58 (1.55, 1.61) |
0.36(0.01)* ** | 1.44 (1.42, 1.46) |
0.49(0.01)* ** | 1.63 (1.60, 1.66) |
-0.00(0.03) | 1.00 (.95, 1.05) |
-0.21(0.07)* * | 0.81 (.71,0.93) |
0.52(0.03)* ** | 1.68 (1.58, 1.80) |
| Year 2 vs. 1 (year 1 ref.) |
-0.93(0.02)* ** | 0.40 (.38,0.41) |
-0.18(0.02)* ** | 0.83 (.79,0.87) |
-0.95(0.02)* ** | 0.39 (.37,0.40) |
-0.57(0.05)* ** | 0.57 (.52,0.62) |
-5.29(0.18)* ** | 0.01 (.00,0.01) |
-1.93(0.07)* ** | 0.15 (.13,0.17) |
| Cohort (comparison cohort ref.) |
0.18(0.02)* ** | 1.20 (1.15, 1.25) |
0.22(0.02)* ** | 1.25 (1.19, 1.31) |
0.22(0.02)* ** | 1.24 (1.19, 1.30) |
0.57(0.06)* ** | 1.78 (1.59, 1.99) |
-0.22(0.12)† | .80 (.63, 1.01) |
0.25(0.07)* ** | 1.28 (1.11, 1.48) |
| Cohort X Year | -1.30(0.02)* ** | 0.27 (.26,0.29) |
2.25(0.03)* ** | 9.53 (8.95, 10.20) |
-0.81(0.03)* ** | 0.45 (.42,0.47) |
-0.65(0.06)* ** | 0.52 (.46,0.59) |
-1.20(0.20)* ** | 0.30 (.20,0.45) |
-0.55(0.10)* ** | 0.58 (.48,0.70) |
| Random effects | SD | SD | SD | SD | SD | SD | ||||||
| Level 2 | ||||||||||||
| Interceptb | 1.70 | 1.28 | 1.80 | 15.92 | 23.00 | 11.49 | ||||||
Note: CCI = Charlson Comorbidity Index; PDG = Psychiatric Diagnosis Groups; Est. = Estimate; SE = Standard Error; adjOR = Adjusted Odds Ratio; 95% CI = 95% Confidence Interval; SD = Standard Deviation
a Centered and standardized to overcome scale differences and facilitate model convergence
b Parameter estimate of between person variance around fixed intercept.
†p < .10, *p < .05, * *p < .01, * **p < .001
Fig. 2.
Unadjusted plots of the percents and counts of patients who received buprenorphine, methadone, or naltrexone by cohort and over time.
3.1.2. Therapy/counseling
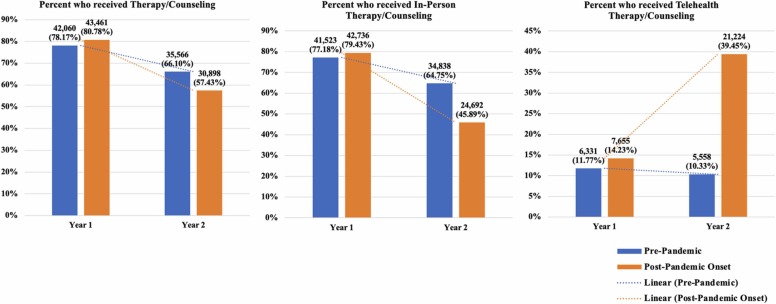
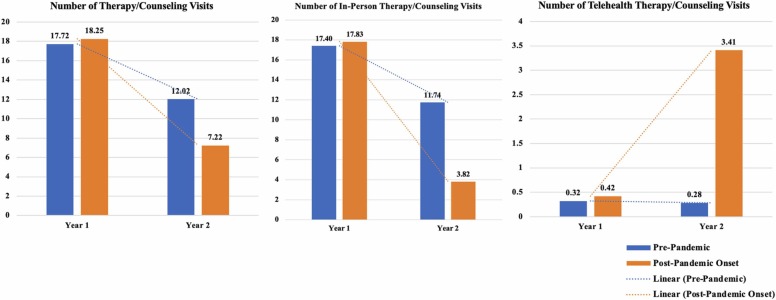
Similar to MOUD, we found that veterans in the pandemic-exposed cohort were significantly less likely to receive in-person therapy/counseling in year two, post-pandemic onset, compared to Veterans in year two in the pre-pandemic cohort (See Table 2, Fig. 3). The opposite was true for telehealth therapy/counseling; the pandemic-exposed cohort experienced an approximate 850% increase, adj OR = 9.53, 95% CI (8.95, 10.20), in the probability of receiving telehealth therapy/counseling in the year post-pandemic onset. However, the rise in telehealth for the pandemic-exposed cohort was not enough to completely offset the drop in in-person care, as indicated by the fact that total therapy/counseling receipt was lowest during COVID-19 among the pandemic-exposed cohort (Fig. 3). In addition, women, Veterans of color, and patients with higher PDG scores were more likely to receive therapy/counseling, whereas older Veterans and Veterans with higher CCI scores were less likely to receive therapy/counseling.
