Abstract
Sensory dysfunction is a common consequence of many forms of neurological injury. Rehabilitative paradigms that incorporate sensory retraining can provide modest benefits, but the majority of patients are left with lasting sensory loss. We have developed a novel strategy that uses closed-loop vagus nerve stimulation (VNS) paired with motor rehabilitation to facilitate recovery after neurological injury. VNS drives robust, phasic activation of neuromodulatory networks concurrent with rehabilitation to enhance synaptic plasticity and support recovery. A clinical case report provides initial evidence that a similar implementation of closed-loop VNS paired with a tactile rehabilitation regimen could improve recovery of somatosensory function. Here, we sought to build on this promising initial clinical data and rigorously evaluate the ability of VNS paired with tactile rehabilitation to improve recovery in an animal model of chronic sensory loss. The study design, including planned sample size, assessments, and statistical comparisons, was preregistered prior to beginning data collection (https://osf.io/xsnj5/). VNS paired with tactile rehabilitation resulted in a significant and nearly complete recovery of mechanosensory withdrawal thresholds. Equivalent tactile rehabilitation without VNS failed to improve sensory function. This VNS-dependent restoration of sensory thresholds was maintained for several months after the cessation of stimulation, illustrating long-term benefits. Moreover, VNS paired with tactile rehabilitation resulted in significant generalized improvements in other measures of forelimb sensorimotor function, including forelimb use asymmetry and paw placement. Given the safety and tolerability of VNS therapy, these findings suggest that incorporating VNS paired with sensory retraining into rehabilitative regimens may represent a fundamentally new method to increase recovery of sensory function after neurological injury.
Keywords: vagal nerve stimulation, peripheral nerve injury, peripheral neuropathy
Introduction
Loss of somatosensation is a common consequence of neurological injury. Damage to peripheral nerves leads to profound impairments in somatosensation in many patients, which typically persist even after surgical repair 1,2. There are no consistently effective methods to restore sensory function, but rehabilitation paradigms that incorporate sensory retraining may provide modest benefits to some patients 3–7. While treatment strategies tend to focus on restoration of motor function after neurological injury, deficits in somatosensation strongly contribute to disability 8–10. Given the prevalence and significance of sensory loss, the development of effective interventions that can restore somatosensory function has the potential to yield substantial benefits for patients suffering from a wide range of neurological disorders.
We have developed a novel strategy using closed-loop vagus nerve stimulation (VNS) to enhance the benefits of rehabilitation 11. VNS drives rapid, phasic activation of multiple neuromodulatory systems 12,13. Engaging these neuromodulatory networks concurrent with training provides pro-plasticity feedback to support synaptic plasticity in the neural circuits activated by training 14,15. An initial study provided a proof-of-principle demonstration that pairing VNS with tones drives robust, specific plasticity in the auditory cortex 16. Subsequent studies have built on this premise, showing that VNS paired with motor training produces similar training-specific plasticity in motor networks 17.
After neurological injury, strategies that support plasticity in spared networks represent a potential strategy to facilitate recovery of function 18. Based on VNS-dependent enhancement of plasticity, a number of studies have evaluated the utility of closed-loop VNS paired with rehabilitation 17. VNS paired with motor rehabilitation improves recovery of motor function in a variety of animal models of neurological injury, as well as in patients 19–29. Moreover, pairing VNS with various auditory sensory stimuli drives robust, stimulus-specific plasticity in auditory cortex, raising the possibility that delivery of VNS with other sensory modalities may produce similar effects 30–32. In support of this hypothesis, a pilot study in a chronic stroke patient with substantial sensory loss reported initial evidence that pairing VNS with tactile rehabilitation improved a number of measures of somatosensory function 33.
In the present study, we sought to build on this promising initial clinical data and rigorously evaluate the ability of VNS paired with tactile rehabilitation to improve recovery in an animal model of chronic sensory loss. Additionally, we evaluated the durability of VNS-dependent effects and whether improvements in somatosensation would generalize to other measures of forelimb function. To do so, rats underwent transection and gap repair of the median and ulnar nerves in the forelimb, which produces lasting deficits in somatosensation in spite of reinnervation. Beginning 16 weeks after nerve injury, animals were randomized to receive either a tactile rehabilitation paradigm that consisted of presentation of a variety of mechanical stimuli to the ventral surface of the injured paw or equivalent tactile rehabilitation with 0.5 s bursts of VNS paired with presentation of each tactile stimulus. Mechanosensory thresholds were measured weekly throughout therapy and for 8 weeks after the cessation of therapy to identify any lasting benefits. Additionally, multiple measures of forelimb sensorimotor function were evaluated throughout the study to examine generalization of recovery. The results from this study corroborate the findings from the pilot human study and suggest that VNS during tactile rehabilitation may improve recovery of sensory function after neurological injury..
Methods
Experimental Design
All experimental procedures, group sizes, outcome measures, statistical comparisons, and exclusion criteria were preregistered on Open Science Framework before data collection began (https://osf.io/xsnj5/). Before injury, all rats underwent baseline assessment of mechanosensory withdrawal thresholds, grip strength, and cylinder testing. All rats then underwent transection and tubular repair of the median and ulnar nerves in the right forearm and implantation of a stimulating cuff electrode on the left cervical vagus nerve. Beginning on week 16 post-injury, rats underwent baseline assessment of sensorimotor function and were dynamically allocated into two balanced groups based on mechanosensory withdrawal thresholds of the impaired forelimb. One group received tactile rehabilitation (Rehab, n = 8), comprised of 6 weeks of daily sessions in which 200 presentations of a range of tactile stimuli, including a paintbrush, a 10g filament, a copper rod, and a puff of air, were applied to the ventral surface of the injured paw. The other group received equivalent tactile rehabilitation, but a 0.5 train of VNS was paired with the delivery of each tactile stimulus (VNS+Rehab, n = 9). Mechanosensory withdrawal thresholds were measured weekly during therapy and every two weeks for two months after the cessation of therapy. Additional measures of forelimb sensorimotor function, including cylinder asymmetry, grip strength, horizontal ladder rung, and footprint analysis, were collected at multiple time points throughout the study (Fig. 1A). Seven rats were excluded from the study based on predefined criteria: mortality (n = 2), VNS device failure (n = 4), and autophagia (n = 1). Data from subjects excluded for VNS device failure are included as an intent to treat (ITT) analysis in the Supplemental information. All source data indexed across animals can be found in Supplementary Tables 1–7.
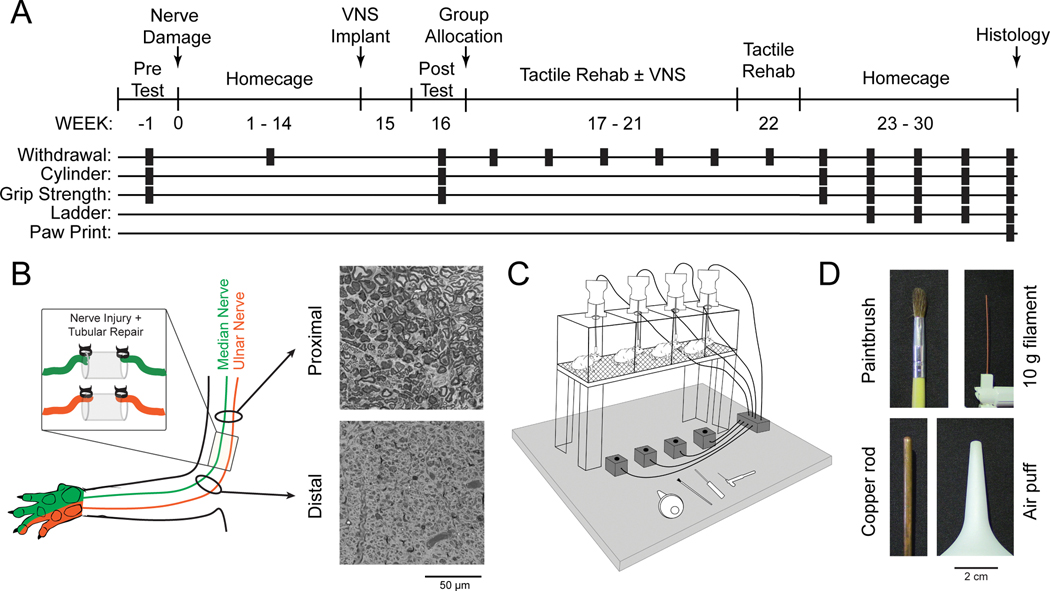
Fig. 1. Experimental Design and Tactile Rehabilitation Paradigm.
