Abstract
The relationship between osteogenesis and angiogenesis is complex. Normal bone development requires angiogenesis, mediated by vascular endothelial growth factor A (VEGFA). Studies have demonstrated through systemic inhibition or genetic modification that VEGFA is indispensable for several types of bone repair, presumably via its role in supporting angiogenesis. But a direct role for VEGFA within osteoblasts, in the absence of angiogenesis, has also been suggested. To address the question of whether VEGFA from osteoblasts supports bone formation directly, we applied anabolic loading to induce lamellar bone formation in mice, a process shown to be independent of angiogenesis. We hypothesized that VEGFA from osteoblasts is required for lamellar bone formation. To test this hypothesis, we applied axial tibial compression to inducible Cre/LoxP mice from three lines. Vegfafl/fl mice were crossed with Ubiquitin C (UBC), Osterix (Osx) and Dentin-Matrix Protein 1 (DMP1) Cre-ERT2 mice to target all cells, (pre)osteoblast-lineage cells, and mature osteoblasts and osteocytes, respectively. Genotype effects were determined by comparing control (Vegfafl/fl) and Cre+ (VegfaΔ) mice for each line. At 5 months of age tamoxifen was injected for 5 days followed by a 3-week clearance prior to loading. Female and male mice (N = 100) were loaded for 5 days to peak forces to engender −3100 με peak compressive strain and processed for dynamic histomorphometry (day 12). Percent MS/BS increased 20–70% as a result of loading, with no effect of genotype in Osx or Dmp1 lines. In contrast, the UBC groups had a significant decrease in relative periosteal BFR/BS in VegfaΔ vs. Vegfafl/fl mice. The UBC line did not have any cortical bone phenotype in non-loaded femurs. In summary, dynamic histomorphometry data confirmed that tibial loading induces lamellar bone formation. Contrary to our hypothesis, there was no decrease in loading-induced bone formation in the Osx or Dmp1 lines in the absence of VEGFA. There was a decrease in bone formation in the UBC line where all cells were targeted. This result indicates that VEGFA from a non-osteoblast cell source supports loading-induced lamellar bone formation, although osteoblast/osteocyte VEGFA is dispensable. These findings support a paracrine model whereby non-osteoblast VEGFA supports lamellar bone formation, independent of angiogenesis.
1. Introduction
Vascular endothelial growth factor A (VEGFA), produced by osteoblasts, leukocytes, and endothelium, is a key biological growth factor in angiogenesis and normal bone development (Hu and Olsen, 2016)(Neufeld et al., 1999)(Lee et al., 2007)(Street et al., 2002). A role for VEGFA in bone repair has also been demonstrated. Following full femur fracture, mice treated with a soluble VEGF receptor exhibited decreased angiogenesis, callus mineralization, and bone formation (Street et al., 2002). In the same study, the addition of VEGF improved healing of radius segmental defects in rabbits (Street et al., 2002). These results demonstrate that modulation of VEGF can alter bone repair outcomes.
More recent studies have used inducible Cre-LoxP mouse models to examine the role of VEGFA from different cell types in bone repair. Using a mouse tibial defect model, Hu et al. showed that both osteogenesis and angiogenesis was compromised when VEGFA was knocked out with Osx Cre (targeting the osteoblast lineage) but not with VECAD Cre (targeting vascular endothelial cells), indicating that VEGFA from osteoblasts is critical for defect repair (Hu and Olsen, 2016). They further showed that optimal levels of VEGFA are required for coupling of angiogenesis and osteogenesis during defect healing. Buettmann et al. evaluated healing of full femur fractures and ulnar stress fractures in mice carrying VEGFA floxed alleles (Vegfafl/fl) crossed with tamoxifen inducible Cre-ERT2 lines driven by Ubiquitin C (VegfaΔUBC), Osterix (VegfaΔOsx), and Dentin matrix protein 1 (VegfaΔDmp1) (Buettmann et al., 2019). This allowed for global (UBC), early osteoblast lineage (Osx), or late osteoblast lineage (Dmp1) conditional deletion of VEGFA at 12-weeks of age. Following fracture, knockout mice had varying amounts of impaired healing that directly correlated to the broadness of Cre expression within the osteoblast lineage. Fractures in VegfaΔUBC mice did not heal, while fractures in VegfaΔOsx mice had moderately impaired healing and VegfaΔDmp1 mice exhibited normal healing (Buettmann et al., 2019). Collectively, these findings support that VEGFA from early osteoblasts is crucial for several types of bone repair.
In studies of osteogenesis in the setting of bone injury and repair, it is difficult to decouple angiogenesis from osteogenesis because the two processes are so tightly linked (Brandi and Collin-Osdoby, 2006; Diomede et al., 2020; Hu and Olsen, 2016; Maes et al., 2008; Matsuzaki et al., 2007). For example, Buettmann et al. used endomucin immunostaining to mark vessels, and found that vessel density in fracture callus of VEGFA knockout mice was reduced in proportion to the reduction in bone formation (Buettmann et al., 2019). Thus, it remains unclear if VEGFA from osteoblasts acts directly (autocrine or intracrine) to promote bone formation, or if it acts indirectly (paracrine) by promoting angiogenesis which in turn supports bone formation by secretion of other factors such as PDGF (Böhm et al., 2019). There is evidence supporting both possibilities (Hu and Olsen, 2016; Liu et al., 2012).
To address the question of whether VEGFA from osteoblasts supports bone formation independent of angiogenesis (i.e., a direct effect), we turned to a model of non-damaging, anabolic loading to induce lamellar bone formation in adult mice. We have previously reported that lamellar bone formation in this context is independent of angiogenesis (Tomlinson et al., 2014), which is in contrast to the vascular-dependent formation of intramembranous (woven) bone that occurs with bone injury and repair (Hausman et al., 2001; Tomlinson et al., 2013).
We hypothesized that VEGFA from osteoblasts is required for lamellar bone formation in adult mice. To test this hypothesis, we performed axial tibial loading in inducible Cre/LoxP mice (UBC, Osx, and Dmp1 Cre-ERT2 drivers crossed with Vegfafl/fl mice) and evaluated loading-induced bone formation in the absence of VEGFA broadly (VegfaΔUBC), from (pre)osteoblast-lineage cells (VegfaΔOsx), and from mature osteoblasts and osteocytes (VegfaΔDmp1). At 5 months of age, mice were given tamoxifen followed by loading and subsequent assessment of dynamic histomorphometry outcomes. We demonstrated that loading-induced lamellar bone formation was decreased in VegfaΔUBC mice, but was not affected in VegfaΔOsx mice or VegfaΔDmp1 mice.
2. Methods
2.1. Study Design
Three separate tamoxifen inducible Cre-ERT2 mouse lines (UBC (Ruzankina et al., 2007), Osx (Maes et al., 2007), Dmp1 (Kim et al., 2012)) were crossed to mice carrying floxed VEGFA (Vegfafl/fl) alleles. Previous work with reporter versions of mouse lines confirmed targeting of the cell types of interest (Buettmann et al., 2019). UBC Cre-ERT2 mice (broad targeting of all cells) were purchased from Jackson Laboratories (catalog #007001, Bar Harbor, ME, USA). Osx Cre-ERT2 mice (targeting osteoblast lineage cells from pre-osteoblast through osteocyte) were shared from the laboratory of Dr. Henry Kronenberg (Mass General). Dmp1 Cre-ERT2 mice (targeting late osteoblast lineage cells including osteocytes) were shared by Dr. Paola Pajevic (Boston University). Vegfafl/fl mice were provided by Dr. Bjorn Olsen (Harvard) with permission from Genentech (South San Francisco, CA, USA). In all breeding pairs both male and female mice contained two floxed VEGFA alleles. Male breeders also had a single copy of either UBC-, Osx-, or Dmp1-CreERT2. Cre-positive (Cre+) and Cre-negative (Control) littermates were used for experiments. Throughout this paper, conditional knockout experimental groups are labeled VegfaΔUBC, VegfaΔOsx, and VegfaΔDmp1, while littermate controls are labeled Vegfafl/fl or Control. Mice were group-housed (maximum five per cage) under a standard 12-hour light/dark cycle and given access to food (Purina LabDiet 5053; Purina) and water ad libitum. All mice at 5 months of age (male and female, N = 128) were given tamoxifen for 5 days (100 mg/kg/day PO; Sigma-Aldrich) for Cre activation followed by a 3-week clearance (Figure 1). Tamoxifen-induced recombination was confirmed by PCR of genomic DNA from tissues of interest. In brief, femurs from Cre+ mice of each line (VegfaΔUBC, VegfaΔOsx, and VegfaΔDmp1) showed recombination, while kidneys from VegfaΔUBC and VegfaΔDmp1 mice also showed recombination (Supplemental Figure 1). Kidneys from Cre+ VegfaΔOsx did not show recombination, and likewise recombination was not seen in tissues from Cre- mice. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis School of Medicine in accordance with the Animal Welfare Act and PHS Policy on Humane Care and Use of Laboratory Animals. Mice were euthanized by CO2 asphyxiation.