Fig. 3.
Unadjusted plots of the percents and counts of patients who received therapy/counseling overall, in-person, or delivered via telehealth by cohort and over time.
3.2. Secondary analyses
Secondary analyses included the subsets of Veterans from each cohort who received any of the respective interventions below. We chose to examine these patients separately to evaluate the degree of coverage and access to MOUD and therapy/counseling once Veterans received them.
3.2.1. Percent days covered (PDC) on MOUD
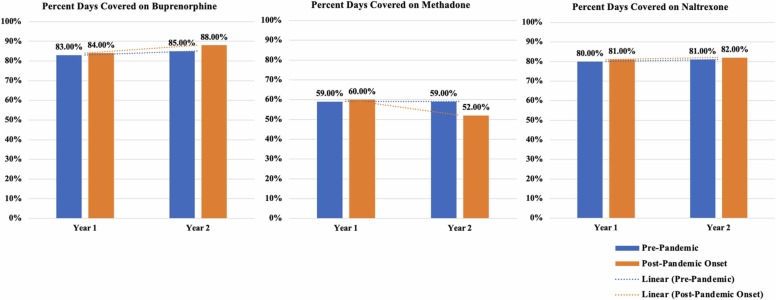
Among Veterans who received any methadone, their PDC decreased more sharply from year one to two for the pandemic-exposed cohort, corresponding to the onset of COVID-19 (See Table 3, Fig. 4). On the other hand, the PDC on buprenorphine was higher in year two among Veterans in the pandemic-exposed cohort compared to the pre-pandemic comparison cohort. Naltrexone PDC did not differ by cohort over time. PDC for buprenorphine and methadone was lower among Veterans of color. Older Veterans had higher PDC on methadone and naltrexone, and rural Veterans had higher methadone and buprenorphine PDC compared to urban Veterans. Buprenorphine and methadone PDC were lower for Veterans with more psychiatric comorbidities (higher PDG scores), and buprenorphine and naltrexone PDC were lower for Veterans with higher CCI scores.
Table 3.
Fixed and random parameter estimates for linear mixed models predicting the percent of days covered on MOUD among those who utilized MOUD care, between cohorts and over time.
| Buprenorphine PDC (n = 23,915) |
Methadone PDC (n = 6394) |
Naltrexone PDC (n = 9126) |
|
|---|---|---|---|
| Fixed Effects | Est. (SE) | Est. (SE) | Est. (SE) |
| Intercept | 0.83(0.00)* ** | 0.58(0.01)* ** | 0.80(0.00)* ** |
| Agea | .00(0.00) | 0.06(0.01)* ** | 0.01(0.00)* ** |
| Gender (male ref.) |
-0.00(0.01) | 0.02(0.02) | 0.01(0.01) |
| Race/Ethnicity (White ref.) |
-0.07(0.00)* ** | -0.11(0.01)* ** | -0.00(0.00) |
| Rural vs. Urban (urban ref.) |
0.02(0.00)* ** | 0.13(0.01)* ** | -0.00(0.00) |
| CCIa | -.03(0.00)* ** | 0.00(0.00) | -0.01(0.00)* ** |
| PDGa | -.02(0.00)* ** | -0.03(0.01)* ** | -0.00(0.00) |
| Year 2 vs. 1 (year 1 ref.) |
-0.01(0.00)* * | -0.02(0.00)* ** | 0.00(0.00) |
| Cohort (comparison cohort ref.) |
0.01(0.00)* ** | 0.01(0.01) | 0.01(0.00)* |
| Cohort X Year | 0.01(0.00)* ** | -0.08(0.01)* ** | 0.01(0.01)† |
| Random effects | SD | SD | SD |
| Level 2 | |||
| Interceptb | .21 | 0.31 | 0.04 |
| Level 1 | |||
| Residual | 0.11 | 0.15 | 0.17 |
Note: CCI = Charlson Comorbidity Index; PDG = Psychiatric Diagnosis Groups; Est. = Estimate; SE = Standard Error; SD = Standard Deviation
a Centered and standardized to overcome scale differences and facilitate model convergence
b Parameter estimate of between person variance around fixed intercept.
