Abstract
Background
Streptococcus constellatus, a commensal, plays an important role in purulent infections. It has been reported as aggressive pathogen causing pleural empyema. But the role of S. constellatus in empyema has not been taken seriously. There are no studies about clinical characteristics of empyema caused by S. constellatus domestically and abroad. This study aimed to explore the clinical features and management of empyema caused by S. constellatus.
Methods
A retrospective review of 9 patients diagnosed with empyema caused by S. constellatus in a hospital between January 2010 and August 2021 was performed.
Results
S. constellatus empyema were mostly seen in old males (66.7%) with comorbid diseases. The high-risk factors include diabetes mellitus, oral infection, and oral surgery. All were unilateral encapsulated empyema (right-side, 55.6%), diagnosed with pneumonia (bilateral pneumonia, 88.9%; ipsilateral lung abscess, 44.4%). 33.3% of patients had S. constellatus and anaerobes co-isolated. S. constellatus were sensitive to penicillin G, linezolid, levofloxacin, vancomycin, ceftriaxone, and chloramphenicol, resistant to erythromycin, tetracycline, and clindamycin. 33.3% of the patients needed ventilator support. The primary treatment to S. constellatus empyema was timely pus drainage, intravenous antibiotics, and enough nutrition support, intrapleural fibrinolytics and surgery (VAST recommended first) in necessity.
Conclusion
S. constellatus may cause pneumonia and lung abscess first and then spread to cause empyema mainly in old males with comorbid diseases. S. constellatus often co-isolated with anaerobes in empyema. Antibiotics should cover simultaneously both S. constellatus and anaerobes.
Keywords: S. constellatus, empyema, clinical features, anaerobes
Introduction
Streptococcus constellatus (S. constellatus) is a gram-positive and catalase test-negative coccus, first isolated from periodontal abscess and described by Guthof in 1956.1 S. constellatus, a member of Streptococcus milleri group (SMG), is commensal in the mouth, upper respiratory tract, and gastrointestinal tract.2 It was considered as pathogen in purulent infections including subdural empyema,3 liver abscess,4 frontal sinus abscess,5 head and neck infections.6 SMG is identified as one of the main pathogens causing community-acquired pleural infections.7 It was reported that 54.5% of patients with SMG pulmonary infections had pleural effusion, and that pleural effusion was more frequently observed in patients with SMG infections than that infected with other pathogens.8 In an animal study of 21 mice with SMG pulmonary infections, 66.7% were found to have empyema, and S. constellatus was commonly observed as pathogen, especially in empyema (8/21).9 A case of S. constellatus causing empyema was also reported in our previous study.10 Empyema caused by S. constellatus has not been taken seriously. The possible reasons are as follows: (1) the special culture conditions of S. constellatus that has access to oxygen and higher concentration of carbon dioxide,11 make it difficult to be detected. (2) As commensal bacteria, the isolated S. constellatus is not generally considered as the pathogen of respiratory infections. The present study aimed to explore the clinical features of S. constellatus empyema and provide some ideas for the diagnosis and treatment of S. constellatus empyema.
Methods
This retrospective review was performed in a hospital. Patient diagnosed with pleural empyema with positive S. constellatus cultures or (and) sequence detected by Next-Generation Sequencing (NGS) from pleural fluid from January 2010 to August 2021 was enrolled. Pleural empyema was defined by pleural effusion indicated by images and macroscopic purulent fluid in this study. We collected clinical information of patients, including epidemiology, past-history, clinical manifestation, laboratory examination results, therapy. To explore the possible mechanism of S. constellatus causing empyema, we focused on the presence of pneumonia, lung abscess, and bronchopleural fistula. The antibiotics susceptibility test (AST) of S. constellatus isolates from pleural fluid was performed by K-B AGAR and minimum inhibitory concentration of antibiotics (MIC) test. It is divided into three grades: sensitive (S), intermediate (I) and resistant (R). The study was approved by the First Affiliated Hospital of Guangxi Medical University Medical Ethics Committee [NO.2022-KY-E-(142)].
Results
Clinical and Laboratory Characteristics
Nine patients diagnosed with empyema with S. constellatus isolated from pleural fluid were enrolled in this study. The clinical features and laboratory findings were summarized in Table 1 (Table 1). The average age was 53.78±11.96 years, and 66.7% of the patients were male. 77.8% had underlying conditions, diabetic mellitus commonly. The average time from onset of clinical symptoms to admission for drainage of purulent pleural effusion was 14.22±6.14 days. All patients had fever, and most had cough, sputum production, dyspnea, chest pain and tightness. On admission, all patients showed hypoproteinemia (30.91±4.09, normal 40–45g/L) with average BMI 23.73±2.16 kg/m2, and the remaining liver function and renal function showed no abnormality. Abdominal and urinary ultrasonography revealed no significant kidney or liver lesion that may cause pleural effusion. Inflammatory markers of all patients were significantly rising. Average WBC count, neutrophil percentage, C-reactive protein, and procalcitonin were 17.37±7.10 (cells 10^9/L), 0.85±0.05, 198.6±56.78(mg/L), 1.55±1.64(ng/mL), respectively. No pathogen was found in the blood cultures of all patients. None of the patients had a history of immunodeficiency or use of hormone or immunosuppressive agents. Lymphocyte subgroup analysis of six patients was unremarkable. Four patients had hypogammaglobulinemia, with IgM 0.787, 0.630, 0.54, and 0.46 g/L, respectively (normal 0.84-1.32 g/L). Unfortunately, we cannot assess the remaining patients’ immune status as they did not receive related laboratory tests.