Figure 1.
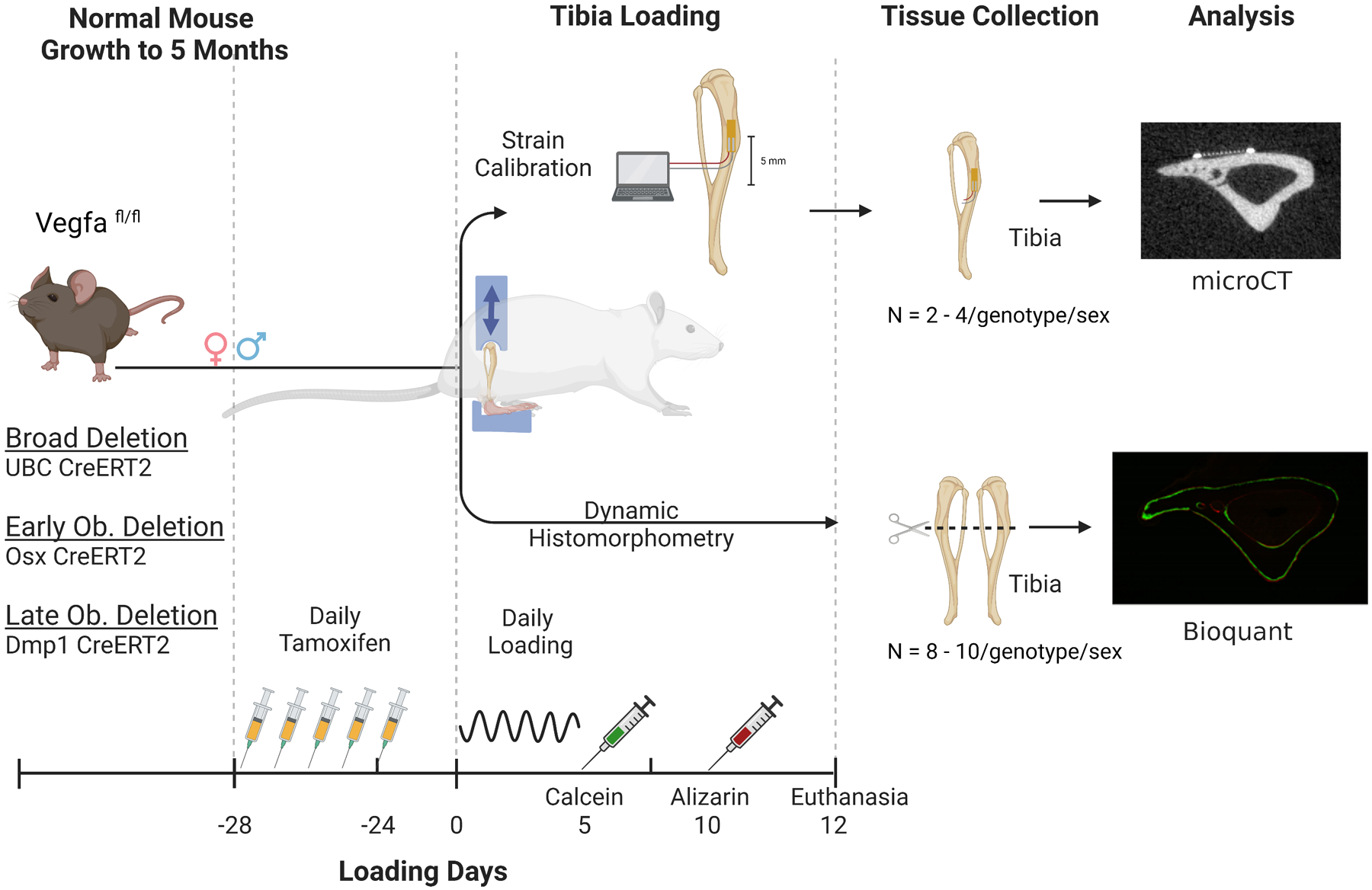
Overview of study design. Three separate tamoxifen inducible Cre-ERT2 mouse lines (UBC, Osx, or Dmp1) were crossed to mice carrying floxed VEGFA (Vegfafl/fl) alleles. At 20 weeks of age (5 mo) mice received 5 daily doses of tamoxifen followed by a 3-week clearance. An initial group was euthanized at 24 weeks of age (day 0) and tibia were used for strain calibration. Separate cohorts of mice were loaded daily for 5 days and euthanized 12 days after the start of loading for dynamic histomorphometry outcomes. (Created with BioRender.com)
2.2. Bone Strain Analysis
Prior to in vivo loading, a subset of mice was used for ex vivo strain gauge analysis (n = 2–4/sex/genotype, N = 28 total, Supplemental Table 1) in order to determine forces for strain-matched loading of male and female mice from each Cre line. These mice were euthanized 28 days after the first tamoxifen dose (corresponding to the first day of in vivo loading for ‘Anabolic Tibial Loading’). Strain data was captured from a single element gauge (FLK-1-11-1LJC, Tokyo Measuring Instruments Lab) placed on the anteromedial (tensile) surface 5 mm proximal to the tibial-fibular junction (corresponding to the section where bone formation was measured). A preload (−0.5 N, Dynamight 8841, Instron) was applied and the right tibias were cyclically loaded (12 cycles, 4-Hz) to compressive forces of 2, 4, 6, 8 and 10 N with 3 minutes of rest between each loading force. In some cases (at high forces) the anterior cruciate ligament was torn during loading and for these samples strain data after the tear was omitted. Compressive strains were extrapolated to the posterolateral apex (site of peak compression) (Patel et al., 2014). Force-strain relationships for both tensile and compressive strains were determined using linear regression. A preliminary study using 8 N loading force in Osx and Dmp1 lines demonstrated lamellar bone formation in male and female Osx mice, with woven bone formation in female Dmp1 mice and no bone formation in male Dmp1 mice. Based on these observations, peak compressive loading forces were selected to induce an anabolic response with minimal woven bone formation (approx. −3100 με).
2.3. Anabolic Tibia Loading
The axial tibial compression model induces bone formation without bone injury. To induce bone formation, the right hind limbs from 100 mice were cyclically loaded at a frequency of 4 Hz with a 7–10 N peak force (Supplemental Table 1) for 60 cycles. The loading was repeated for 5 consecutive days. Mice received buprenorphine (0.1 mg/kg s.c.) following each loading bout for analgesia, were returned to their cages to resume normal activity, and were monitored daily. The left tibias were not loaded and served as contralateral controls. Calcein (10 mg/kg, i.p., Sigma) was administered on day 5 and alizarin red (30 mg/kg i.p., Sigma) on day 10 to label newly forming mineralizing bone. Following loading, mice were sacrificed on day 12, allowing time for post-loading bone formation to occur. After dissection from soft tissues, bilateral tibias were embedded for plastic histology.