†p < .10, *p < .05, * *p < .01, * **p < .001
Fig. 4.
Unadjusted plots of the percent of days covered on buprenorphine, methadone, or naltrexone during months in which the medication was prescribed, by cohort and over time.
3.2.2. Number of months prescribed/administered MOUD
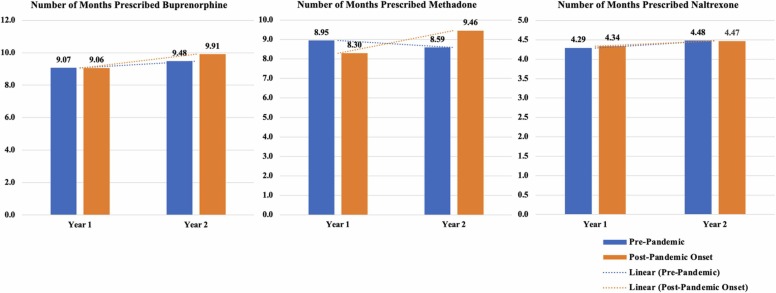
Among Veterans who received any buprenorphine, the number of months prescribed increased from year one to two for the pandemic-exposed cohort relative to the pre-pandemic cohort (See Table 4, Fig. 5). The number of months Veterans received methadone appeared to increase from year one to two for Veterans in the pandemic-exposed cohort, and decrease from years one to two for the pre-pandemic cohort (see unadjusted output in Fig. 5). The number of months Veterans received naltrexone did not differ between cohort over time. Veterans of color had fewer months of buprenorphine and methadone receipt. Veterans with higher CCI scores had fewer months of any MOUD; Veterans with higher PDG scores received naltrexone for a greater number of months and received methadone for a fewer number of months. Older Veterans had a higher number of months prescribed each MOUD.
Table 4.
Fixed and random parameter estimates for linear mixed models predicting the total number of months of MOUD utilization among those who utilized care, between cohorts and over time.
| Buprenorphine Number of Months (n = 23,915) |
Methadone Number of Months (n = 6394) |
Naltrexone Number of Months (n = 9126) |
|
|---|---|---|---|
| Fixed Effects | Est. (SE) | Est. (SE) | Est. (SE) |
| Intercept | 8.76(0.06)* ** | 8.86(0.10)* ** | 4.19(0.07)* ** |
| Agea | .12(0.03)* ** | 1.00(0.07)* ** | 0.19(0.04)* ** |
| Gender (male ref.) |
-0.04(0.11) | -0.22(0.29) | 0.18(0.12) |
| Race/Ethnicity (White ref.) |
-1.08(0.07)* ** | -0.80(0.13)* ** | -0.03(0.07) |
| Rural vs. Urban (urban ref.) |
0.23(0.07)* ** | 0.03(0.15) | -0.33(0.08)* ** |
| CCIa | -.66(0.04)* ** | -0.26(0.06)* ** | -0.20(0.04)* ** |
| PDGa | -.01(0.03) | -0.23(0.07)* ** | 0.24(0.03)* ** |
| Year 1 vs. 2 (year 1 ref.) |
0.15(0.05)* * | -1.49(0.09)* ** | -0.01(0.08) |
| Cohort (comparison cohort ref.) |
0.03(0.07) | -0.77(0.13)* ** | 0.08(0.08) |
| Cohort X Year | 0.27(0.07)* ** | 1.18(0.14)* ** | -0.10(0.11) |
| Random effects | SD | SD | SD |
| Level 2 | |||
| Interceptb | 3.70 | 3.95 | 1.99 |
| Level 1 | |||
| Residual | 3.11 | 2.92 | 2.64 |
Note: CCI = Charlson Comorbidity Index; PDG = Psychiatric Diagnosis Groups; Est. = Estimate; SE = Standard Error; SD = Standard Deviation
a Centered and standardized to overcome scale differences and facilitate model convergence
b Parameter estimate of between person variance around fixed intercept.