(A) Timeline of experimental design illustrating when each assessment is performed. (B) Schematic and representative images from proximal and distal cross-sections of the median nerve approximately 30 weeks after nerve transection and tubular repair. Reinnervation takes place, but the procedure results in chronic deficits in nerve architecture distal to the injury site. (C) Schematic of the tactile rehabilitation apparatus. Rats were placed in individual cages with a wire mesh floor. A variety of tactile stimuli were applied to the ventral surface of the right (injured) forepaw. A button press coincident with the delivery of the tactile stimuli initiated a 500 ms train of VNS in the appropriate group. (D) Detailed view of the devices utilized during tactile rehabilitation. The stimuli were selected to encompass a wide range of somatosensory features.
Subjects
Adult female Sprague Dawley rats (n = 24) weighing approximately 300g when they entered the study were obtained from Charles River Laboratories. The rats were housed in a 12:12 reversed light cycle environment, and behavioral training was performed during the dark cycle to increase daytime activity levels. All procedures performed in the study were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee (Protocols: 14–10 and 99–06).
Forelimb Nerve Injury
Complete transection of both the median and ulnar nerves proximal to the elbow followed by tubular repair was performed as previously described 34. Animals were deeply anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/k, i.p.), and acepromazine (5 mg/kg, i.p.) and were given supplemental doses as needed to maintain anesthesia levels. A small incision proximal to the elbow of the right forelimb was made, and the median and ulnar nerves were carefully isolated and exposed. Both nerves were transected 1cm proximal to the elbow. Immediately following transection, the proximal and distal stumps of each nerve were sutured 1 mm from the ends of a 8 mm saline-filled polyurethane tube (Micro-Renathane 0.095” I.D 0.066” O.D., Braintree Scientific, Inc., Braintree, MA), resulting in a 6 mm gap between nerve stumps. The skin incision was sutured and treated with antibiotic ointment. All animals were given enrofloxacin (10 mg/kg) immediately following surgery and sustained release buprenorphine (1.2 mg/kg) for 6 days following injury. Animals were placed in Elizabethan collars for approximately 1 week following injury to limit autophagia.
Vagus Nerve Stimulation Implantation Surgery
VNS implantation procedures were performed as described in previous studies 20,22–28,35. All rats underwent surgical implantation procedures to ensure blinding. Fifteen weeks after transection of the median and ulnar nerves, rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.p.), xylazine (20 mg/kg, i.p.), and acepromazine (5 mg/kg, i.p.), and were placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lamboid suture and over the cerebellum. A two-channel connector was mounted to the screws using acrylic. The rat was then removed from the stereotaxic apparatus and placed in a supine position. An incision was made on the left side of the neck and the overlying musculature was blunt dissected to isolate the vagus nerve. The nerve was placed into a bipolar stimulating cuff electrode, and the electrode leads were tunneled subcutaneously and connected with the two-channel skull-mounted connector. Incised skin was then sutured closed. All rats received enrofloxacin (s.c., 10 mg/kg) following surgery. Regardless of group assignment, all rats underwent implantation of the headmount and cuff electrode. To confirm cuff electrode functionality and proper placement, VNS-dependent activation of the Hering-Breuer reflex was assessed as in previous studies 36,37. To do so, blood oxygenation saturation during trains of VNS (0.8 mA, 30 Hz, 100 μs pulse width, up to 5 s train duration) was monitored via pulse oximetry during cuff implant. The cuff electrode was replaced if rats failed to demonstrate a reliable drop in oxygen saturation during the implant surgery.
Tactile Rehabilitation and Delivery of Vagus Nerve Stimulation
Tactile rehabilitation began 17 weeks post-forelimb nerve injury and continued for 6 weeks. Sessions of tactile rehabilitation were performed once daily, four days per week, with each session lasting approximately 1.5 hours. During each session, up to 8 animals were placed in individual acrylic chambers (14 × 15 cm) with a mesh floor (Fig. 1C). Each session consisted of 200 touches to the ventral surface of the right (injured) forepaw with diverse mechanical stimuli (Fig. 1D and Supplemental Video 1): a 10g von Frey filament (North Coast Medical, Gilroy, CA), a paintbrush (Kiss Products, Port Washington, NY), a 4 mm diameter copper rod (Everbilt, Atlanta, GA), and puffs of air delivered with a handheld bulb (Innovo Medical, Stafford, TX). Individual stimuli were presented in blocks of 10 with at least 10 seconds between each delivery, resulting in a total of 50 touches with each stimulus per session. Each tactile stimulation was typically 1 s in duration. Stimuli were chosen to span the range from below to above forelimb withdrawal threshold. The von Frey filament was applied perpendicularly to the paw and the digits. The paintbrush was applied across the paw and digits in varying directions and with an approximate upward force of 50g. The copper rod was applied to the paw with the minimal force sufficient to slightly raise the paw off the mesh floor. The handheld bulb was positioned approximately 4 cm below the paw and puffs of air were applied from multiple angles.
In the appropriate group, a train of VNS was triggered by a button press to coincide with delivery of each mechanical stimulus during tactile rehabilitation sessions. VNS parameters were equivalent to previous studies 19,22,24,25,28. Each 0.5 s stimulation train consisted of 0.8 mA 100 μsec biphasic pulses delivered at 30 Hz. No VNS was delivered after week 21 to assess effects lasting after the cessation of stimulation. Experimenters delivering tactile therapy were blinded to group assignment. All subjects in the study, regardless of group, were implanted with the same vagus nerve stimulation (VNS) device and headmount and were connected to a stimulator cable during therapy to ensure that they were indistinguishable in appearance. As a result, there were no visible differences between subjects to bias the experimenter administering the assessments.
Mechanosensory Withdrawal Threshold Testing
Mechanosensory detection thresholds were assessed in all animals according to standard procedures 38. Testing was performed in an acrylic chamber (19.5 × 9.6 cm) on a wire mesh floor. For each session, animals were allowed to acclimate to the behavioral chamber for 30 min before testing commenced. Mechanical withdrawal thresholds of the left and right forelimbs were tested using a dynamic plantar aesthesiometer (Cat. No. 37450, Ugo Basile, Switzerland). The actuator filament (0.5 mm diameter) was applied to the plantar surface of the forepaw, and a linearly increasing force was applied (20 s ramp time, 50 g maximal force). The force at which paw withdrawal occurred was captured for analysis. The left and right forelimbs were alternately tested with a minimum of 1 min between consecutive tests. Trials resulting in paw withdrawal due to spontaneous exploratory activity were excluded from analysis. Assessments were performed before injury (Week -1), before therapy (Weeks 8 & 16), weekly during therapy (Weeks 17–22), and biweekly after the conclusion of therapy (Weeks 22, 24, 26, 28, and 30) by experimenters blinded to group.