Table 1.
The Clinical and Laboratory Features of Patients with S. constellatus Empyema
| Term | Total (n = 9) |
|---|---|
| Age (y); mean ± SD | 53.78±11.96 |
| Gender-male n (%) | 6 (66.7%) |
| BMI; mean ± SD (kg/m^2) | 23.73±2.16 |
| Comorbidity diseases n (%) | 7 (77.8%) |
| Chronic pulmonary disease and heart failure | 1 (11.1%) |
| Atrial septal defect | 1 (11.1%) |
| Diabetes mellitus | 4 (44.4%) |
| Chronic anemia (hemoglobin <90g/L) | 1 (11.1%) |
| Hypoalbuminemia n (%) | 9 (100%) |
| Smoking history n (%) | 4 (44.4%) |
| Achohol n (%) | 1 (11.1%) |
| From symptom onset to purulent pleural fulid drained (days) | 14.2±6.14 |
| Symptoms at presentation n (%) | |
| Fever | 9 (100%) |
| Cough | 8 (88.9%) |
| Sputum | 6 (66.7%) |
| Chest pain | 6 (66.7%) |
| Chest distress | 2 (22.2%) |
| Dyspnea | 7 (77.8%) |
| Previous antibiotic treatment n (%) | 2 (22.2%) |
| Laboratory findings at presentation | |
| White blood cell counts (10^9cells/L) | 17.37±7.10 |
| Neutrophil percentage | 0.85±0.05 |
| c-reactive protein (normal 0–10 mg/L) | 198.6±56.78 |
| Procalcitonin (normal 0–0.05ng/mL) | 1.55±1.64 |
| Rrythrocyte sedimentation rate (normal 0–20mm/h) | 89.57±14.50 |
| serum albumin (normal 40–45 g/L) | 30.91±4.09 |
| Bacteriology of thoracic drainage fluid n (%) | |
| Only S. constellatus | 6 (66.7%) |
| Mixed infection | 3 (33.3%) |
| Blood culture n (%) | 8 |
| Bacteremia | 0 |
| No bacteria cultured | 8 (88.9%) |
Characteristics of Pleural Fluid
As the table showed (Supplementary Table 1), all were macroscopically purulent and turbid liquid with Rivalta test positive, and the WBC counts were more than 500*10^6 cells/L, mainly multinucleated cells. Pleural effusion was exudate in all patients, according to the Light standard that LDH level of pleural effusion was more than 2/3 of the upper limit of serum normal value (>245U/L). S. constellatus can be isolated from primal pleural fluid in all patients, except case 9 of whom sequences of S. constellatus was detected by NGS of pleural fluid. As shown in Table 1 (Table 1), S. constellatus was mainly found as a solitary isolate from pleural fluid of 66.7% of patients. S. constellatus and anaerobes were co-isolated from pleural fluid of three patients (two co-isolated with P oris, one co-isolated with P buccae) (Supplementary Table 1). Based on clinical experience and previous published studies, we considered S. constellatus was a significant isolate rather than a commensal. The results of AST of S. constellatus isolates of patients were showed (Table 2). Antibiotics 100% efficacy against S. constellatus were penicillin G, linezolid, levofloxacin and vancomycin. Ceftriaxone (87.5%) and chloramphenicol (75%) showed relatively high efficiency. S. constellatus was observed resistant to erythromycin (57%), tetracycline (50%) and clindamycin (62.5%).
Table 2.
Antibiotics Susceptibility Test of S. constellatus Isolates from Pleural Fluid
| ID | Penicillin G | Ceftriaxone | Linezolid | Levofloxacin | Vancomycin | Erythromycin | Tetracyclines | Clindamycin | Chloramphenicol |
|---|---|---|---|---|---|---|---|---|---|
| Case1 | S | S | S | S | S | R | R | R | S |
| Case2 | S | S | S | S | S | S | S | S | S |
| Case3 | S | S | S | S | S | R | R | R | R |
| Case 4 | S | R | S | S | S | / | I | R | S |
| Case 5 | S | S | / | S | S | R | S | R | S |
| Case 6 | S | S | S | S | S | S | S | S | S |
| Case 7 | S | S | S | S | S | S | R | S | S |
| Case 8 | S | S | S | S | S | R | R | R | I |
| Case 9 | / | / | / | / | / | / | / | / | / |
| Sensitive (%) | 100% | 87.5% | 100% | 100% | 100% | 42.9% | 37.5% | 37.5% | 75.0% |
Image Features
As shown in Table 3 (Table 3), all patients had unilateral empyema, mostly right-side (55.6%) and encapsulated empyema. The average pleural fluid thickness was 10.71±2.88 cm. All patients with empyema were diagnosed with pneumonia, bilateral pneumonia especially (88.9%). One patient had left-side pneumonia, and his empyema was also left-side. 44.4% of the patients had lung abscess, which was consistently ipsilateral to empyema. Chest CT indicated that more than half of patients had intrathoracic lymphadenectasis, including hilus pulmonic and mediastinal lymph nodes. Two patients had bronchopleural fistula and two patients had pyopneumothorax.
Table 3.