†p < .10, *p < .05, * *p < .01, * **p < .001
Fig. 5.
Unadjusted plots of the number of months prescribed buprenorphine, methadone, or naltrexone by cohort and over time.
3.2.3. Number of therapy/counseling encounters
The number of in-person and total therapy/counseling encounters decreased significantly more from years one to two in the pandemic-exposed relative to the pre-pandemic cohort, coinciding with COVID-19 onset for the former (See Table 5, Fig. 6). As before, while utilization of telehealth increased significantly for the pandemic-exposed cohort in year two, this was not enough to totally offset the loss in therapy/counseling session attendance during COVID-19. Whereas women were more likely to have any therapy/counseling utilization in the prior therapy/counseling models, the current model that they attended fewer in-person and total therapy/counseling sessions overall.
Table 5.
Fixed and random parameter estimates for linear mixed models predicting the number of therapy/counseling sessions attended between cohorts and over time.
| Therapy/Counseling Number of Sessions Attended |
|||
|---|---|---|---|
| In Person |
Telehealth |
Total |
|
| Fixed Effects | Est. (SE) | Est. (SE) | Est. (SE) |
| Intercept | 17.43(0.16)* ** | 0.18(0.03)* ** | 17.61(0.17)* ** |
| Agea | -2.58(0.09)* ** | -0.17(0.01)* ** | -2.75(0.10)* ** |
| Gender (male ref.) |
-1.46(0.34)* ** | 0.41(0.05)* ** | -1.05(0.35)* * |
| Race/Ethnicity (White ref.) |
3.43(0.19)* ** | 0.07(0.03)* * | 3.51(0.19)* ** |
| Rural vs. Urban (urban ref.) |
-3.76(0.19)* ** | -0.14(0.03)* ** | -3.91(0.20)* ** |
| CCIa | -.60(0.09)* ** | -0.09(0.01)* ** | -0.70(0.09)* ** |
| PDGa | 3.97(0.09)* ** | 0.34(0.01)* ** | 4.31(0.09)* ** |
| Year 2 vs. 1 (year 1 ref.) |
-5.68(0.14)* ** | -0.03(0.03) | -5.71(0.14)* ** |
| Cohort (comparison cohort ref.) |
-0.23(0.19) | 0.09(0.03)* * | -0.14(0.20) |
| Cohort X Year | -6.92(0.20)* ** | 2.61(0.04)* ** | -4.30(0.20)* ** |
| Random effects | SD | SD | SD |
| Level 2 | |||
| Interceptb | 21.90 | 0.93 | 23.07 |
| Level 1 | |||
| Residual | 23.20 | 5.08 | 23.09 |
Note: CCI = Charlson Comorbidity Index; PDG = Psychiatric Diagnosis Groups; Est. = Estimate; SE = Standard Error; SD = Standard Deviation
a Centered and standardized to overcome scale differences and facilitate model convergence
b Parameter estimate of between person variance around fixed intercept.
†p < .10, *p < .05, * *p < .01, * **p < .001
Fig. 6.
Unadjusted plots of the number of therapy/counseling sessions received by cohort and over time.
4. Discussion
The COVID-19 pandemic challenged the healthcare system at a time when access to life-saving treatment for OUD became more critical than ever. Using nationwide VHA electronic health record data, we found that the likelihood of Veterans receiving any form of MOUD in VHA decreased significantly following the pandemic onset, relative to trends observed among a matched cohort of patients observed leading up to, but not during, COVID-19. Similarly, therapy/counseling receipt during the year post-pandemic onset was significantly impacted, with a net loss in care delivered despite exponential increases in telehealth offerings across VHA (Gustavson et al., 2020). However, among Veterans who received treatment, buprenorphine PDC and the number of months prescribed buprenorphine or methadone appeared to improve relative to the pre-pandemic comparison cohort. Taken together, these data suggest negative impacts regarding receipt of any MOUD or therapy/counseling for Veterans with an existing diagnosis of OUD, but less disruption, or perhaps even improved coverage, among Veterans who received any treatment during COVID-19.