2.4. Dynamic Histomorphometry
Following axial tibial compression loading, bilateral mouse tibias (N = 200 combined loaded and non-loaded) were embedded in methylmethacrylate plastic (Sigma). Transverse sections (100-μm-thick) were cut from the diaphysis, 5 mm proximal to the tibial-fibular junction on a saw-microtome (Leica 1600SP) and imaged using a confocal microscope (Leica TCS SPE). Dynamic histomorphometric analysis was performed on sections (one section/bone) using calcein and alizarin red labeling analyzed with commercial software (Osteo II, BIOQUANT). Following published guidelines (Dempster et al., 2013), standard lamellar and woven bone formation indices were determined for the entire periosteal (Ps) and endocortical (Ec) surfaces separately. Primary outcome variables were 1) mineralizing surface per bone surface (MS/BS): percentage of the perimeter that has new bone formation (an indication of the number of active osteoblasts), 2) mineral apposition rate (MAR): the amount of space between labels (a surrogate for rate of osteoblast output), and 3) bone formation rate (BFR/BS): a multiplication of MS/BS and MAR (total osteoblast output). There were small amounts of woven bone on some samples, and to include that, total MS/BS was computed as (0.5sLS + dLS + Wo.S)/BS. Measurements of mineral apposition rate (MAR) and bone formation rate per bone surface (BFR/BS) including woven bone are denoted MAR+ and BFR/BS+, respectively (De Souza et al., 2005). Some samples had no visible double-labeled surface, so a minimum value of 0.3 μm/day was assigned for MAR and was used to calculate BFR/BS (Dempster et al., 2013); this approach avoids excluding samples with no double-label from data analysis.
2.5. Bone phenotyping and mechanical testing of VegfaΔUBC
Right femurs were harvested at euthanasia (post loading day 12) from male and female VegfaΔUBC and Vegfafl/fl mice for cortical bone analysis and three-point bending tests (n = 8–10/group, 38 total). Spinal segments (L5) were harvested for cancellous bone analysis from the same mice. After dissection and muscle removal, bones were wrapped in saline-soaked gauze and stored frozen at −20 °C until use. Bones were scanned by microCT (vivaCT 40, Scanco Medical AG, 10.5 μm, 1000 projections, 300 ms, 70 kVp, 114 μA, high resolution). The femoral mid-diaphysis (1.05 mm, 100 slices) was analyzed using the Scanco software with contours drawn on the periosteal surface of the bone (analysis parameters: sigma 0.4, support 1, threshold 350 mg HA/cm3). Cortical analysis parameters included total area (Tt.Ar), bone area (Ct.Ar), bone area fraction (Ct.Ar/Tt.Ar), medullary area (Ma.Ar), cortical bone thickness (Ct.Th), polar moment of inertia (pMOI), minimum moment of inertia (Imin), maximum moment of inertia (Imax), and tissue mineral density (TMD). For cancellous analysis, the analysis region was defined as the entirety of L5 vertebral body (average length 2.4 mm). Contours were drawn on the endosteal surface of the cortical bone (analysis parameters: sigma 0.4, support 1, threshold 200 mg HA/cm3). Cancellous analysis parameters included total volume (TV), bone volume (BV), bone volume fraction (BV/TV), connectivity density (Conn-Dens), structure model index (TRI-SMI), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and volumetric bone mineral density (vBMD). After microCT, femur lengths were measured with a digital caliper and the midpoint marked with pencil. Following one additional freeze/thaw cycle, the right femurs were subjected to three-point bending according to published guidelines (Jepsen et al., 2015) (span = 7 mm, monotonic ramp, 0.1 mm/s; Dynamight 8841, Instron). Force-displacement plots were analyzed to determine structural properties: stiffness, yield load, maximum (ultimate) load, post-yield displacement, and work to fracture. Material properties were estimated using simple beam theory (Jepsen et al., 2015): yield stress, ultimate stress, and Young’s modulus.
2.6. Statistics
Data are presented as means and standard deviation with individual data points plotted. For periosteal dynamic histomorphometry outcomes (total MS/BS, MAR, MAR+, BFR/BS, and BFR/BS+), each parameter was initially analyzed using a repeated measures 3-way analysis of variance (ANOVA) with factors of loading (loaded vs non-loaded limb), sex, and genotype (Vegfafl/fl vs VegfaΔ). Due to significant sex effects and sex-loading interactions, males and females were subsequently analyzed separately using repeated measures 2-way ANOVA with factors of limb (loaded vs non-loaded) and genotype with Tukey post hoc tests. In addition, the data were analyzed using relative values (loaded minus non-loaded) with 1-way ANOVA. Endocortical results were analyzed using 2-way ANOVA (factors of genotype and sex). Bone phenotyping data from microCT and mechanical testing was analyzed using 2-way ANOVA. Significance was defined at p < 0.05. Analysis was performed with Prism (GraphPad, La Jolla, CA).
3. Results
3.1. Load-strain relationships differed between Cre lines
Ex vivo bone strain measurements were taken from the region of peak tensile strain and extrapolated to the peak compressive region (Figure 2). Each Cre line was analyzed independently. For all mouse lines, genotypes were pooled between sexes, as force-strain relationships were not significantly different between VegfaΔ and Vegfafl/fl. In both the UBC and Osx-Cre lines there were no sex differences in the load-strain relationships and data were pooled. In the Dmp1-Cre line there were significant differences between sexes. Loading parameters were strain-matched for each Cre-line (approx. −3100 με compression, +1800 με tension) (Supplemental Table 1) following a pilot study to verify loading-induced bone formation at these strains. Accordingly, UBC and Osx-Cre lines (both males and females) were loaded to −10 N and −8 N, respectively. Male Dmp1 mice were loaded to −10 N while females were loaded to −7 N. Importantly, within each sex, control (Vegfafl/fl) and VegfaΔ mice received similar bone strain stimuli. This allowed for a direct comparison to see the effect of loading-induced bone formation with reduced VEGFA expression in specific cell types.
Figure 2.