The Image Features of 9 Patients with Empyema Caused by S. constellatus
| Term | Total (n = 9) |
|---|---|
| Empyema n (%) | |
| Left | 4 (44.4%) |
| Right | 5 (55.6%) |
| Encapsulated empyema | 9 (100%) |
| Pyopneumothorax | 2 (22.2%) |
| Pleural fluid thickness (cm) | 10.71±2.88 |
| Pneumonia (n/%) | 9 (100%) |
| Left/right/both | 1/0/8 |
| Lung abscess | 4 (44.4%) |
| Lymphadenectasis(n/%) | 6 (66.7%) |
| Only mediastinal lymph node | 3 (33.3%) |
| Hilus pulmonis and mediastinal lymph nodes | 3 (33.3%) |
| Bronchopleural fistula (n/%) | 2 (22.2%) |
Treatment and Outcome
Treatment and prognosis of patients were shown in Table 4. All patients received intravenous antibiotic and pleural effusion drainage, and 33.3% received additional therapy, including intrapleural urokinase and surgery. The average duration of antibiotic use was 44.3±22.5 days. 55.6% of patients developed respiratory failure. And three patients developed severe pneumonia requiring ventilator support and two developed acute respiratory distress syndromes (ARDS). After treatment, 88.9% of patients were significantly cured. Only one patient (case 6) developed septic shock without significant improvement after comprehensive treatment and died finally. As shown in table (Supplementary Table 2), the inflammatory markers of case 6 who died after treatment were significantly higher than that before treatment. Inflammatory markers in the other 8 patients were significantly lower after treatment. To some extent, these inflammatory indicators can effectively evaluate the therapy efficacy of S. constellatus empyema.
Table 4.
The Treatment and Prognosis of 9 Patients with S. constellatus Empyema
| Term | Total (n = 9) |
|---|---|
| Therapy (n/%) | |
| Antibiotics+ drainage | 6 (66.7%) |
| Antibiotics+ drainage + Intrapleural fibrinolytics | 1 (11.1%) |
| Antibiotics+ drainage+ Intrapleural fibrinolytics + surgery | 1 (11.1%) |
| Antibiotics+ drainage+ surgery | 1 (11.1%) |
| Duration of antibiotic use (days) | 44.3±22.5 |
| Respiratory failure (n/%) | 5 (55.6%) |
| Sever pneumonia | 3 (33.3%) |
| ARDS | 2 (22.2%) |
| Ventilator using (n/%) | 3 (33.3%) |
| Hospital stays(days) | 23±7.02 |
| Outcome(n/%) | |
| Cured and survival | 8 (88.9%) |
| Worsen and death | 1 (11.1%) |
Literature Review
We searched relevant articles in databases with search terms (“Streptococcus constellatus” and empyema) with no language restrictions. At present, no original clinical studies on empyema caused by S. constellatus have been published, but case reports. 14 patients in the literature with empyema with S. constellatus cultured from pleural fluid was enrolled.12–23 The clinical information was shown in the table (Supplementary Table 3). 92.9% of patients were male. Mostly were middle-aged and elderly patients, with an average age of 50.21±11.37 years. Almost all patients had underlying conditions or risk factors including alcoholism, diabetes. Three had recently exodontia. One patient developed a mediastinitis who had gastroesophageal reflux and recently underwent gastroscopy. 55.6% of patients had purulent infections besides empyema, including liver abscess, lung abscess, mandibular tissue abscess, and gingival abscess. The right-side empyema was more common (72.8%), and two patients had pyopneumothorax. 71.4% were monoinfection involving only S. constellatus, while 28.6% were pleural polyinfection involving S. constellatus and other organisms, most of which were anaerobes. The primary treatment was intravenous antibiotics and pleural fluid drainage, and nearly half of the patients also received other treatments, including intrapleural fibrinolytic agents (urokinase, streptokinase) and surgery (decortication, partial excision of lobe with lung abscess). The average duration of antibiotic use was 43.5±9.25 days. All patients were cured after treatment.
Case Presentation of Case 6
On 19 October 2020, a 55-year-old man referred to emergency ward of our hospital with a 9-days history of progressively worsening right chest pain, dyspnea, right upper abdominal pain and a low-grade fever. He broken his right foot and recovered after surgery in 2015. He has been a smoker for more than 30 years, never taken hormones or immunosuppressive agents, with unremarkable family medical history. On admission, heart rate 105 beats/min; respiratory rate 23 breaths/min; temperature 37.5°C; blood pressure 152/86mmHg, PaCO2 32.2mmHg, PaO2 71.8mmHg. No lymphadenopathy was detected. Weakened breath sounds were detected in the right hemithorax with small wet rales and stony-related dullness to percussion. The abdomen was soft, with tenderness in the right upper abdomen and under the xiphoid process, no rebound tenderness. The rest physical examination was unremarkable.