In another recent study using VHA data, Lin et al. (2022) found that the number of Veterans receiving buprenorphine in VHA averaged 103 patients per month in the year prior to COVID-19 and dropped to an average of 47 patients per month post-pandemic onset. This suggests that buprenorphine treatment continued to expand during COVID-19 but at a slower rate during the pandemic relative to the prior year. Lin et al. (2022) characterize their results as evidence of continued buprenorphine expansion during the pandemic and attribute this to increases in telehealth appointments, consistent with our findings of significant telehealth expansion. Despite increases in telehealth, and using a different cohort definition and approach than Lin et al. (2022), we found that Veterans with OUD were less likely to receive buprenorphine in the post-pandemic period overall. These findings are consistent with another VHA study showing that rates of prescription drugs, including buprenorphine, had not yet returned to pre-pandemic levels by August 2020 (Myers et al., 2021).
Our finding that PDC on buprenorphine was less and PDC on methadone was more negatively impacted during the pandemic, relative to the pre-pandemic comparison cohort, suggests that COVID-19 may have been more disruptive to Veterans being treated with methadone than it was for Veterans receiving buprenorphine. Consistently, the policy exemptions that went into effect in February-March 2020 were designed to prevent disruption to MOUD treatment and were more generous for buprenorphine than for methadone (e.g., the requirement for in-person evaluation was no longer required for buprenorphine initiation but it was still required for methadone). Additional research is needed to further examine methadone access barriers, particularly with respect to potential barriers to implementing the revised MOUD treatment guidelines during COVID-19 and beyond. Since methadone may be more efficacious or preferred over buprenorphine for the treatment of some patients with OUD, who use higher potency opioids such as fentanyl, expanding access is critical (Simon et al., 2022). Interestingly, PDC on naltrexone was unrelated to time or cohort, consistent with the fact that COVID-19-related MOUD policy exemptions were not relevant to naltrexone prescriptions.
The number of months prescribed buprenorphine increased over time, especially among Veterans treated during the pandemic. The number of months prescribed methadone decreased from year one to two overall, but less so for Veterans treated during the pandemic. These trends are consistent with the aims of the MOUD policy exemptions and may signal that the expansion of care during COVID-19 had net positive effects regarding MOUD maintenance among those already receiving MOUD. Additional research is needed to also examine the possibility of medication switching during the pandemic, such as patients being transferred from methadone to buprenorphine, which could help explain some of the medication trends observed here.
Despite being ancillary to the primary aims of the current investigation, several observed covariate effects are worth specific mention. For example, we found that women were more likely than men to receive any therapy/counseling but received fewer sessions overall. Veterans of color were also more likely to receive any therapy/counseling or naltrexone but were less likely to receive buprenorphine. Veterans of color also had lower methadone and buprenorphine PDC and fewer prescription months compared to White Veterans. These findings support the need for further research to identify unmet treatment needs and to promote health equity. We also found that older Veterans were more likely to receive methadone and less likely to receive buprenorphine and naltrexone, although among Veterans who received treatment, older Veterans had higher methadone and naltrexone PDC and the number of months prescribed each MOUD. In addition to considering whether additional research or outreach are needed to bridge apparent age disparities, continued efforts to extend MOUD access to Veterans with more medical or psychiatric comorbid conditions, and to rural Veterans who are not receiving MOUD, seems warranted. On the other hand, there may be important lessons to be learned about maintaining rural Veterans on MOUD given that rural Veterans in our study evidenced greater PDC on their prescriptions.
4.1. Limitations
Limitations of the current study include the potential for informed presence bias, which may have resulted in having more/less complete information on patients who utilized more/fewer VHA services, respectively. We required Veterans to have documentation of an OUD in their medical record during specified date ranges prior to the pandemic. This was intention for the current analysis, as we were primarily interested in evaluating healthcare impacts on Veteran patients with documented OUD by the start of COVID-19. As such, it is not possible in the current study to evaluate impacts of COVID-19 or MOUD policy exemptions on Veteran patients who were newly diagnosed with OUD post-pandemic onset. Lastly, inferences drawn from this study are limited by the quasi-experimental design yet strengthened to the extent that we achieved balance between cohorts at baseline across key variables, built the comparator group using a date range that entirely preceded COVID-19, and included a full 27 months (divided into two years) of data for each cohort to account for time effects within cohorts. Nevertheless, MOUD policy exemptions are linked in time with COVID-19 onset so it is impossible to separate the effects of policy changes from the effects of COVID-itself. In future research, it may be beneficial to examine additional patterns of therapy/counseling and MOUD utilization that were made possible through implementation of the MOUD policy exemptions specifically. For example, future studies could examine whether rates of MOUD initiation via telehealth changed post-pandemic onset, or to evaluate changes in prescription length and take-home dose amounts, and other measures consistent with the leniencies outlined by the MOUD policy exemptions. Given VHA’s telehealth capabilities prior to COVID-19, it is possible that it was better equipped to respond to the COVID-19 public health emergency compared to other healthcare systems, or relative to data contained in commercial, Medicaid, or Medicare databases. This is an untested possibility worthy of future research attention. Additional research would also benefit from greater granularity in examining the impacts of MOUD policy effects on patients with OUD, including qualitative accounts of patients, providers, and policy makers, who can speak to the impacts of these policies on treatment access and patient outcomes.