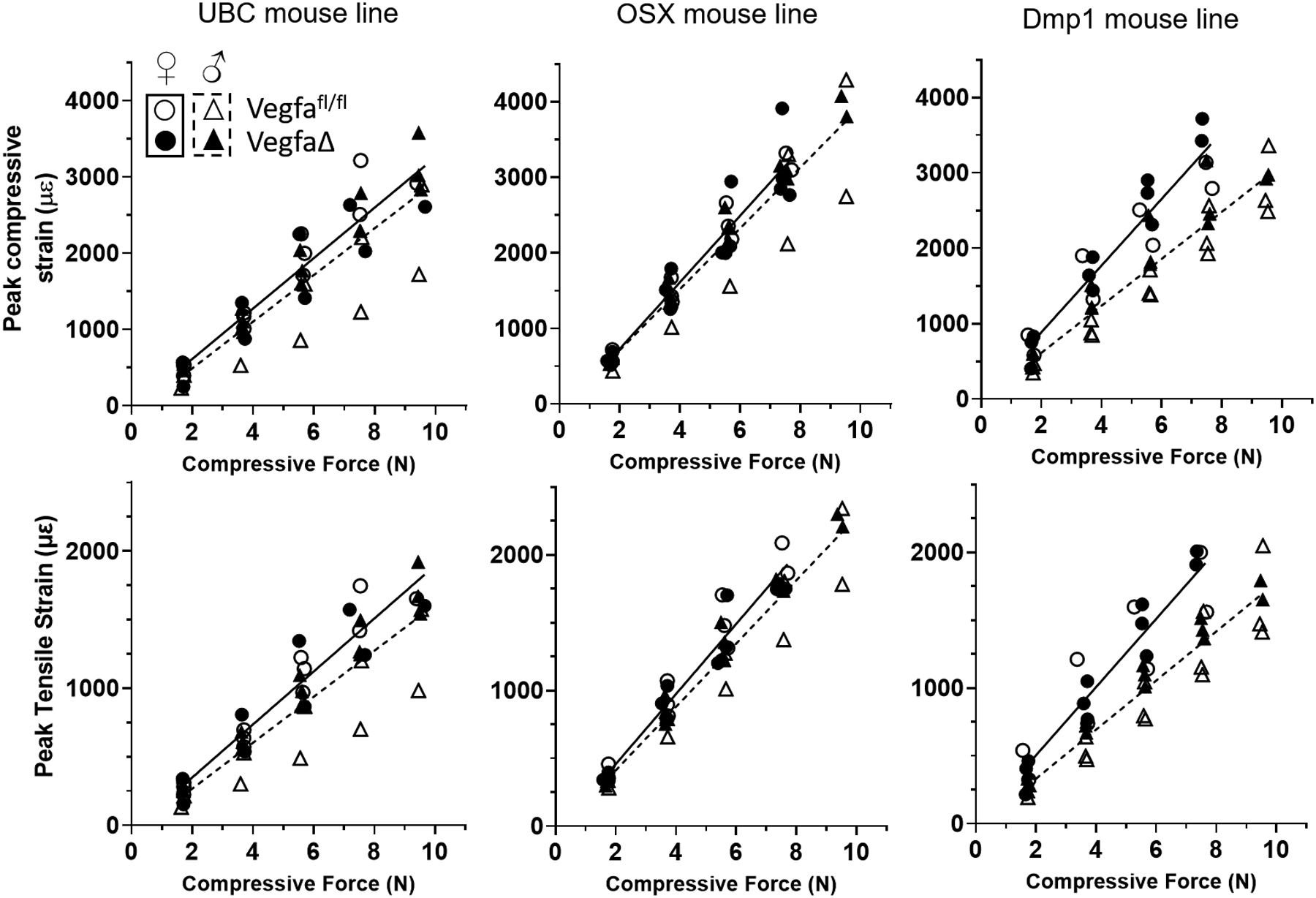
Strain gauge data from loaded tibia. Peak tensile strain was collected and peak compressive strain was calculated after microCT imaging. In all lines of mice, there were no genotype (Vegfafl/fl (control) vs VegfaΔ (Cre+)) related differences within each sex, indicating that short-term deletion of Vegfa did not alter force-strain relationships. For linear regression analysis, data for the two genotypes of each mouse line were pooled and analyzed separately for females (solid line) and males (dashed line). In the UBC line, the strain profiles were similar for all mice. Trends for the Osx line matched those of UBC, with the exception that the slope of the curve fit was higher. In the Dmp1 line there were sex differences. In all groups of mice a target peak compressive strain of −3100 microstrain was used for in vivo loading.
3.2. Body weight was moderately influenced by VEGFA loss in UBC mice
The tamoxifen injections and loading protocols (Figure 1) were well tolerated. All three Cre mouse lines demonstrated similar changes in body weight throughout the experiment with no major weight losses as a result of tamoxifen (TMX) administration or loading (Supplemental Table 2, Supplemental Figure 2). In the UBC mouse line there was moderate weight loss in three of four groups in the 24 days between the end of TMX administration and loading (<5%). There were no differences in weight loss between Vegfafl/fl and VegfaΔOsx mice; however, there was a sex effect with females losing weight (−1.5%) and male mice gaining weight (1.5%). There were no sex or genotype differences between Vegfafl/fl and VegfaΔDmp1 mice related to body weight.
3.3. Loading-induced bone formation was impaired in mice with global (UBC) deletion of Vegfa
To investigate the role of VEGFA from all cells in the anabolic effect of skeletal loading, 5-month-old male and female mice were subjected to 5 bouts of axial compression on the right tibia while contralateral left tibias served as controls. Cre expression for global deletion (UBC) was induced by tamoxifen administration 4 weeks prior to loading (Figure 1). Tibial loading stimulated periosteal bone formation in both control (Vegfafl/fl) and conditional knockout (VegfaΔUBC) mice, as evidenced by significantly greater indices of bone formation in loaded versus contralateral non-loaded tibias (Figure 3, Supplemental Tables 3–4). However, the periosteal loading response was slightly diminished in conditional knockout mice. For female mice, Ps.MS/BS (indication of number of active osteoblasts) of loaded tibias was 18% higher in VegfaΔUBC mice relative to Vegfafl/fl control mice (p<0.05, Figure 3B, Supplemental Table 4), whereas Ps.MAR (spacing between labels) and Ps.BFR/BS (total osteoblast output) were 13% and 15% lower, respectively. In male mice, Ps.MS/BS of loaded tibias was 12% lower in VegfaΔUBC mice relative to Vegfafl/fl control mice, and Ps. MAR and Ps.BFR/BS were 18% and 28% lower, respectively (Figure 3C, Supplemental Table 4). Importantly, a significant loading-genotype interaction was detected for Ps.BFR/BS in both sexes, indicating that the effect of loading on periosteal bone formation differed between control and knockout mice with a global deletion of VEGFA (p<0.05). Similarly, when the effect of loading was expressed as a relative measure (i.e., loaded minus non-loaded control), periosteal bone formation rate was reduced by 22% (p<0.05) and 28% (p<0.05) in female and male mice, respectively, in the VegfaΔUBC group compared to Vegfafl/fl.
Figure 3.
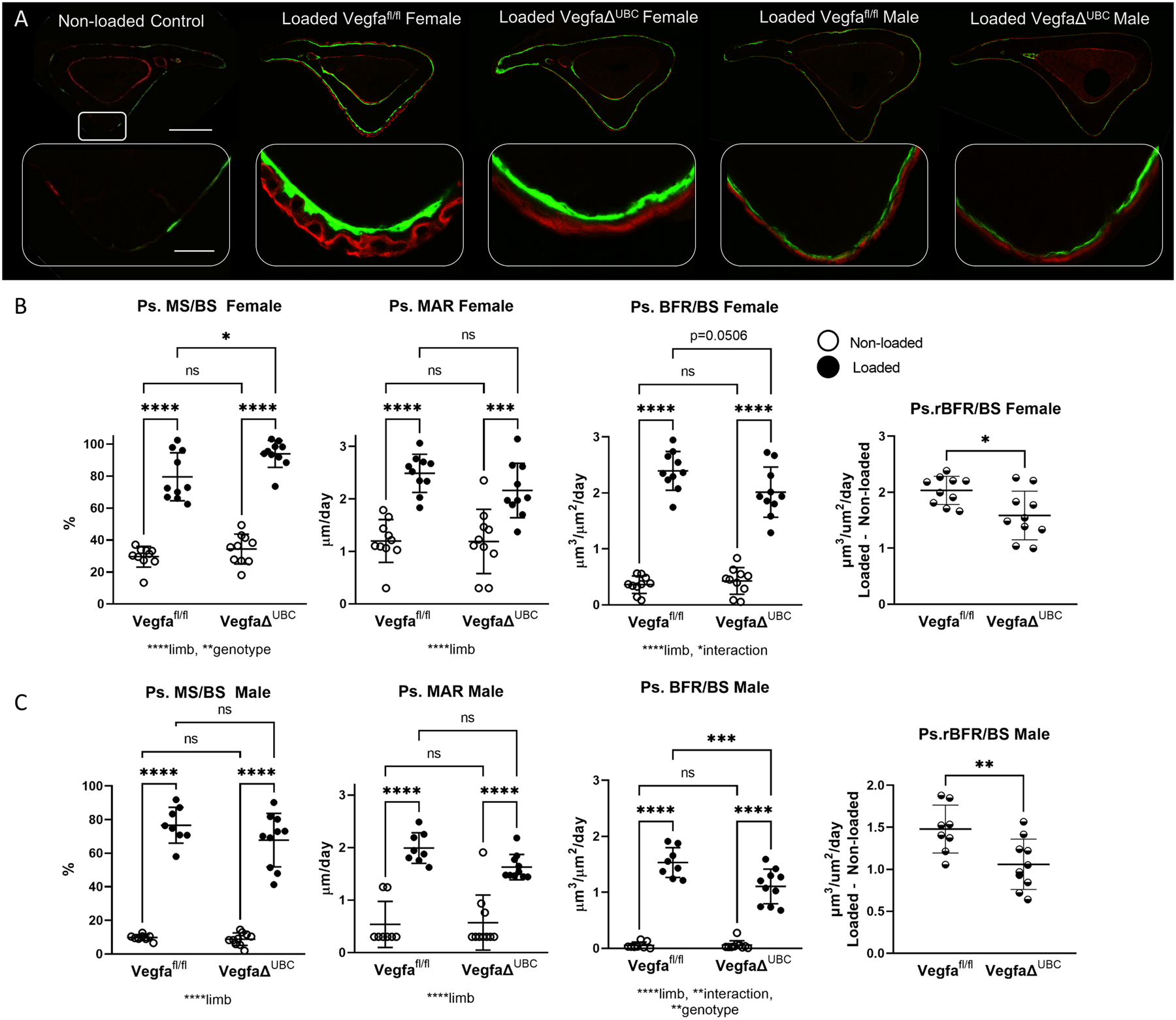
Loading-induced periosteal bone formation is impaired in mice with a global deletion of VEGFA using UBC Cre. A) Representative images from control and loaded groups demonstrate increased periosteal label around the entire tibia after loading (scale bar 500 micron). Magnified images of the area of peak compression demonstrate increased label and spacing between labels on loaded bones (scale bar 50 micron). B) Indices of periosteal bone formation in female control (Vegfafl/fl) and conditional knockout (VegfaΔUBC) mice (n = 10). C) Indices of periosteal bone formation in male control (Vegfafl/fl) and conditional knockout (VegfaΔUBC) mice (n = 8–10). Two-factor ANOVA was used to evaluate the effects of limb (loading) and genotype, with Tukey post hoc comparisons. One-way ANOVA was used to compare relative bone formation rate (BFR/BS Loaded minus BFR/BS Non-loaded), which is an index of loading-induced bone formation. Bars depict mean ± SD, with individual data points shown. Parameters shown do not include woven bone. * p<0.05 ** p<0.01, *** p<0.001, **** p<0.0001.