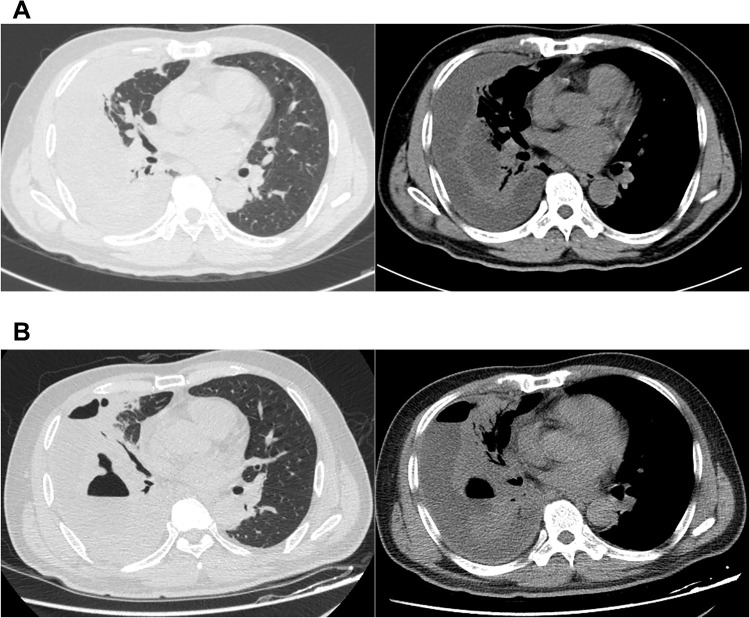
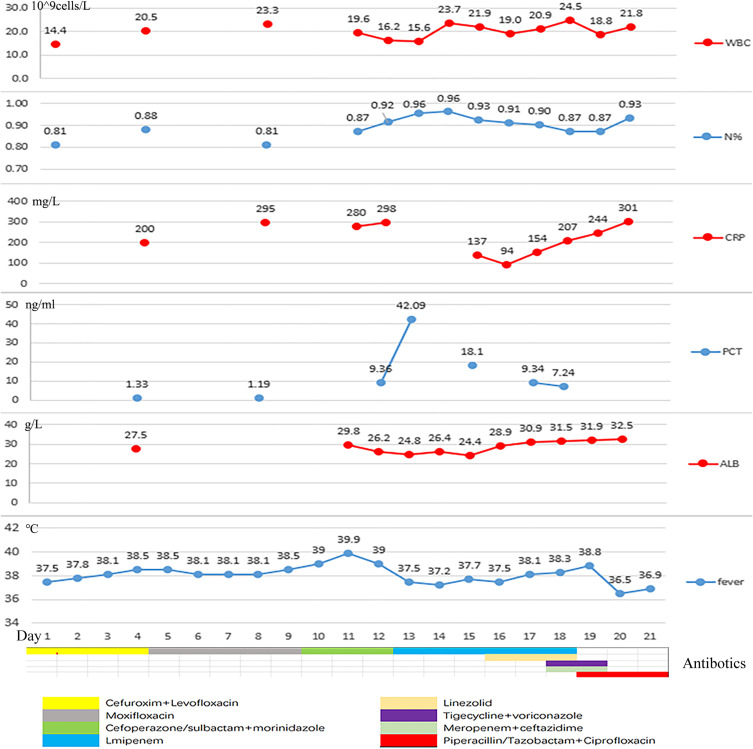
Results of laboratory examination on admission were as follows: WBC count, 14.43*109 cells/L; neutrophils percent, 0.81; C-reactive protein, >192 mg/L; procalcitonin, 1.33 ng/mL, serum albumin 27.5 g/L. Liver and kidney functions test were normal. Serum immunoglobulin test indicated hypogammaglobulinemia with IgM 0.46 g/L (normal 0.84–1.32 g/L) and lymphocyte subgroup test demonstrated no abnormality. Ultrasound suggested an atrial septal defect with a small amount of left to right shunt at the atrial level. A large right-sided pleural effusion was identified in chest radiograph. Consistently, chest CT showed bilateral pneumonia and a large right-sided encapsulated and separate pleural effusion, with almost collapsed right lung and lightly mediastinal lymphadenectasis (Figure 1A). Abdominal ultrasound and CT were unremarkable. Empirical antibiotics (cefuroxime, levofloxacin, moxifloxacin, cefodizime) were administered. And we punctured ultrasound-guided to drain empyema repeatedly, in view of encapsulated and separate pleural empyema difficult to drain via chest tube drainage.
Figure 1.
CT images the dead patient (case 6). (A) CT on admission showed bilateral pneumonia and a large right-sided encapsulated and separate pleural effusion. (B) CT repeated in the 10th day showed empyema and pneumonia aggravated, lung abscess and bronchopleural fistula were found.
G+ cocci, G- bacillus and pharyngeal bacteria could be found in sputum smear, with no pathogen cultured. A small amount of G+ coccus was found in pleural fluid smear. S. constellatus and Prevotella buccae were cultured from initial yellow pleural fluid. AST showed the isolated S. constellatus susceptive to all the following antibiotics: penicillin G, ceftriaxone, linezolid, levofloxacin, vancomycin, erythromycin, tetracyclines, clindamycin, chloramphenicol. And the antibiotic regimen was adjusted (cefoperazone/sulbactam against S. constellatus, and morinidazole against Prevotella buccae).