5. Conclusion
In a nationwide sample of Veterans with current OUD, we found a reduced likelihood of receiving buprenorphine, methadone, naltrexone, or therapy/counseling following the onset of COVID-19. However, among patients who did receive care, we found some evidence of improved adherence to buprenorphine and methadone during the pandemic compared to prior years.
The adverse effects of the pandemic on patients with OUD, during an ongoing opioid epidemic with surging overdose deaths (Friedman et al., 2021), have been substantial and suggest that changes to policy, practice, and outreach are needed to better engage Veterans with OUD who are not receiving adequate care. Indeed, greater access to MOUD has been shown to be both cost-effective but also associated with significant reductions on overdose incidence and mortality (Fairley et al., 2021). On the other hand, the MOUD policy exemptions that went into effect during the pandemic, with the purpose of preventing disruption to live-saving care, may have prevented further treatment disruption and, in some cases, improved access to MOUD at a time when it was most critical. We recommend that these policy exemptions remain in effect but also with an eye toward other strategies for expanding access to treatment for OUD. Further exploration of Veteran outcomes associated with COVID-19 and these policy exemptions is warranted, including qualitative research to highlight perspectives of policy makers, clinicians, and Veteran patients with OUD to guide data-driven MOUD policy.
Funding
This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Project Program Award (20–11081; dual-PIs Livingston & Weisberg).
CRediT authorship contribution statement
Nicholas A. Livingston: conception and design, data analysis, interpretation of data, writing and critical revision of the article. Michael Davenport: data management, data analysis, interpretation of data, and critical revision of the article. Michael Head: conception and design, interpretation of data, and critical revision of the article. Rachel Henke: conception and design, interpretation of data, and critical revision of the article. Lavonia Smith LeBeau: interpretation of data and critical revision of the article. Teresa B. Gibson: conception and design, interpretation of data, and critical revision of the article. Anne N. Banducci: interpretation of data and critical revision of the article. Alexis Sarpong: interpretation of data and critical revision of the article. Saketh Jayanthi: interpretation of data and critical revision of the article. Clara Roth: interpretation of data and critical revision of the article. Jessica Camacho-Cook: interpretation of data and critical revision of the article. Frank Meng: interpretation of data and critical revision of the article. Justeen Hyde: interpretation of data and critical revision of the article. Norah Mulvaney-Day: interpretation of data and critical revision of the article. Mackenzie White: interpretation of data and critical revision of the article. Daniel Chen: interpretation of data and critical revision of the article. Michael Stein: interpretation of data and critical revision of the article. Risa Weisberg: conception and design, interpretation of data, and critical revision of the article.
Author note
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee, the U.S. Department of Veterans Affairs, or other listed affiliates.
Author agreement
We declare that this manuscript is original and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Conflict of Interest
Authors declared no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2022.109678.
Appendix A. Supplementary material
Supplementary material
.
References
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders (5th ed.).
- Ashcraft M.L., Fries B.E., Nerenz D.R., Falcon S.P., Srivastava S.V., Lee C.Z., Berki S.E., Errera P. A psychiatric patient classification system. An alternative to diagnosis-related groups. Med. Care. 1989;27(5):543–557. doi: 10.1097/00005650-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. [Google Scholar]
- Bohnert A.S., Ilgen M.A., Galea S., McCarthy J.F., Blow F.C. Accidental poisoning mortality among patients in the department of veterans affairs health system. Med. Care. 2011;49(4):393–396. doi: 10.1097/MLR.0b013e318202aa27. [DOI] [PubMed] [Google Scholar]
- Center for Ethics and the Role of Law. The Intersection of Opioid Overuse and Veteran Mental Health Challenges, 2017. Retrieved August 17 from. 〈https://www.law.upenn.edu/live/files/6192-cerl-report-on-ptsd-and-opioid-addiction-final〉.