While the goal of tibial loading was to only stimulate lamellar bone formation, results show that loading did occasionally produce woven bone in the area of peak compression. There was evidence of periosteal woven bone formation in 7/38 samples from the UBC line, with 6 of those samples in the female Vegfafl/fl group. Data trends were similar when evaluating bone measurements including or excluding the contribution of woven bone (both are provided in Supplemental Table 4; data in Figure 3 do not include woven bone). Endocortical measurements were also analyzed, but were not significantly different for any outcome between genotypes for non-loaded or loaded limbs. In summary, the anabolic response to loading was significantly impaired in the periosteum of female and male mice by the absence of VEGFA globally.
3.4. Loading induced bone formation was not significantly impacted by VEGFA deletion in osteoblast lineage (Osx+) cells
Similar to the UBC line, 5 days of tibial loading of mice from the Osx line stimulated periosteal bone formation in both control (Vegfafl/fl) and conditional knockout (VegfaΔOsx) mice (Figure 4, Supplemental Tables 3, 5). The periosteal loading response was less in male compared to female mice, and only in females did we observe any significant effects of knockout. For female mice, in loaded tibias Ps.MS/BS was 19% lower in VegfaΔOsx mice relative to Vegfafl/fl control mice (p<0.05, Figure 4B, Supplemental Table 4), whereas Ps.MAR was increased 60% (p = 0.07) and Ps.BFR/BS was not different between genotypes. For male mice, in loaded tibias Ps.MS/BS, Ps.MAR and Ps.BFR/BS were not different in VegfaΔOsx mice relative to Vegfafl/fl control mice (Figure 4C). Moreover, a significant loading-genotype interaction was not detected for Ps.BFR/BS in either sex, indicating that the effect of loading on periosteal bone formation was not impacted by osteoblast lineage deletion of VEGFA. Similarly, when the effect of loading was expressed as a relative measure (i.e., loaded minus non-loaded control), there were no genotype differences.
Figure 4.
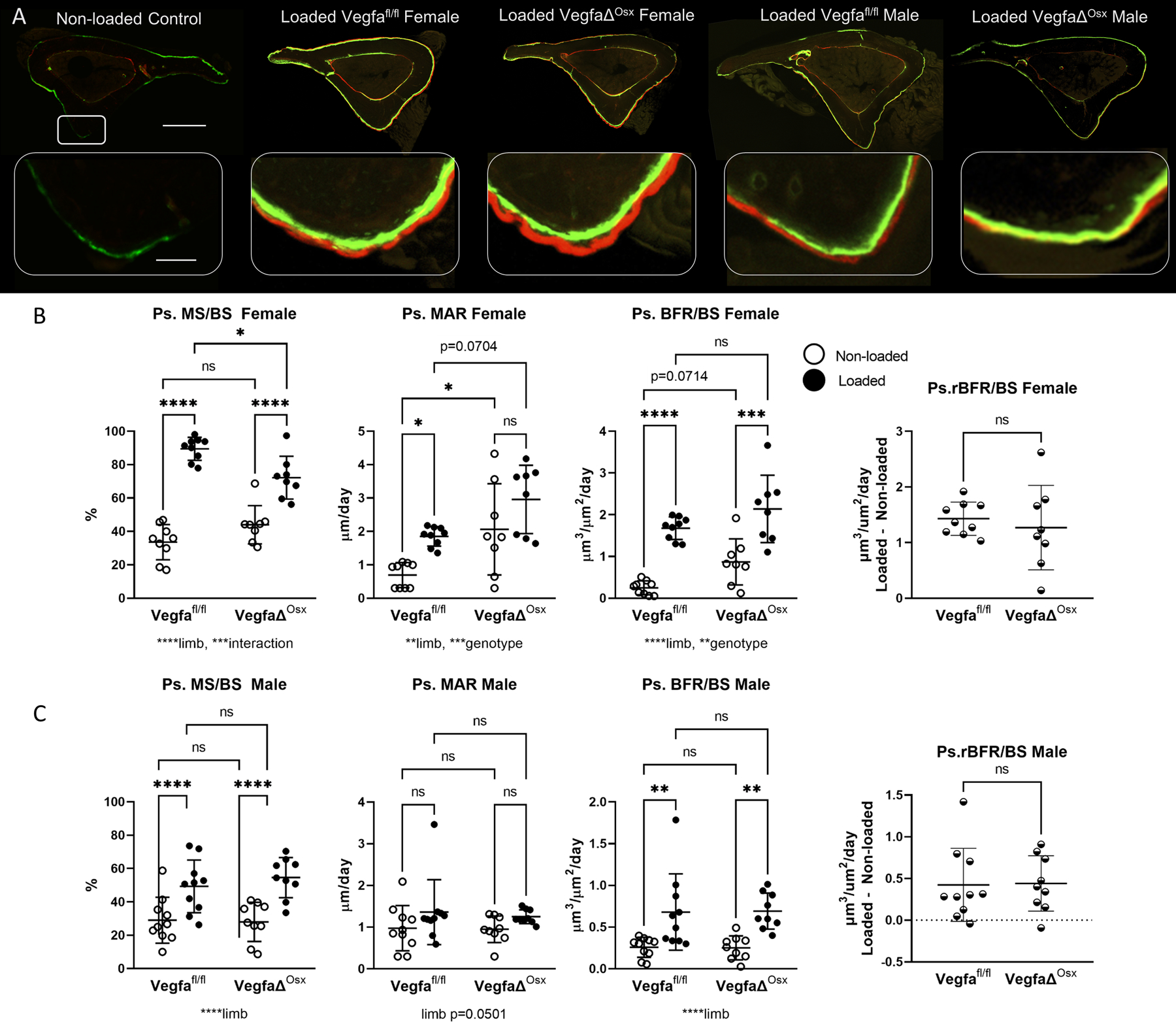
Loading-induced periosteal bone formation is maintained in mice with osteoblast lineage deletion of VEGFA using Osx Cre. A) Representative images from control and four loaded groups demonstrate increased periosteal label around the entire tibia after loading (scale bar 500 micron). Magnified images of the area of peak compression demonstrate increased label and spacing between labels on loaded bones (scale bar 50 micron). B) Indices of periosteal bone formation in female control (Vegfafl/fl) and conditional knockout (VegfaΔOsx) mice (n = 8–9). C) Indices of periosteal bone formation in male control (Vegfafl/fl) and conditional knockout (VegfaΔOsx) mice (n = 9–10). Two-factor ANOVA was used to evaluate the effects of limb (loading) and genotype, with Tukey post hoc comparisons. One-way ANOVA was used to compare relative bone formation rate (BFR/BS Loaded minus BFR/BS Non-loaded), which was used as an index of loading-induced bone formation. Bars depict mean ± SD, with individual data points shown. Parameters shown do not include woven bone. * p<0.05
Some woven bone formation occurred in Osx mice in the area of peak compression. Periosteal woven bone formation was observed in 6/36 samples, with 5 of those samples in the female VegfaΔOsx group. Data trends were similar for bone measurements including or excluding the contribution of woven bone (both are provided in Supplemental Table 5; data in Figure 4 do not include woven bone). Endocortical measurements were also analyzed, but were not significantly different for any variable between genotypes for non-loaded or loaded limbs. Overall, the anabolic response to tibial loading was not impaired by the absence of VEGFA from osteoblast lineage (Osx+) cells.