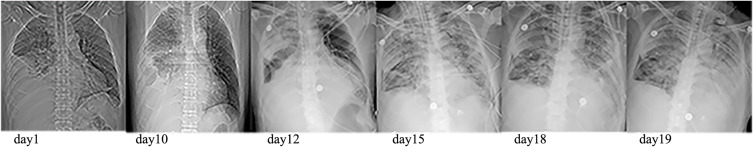
Although the patient had received intravenous antibiotics, albumin supplementation and pleural fluid drainage since admission, the condition was getting worse. Repeated CT showed empyema and pneumonia intensified, and lung abscess and bronchopleural fistula were found (Figure 1B). The patient developed into severe pneumonia with respiratory failure. Since the patient was too serious infected to tolerate surgery, we performed chest tube drainage and drained green-yellow purulent fluid in the 13th day. He was transferred to intensive care unit for further treatment, needing tracheal intubation and mechanical ventilation to maintain respiratory function. After several days, the patient eventually developed into severe ARDS (PaO2/FiO2: 78 mmHg) and septic shock. It required crystals and colloidal solution, continuous norepinephrine pumping (0.5μg/kg min) to boost blood pressure. Patient’s dyspnea exacerbated, and aspiration of sputum and bronchoalveolar lavage were performed repeatedly via fiberoptic bronchoscopy. During ICU stays, antibiotics were adjusted several times depending on the disease. Repeated chest X-ray showed that the patient’s empyema and pneumonia gradually worsened (Figure 2) and the patient demand to be discharged in the 21st day. He died shortly after leaving hospital. After the patient was discharged from the hospital, Pseudomonas aeruginosa (P. aeruginosa) was isolated from alveolar lavage fluid and sputum samples that were sent on the 19th day. The patient had already received antimicrobial treatment strongly against P. aeruginosa throughout clinical course, but the condition deteriorated (Figure 3). So, we considered that the isolated was difficult-to-treat resistance (DTR) P. aeruginosa.
Figure 2.
Chest radiograph of the patient during hospitalization.
Figure 3.
During hospitalization, the changes of body temperature, inflammatory indicators (including white blood cell count, neutrophil percentage, C-reactive protein, procalcitonin), nutritional status (plasma albumin), and antibiotic regimen adjustment process.
Discussion
In a large trial of 434 patients from over 40 centers in the UK, Gram-positive aerobic organisms were the most frequent pathogens identified in community acquired pleural infection.24 Streptococcal species including the SMG and S. aureus account for approximately 65% of cases.7 S. constellatus, as a member of SMG, can reach the original sterile parts of individuals with lower immunity and cause suppurative infection. It is reported that S. constellatus is a common pathogen in empyema in some studies of SMG pulmonary infections. But there is no clinical study on empyema caused by S. constellatus. In the present study, we retrospectively analyzed the clinical characteristics of 9 patients diagnosed with empyema caused by S. constellatus. We found that the patients tended to be quinquagenarian men with comorbid diseases and lower immunity. The high-risk factors for S. constellatus empyema include diabetes mellitus, oral infection, and oral surgery. The mean time from symptom onset to purulent pleural fluid drained was about two weeks. It was demonstrated that SMG infections occur mainly in male patients and patients with comorbid diseases, and the mean time to pus formation is 18 days.25–27 This was consistent with our study.
SMG was considered causing thoracic infections via some pathways, including aspiration of oral secretions, direct implantation by trauma or surgery, extension by contiguity, and hematogenous dissemination.26,28,29 In a retrospective study of 30 patients, SMG pneumonia tended to more frequently occur in males with comorbid diseases and involve pleural effusion with purulent formation, and S. intermadius was the most common organism followed by S. constellatus.30 Okada et al reported that pleural effusion was observed in 54.5% of patients with SMG infections, and that pleural effusion was more frequently observed in patients with SMG infections than that were infected with other pathogens, including S. pneumoniae.8 It was showed a low SMG detection rate in patients with pneumonia without pleural effusion.31 In the present study, all patients had pneumonia, nearly half of the patients had lung abscess, and some had bronchopleural fistula. We observed right-side empyema was more common in S. constellatus empyema in this study. As we known, the right bronchus is wider, more vertical, and shorter than the left one. Therefore, we speculated that S. constellatus mainly cause pneumonia and lung abscess, and then invades the pleura and causes empyema by aspiration. Most of the patients reported in the literature had a history of recent exodontia and extra-thoracic abscesses. Hematogenous dissemination may be another important way of S. constellatus to cause pleural empyema. And a history of recent exodontia may be an important indication for the diagnosis of S. constellatus empyema.
S. constellatus were reported more likely to be polymicrobial in suppurative infections.31 About 33.3% of empyema in our study were mixed infections involving S. constellatus and anaerobes. The reported rates of SMG polymicrobial infection with other bacteria ranged from 13% to 45% in respiratory infections caused by SMG.8,28,29,32 And the rates of mixed infections involving the SMG combined with anaerobes range from 14% to 24%.25,33 A study of in vitro and in vivo experiments demonstrated that the presence of anaerobes can positively enhance infection of S. constellatus related to high rate of lung abscess development and mortality. The potential mechanism was that anaerobes itself and its metabolite may impair the function of polymorphonuclear leukocytes (PMNLs) to inhibit the bactericidal activity of the host and stimulate the growth of S. constellatus.34