- Centers for Disease Control. Healthcare Facilities: Preparing for Community Transmission, 2020. Retrieved May 20, 2022 from. 〈https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html〉.
- Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Connery H.S. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv. Rev. Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Fairley M., Humphreys K., Joyce V.R., Bounthavong M., Trafton J., Combs A., Oliva E.M., Goldhaber-Fiebert J.D., Asch S.M., Brandeau M.L., Owens D.K. Cost-effectiveness of treatments for opioid use disorder. JAMA Psychiatry. 2021;78(7):767–777. doi: 10.1001/jamapsychiatry.2021.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Beletsky L., Schriger D.L. Overdose-related cardiac arrests observed by emergency medical services during the US COVID-19 Epidemic. JAMA Psychiatry. 2021;78(5):562–564. doi: 10.1001/jamapsychiatry.2020.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, B., 2017. Opioid abuse crisis takes heavy toll on U.S. veterans. Reuters. Retrieved August 16 2022 from. 〈https://www.reuters.com/article/us-usa-veterans-opioids/opioid-abuse-crisis-takes-heavy-toll-on-u-s-veterans-idUSKBN1DA1B2〉.
- Gomes T., Campbell T.J., Kitchen S.A., Garg R., Bozinoff N., Men S., Tadrous M., Munro C., Antoniou T., Werb D., Wyman J. Association between increased dispensing of opioid agonist therapy take-home doses and opioid overdose and treatment interruption and discontinuation. JAMA. 2022;327(9):846–855. doi: 10.1001/jama.2022.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.J., Drexler K., Hawkins E.J., Burden J., Codell N.K., Mhatre-Owens A., Dungan M.T., Hagedorn H. Stepped care for opioid use disorder train the trainer (SCOUTT) initiative: expanding access to medication treatment for opioid use disorder within veterans health administration facilities. Subst. Abus. 2020;41(3):275–282. doi: 10.1080/08897077.2020.1787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson A.M., Gordon A.J., Kenny M.E., McHenry H., Gronek J., Ackland P.E., Hagedorn H.J. Response to coronavirus 2019 in veterans health administration facilities participating in an implementation initiative to enhance access to medication for opioid use disorder. Subst. Abus. 2020;41(4):413–418. doi: 10.1080/08897077.2020.1809609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K.A., Foot C., Levander X.A., Cook R., Terashima J.P., McIlveen J.W., Korthuis P.T., McCarty D. Treatment retention, return to use, and recovery support following COVID-19 relaxation of methadone take-home dosing in two rural opioid treatment programs: a mixed methods analysis. J. Subst. Abus. Treat. 2022 doi: 10.1016/j.jsat.2022.108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iheanacho T., Stefanovics E., Rosenheck R. Opioid use disorder and homelessness in the veterans health administration: the challenge of multimorbidity. J. Opioid Manag. 2018;14(3):171–182. doi: 10.5055/jom.2018.0447. [DOI] [PubMed] [Google Scholar]
- Jercich, K., 2022. How the VA laid the groundwork for a pandemic-fueled telehealth spike. Healthcare IT News. Retrieved August 8 2022 from. 〈https://www.healthcareitnews.com/news/how-va-laid-groundwork-pandemic-fueled-telehealth-spike〉.