3.5. Loading induced bone formation was not altered by VEGFA deletion in mature osteoblasts and osteocytes (Dmp1+)
As with the other Cre lines, 5 days of tibial loading in mice of the Dmp1 line stimulated periosteal bone formation in both control (Vegfafl/fl) and conditional knockout (VegfaΔDmp1) mice (Figure 5, Supplemental Tables 3, 6). Unlike the other Cre lines, there were no genotype or sex related differences in VegfaΔDmp1 mice relative to Vegfafl/fl control mice (Figure 5B,C), indicating that loading-induced periosteal bone formation was unaffected by deletion of VEGFA in mature osteoblasts and osteocytes. Similarly, when the effect of loading is expressed as a relative measure (i.e. loaded minus non-loaded control), there were no genotype or sex differences for the Vegfafl/fl and VegfaΔDmp1 groups.
Figure 5.
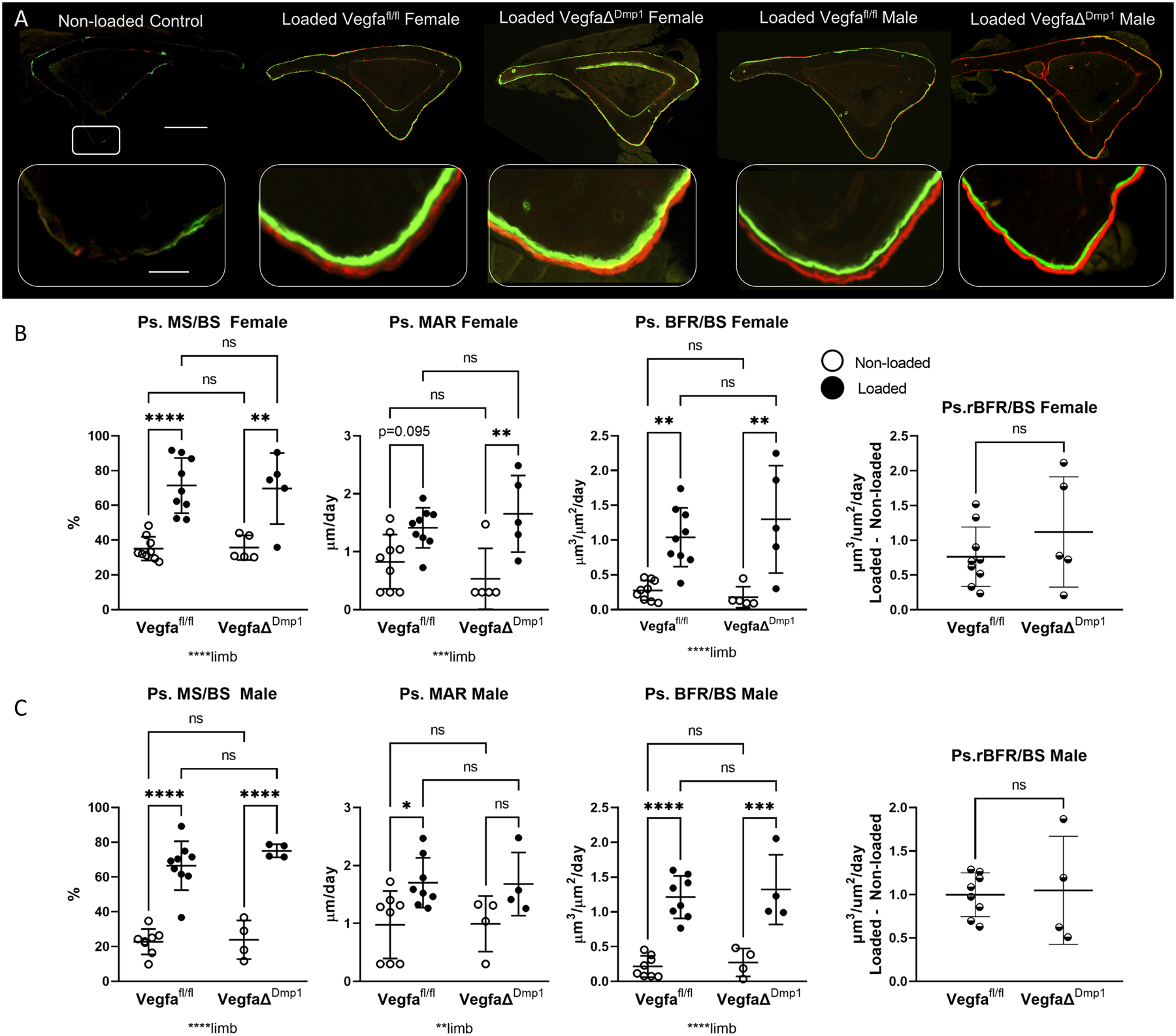
Loading-induced periosteal bone formation is maintained in mice with mature osteoblast lineage deletion of VEGFA using Dmp1 Cre. A) Representative images from control and four loaded groups demonstrate increased periosteal label around the entire tibia after loading (scale bar 500 micron). Magnified images of the area of peak compression demonstrate increased label and spacing between labels on loaded bones (scale bar 50 micron). B) Indices of periosteal bone formation in female control (Vegfafl/fl) and conditional knockout (VegfaΔDmp1) mice (n = 5–9). C) Indices of periosteal bone formation in male control (Vegfafl/fl) and conditional knockout (VegfaΔDmp1) mice (n = 4–7). Two-factor ANOVA was used to evaluate the effects of limb (loading) and genotype, with Tukey post hoc comparisons. One-way ANOVA was used to compare relative bone formation rate (BFR/BS Loaded minus BFR/BS Non-loaded), which was used as an index of loading-induced bone formation. Bars depict mean ± SD, with individual data points shown. Parameters shown do not include woven bone. * p<0.05
Tibial loading produced a small amount of woven bone in the area of peak compression in 3/26 Dmp1 mice. Data trends were similar for bone measurements including or excluding the contribution of woven bone (both are provided in Supplemental Table 6; data in Figure 5 do not include woven bone). Endocortical measurements were also analyzed but were not significantly different for any variable between genotypes for non-loaded or loaded limbs. In summary, the anabolic response to tibial loading was not impaired by the absence of VEGFA from late-stage osteoblast lineage cells.