Although low mortality (11.1%) was showed in patients with S. constellatus empyema, more than half developed respiratory failure and 33.3% develop severe pneumonia, even ARDS and septic shock. It caused huge medical economic burden. Early diagnosis of S. constellatus empyema and timely treatment is very important. It is recommended all patients with pleural infection should early receive antibiotics.7 When a patient is suspected to have empyema due to S. constellatus, it is vital to administer empirical antibiotics before bacterial culture and AST results available. As we known, there is no study on AST of S. constellatus related to pleural infection so far. In this study, S. constellatus isolates were completely sensitive to penicillin G, linezolid, levofloxacin and vancomycin. Ceftriaxone, chloramphenicol showed relatively low efficiency against S. constellatus. More than half of isolates were resistant to erythromycin, tetracycline and clindamycin. In a study involving 33 periodontal S. constellatus isolates, Thomas et al reported clindamycin was an effective antibiotic against S. constellatus and about 30% of the isolates were resistant to doxycycline.35 Piotr et al observed that S. constellatus had the highest resistance rate to clindamycin (49%) and all isolates were susceptible to vancomycin in an AST data of 779 viridians group Streptococcus isolates from orofacial infections.36 This was different from our results, which may be caused by the different sampling sources, local antibiotic policies and resistance patterns. These may have certain guiding significance for the empirically antibiotics therapy to S. constellatus empyema. Unfortunately, small sample sizes and frequent antibiotic adjustments in patients made it difficult to analyze the characteristics of antimicrobial treatment in patients with empyema caused by S. constellatus. Therefore, the use of antibiotics in the nine enrolled patients was not specified in this study. We found that S. constellatus was quite often co-isolated with anaerobes in empyema. Interestingly, in the process of data collection, we found that almost all patients were simultaneously treated with ornidazole, morinidazole or metronidazole against anaerobic bacteria in addition to antibiotics against S. constellatus. Thus, we held the opinion that antibiotics should cover both S. constellatus and anaerobes. In the present study, all patients received intravenous antibiotics and pleural fluid drainage, and favorable outcomes achieved in most of the cases. Duration of antibiotic use was about six weeks. It was argued that delayed pleural drainage probably leads to increased morbidity, duration of hospital stays and increased mortality of pleural infection.7 In addition to antibiotic treatment, early and adequate pleural drainage is very important for the treatment of S. constellatus empyema. Hypoproteinemia was very common in patients with S. constellatus empyema. And hypoalbuminaemia was considered associated with a poor outcome from pleural infection,7 so enough nutritional support should be emphasized in the treatment of S. constellatus empyema. In addition to antibiotics and drainage, two patients in our study and six patients with S. constellatus empyema in the literature were cured by surgical treatment, including decortication and excision of the lung lobe with lung abscess. And most of them received Video-assisted Thoracoscopic Surgery (VATS) rather than open thoracotomy. Surgical treatment should be taken into consideration when persistent purulent pleural effusion cannot be effectively resolved after adequate antibiotics and drainage, and VATS may be the preferred procedure.
We explored the cause of the patient’s death and concluded that empyema caused by S. constellatus and anaerobes and subsequently severe pneumonia caused by DTR P. aeruginosa contributed to the patient’s death. First, the patient had separate and encapsulated empyema, making the pus difficult to be drained effectively. Pneumonia caused by DTR P. aeruginosa rapidly aggravated, and the result of AST was reported too late to adjust antibiotic regimen in time. Lung abscess and bronchopleural fistulas exacerbated empyema. Secondly, surgical treatment could not be performed timely to remove the infection lesions. Finally, the patient had persistent hypoproteinemia despite protein supplementation, and his serum IgM levels were low, resulting in compromised immunity. Together, the patient’s infection could not be effectively controlled, and septic shock gradually worsened, leading to multiple organ failure and death.
We acknowledged several limitations of this study: (1) S. constellatus empyema is rare and S. constellatus is difficult to cultivate. This was a single-center study and sample size was small, may lead to poor reliability of this research. (2) This was a retrospective study, and we could not trace the patient’s recent tooth extraction and periodontal inflammation without records in the hospital system. (3) We could not know the sequence of empyema, pneumonia/lung abscess and bronchopleural fistula and lack of microbiology of pneumonia and lung abscess. And no S. constellatus isolates was identified in sputum specimens of all patients. The conjecture that S. constellatus caused pneumonia and lung abscess first and then empyema remains further investigation to elucidate. (4) Anaerobes was reported to strengthen pathogenicity of S. constellatus in pulmonary infection. We did not compare the difference in disease severity and prognosis between the two groups of single S. constellatus and polymicrobial combined with anaerobes. (5) it was confirmed that Pseudomonas aeruginosa can form biofilms in the pleural cavity,37 and we can further investigate whether S. constellatus can form biofilms in the pleural cavity.
Conclusions
S. constellatus empyema was found more frequently in old males with comorbid diseases, and most were right-side encapsulated empyema with bilateral pneumonia and abscess. We speculated that S. constellatus mainly caused pneumonia and lung abscess first and then spread to cause empyema. S. constellatus empyema can lead to large medical economic burden. Timely pus drainage, antibiotics (intravenous antibiotic first) and enough nutrition support are crucial. Anaerobes may involve and aggravate S. constellatus empyema, antibiotics should against both S. constellatus and anaerobes. Intrapleural fibrinolytics and surgical treatment (VAST recommended first) should be considered if necessary.
Funding Statement
This project was supported by the National Natural Science Foundation of China (No. 82104499, No. 82160783, No. 81760743, No. 81760024, No. 82260023); the National Natural Science Foundation of Guangxi (2022GXNSFAA035646); the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region, self-funded projects (No. Z20200825); the Key Research Program of Guangxi Science and Technology Department (No. AB21196010); Guangxi Health Commission Key Lab of Fungi and Mycosis Research and Prevention (No. ZZH2020004); The First Affiliated Hospital of Guangxi Medical University Provincial and Ministerial Key Laboratory Cultivation Project: Guangxi Key Laboratory of Tropical Fungi and Mycosis Research (No. YYZS2020006); Guangxi Medical and Health Suitable Technology Development and Popularization Application Project (No. S2019090); the First Affiliated Hospital of Guangxi Medical University Clinical Research Climbing Program Youth Science and Technology Morning Star Program (No. 201903032); Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University.