- Joudrey P.J., Adams Z.M., Bach P., Van Buren S., Chaiton J.A., Ehrenfeld L., Guerra M.E., Gleeson B., Kimmel S.D., Medley A., Mekideche W., Paquet M., Sung M., Wang M., You Kheang R.O.O., Zhang J., Wang E.A., Edelman E.J. Methadone access for opioid use disorder during the COVID-19 pandemic within the United States and Canada. JAMA Netw. Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.18223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Fernandez A.C., Bonar E.E. Telehealth for substance-using populations in the age of coronavirus disease 2019: recommendations to enhance adoption. JAMA Psychiatry. 2020;77(12):1209–1210. doi: 10.1001/jamapsychiatry.2020.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.A., Zhang L., Kim H.M., Frost M.C. Impact of COVID-19 telehealth policy changes on buprenorphine treatment for opioid use disorder. Am. J. Psychiatry appiajp. 2022:21111141. doi: 10.1176/appi.ajp.21111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston N.A., Ameral V., Banducci A.N., Weisberg R.B. Unprecedented need and recommendations for harnessing data to guide future policy and practice for opioid use disorder treatment following COVID-19. J. Subst. Abus. Treat. 2021;122 doi: 10.1016/j.jsat.2020.108222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midboe A.M., Byrne T., Smelson D., Jasuja G., McInnes K., Troszak L.K. The opioid epidemic in veterans who were homeless or unstably housed. Health Aff. (Millwood) 2019;38(8):1289–1297. doi: 10.1377/hlthaff.2019.00281. [DOI] [PubMed] [Google Scholar]
- Morgan J.R., Quinn E.K., Chaisson C.E., Ciemins E., Stempniewicz N., White L.F., Linas B.P., Walley A.Y., LaRochelle M.R. Variation in initiation, engagement, and retention on medications for opioid use disorder based on health insurance plan design. Med. Care. 2022;60(3):256–263. doi: 10.1097/MLR.0000000000001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers U., Bishu K., Gauthier-Wetzel H., Grubaugh A., Axon R.N., Ana E.S., Gebregziabher M. Impact of COVID-19 on prescriptions for controlled substances for Veterans with opioid use disorder. Health Serv. Res. 2021;56:5–6. [Google Scholar]
- Office of Public and Intergovernmental Affairs, 2018. VA Expands Telehealth by Allowing Health Care Providers to Treat Patients Across State Lines. Retrieved August 8, 2022 from 〈https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054〉.
- Rhee T.G., Rosenheck R.A. Comparison of opioid use disorder among male veterans and non-veterans: Disorder rates, socio-demographics, co-morbidities, and quality of life. Am. J. Addict. 2019;28(2):92–100. doi: 10.1111/ajad.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA, 2020c. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Retrieved August 5 2022 from. 〈https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf〉.
- SAMHSA, 2020a. Medication-assisted treatment. Retrieved May 10 2022 from. 〈https://www.samhsa.gov/medication-assisted-treatment〉.
- SAMHSA, 2020d. Opioid Treatment Program (OTP) Guidance. Retrieved August 5 2022. from. 〈https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf〉.
- SAMHSA, 2020b. Statutes, regulations, and guidelines. Retrieved August 5 2022 from. https://www.samhsa.gov/medication-assisted-treatment/statutes-regulations-guidelines.
- SAMHSA, 2015. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. Retrieved August 5 2022 from. 〈https://store.samhsa.gov/sites/default/files/d7/priv/sma15–4907.pdf〉.
- SAMHSA Key substance use and mental health indicators in the United States: Results from the 2018 national survey on drug use and health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: center for behavioral health statistics and quality. Subst. Abus. Ment. Health Serv. Adm. 2019:2019. [Google Scholar]
- SAMHSA, 2022. Statutes, Regulations, and Guidelines. Retrieved August 5 2022 from. 〈https://www.samhsa.gov/medication-assisted-treatment/statutes-regulations-guidelines〉.
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths - United States. 2013-2017. Mmwr. 2018;67(5152):1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C., Vincent L., Coulter A., Salazar Z., Voyles N., Roberts L., Frank D., Brothers S. The methadone manifesto: treatment experiences and policy recommendations from methadone patient activists. Am. J. Public Health. 2022;112(S2):S117–S122. doi: 10.2105/AJPH.2021.306665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkalanka P., Lund B.C., Arndt S., Field W., Charlton M., Ward M.M., Carnahan R.M. Association between buprenorphine for opioid use disorder and mortality risk. Am. J. Prev. Med. 2021;61(3):418–427. doi: 10.1016/j.amepre.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will J., Abare M., Olson M., Chyorny A., Wilhelm-Leen E. Emergency department utilization by individuals with opioid use disorder who were recently incarcerated. J. Subst. Abus. Treat. 2022;141 doi: 10.1016/j.jsat.2022.108838. [DOI] [PubMed] [Google Scholar]
- World Health Organization . ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. second ed. World Health Organization; 2004. [Google Scholar]
- Zhang P., Tossone K., Ashmead R., Bickert T., Bailey E., Doogan N.J., Mack A., Schmidt S., Bonny A.E. Examining differences in retention on medication for opioid use disorder: an analysis of Ohio Medicaid data. J. Subst. Abus. Treat. 2022;136 doi: 10.1016/j.jsat.2021.108686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material