3.6. Baseline bone size or strength was not altered in VegfaΔUBC mice
To assess the possible effects of global VEGFA deletion on bone homeostasis, we evaluated phenotypes of Vegfafl/fl and VegfaΔUBC bones not subjected to mechanical loading. Vertebral segments (L5) were removed to analyze cancellous bone using microCT, and right femurs were harvested for cortical bone midshaft analysis by microCT and three-point bending. There were some differences in cancellous bone parameters by 2-way ANOVA with respect to sex and genotype (Supplemental Table 7). Female VegfaΔUBC mice had significantly lower BV, BV/TV, Tb.N, and vBMD (12 to 20%) compared to controls (Vegfafl/fl). There were no differences between male VegfaΔUBC mice and Vegfafl/fl mice. For cortical bone analysis, there were some modest differences attributed to sex; female femurs had slightly lower total area, but increased cortical thickness compared to males (Supplemental Table 7). Nonetheless, in both males and females, there were no significant differences in post-hoc testing comparing control versus conditional knockout mice for any of the six cortical parameters evaluated (T.Ar, B.Ar, B.Ar/T.Ar, C.Th, pMOI, and TMD). Mechanical testing of femurs revealed no genotype differences in whole-bone (structural) properties and only modest sex differences. In post-hoc testing there were no significant differences in any femur structural or material property between VegfaΔUBC mice in comparison to Vegfafl/fl mice. These results indicate that short-term global deletion of VEGFA had a mild effect on cancellous bone in female mice, but overall did not impact cortical bone morphology or mechanical properties.
4. Discussion
VEGF is essential to blood vessel formation, and promotes normal bone development accompanied by angiogenesis (Hu and Olsen, 2016; Lee et al., 2007; Neufeld et al., 1999; Street et al., 2002). Following skeletal injury, VEGFA in bone is upregulated (McKenzie et al., 2011; Wohl et al., 2009) and its expression by (pre)osteoblasts (Osx+) is required for intramembranous and endochondral healing exhibited in a range of bone injuries, from small drill holes, to non-displaced stress fractures, to full fractures (Buettmann et al., 2019; Hu and Olsen, 2016). However, because bone formation during healing depends on, and is tightly coupled to angiogenesis (Hausman et al., 2001; Jacobsen et al., 2008; Maes et al., 2010; Street et al., 2002; Tomlinson et al., 2013), it has been difficult to determine whether osteoblast VEGFA stimulates bone formation directly or does so by promoting angiogenesis.
In a previous study, we showed that inhibition of angiogenesis blocked woven bone formation after stress fracture but did not inhibit lamellar bone formation induced by non-injurious, anabolic bone loading even though lamellar bone formation was associated with modest angiogenesis (Tomlinson et al., 2014). In the current study, we turned to non-damaging axial tibial loading to induce lamellar bone formation in adult mice with inducible deletion of VEGFA in different cell types; we hypothesized that VEGFA from osteoblasts is required for lamellar bone formation. Results indicated that mice with conditional deletion of VEGFA in (pre)osteoblasts (VegfaΔOsx) and mature osteoblasts and osteocytes (VegfaΔDmp1) had comparable loading-induced lamellar bone formation as control (Vegfafl/fl) mice, which does not support our hypothesis. On the other hand, loading-induced bone formation was decreased in mice where VEGFA was deleted from all cells (VegfaΔUBC). Our findings indicate that VEGFA from a non-osteoblast cell source supports lamellar bone formation in a setting where angiogenesis is not required, whereas osteoblast/osteocyte derived VEGFA is not required. This supports a model whereby osteoblasts are responding in a paracrine manner to VEGFA from local non-osteolineage cells. Identifying the cell type responsible for this effect will require additional studies.
We used strain gauge analysis of the different mouse genotypes to estimate forces needed for strain-matched mechanical loading. Despite being on similar genetic backgrounds, the different Cre lines required different forces to strain match, and there was a sex effect in one of the three lines. Specifically, UBC- and Osx-Cre lines (both male and female) were loaded to −10 N and −8 N, respectively, while male Dmp1 mice were loaded to −10 N and females were loaded to −7 N. The overall goal of this approach is to deliver a comparable mechanical stimulus across gene and sex groups (Main et al., 2020), and the consistent formation of lamellar bone observed by dynamic histomorphometry indicates that strain-matched loading induced a comparable anabolic response. However, there were modest differences between the different genetic backgrounds most notably that the UBC line had the greatest loading response (based on Ps.rBFR/BS). In addition, female mice from the UBC and Osx lines had a greater response compared to the respective males. Importantly, the strain analysis indicated that there were no differences between control (Vegfafl/fl) and conditional knockout (VegfaΔ) mice of the same sex and Cre line, consistent with the lack of a cortical bone phenotype caused by short-term deletion of VEGFA in our study. Thus, for the primary comparison of interest (control vs cKO) mice were loaded to equal forces.
This study has several limitations. First, it was observed that 16% of loaded tibias responded to loading by producing some woven bone. Specifically, 7/38 samples from UBC mice, 6/36 samples from Osx mice, and 3/26 samples from Dmp1 mice had small amounts of woven bone at the region of peak compressive strain. The presence of woven bone indicates that a more robust bone formation process has occurred, and indicates that our loading stimulus was near the woven-lamellar transition (Turner et al., 1994). Woven bone formation has been linked to angiogenesis (Tomlinson et al., 2014). Because we wanted to test the role of VEGFA independent of angiogenesis, the parameters shown in Figures 3–5 are based solely on the lamellar bone contributions to bone formation. Nonetheless, data analysis including woven bone contributions leads to similar overall conclusions about the effect of VEGFA conditional deletion (Suppl. Tables S3–S5). Second, when mice were exposed to tamoxifen, weight was found to vary based on genotype where the UBC line lost slightly more weight than their control counterparts, the Osx line had modest effects of tamoxifen on weight when stratified by sex, and the Dmp1 line were found to have a slight difference in weight stratified by genotype. While differences in body weight were observed, the tamoxifen effects appeared to be modest. The UBC line underwent additional phenotyping analysis and found that baseline cortical bone morphology and mechanical properties were not affected in knockout mice.
In summary, axial tibial loading induced lamellar bone formation in 5-month-old mice from three Cre/Vegfa floxed lines. Only VegfaΔUBC mice had diminished loading-induced bone formation, indicating that VEGFA from a non-osteoblast cell source supports osteoblast bone formation in a context (lamellar bone formation) where angiogenesis is not required. In contrast, VEGFA derived from osteoblasts and osteocytes (Osx+ or Dmp1+) is not necessary for lamellar bone formation to occur in response to an anabolic loading stimulus. This latter result is different from prior findings that VEGFA from osteoblasts (Osx+) contributes to woven bone formation during fracture healing. Taken together, the data support a model whereby VEGFA from osteoblasts supports bone formation indirectly by coupling osteogenesis and angiogenesis, and thus does not play a role in settings where bone formation is independent of angiogenesis.
Supplementary Material
Supplemental Figure 1. Inducible Cre lines show DNA recombination indicating VEGFA deletion in bone. DNA recombination for exon 3 of the Vegfa gene was assayed by PCR using DNA extracted from femur (with marrow) and kidney. Cre-ERT2 positive (+) and negative (−) mice received 1 week of tamoxifen; all mice were Vegfafl/fl. A female and male mouse from each genotype are represented. In femurs, Cre+ conditional knockout mice had the shorter amplicon of 560 base pairs (Mutant band), thereby showing Vegfa recombination in bones of Cre+ mice from each mouse line. On the other hand, Cre- littermates only had the larger amplicon of 2160 base pairs (WT), indicative of no recombination at exon 3. In kidney, UBC-CreERT2+ showed recombination, as expected, while Dmp1-CreERT2+ kidneys showed recombination, indicating non-bone targeting.
Supplemental Figure 2. Body weight data taken over the course of the experiment. VegfaΔUBC (Cre+) male and female mice lost 3–4% body weight between the start of tamoxifen injections and euthanasia (Supplemental Table 2). Based on body weight measurements taken during dosing and loading, most of the weight loss occurred in the 3-week window between tamoxifen injections and the beginning of loading. Weight loss due to loading was negligible and not different between Vegfafl/fl (Control) and VegfaΔUBC (Cre+) mice (Supplemental Table 2). There were no differences in weight loss between Vegfafl/fl (Control) and VegfaΔOsx (Cre+) mice, however there was a sex effect with females losing weight (−1.5%) and male mice gaining weight (1.5%). There were no sex or genotype differences between Vegfafl/fl (Control) and VegfaΔDmp1 (Cre+) mice related to body weight. The Dmp1 mice held a steady weight throughout the 5 weeks of the experiment.