Cylinder Forelimb Asymmetry Testing
Spontaneous use of the forelimbs during exploratory activity was measured in a subset of animals using the cylinder forelimb asymmetry task, similar to previous descriptions 39. Animals were placed in a transparent cylinder (20 cm diameter) and allowed to freely explore for two minutes. Video was be recorded from directly underneath the cylinder through a clear sheet of acrylic. The total number of both left and right forepaw contacts with the wall of the cylinder were recorded. An asymmetry index, describing the relative use of the injured forelimb, was calculated as [(right/(left + right)) × 100]. Assessments were performed before injury (Week -1), before therapy (Week 16), and biweekly after the conclusion of therapy (Weeks 22, 24, 26, 28, and 30) by experimenters blinded to group.
Grip Strength Testing
A custom-made grip strength meter was used to measure the grip strength of the right and left forepaws independently, similar to previous descriptions 40. The rat was positioned over the two horizontal bars attached to separate force transducers such that each forepaw grasped a single bar. During testing, rats were held by the hindquarters while horizontally suspended and slowly pulled away from the module until grip broke. The peak force at which grip is released from the bar was recorded for each paw individually. Five trials were performed at each assessment, and the average of the peak grip forces were recorded. Assessments were performed before injury (Week -1), before therapy (Week 16), and biweekly after the conclusion of therapy (Weeks 22, 24, 26, 28, and 30) by experimenters blinded to group.
Horizontal Ladder Rung Testing
Horizontal ladder rung walking task was performed to assess forelimb placing, similar to previous studies 41,42. The test apparatus consisted of Plexiglas walls that created a 1 m long alley. Metal rungs (3 mm diameter) were inserted into the base of the walls to create an irregular pattern that varied the distance of the rungs from 1 to 5 cm. The same pattern was kept consistent across all animals. The width of the alley was adjusted to approximately 1 cm wider than an animal to prevent turning around. During testing, the apparatus was elevated 30 cm above the ground, and animals spontaneously walked the length of the alley. A video camera was positioned slightly below the horizontal plane so that paw positions could be easily visualized. Three to five trials were performed at each assessment to ensure data was collected during continuous walking. Frame-by-frame analysis of videos was performed offline and scored by a blinded experimenter, as in previous studies 42,43. The percentage of misses or slips was calculated as the number of steps with a score of 0–2 divided by the total number of steps scored. Assessments were performed biweekly after the conclusion of therapy (Weeks 24, 26, 28, and 30) by experimenters blinded to group.
Pawprint Analysis
Pawprint analysis was performed using the stamp and paper method as previously described 41. The forepaws of the animals were pressed into non-toxic ink, and the animals walked down a Plexiglas corridor (24 in × 4 in) with paper lining the floor. Each animal performed 1–3 trials to ensure three footprints from each paw could be analyzed. The paper was scanned and digitized, and three footprints from both the left and right paw were analyzed by a blinded experimenter using ImageJ software. Toe spread, the distance between the center of the second and fifth digits, was measured and recorded. Due to technical complications, footprint data was not collected in one rat. Assessments were performed at conclusion of therapy (Week 30) by experimenters blinded to group.
Histology
After completion of behavioral testing, segments of the median and ulnar nerves proximal and distal to the injury site were removed for histological analysis. Animals were deeply anesthetized (ketamine hydrochloride, 80 mg/kg, i.p. and xylazine, 10 mg/kg, i.p.) and the median and ulnar nerves in the right forelimb were identified. Segments (5–10 mm) were dissected from both proximal and distal segments of each nerve and were post-fixed in 4% paraformaldehyde. After 24 hours, the nerve segments were transferred to a solution of 4% PFA and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. Four hours later, the segments were transferred to 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. Tissue segments were blocked and sliced into 4–5 um sections. Sections were mounted on slides and stained with toluidine blue before microscopic imaging at 40x magnification. The images were then analyzed for g-ratio and fiber count by blinded experimenters using ImageJ software (NIH). G-ratio was calculated as the ratio of the inner fiber diameter to the total outer diameter of the fiber.
Statistical Analysis
All group sizes, outcome measures, and planned statistical comparisons were included in the study pre-registration prior to beginning data collection. Mechanical withdrawal thresholds, cylinder task right forelimb use, and grip strength were analyzed using a two-way repeated measures ANOVA to assess effect of group, followed by post hoc Bonferroni-corrected unpaired t-tests where appropriate. Paired t-tests were used to compare measures within subjects from pre-injury to week 8 and week 16 pre-therapy time points, where applicable. Two-way ANOVA was used to compare footprint data, followed by unpaired t-tests. Ladder walking data was compared with an unpaired t-test. Statistical tests for each comparison are noted in the text. Figures depict mean ± standard error of the mean.
Results
All animals underwent pre-injury baseline assessment of forelimb sensory motor function, including evaluation of mechanical withdrawal thresholds, forelimb use asymmetry, and forepaw grip strength test. Following baseline testing, all animals underwent transection and tubular repair of the median and ulnar nerves in the right forelimb. This procedure results in total denervation of the mechanoreceptors in the ventral surface of the forepaw while sparing the vast majority of innervation to the dorsal surface of the forepaw innervated by the radial nerve (Meyers 2017, Meyers 2019). Although reinnervation occurs, animals exhibit chronic disruption of nerve morphology and lasting impairments in somatosensation (Fig. 1B). Mechanosensory withdrawal thresholds in the right forelimb were significantly elevated 8 weeks post-injury (Fig. 2; PRE v. Wk 8; Paired t-test, t(16) = 29.61, p = 2.11 × 10−15). Impaired somatosensation was stable when assessed at 16 weeks post-injury (PRE v. Wk 16; Paired t-test, t(16) = 29.67, p = 2.04 × 10−15). No differences in withdrawal thresholds were observed between groups prior to beginning therapy (Rehab v. VNS+Rehab at Wk 16; Unpaired t-test, t(15) = 0.01, p = 0.99).
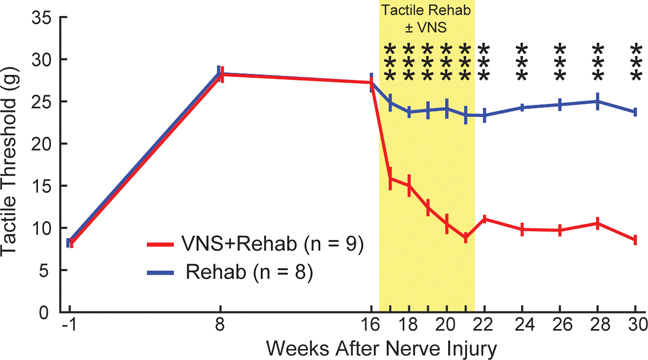
Fig. 2. VNS paired with tactile rehabilitation restores somatosensory thresholds.
Nerve damage results in chronic impairments in somatosensation in the forepaw, as indicated by a lasting increase in mechanical withdrawal thresholds. VNS paired with tactile rehabilitation (VNS+Rehab) drives robust, significant improvements in somatosensory thresholds compared to equivalent tactile rehabilitation without VNS (Rehab). The yellow shaded region denotes when tactile therapy with or without VNS was delivered. VNS-dependent restoration of somatosensory thresholds is stable, lasting many weeks after the cessation of stimulation. Unpaired t-tests across groups at each time point; *** denotes p < 0.001. Error bars indicate mean ± SEM.