Abbreviations
S. constellatus, Streptococcus constellatus; SMG, Streptococcus milleri group; NGS, Next-Generation Sequencing; CT, computed tomography; AST, antibiotics susceptibility test; MIC, minimum inhibitory concentration; ARDS, acute respiratory distress syndromes; VATS, video-assisted thoracoscopic surgery; WBC, white blood cell counts; ALB, albumin; ADA, adenosine deaminase; GLU, glucose; PRO, protein; LDH, lactate dehydrogenase; NEU%, neutrophil percentage; CRP, C-reactive protein; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; DVT, deep venous thrombosis; PE, pulmonary embolism; DTR, difficult-to-treat resistance; P. aeruginosa, Pseudomonas aeruginosa.
Ethics Approval and Consent to Participate
The study was approved by the First Affiliated Hospital of Guangxi Medical University Medical Ethics Committee [NO.2022-KY-E-(142)]. Written informed consent was obtained from study participants. This study complies with the Declaration of Helsinki.
Consent for Publication
Written informed consent for publication of the clinical details was obtained from the patient (case 6).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Guthof O. [Pathogenic strains of Streptococcus viridans; streptocci found in dental abscesses and infiltrates in the region of the oral cavity]. Zentralbl Bakteriol Orig. 1956;166(7–8):553–564. German. [PubMed] [Google Scholar]
- 2.Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257–285. doi: 10.1093/clinids/10.2.257 [DOI] [PubMed] [Google Scholar]
- 3.Sahin S, Yazar U, Cansu A, Kul S, Kaya S, Ozdogan EB. Is Sinusitis innocent?--unilateral subdural empyema in an immunocompetent child. Indian J Pediatr. 2015;82(11):1061–1064. doi: 10.1007/s12098-015-1771-x [DOI] [PubMed] [Google Scholar]
- 4.Atemnkeng F, Al-Ttkrit A, David S, et al. An unusual case of intraabdominal abscess after a colonoscopy with polypectomy. J Med Cases. 2021;12(8):301–305. doi: 10.14740/jmc3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrera W, Lewis WB, Silkiss RZ. Frontal sinus abscess with cutaneous fistula secondary to Streptococcus constellatus. Orbit. 2021;40(2):171. doi: 10.1080/01676830.2020.1760314 [DOI] [PubMed] [Google Scholar]
- 6.Hirai T, Kimura S, Mori N. Head and neck infections caused by Streptococcus milleri group: an analysis of 17 cases. Auris Nasus Larynx. 2005;32(1):55–58. doi: 10.1016/j.anl.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Davies HE, Davies RJ, Davies CW; Group BTSPDG. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–53. doi: 10.1136/thx.2010.137000 [DOI] [PubMed] [Google Scholar]
- 8.Okada F, Ono A, Ando Y, et al. High-resolution CT findings in Streptococcus milleri pulmonary infection. Clin Radiol. 2013;68(6):e331–337. [DOI] [PubMed] [Google Scholar]
- 9.Kanamori S, Kusano N, Shinzato T, Saito A. The role of the capsule of the Streptococcus milleri group in its pathogenicity. J Infect Chemother. 2004;10(2):105–109. doi: 10.1007/s10156-004-0305-7 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Cheng H, Cai Z, et al. Identification of Microbiome Etiology Associated With Drug Resistance in Pleural Empyema. Front Cell Infect Microbiol. 2021;11:637018. doi: 10.3389/fcimb.2021.637018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiley RA, Hall LM, Hardie JM, Beighton D. A study of small-colony, beta-haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int J Syst Bacteriol. 1999;49(Pt 4):1443–1449. doi: 10.1099/00207713-49-4-1443 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Xiao B, Liang Z. Successful treatment of pyopneumothorax secondary to Streptococcus constellatus infection with linezolid: a case report and review of the literature. J Med Case Rep. 2020;14(1):180. doi: 10.1186/s13256-020-02475-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrastek D, Hickman S, Sitaranjan D, et al. Streptococcus constellatus Causing Empyema and Sepsis, Necessitating Early Surgical Decortication. Case Rep Infect Dis. 2020;2020:4630809. doi: 10.1155/2020/4630809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Che Rahim MJ, Mohammad N. Pyopneumothorax secondary to Streptococcus milleri infection. BMJ Case Rep. 2016;2016:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schimmel T, Trawinski H, Karlas T, Wendt S, Lubbert C. Polymikrobielle Leberabszesse und Pleuraempyem bei einem 40-jährigen Mann nach Zahnextraktion und geschlossener Parodontitis-Behandlung: Ein Fallbericht[Polymicrobial liver abscesses and pleural empyema in a 40-year-old male after tooth extraction and closed periodontal treatment: a case report]. Z Gastroenterol. 2019;57(5):600–605. German. doi: 10.1055/a-0829-7017 [DOI] [PubMed] [Google Scholar]