Highlights.
Tibial loading stimulated lamellar bone formation in mice with inducible deletion of VEGFA
Loading-induced bone formation was unaffected by loss of VEGFA in osteoblasts/osteocytes (Osx+, Dmp1+)
Loading-induced bone formation was diminished by global loss of VEGFA (UBC+)
Lamellar bone formation in adult mice does not require VEGFA from osteoblasts/osteocytes
Acknowledgements
This work was supported by funding from NIH/NIAMS (R01 AR050211, P30 AR074992), NIH (S10 RR023660). The authors would like to thank the Washington University in St. Louis Musculoskeletal Research Center (MRC) Cores and staff for the facilities and equipment maintenance. VEGFAfl/fl mice from Genentech (Roche Holding AG) were kindly provided by the lab of Bjorn Olsen (Harvard). Inducible Osx Cre-ERT2 were kindly provided by the lab of Henry Kronenberg (Harvard). Inducible Dmp1 Cre-ERT2 from Paolo Pajevic (Boston University) were kindly provided by the lab of Alexander Robling (Indiana University Medical School).
Footnotes
Appendix A. Supplementary Data
Supplementary Tables can be found in the excel file.
References:
- Böhm A-M, Dirckx N, Tower RJ, Peredo N, Vanuytven S, Theunis K, Nefyodova E, Cardoen R, Lindner V, Voet T, Van Hul M, Maes C, 2019. Activation of Skeletal Stem and Progenitor Cells for Bone Regeneration Is Driven by PDGFRβ Signaling. Dev. Cell 51, 236–254.e12. 10.1016/j.devcel.2019.08.013 [DOI] [PubMed] [Google Scholar]
- Brandi ML, Collin-Osdoby P, 2006. Vascular biology and the skeleton. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 21, 183–192. 10.1359/JBMR.050917 [DOI] [PubMed] [Google Scholar]
- Buettmann EG, McKenzie JA, Migotsky N, Sykes DA, Hu P, Yoneda S, Silva MJ, 2019. VEGFA From Early Osteoblast Lineage Cells (Osterix+) Is Required in Mice for Fracture Healing. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 34, 1690–1706. 10.1002/jbmr.3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza RL, Matsuura M, Eckstein F, Rawlinson SCF, Lanyon LE, Pitsillides AA, 2005. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37, 810–818. 10.1016/j.bone.2005.07.022 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM, 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 28, 2–17. 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P, Mazzon E, Trubiani O, 2020. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci 21, E3242. 10.3390/ijms21093242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman MR, Schaffler MB, Majeska RJ, 2001. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29, 560–564. 10.1016/s8756-3282(01)00608-1 [DOI] [PubMed] [Google Scholar]
- Hu K, Olsen BR, 2016. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest 126, 509–526. 10.1172/JCI82585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC, 2008. Bone Formation During Distraction Osteogenesis Is Dependent on Both VEGFR1 and VEGFR2 Signaling. J. Bone Miner. Res 23, 596–609. 10.1359/jbmr.080103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Silva MJ, Vashishth D, Guo XE, van der Meulen MCH, 2015. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 30, 951–966. 10.1002/jbmr.2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Pajevic PD, Selig M, Barry KJ, Yang J-Y, Shin CS, Baek W-Y, Kim J-E, Kronenberg HM, 2012. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 27, 2075–2084. 10.1002/jbmr.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML, 2007. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703. 10.1016/j.cell.2007.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR, 2012. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Invest 122, 3101–3113. 10.1172/JCI61209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Carmeliet G, Ruhrberg C, 2008. Vascular and Nonvascular Roles of VEGF in Bone Development, in: VEGF in Development. pp. 79–90. 10.1007/978-0-387-78632-2_7 [DOI] [Google Scholar]
- Maes C, Kobayashi T, Kronenberg HM, 2007. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann. N. Y. Acad. Sci 1116, 149–164. 10.1196/annals.1402.060 [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM, 2010. Osteoblast Precursors, but Not Mature Osteoblasts, Move into Developing and Fractured Bones along with Invading Blood Vessels. Dev. Cell 19, 329–344. 10.1016/j.devcel.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main RP, Shefelbine SJ, Meakin LB, Silva MJ, van der Meulen MCH, Willie BM, 2020. Murine Axial Compression Tibial Loading Model to Study Bone Mechanobiology: Implementing the Model and Reporting Results. J. Orthop. Res. Off. Publ. Orthop. Res. Soc 38, 233–252. 10.1002/jor.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Wohl GR, Novack DV, Lynch JA, Silva MJ, 2007. Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna. Calcif. Tissue Int 80, 391–399. 10.1007/s00223-007-9031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Bixby EC, Silva MJ, 2011. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PloS One 6, e29328. 10.1371/journal.pone.0029328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z, 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 13, 9–22. [PubMed] [Google Scholar]
- Patel TK, Brodt MD, Silva MJ, 2014. Experimental and finite element analysis of strains induced by axial tibial compression in young-adult and old female C57Bl/6 mice. J. Biomech 47, 451–457. 10.1016/j.jbiomech.2013.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ, 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126. 10.1016/j.stem.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RAD, Filvaroff EH, 2002. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. U. S. A 99, 9656–9661. 10.1073/pnas.152324099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ, 2013. Angiogenesis is required for stress fracture healing in rats. Bone 52, 212–219. 10.1016/j.bone.2012.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RE, Schmieder AH, Quirk JD, Lanza GM, Silva MJ, 2014. Antagonizing the αv β3 integrin inhibits angiogenesis and impairs woven but not lamellar bone formation induced by mechanical loading. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 29, 1970–1980. 10.1002/jbmr.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CH, Forwood MR, Rho JY, Yoshikawa T, 1994. Mechanical loading thresholds for lamellar and woven bone formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 9, 87–97. 10.1002/jbmr.5650090113 [DOI] [PubMed] [Google Scholar]
- Wohl GR, Towler DA, Silva MJ, 2009. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone 44, 320–330. 10.1016/j.bone.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Inducible Cre lines show DNA recombination indicating VEGFA deletion in bone. DNA recombination for exon 3 of the Vegfa gene was assayed by PCR using DNA extracted from femur (with marrow) and kidney. Cre-ERT2 positive (+) and negative (−) mice received 1 week of tamoxifen; all mice were Vegfafl/fl. A female and male mouse from each genotype are represented. In femurs, Cre+ conditional knockout mice had the shorter amplicon of 560 base pairs (Mutant band), thereby showing Vegfa recombination in bones of Cre+ mice from each mouse line. On the other hand, Cre- littermates only had the larger amplicon of 2160 base pairs (WT), indicative of no recombination at exon 3. In kidney, UBC-CreERT2+ showed recombination, as expected, while Dmp1-CreERT2+ kidneys showed recombination, indicating non-bone targeting.
Supplemental Figure 2. Body weight data taken over the course of the experiment. VegfaΔUBC (Cre+) male and female mice lost 3–4% body weight between the start of tamoxifen injections and euthanasia (Supplemental Table 2). Based on body weight measurements taken during dosing and loading, most of the weight loss occurred in the 3-week window between tamoxifen injections and the beginning of loading. Weight loss due to loading was negligible and not different between Vegfafl/fl (Control) and VegfaΔUBC (Cre+) mice (Supplemental Table 2). There were no differences in weight loss between Vegfafl/fl (Control) and VegfaΔOsx (Cre+) mice, however there was a sex effect with females losing weight (−1.5%) and male mice gaining weight (1.5%). There were no sex or genotype differences between Vegfafl/fl (Control) and VegfaΔDmp1 (Cre+) mice related to body weight. The Dmp1 mice held a steady weight throughout the 5 weeks of the experiment.