We sought to identify whether pairing tactile rehabilitation with VNS could improve recovery of forelimb somatosensation in animals with chronic sensory deficits. To do so, all animals underwent six weeks of tactile rehabilitation, four sessions per week, beginning on week 17 post-injury. Each session was designed based on clinical sensory retraining and consisted of delivery of 200 touches to the ventral surface of the injured forepaw with a range of mechanical stimuli (Fig. 1C&D). Animals received either tactile rehabilitation without VNS (Rehab, n = 8) or equivalent tactile rehabilitation with a 0.5 train of VNS delivered concurrent with the delivery of each mechanical stimulus (VNS+Rehab, n = 9). VNS paired with tactile rehabilitation resulted in significant reductions of somatosensory withdrawal thresholds compared to equivalent tactile rehabilitation without VNS, consistent with improvements in somatosensory function (Fig. 2, Rehab v. VNS+Rehab; Two-way repeated measures ANOVA, F[1,15] = 384.19; p = 4.23 × 10−12). Post hoc tests revealed a significant improvement in somatosensory thresholds in the VNS+Rehab group on the first week of therapy that was maintained throughout the remainder (Fig. 2, Rehab v. VNS+Rehab; Bonferroni-corrected unpaired t-tests, wks 17–21 all p < 8.33 × 10−3). VNS-dependent improvements in withdrawal thresholds were maintained on week 22 after the cessation of VNS (Wk. 22; Rehab v. VNS+Rehab; Unpaired t-test, t(15) = 12.61, p = 2.19 × 10−9). Moreover, improved somatosensory function was observed in the VNS+Rehab group for two months after the conclusion of tactile rehabilitation, indicative of a lasting restoration of sensation (Rehab v. VNS+Rehab; Bonferroni-corrected unpaired t-tests, wks 22–30 all p < 8.33 × 10−3). An intent to treat analysis including available data from the four excluded subjects reveals similar findings (Table S2). No differences in sensory thresholds were observed in the uninjured forepaw at any time point, indicating that VNS-dependent changes in withdrawal thresholds are specific to the rehabilitated paw (Two-way repeated measures ANOVA, F[1,15] = 1.14; p = 0.30). These findings demonstrate that VNS paired with tactile rehabilitation produces substantial, stable improvements in somatosensory function in animals with chronic sensory loss.
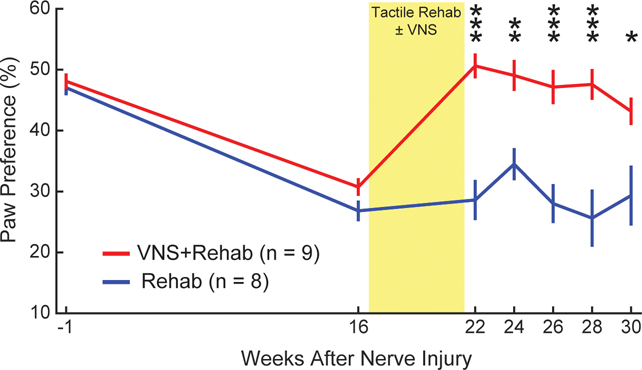
Sensory and motor function are highly integrated, and neurological injury often produces impairments in both. We sought to determine whether the observed VNS-dependent improvements in somatosensory function would generalize to a number of other measures of forelimb sensorimotor function. First, we assessed spontaneous volitional forelimb use during exploratory behavior with the cylinder task. As expected, nerve injury produced a dramatic asymmetry of forelimb use favoring the uninjured paw (Fig. 3, PRE v. Wk 16; Paired t-test, t(16) = 12.69, p = 9.06 × 10−10). No differences were observed across groups before therapy (Rehab v. VNS+Rehab at Wk 16; Unpaired t-test, t(15) = 1.77, p = 0.097). At each time point after the conclusion of therapy, animals that received VNS+Rehab demonstrated significantly greater use of the injured forelimb compared to animals that received Rehab, as demonstrated by a reduction in paw preference asymmetry (Two-way repeated measures ANOVA, F[1,15] = 29.48; p = 6.95 × 10−5; Bonferroni-corrected unpaired t-tests; wks 22–28 all p < 0.01).
Fig. 3. VNS-dependent recovery generalizes to an untrained sensorimotor forelimb task.
Nerve damage and resultant sensory loss produces an overreliance on the use of the uninjured forelimb during exploration, demonstrated by a reduction in preference for the injured paw. Rats that received VNS paired with tactile rehabilitation (VNS+Rehab) exhibited a restoration of paw preference compared to equivalent tactile rehabilitation without VNS (Rehab) after the completion of therapy, indicating greater volitional use of the injured forelimb. Improved forelimb use was observed for many weeks after the cessation of therapy. The yellow shaded region denotes when tactile therapy with or without VNS was delivered. Unpaired t-tests across groups at each time point; * denotes p < 0.05, ** p < 0.01, *** p < 0.001. Error bars indicate mean ± SEM.
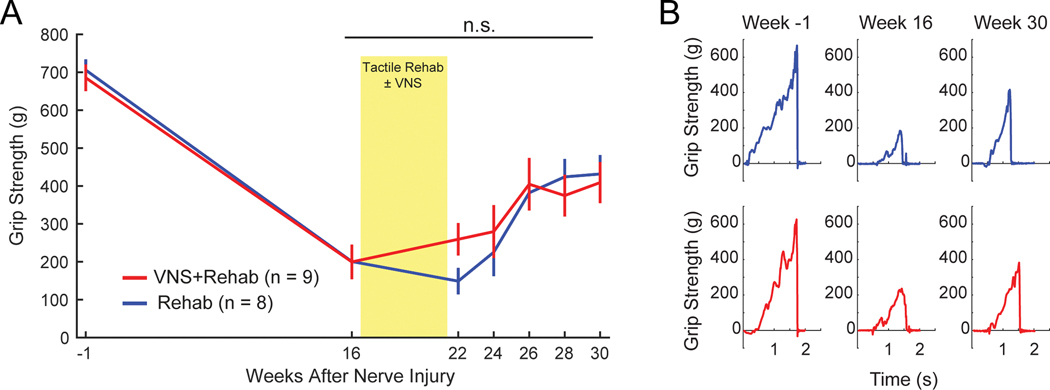
Next, we assessed grip strength. Nerve injury produced a significant decrease in grip strength in the injured forepaw, consistent with observations from previous studies (Fig. 4, PRE v. Wk 16; Paired t-test, t(16) = 10.17, p = 2.15 × 10−8) 41. No differences were observed across groups before therapy (Rehab v. VNS+Rehab at Wk 16; Unpaired t-test, t(16) = 0.02, p = 0.98). No differences in recovery of grip strength in the injured forepaw were observed between the VNS+Rehab and Rehab groups, suggesting that VNS paired with tactile rehabilitation does not directly improve recovery of forelimb strength (Rehab v. VNS+Rehab; Two-way repeated measures ANOVA, F[1,15] = 0.15; p = 0.69).
Fig. 4. VNS paired with tactile rehabilitation does not restore motor function.
(A) Grip strength is substantially reduced following nerve damage. VNS paired with tactile rehabilitation did not yield significant benefits in recovery of grip strength compared to equivalent tactile rehabilitation without VNS, suggesting that VNS therapy explicitly does not restore motor function. The yellow shaded region denotes when tactile therapy with or without VNS was delivered. (B) Representative examples illustrating grip strength at multiple time points during therapy. Unpaired t-tests across groups at each time point; n.s. denotes not significant. Error bars indicate mean ± SEM.