- 16.Revilla-Martí P. Empiema pleural por Streptococcus constellatus[Pleural empyema caused by Streptococcus constellatus]. Rev Clin Esp. 2011;211(11):612–613. Spanish. doi: 10.1016/j.rce.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Rai K, Matsuo K, Yonei T, Sato T. 抜歯後に口腔内常在菌2種により難治性膿胸を発症し, 胸腔鏡下搔爬術を要した1例[Treatment-refractory-dental-extraction-associated pyothorax involving infection by 2 species of oral originated bacteria requires surgical debridement by video assisted thoracoscopic surgery (VATS)]. Kansenshogaku Zasshi. 2008;82(5):461–465. Japanese. doi: 10.11150/kansenshogakuzasshi1970.82.461 [DOI] [PubMed] [Google Scholar]
- 18.Peromingo JA, Sánchez Leira J. Streptococcus constellatus: agente etiológico asociado en empiema pleural[Streptococcus constellatusas a causative agent of empyema. Report of one case]. Rev Med Chil. 2006;134(8):1030–1032. Spanish. doi: 10.4067/s0034-98872006000800013 [DOI] [PubMed] [Google Scholar]
- 19.Ortiz de Saracho J, Barbancho S, Mostaza JL. Mediastinitis y empiema pleural por Streptococcus constellatus[Mediastinitis and pleural empyema caused by Streptococcus constellatus]. Arch Bronconeumol. 2004;40(12):602. Spanish. doi: 10.1016/S0300-2896(04)75599-4 [DOI] [PubMed] [Google Scholar]
- 20.Kim DH. Empyema caused by transdiaphragmatic extension of pyogenic liver abscess. Clin Case Rep. 2019;7(1):240–241. doi: 10.1002/ccr3.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vulisha AK, Sam R, Nur H, Bhardwaj N, Sirineni S. Aggressive Presentation of Streptococcus constellatus. Cureus. 2021;13(4):e14534. doi: 10.7759/cureus.14534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alejandro MH, Perez Laura P. Empyema Caused by Streptococcus constellatus: atypical Presentation of a Typical Pneumonia. J Clin Case Rep. 2018;08:02. doi: 10.4172/2165-7920.10001079 [DOI] [Google Scholar]
- 23.Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21(Suppl 3):S238–243. doi: 10.1093/clind/21.Supplement_3.S238 [DOI] [PubMed] [Google Scholar]
- 24.Maskell NA, Batt S, Hedley EL, Davies CW, Gillespie SH, Davies RJ. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med. 2006;174(7):817–823. doi: 10.1164/rccm.200601-074OC [DOI] [PubMed] [Google Scholar]
- 25.Wong CA, Donald F, Macfarlane JT. Streptococcus milleri pulmonary disease: a review and clinical description of 25 patients. Thorax. 1995;50(10):1093–1096. doi: 10.1136/thx.50.10.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porta G, Rodriguez-Carballeira M, Gomez L, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J. 1998;12(2):357–362. doi: 10.1183/09031936.98.12020357 [DOI] [PubMed] [Google Scholar]
- 27.Molina JM, Leport C, Bure A, Wolff M, Michon C, Vilde JL. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand J Infect Dis. 1991;23(6):659–666. doi: 10.3109/00365549109024289 [DOI] [PubMed] [Google Scholar]
- 28.Kobashi Y, Mouri K, Yagi S, Obase Y, Oka M. Clinical analysis of cases of empyema due to Streptococcus milleri group. Jpn J Infect Dis. 2008;61(6):484–486. [PubMed] [Google Scholar]
- 29.Jerng JS, Hsueh PR, Teng LJ, Lee LN, Yang PC, Luh KT. Empyema thoracis and lung abscess caused by viridans streptococci. Am J Respir Crit Care Med. 1997;156(5):1508–1514. doi: 10.1164/ajrccm.156.5.97-03006 [DOI] [PubMed] [Google Scholar]
- 30.Noguchi S, Yatera K, Kawanami T, et al. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm Med. 2015;15:133. doi: 10.1186/s12890-015-0128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claridge JE, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (Streptococcus milleri group) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32(10):1511–1515. doi: 10.1086/320163 [DOI] [PubMed] [Google Scholar]
- 32.Siegman-Igra Y, Azmon Y, Schwartz D. Milleri group streptococcus--a stepchild in the viridans family. Eur J Clin Microbiol Infect Dis. 2012;31(9):2453–2459. doi: 10.1007/s10096-012-1589-7 [DOI] [PubMed] [Google Scholar]
- 33.Takayanagi N, Kagiyama N, Ishiguro T, Tokunaga D, Sugita Y. Etiology and outcome of community-acquired lung abscess. Respir Int Rev Thoracic Dis. 2010;80(2):98–105. doi: 10.1159/000312404 [DOI] [PubMed] [Google Scholar]
- 34.Shinzato T, Saito A. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J Med Microbiol. 1994;40(2):118–123. doi: 10.1099/00222615-40-2-118 [DOI] [PubMed] [Google Scholar]
- 35.Rams TE, Feik D, Mortensen JE, Degener JE, van Winkelhoff AJ. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J Periodontol. 2014;85(12):1792–1798. doi: 10.1902/jop.2014.130291 [DOI] [PubMed] [Google Scholar]
- 36.Leszczynski P, Sokol-Leszczynska B, Mlynarczyk A, Sawicka-Grzelak A, Mlynarczyk G. An Analysis of Resistance Patterns of Oral Streptococci Obtained from Orofacial Infections Against Beta-lactams, Clindamycin and Vancomycin over 2014-2018. Oral Health Prev Dent. 2019;17(6):585–589. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Li J, Liang J, Zhang Z, Wei Q, Wang K. The effect of Cyclic-di-GMP on biofilm formation by Pseudomonas aeruginosa in a novel empyema model. Ann Translational Med. 2020;8(18):1146. doi: 10.21037/atm-20-6022 [DOI] [PMC free article] [PubMed] [Google Scholar]