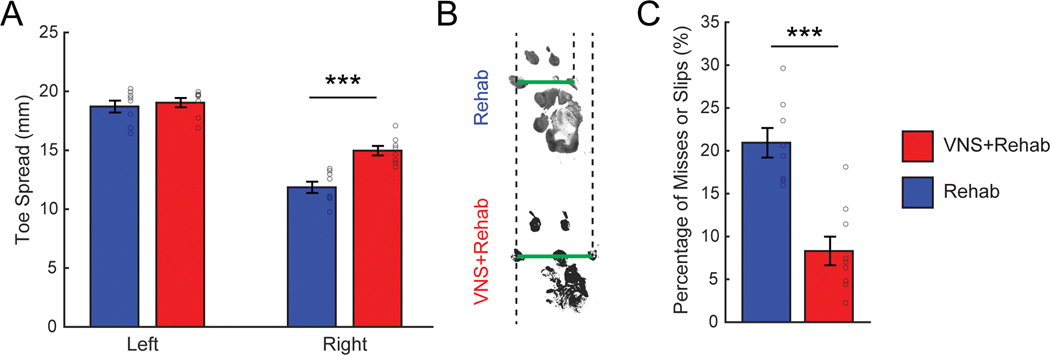
Impairments in locomotion, reflecting both sensory and motor dysfunction, arise from nerve damage 41,44. We tested whether VNS-dependent increases in somatosensory withdrawal thresholds would improve recovery of toe spread width during walking. Nerve injury significantly decreased the length of toe spread in the impaired right forepaw compared to the uninjured left forepaw, consistent with a loss of sensorimotor function in the paw (Fig. 5A&B, Two-way ANOVA, F[1,31] = 147.48, p = 1.12 × 10−12). VNS+Rehab resulted in significant improvements in toe spread distance in the impaired forepaw compared to Rehab alone (Fig. 5A, Right paw, Rehab v. VNS+Rehab; Unpaired t-test, t(15) = 4.94, p = 2.19 × 10−4). Additionally, we assessed skilled forelimb placing using the horizontal ladder walking task 41,42. Animals that received VNS+Rehab demonstrated better forepaw placement accuracy compared to Rehab, as evidenced by significantly fewer missed placements and slips (Fig. 5C, Rehab v. VNS+Rehab; Unpaired t-test, t(15) = 5.25, p = 9.62 × 10−5). The improvements in both measures suggest generalization of the benefits of VNS paired with tactile therapy.
Fig. 5. Skilled forelimb use during locomotion is improved by VNS paired with tactile rehabilitation.
(A) Forelimb toe spread in the injured right forepaw was reduced compared to the intact left forepaw after nerve damage, consistent with sensorimotor dysfunction. VNS paired with tactile rehabilitation (n = 8) significantly increased toe spread compared to equivalent tactile rehabilitation without VNS (n = 8) on Week 30. (B) Representative examples of footprints collected from the injured right forepaw after the completion of tactile rehabilitation with or without VNS. Green lines illustrate the toe spread measurement, and dotted lines are shown for alignment. (C) Additionally, rats that received VNS paired with tactile rehabilitation (n = 9) demonstrate significantly fewer misses or slips during the ladder walking assessment compared to rats that received tactile rehabilitation without VNS (n = 8). Together, these findings indicate that the benefits of VNS paired with tactile rehabilitation generalize to measures of forelimb use during locomotion. Unpaired t-tests across groups at each time point; *** denotes p < 0.001. Circles depict data from individual subjects. Error bars indicate mean ± SEM.
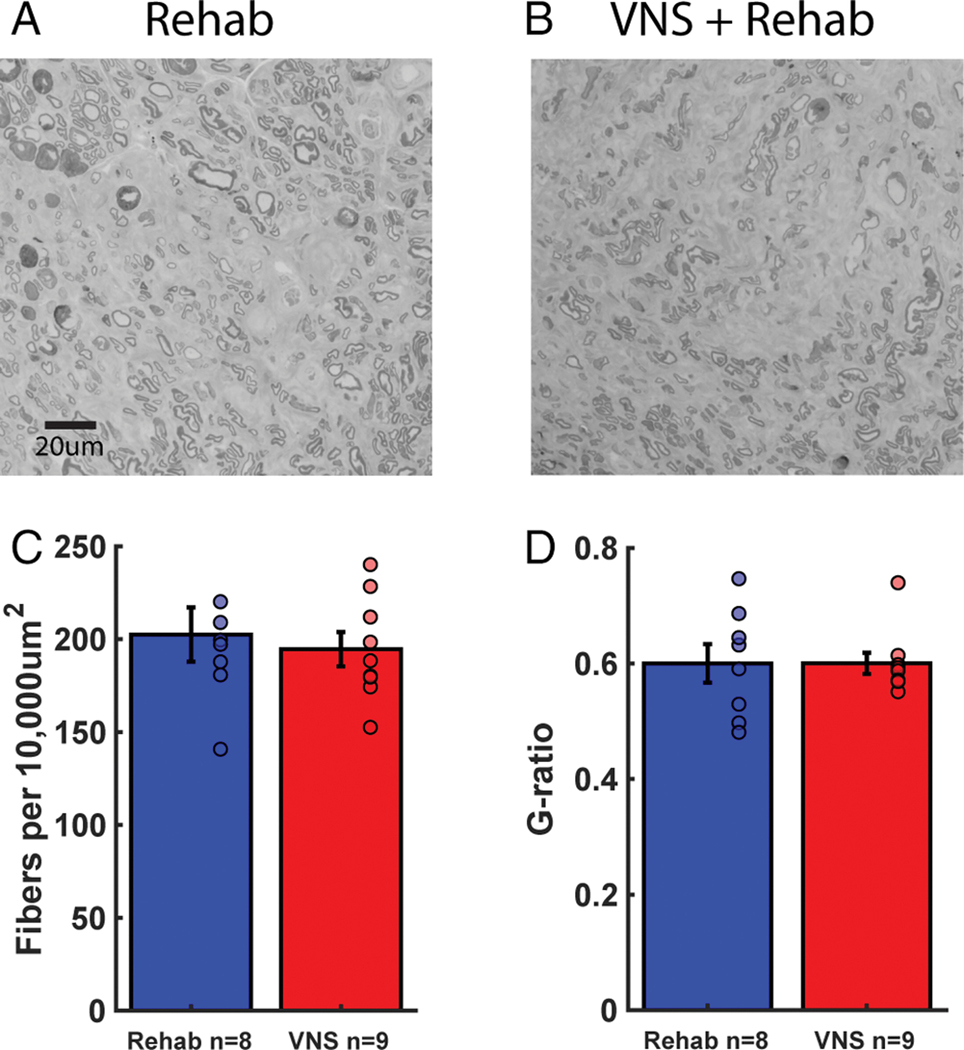
Changes in reinnervation could lead to improved sensory function after injury. We explored whether VNS influenced the degree of axonal regrowth in the distal nerve segment after injury. No differences in axon fiber number or g-ratio, a metric of remyelination, were observed across groups (Fig. 6, Rehab v. VNS+Rehab; Fiber number, Unpaired t-test, t(15) = 0.47, p = 0.65; G-ratio; Unpaired t-test, t(15) = −4.70 × 10−3, p = 0.99). These findings are consistent with previous studies that VNS does not influence peripheral nerve health or regeneration and demonstrate that peripheral changes cannot account for improved recovery 45. Rather, this supports the notion that VNS paired with rehabilitative therapy enhances synaptic plasticity in central networks to support recovery of function 17.
Fig. 6. VNS does not influence peripheral nerve regeneration or health.
(A, B) Example images of fibers in the median nerve distal to the site of injury in rats that received Rehab or VNS+Rehab. (C) The number of fibers in the distal segment of the median nerve is comparable between groups. (D) Additionally, g-ratio, a metric of remyelination, is not different between groups. These findings indicate that differences in peripheral nerve regeneration cannot account for VNS-dependent improvements in sensory function. Unpaired t-tests across groups at completion of study. Circles depict data from individual subjects. Error bars indicate mean ± SEM.
Discussion
Sensory loss commonly occurs following neurological injury, and there are no consistently effective methods to restore function. Here, we present findings from the first preregistered, well-controlled study demonstrating that pairing closed-loop VNS with a sensory retraining paradigm can enhance recovery of sensory function after neurological damage. VNS paired with tactile rehabilitation resulted in robust, significant improvements in mechanosensory withdrawal thresholds compared to equivalent tactile rehabilitation without VNS in a rat model of chronic sensory loss. VNS-dependent restoration of somatosensory function was maintained for months after the cessation of therapy. Additionally, delivery of VNS paired with tactile rehabilitation significantly improved recovery on other sensorimotor measures. These findings build on the clinical success of closed-loop VNS therapy and position VNS paired with tactile rehabilitation as a novel strategy to restore somatosensory function after neurological injury.
The present study was motivated by findings from a clinical case study and highlights the utility of rigorous two way translation. This case study provided initial evidence that VNS delivered during sensory retraining therapy may improve somatosensory function in a chronic stroke patient with profound sensory loss 33. While encouraging, the inclusion of a single subject and open-label design limit the broad applicability of these findings. Thus, we sought to replicate or disprove these findings in the present well-controlled, preregistered animal study. Corroborating the initial clinical data, the current results provide confirmatory evidence that VNS paired with tactile rehabilitation can significantly improve recovery of somatosensory function. Further highlighting the clinical feasibility of this strategy, the VNS parameters employed in this study match those currently in clinical use for rehabilitation 21,29. Considered together with the track record of safety, the findings described here support investigation of VNS paired with tactile rehabilitation to restore sensory function in a larger, controlled clinical study.
Somatosensory and motor function are fundamentally integrated, thus improvements in sensory function after neurological injury may produce concomitant benefits in motor control. Indeed, clinical evidence suggests that sensory stimulation may yield increases in motor function in stroke patients 4,46,47. To address whether VNS-dependent enhancement of somatosensory recovery would similarly improve motor function, we evaluated a number of sensorimotor measures in animals that received VNS paired with tactile rehabilitation. Spontaneous forelimb use, as well as placement and weight bearing during locomotion, were significantly improved in animals that received VNS paired with tactile rehabilitation compared to equivalent tactile rehabilitation without VNS. This is consistent with the notion that improved somatosensory function generalizes to subsequent improvements in motor control. Alternatively, VNS paired with tactile rehabilitation did not yield benefits for recovery of grip strength. Unlike the other measures, grip strength assessment minimizes the express need for volitional motor control and coordination, instead emphasizing simple forelimb strength. The absence of a VNS-dependent improvement in grip strength may reflect the minimal contribution of sensory integration in this task, whereas measures of stepping and locomotion rely more on sensorimotor integration. Despite this lack of a somatosensory-dependent improvement in grip strength, a substantial amount of evidence in both animal models and humans demonstrates that VNS paired with motor rehabilitation can improve recovery of other aspects of motor function and control 19–29. In response to tactile stimulation during therapy in the present study, rats would occasionally withdraw their forelimb. Although trials in which withdrawal occurred comprise a small minority of the total number of stimulations, it is plausible that VNS acts to drive plasticity in both cutaneous sensory networks and motor reflex loops engaged by tactile therapy. Thus, recovery of withdrawal thresholds may be driven in part, by improvements in motor function. Ultimately, the optimal implementation of VNS therapy may thus involve co-delivery of both specific sensory retraining as well as motor rehabilitation. This is consistent with evidence from the initial case study, in which the subject received sequential motor rehabilitation for six weeks followed later by tactile rehabilitation for five weeks 33. Subsequent tactile therapy provided improvements in measures of somatosensation, including stereognosis, proprioception, and detection of light touch. Thus, while a single rehabilitative regiment may provide some utility, an individualized therapy that incorporates both sensory and task-specific motor training paired with VNS is likely to provide optimal benefits for patients with both sensory and motor dysfunction.
VNS paired with rehabilitation enhances synaptic plasticity, which is believed to underlie its therapeutic benefits. VNS drives rapid engagement of the cholinergic and noradrenergic neuromodulatory networks during training to enhance training-specific plasticity 12,13. Either depleting these neuromodulatory networks or degrading the temporal association of VNS and training prevents the effects of VNS on both plasticity and recovery, highlighting the importance of VNS-dependent plasticity in restoration of function 14–16,22,25,45. Additionally, VNS drives other molecular changes in the central nervous system, including increased expression of brain derived-neurotrophic factor (BDNF), that may contribute to its effects 48,49. Closed-loop VNS therapies leverage this activation of pro-plasticity neuromodulatory networks concurrent with neural activity evoked by rehabilitation to promote rehabilitation-specific changes in circuits in the central nervous system. Indeed, VNS paired with motor rehabilitation drives extensive synaptic reorganization in spared motor networks that is associated with recovery 26,27. Moreover, VNS paired with auditory stimuli enhances stimulus-specific plasticity at multiple stations throughout the auditory network 31. Similarly, the improved somatosensory thresholds observed in the present study are likely subserved by VNS-dependent enhancement of plasticity throughout cortical and subcortical somatosensory networks. Tactile rehabilitation produces neural activity in these somatosensory networks, and delivery of VNS drives concurrent neuromodulatory release to facilitate synaptic plasticity in neurons activated by the tactile rehabilitation. Consistent with previous findings 45, we did not observe VNS-dependent changes in median nerve health or regeneration with VNS. These findings indicate that VNS does not act through a peripheral restorative mechanism to improve sensory function. Rather, they provide further that VNS likely drives synaptic plasticity throughout the central nervous system to improve recovery 45. Future studies directed at identifying the nature and contribution of VNS-dependent plasticity in somatosensory networks would provide a greater understanding of the underpinnings of VNS therapy and may be useful to develop individualized interventions.
Optimization of therapies is necessary for effective clinical translation. Although a robust effect of VNS paired with tactile rehabilitation was observed in this study, parametric optimization of both the electrical stimulation parameters of VNS and the mechanical stimuli utilized for tactile rehabilitation may need to be leveraged to maximize therapeutic benefits. The present study employed equivalent electrical stimulation parameters to those utilized in clinical studies of VNS paired with rehabilitation 21,29,33,50,51. Although these parameters are effective and have been extensively optimized in other contexts 52–57, it remains possible that alternative VNS intensities or durations may yield greater benefits when paired with tactile rehabilitation. Beyond electrical stimulation parameters, previous experiments in auditory cortex demonstrate that VNS-dependent plasticity is shaped by the features of the paired sensory stimulus 16,30,58. Thus, the specific features of the mechanosensory stimuli presented with VNS are likely to influence the effects of the therapy. In the present study, the mechanosensory stimuli utilized in tactile therapy were selected to encompass a wide range of features and thereby activate a variety of cutaneous receptors in the paw. Although this implementation yielded a rapid, robust improvement in recovery of mechanosensory thresholds, it remains unclear whether these benefits could be replicated with a less diverse set of stimuli or could be further improved with greater stimulus diversity. Additionally, the procedural aspects of delivering tactile therapy with VNS represent an opportunity for optimization. In the initial clinical case study, the therapist used a push button to initiate VNS during manual delivery of passive tactile stimuli or during patient-initiated active object exploration 33. The present study utilized a congruent implementation of timed VNS during manual passive tactile stimulation of the paw delivered by an experimenter. Although the current applications largely rely on manual delivery of VNS and sensory stimuli, this intervention is highly amenable to automation. Closed-loop VNS could be triggered during active object exploration either by camera tracking or acceleration sensors or during passive training by automated mechanical and vibrotactile stimuli or cutaneous electrical stimulation. An automated sensory rehabilitation paradigm, likely employed in conjunction with sensory retraining with a therapist, would provide consistency, reduced cost, and greater access to the therapy, yielding clear advantages for patients.
While the present study reveals insight into VNS-dependent restoration of somatosensory function after neurological injury, a number of limitations merit consideration. First, the primary outcome of the present study evaluated mechanosensory withdrawal thresholds, which clearly fail to capture the full complexity of somatosensory function. Similarly, while the findings reported here indicate that VNS can improve somatosensory detection, we did not evaluate sensory discrimination, as there are few, if any, reliable assessments of forepaw tactile discrimination in rodents. However, despite the limitations of the rodent model, the restoration of tactile thresholds observed in this study corroborates the improvements in somatosensory function reported in the preceding case study, lending validity to the current results. Second, altered somatosensory perception of temperature is a frequent consequence of neurological injury 59,60. While the present study was not designed to evaluate changes in temperature detection, VNS paired with an appropriate regimen of sensory retraining that incorporates delivery of stimuli of variable temperatures may provide a means to target restoration of temperature perception. Future studies that directly test this hypothesis, either in animal models or human subjects, should be considered. Finally, the present study does not delineate the mechanisms by which VNS therapy improves recovery of somatosensory function. A preponderance of literature suggests that VNS-dependent recovery arises from engagement of neuromodulatory circuits and subsequent enhancement of plasticity in central networks 12–15,26,27. Future studies should prioritize identifying the nature of the synaptic changes that underlie the effects of VNS paired with tactile rehabilitation in order to optimize delivery of the therapy.
Here, we present evidence that VNS paired with tactile rehabilitation significantly improves recovery of somatosensory function after neurological injury, corroborating findings from an initial case report. VNS-dependent restoration of sensory thresholds was maintained for several months after the cessation of therapy and generalized to other measures of sensorimotor function. These findings extend previous preclinical and clinical studies showing that VNS paired with motor rehabilitation enhances recovery of motor function and raise the prospective utility of a combinatorial approach employing both sensory and motor rehabilitation with VNS 11,21,29. Together, these studies position VNS as a novel strategy to target sensory recovery and support the need for a well-controlled clinical study to evaluate VNS paired with tactile rehabilitation in patients with sensory dysfunction resulting from neurological injury. Additionally, future studies are needed to validate the use of this strategy in other clinically-relevant models of neurological insult that reduce somatosensory function, as well as uncover the precise neural mechanisms that support recovery.
Supplementary Material
Acknowledgments:
We would like to thank Maria Sosa, Yasir Mian Bilal, Eric Meyers, Mays Alshaikhsalama, Wongani Kalengamaliro, Jimmy Tran, and Ashleigh Abusomwan, Matt Buell, Khalil Abed Rabbo, and Nikki Simmons. This work was supported by The National Institutes of Health NIH R01 NS094384 (SAH), R01 NS085167 (MPK), and R01 NS103803 (RLR).
Footnotes
Potential Conflicts of Interest: MPK has a financial interest in MicroTransponder, Inc., which is developing VNS for stroke. RLR is an owner of Teliatry, which is developing a VNS device. All other authors declare no conflicts of interest.
References
- 1.Lundborg G, Rosén B. Sensory relearning after nerve repair. Lancet 2001;358(9284):809–810. Available from: https://www.sciencedirect.com/science/article/pii/S0140673601060019 [DOI] [PubMed] [Google Scholar]
- 2.Duff S V Impact of Peripheral Nerve Injury on Sensorimotor Control. J. Hand Ther. 2005;18(2):277–291. Available from: https://www.sciencedirect.com/science/article/pii/S0894113005000475 [DOI] [PubMed] [Google Scholar]
- 3.Carey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation. Neurorehabil. Neural Repair 2011;25(4):304–313. Available from: 10.1177/1545968310397705 [DOI] [PubMed] [Google Scholar]
- 4.Schabrun S, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin. Rehabil. 2009;23(1):27–39. Available from: 10.1177/0269215508098897 [DOI] [PubMed] [Google Scholar]
- 5.Doyle S, Bennett S, Fasoli SE, McKenna KT. Interventions for sensory impairment in the upper limb after stroke. Cochrane Database Syst. Rev. 2010;(6) Available from: 10.1002/14651858.CD006331.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byl N, Roderick J, Mohamed O, et al. Effectiveness of Sensory and Motor Rehabilitation of the Upper Limb Following the Principles of Neuroplasticity: Patients Stable Poststroke. Neurorehabil. Neural Repair 2003;17(3):176–191. Available from: 10.1177/0888439003257137 [DOI] [PubMed] [Google Scholar]
- 7.Turville ML, Matyas TA, Blennerhassett JM, Carey LM. Initial severity of somatosensory impairment influences response to upper limb sensory retraining post-stroke. NeuroRehabilitation 2019;43(4):413–423. Available from: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/NRE-182439 [DOI] [PubMed] [Google Scholar]
- 8.Sullivan JE, Hedman LD. Sensory Dysfunction Following Stroke: Incidence, Significance, Examination, and Intervention. Top. Stroke Rehabil. 2008;15(3):200–217. Available from: 10.1310/tsr1503-200 [DOI] [PubMed] [Google Scholar]
- 9.Tyson SF, Hanley M, Chillala J, et al. Sensory Loss in Hospital-Admitted People With Stroke: Characteristics, Associated Factors, and Relationship With Function. Neurorehabil. Neural Repair 2008;22(2):166–172. Available from: 10.1177/1545968307305523 [DOI] [PubMed] [Google Scholar]
- 10.Jaquet J, Shreuders T, Kalmijn S, et al. Median and Ulnar Nerve Injuries: Prognosis and Predictors for Clinical Outcome. J. Reconstr. Microsurg. 2001;51(4):687–692. Available from: https://journals.lww.com/jtrauma/fulltext/2001/10000/median,_ulnar,_and_combined_median_ulnar_nerve.11.aspx [Google Scholar]
- 11.Engineer ND, Kimberley TJ, Prudente CN, et al. Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke. Front. Neurosci. 2019;13 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6449801/ [DOI] [PMC free article] [PubMed]
- 12.Hulsey DR, Riley JR, Loerwald KW, et al. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol. 2017;289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols JA, Nichols AR, Smirnakis SM, et al. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors [Internet]. Neuroscience 2011;189:207–214. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21627982 [DOI] [PubMed] [Google Scholar]
- 14.Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul. 2016;9(2):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulsey DR, Shedd CM, Sarker SF, et al. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp. Neurol. 2019;320:112975. Available from: https://www.sciencedirect.com/science/article/pii/S001448861930127X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays SA. Enhancing Rehabilitative Therapies with Vagus Nerve Stimulation. Neurotherapeutics 2016;13(2):382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain 2011;134(6):1591–1609. Available from: 10.1093/brain/awr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis. 2013;60. [DOI] [PubMed] [Google Scholar]
- 20.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil. Neural Repair 2014;28(7):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimberley TJ, Pierce D, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke 2018;49(11):2789–2792. Available from: 10.1161/STROKEAHA.118.022279 [DOI] [PubMed] [Google Scholar]
- 22.Hays SA, Khodaparast N, Ruiz A, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 2014;25(9):682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014;45(10):3097–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hays SA, Ruiz A, Bethea T, et al. Vagus Nerve Stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol. Aging 2016;43:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodaparast N, Kilgard MP, Casavant R, et al. Vagus Nerve Stimulation during Rehabilitative Training Improves Forelimb Recovery after Chronic Ischemic Stroke in Rats. Neurorehabil. Neural Repair 2016;30(7):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers EC, Solorzano BR, James J, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 2018;49(3):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganzer PD, Darrow MJ, Meyers EC, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 2018;7 Available from: https://elifesciences.org/articles/32058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruitt DT, Schmid AN, Kim LJ, et al. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J. Neurotrauma 2016;33(9):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016;47(1):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engineer CT, Shetake JA, Engineer ND, et al. Temporal plasticity in auditory cortex improves neural discrimination of speech sounds. Brain Stimul. 2017;10(3):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borland M, Vrana WA, Moreno NA, et al. Pairing vagus nerve stimulation with tones drives plasticity across the auditory pathway. J. Neurophysiol. 2019;jn.00832.2018. Available from: 10.1152/jn.00832.2018 [DOI] [PMC free article] [PubMed]
- 32.Vanneste S, Martin J, Rennaker RL, Kilgard MP. Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: An exploratory retrospective study. Sci. Rep. 2017;7(1):17345. Available from: http://www.nature.com/articles/s41598-017-17750-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilgard MP, Rennaker RL, Alexander J, Dawson J. Vagus nerve stimulation paired with tactile training improved sensory function in a chronic stroke patient. NeuroRehabilitation 2018;42(2):159–165. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29562561 [DOI] [PubMed] [Google Scholar]
- 34.Meyers EC, Granja R, Solorzano BR, et al. Median and ulnar nerve injuries reduce volitional forelimb strength in rats. Muscle Nerve 2017;56(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hays SA, Khodaparast N, Sloan AM, et al. The isometric pull task: A novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods 2013;212(2) [DOI] [PubMed] [Google Scholar]
- 36.Rios M, Bucksot J, Rahebi K, et al. Protocol for Construction of Rat Nerve Stimulation Cuff Electrodes. Methods Protoc. 2019;2(1):19. Available from: http://www.mdpi.com/2409-9279/2/1/19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAllen RM, Shafton AD, Bratton BO, et al. Calibration of thresholds for functional engagement of vagal A, B and C fiber groups in vivo. Bioelectron. Med. 2018;1(1):21–27.Available from: 10.2217/bem-2017-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikh S, Shortland P, Lauto A, et al. Sensory perturbations using suture and sutureless repair of transected median nerve in rats. Somatosens. Mot. Res. 2016;33(1):20–28. [DOI] [PubMed] [Google Scholar]
- 39.Schallert T, Fleming SM, Leasure JL, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000;39(5):777–787. Available from: https://www.sciencedirect.com/science/article/pii/S0028390800000058 [DOI] [PubMed] [Google Scholar]
- 40.Dunnett SB, Torres EM, Annett LE. A lateralised grip strength test to evaluate unilateral nigrostriatal lesions in rats. Neurosci. Lett. 1998;246(1):1–4. Available from: https://www.sciencedirect.com/science/article/pii/S0304394098001943 [DOI] [PubMed] [Google Scholar]
- 41.Galtrey CM, Fawcett JW. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J. Peripher. Nerv. Syst. 2007;12(1):11–27. Available from: 10.1111/j.1529-8027.2007.00113.x [DOI] [PubMed] [Google Scholar]
- 42.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J. Neurosci. Methods 2002;115(2):169–179. Available from: https://www.sciencedirect.com/science/article/pii/S0165027002000122 [DOI] [PubMed] [Google Scholar]
- 43.Bolton DAE, Tse ADY, Ballermann M, et al. Task specific adaptations in rat locomotion: Runway versus horizontal ladder. Behav. Brain Res. 2006;168(2):272–279. Available from: https://www.sciencedirect.com/science/article/pii/S0166432805004985 [DOI] [PubMed] [Google Scholar]
- 44.Bontioti EN, Kanje M, Dahlin LB. Regeneration and functional recovery in the upper extremity of rats after various types of nerve injuries. J. Peripher. Nerv. Syst. 2003;8(3):159–168. Available from: 10.1046/j.1529-8027.2003.03023.x [DOI] [PubMed] [Google Scholar]
- 45.Meyers EC, Kasliwal N, Solorzano BR, et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat. Commun. (2019) In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celnik P, Hummel F, Harris-Love M, et al. Somatosensory Stimulation Enhances the Effects of Training Functional Hand Tasks in Patients With Chronic Stroke. Arch. Phys. Med. Rehabil. 2007;88(11):1369–1376. Available from: https://www.sciencedirect.com/science/article/pii/S000399930701338X [DOI] [PubMed] [Google Scholar]
- 47.Wu CW, Seo H-J, Cohen LG. Influence of Electric Somatosensory Stimulation on Paretic-Hand Function in Chronic Stroke. Arch. Phys. Med. Rehabil. 2006;87(3):351–357. Available from: https://www.sciencedirect.com/science/article/pii/S0003999305014309 [DOI] [PubMed] [Google Scholar]
- 48.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179(1):28–34. Available from: https://www.sciencedirect.com/science/article/pii/S0006899307019488 [DOI] [PubMed] [Google Scholar]
- 49.Furmaga H, Carreno FR, Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One 2012;7(5):e34844. Available from: 10.1371/journal.pone.0034844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Ridder D, Vanneste S, Engineer ND, et al. Safety and Efficacy of Vagus Nerve Stimulation Paired With Tones for the Treatment of Tinnitus: A Case Series. Neuromodulation Technol. Neural Interface 2013;17(2):170. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24255953 [DOI] [PubMed] [Google Scholar]
- 51.Tyler R, Cacace A, Stocking C, et al. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci. Rep. 2017;7(1):11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borland MS, Vrana WA, Moreno NA, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul. 2016;9:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loerwald KW, Borland MS, Rennaker RL, et al. The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation [Internet]. Brain Stimul. 2017;11(2):271–277. Available from: https://www.sciencedirect.com/science/article/pii/S1935861X17309634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loerwald KW, Buell EP, Borland MS, et al. Varying Stimulation Parameters to Improve Cortical Plasticity Generated by VNS-tone Pairing. Neuroscience 2018;388:239–247. Available from: https://www.sciencedirect.com/science/article/pii/S030645221830513X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buell EP, Borland MS, Loerwald KW, et al. Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity. Neuroscience 2019;406:290–299. Available from: https://www.sciencedirect.com/science/article/pii/S0306452219301708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buell EP, Loerwald KW, Engineer CT, et al. Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul. 2018; Available from: https://www.sciencedirect.com/science/article/pii/S1935861X18302493 [DOI] [PMC free article] [PubMed]
- 57.Morrison RA, Hulsey DR, Adcock KS, et al. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shetake JA, Engineer ND, Vrana WA, et al. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp. Neurol. 2012;233(1):342–349. [DOI] [PubMed] [Google Scholar]
- 59.Misra UK, Kalita J, Kumar B. A Study of Clinical, Magnetic Resonance Imaging, and Somatosensory-Evoked Potential in Central Post-Stroke Pain. J. Pain 2008;9(12):1116–1122. Available from: https://www.sciencedirect.com/science/article/pii/S1526590008006457 [DOI] [PubMed] [Google Scholar]
- 60.Collins ED, Novak CB, Mackinnon SE, Weisenborn SA. Long-term follow-up evaluation of cold sensitivity following nerve injury. J. Hand Surg. Am. 1996;21(6):1078–1085. Available from: https://www.sciencedirect.com/science/article/pii/S0363502396803194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.